Abstract
Objectives
The aim of this study was to examine the colonization of Streptococcus mutans and Streptococcus sobrinus in supra-gingival plaque samples and to determine their correlation with the prevalence of early childhood caries (ECC) in Thai children.
Materials and methods
A total of 344 Thai children, ages 3 and 5 years, were invited to participate in this study. Caries status of the children was examined. Supra-gingival plaque samples were collected. Quantitative real-time PCR was performed to evaluate DNA levels of S. mutans and S. sobrinus.
Results
Eighty-five percent of the children were colonized by S. mutans and 50.9 % of them were colonized by S. sobrinus. The prevalence of ECC was 43.8 % and 56.2 % among 3- and 5-year-old children, respectively, and was significantly associated with the presence of S. mutans and S. sobrinus. The severity of ECC was significantly correlated with increased DNA levels of the two bacteria. Children who were positive for S. mutans and S. sobrinus (Sm+/Sb+) were 8 times or 44 times more likely to experience ECC than children who were Sm−/Sb+or were Sm−/Sb−.
Conclusions
The study evidence further suggest that children colonized by both S. mutans and S. sobrinus are at the higher risk for ECC.
Clinical relevance
Molecular-based qPCR can be used to detect and quantify S. mutans and S. sobrinus colonization for epidemiological and clinical studies for ECC risk assessment.
Keywords: Mutans streptococci, Early childhood caries, Quantitative real-time PCR, Preschool children, Caries risk, Epidemiology
Introduction
Early childhood caries (ECC) is a particularly destructive form of tooth decay that significantly affects oral health-related quality of life for preschool children [1]. It is one of the most prevalent chronic childhood diseases in many regions worldwide including children in Thailand. According to the Sixth Thai National Oral Health Survey, ECC prevalence ranged from 56 % to over 95 % among Thai preschool children [2, 3] and was 89 % in the northeastern region, with an increasing trend being observed during the past two decades [4]. The lack of effective public health preventive measures, limited dental care providers, low household income, lack of oral health education, and frequent consumption of sweet foods are reported to be the primary contributing factors [4–6]. Therefore, ECC continues to be a major public health problem for Thai young children [7].
Epidemiological studies have consistently demonstrated that mutans streptococci (MS), primarily Streptococcus mutans and Streptococcus sobrinus, are the primary cariogenic microorganisms associated with dental caries [8–10]. Children who were either colonized early by MS or presented with persistently higher levels of MS experienced significantly more ECC [11, 12]. Although S. sobrinus is less prevalent than S. mutans, studies have suggested that the presence of both species in dental plaque is positively correlated with ECC development [13, 14]. Because of their significant cariogenic roles in caries formation, a variety of methods have been developed to identify and measure their colonization. Many of those methods are laboratory culture-based and hence are time-consuming, laborious, and not always accessible in field epidemiological studies.
As an alternative approach, commercially available chairside test kits have been developed for easy detection of MS in the saliva or dental plaque. Previously, using a Dentocult® SM Strip mutans test (Orion Diagnostica, Espoo, Finland), we reported that 77 % of 3- and 5-year-old Thai children (N=350) were MS positive. The distribution of the children's SM Strip scores was significantly correlated with their age, vaginal birth, failure to brush teeth, mother's MS score, mother who practiced pre-chewing feeding habits, and mother who used alcohol regularly [3]. However, the chairside test could neither differentiate S. mutans from S. sobrinus nor yield accurate quantifications of MS colonization.
In recent years, various molecular-based methods have been developed to detect MS and other oral pathogens without in vitro cultivation. For instance, quantitative real-time PCR (qPCR) is one of the most commonly used techniques for detecting and evaluating MS infection in clinical studies [15–17]. Using qPCR methods, the aim of the present study was to qualitatively and quantitatively examine the association between S. mutans and S. sobrinus colonization and ECC experience in a young cohort of Thai preschool children. The hypothesis was that S. mutans and S. sobrinus colonization play synergistic roles in the development of ECC.
Materials and methods
Subject selection
Three hundred forty-four preschool children, 3 or 5 years of age, were invited to participate in this study. The children were scheduled for their required immunization visits at the Health Promotion Hospital in Chiang Mai, Thailand during the summer of 2009. The number of children was 180 in the 3-year-old group and 164 in the 5-year-old group, with boys and girls being evenly distributed in each age group. This study using human subjects received approval from the Ethical Committee of the Faculty of Dentistry, Chiang Mai University, Thailand (No. 12/2008). Written informed consent was obtained from all parents before the study. Subject recruitment criteria and a questionnaire used to collect data about the children's delivery mode and birth weight, medical history, childcare history, dietary habits, and oral health practices have been previously reported [3]. This observational study was designed and conducted following the STROBE guidelines (Strengthening the Reporting of Observational Studies in Epidemiology, http://www.strobe-statement.org).
Oral examination and sample collection
The children were examined for caries status by two standardized pediatric dentists using criteria of the World Health Organization Oral Health Survey Methods for Field Studies [18]. Clinical oral health status was measured using the decay–missing–filled tooth surface (dmfs) or decay–missing– filled teeth (dmft) index for deciduous dentitions. Early childhood caries was classified according to the American Academy of Pediatric Dentistry definition (http://www.aapd.org/media/Policies_Guidelines/P_ECCClassifications.pdf). Supra-gingival plaque samples were collected from all children. They were first asked to rinse their mouth with drinking water and then brush their teeth using a sterile toothbrush for 2 min. The toothbrush was immediately washed in a 50-ml test tube containing 10 ml sterile phosphate buffered saline (PBS). Two milliliters of the bacterial sample was transferred into an Oragene-DNA collection container (Oragene, Ontario, Canada). The container with the bacterial samples was sealed, mixed well, and processed according to the manufacturer's instructions. All of the bacterial samples were transferred to the microbiology laboratory at the Chiang Mai University Faculty of Dentistry within 4 hours, and stored at −20 °C for further processing. After the sample collection, children participated in the study received a professional prophylaxis followed by a topical fluoride treatment and oral hygiene instruction.
Bacterial genomic DNA isolation
Bacterial genomic DNAwas extracted from 1 ml of the plaque samples by a modified DNA purification kit (Epicentre, Madison, WI), according to the protocol described by Li et al. [19]. An additional 10 μl proteinase K (10 mg/ml in TES buffer-10 mM Tris–HCl, pH 8.0; 1 mM EDTA; 100 mM NaCl), 10 μl lysozyme stock solution (100 mg/ml in TES buffer), and 2 μl mutanolysin (5,000 U/ml in PBS) were added to the sample followed by a phenol/chloroform/isoamyl alcohol extraction procedure. The quality and quantity of the DNA was measured with a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). The final concentration of each DNA sample was adjusted to 10 ng/μl for qPCR applications.
Quantitative real-time PCR
All of the qPCRs were performed using MyiQ2 (Bio-Rad, Hercules, CA) with SYBR Green dye. A set of S. mutans-specific primers (forward primer Sm479F, 5′-TCGCGAAA AAGATAAACAAACA-3′ and reverse primer Sm479R, 5′-GCCCCTTCACAGTTGGTTAG-3′) were used to generate a 479-bp amplicon [20]; the positive control for qPCR was S. mutans UA159 (ATCC 700610). A set of S. sobrinus-specific primers (forward primer Ssob287F, 5′-TTCAAAGCCA AGACCAAGCTAGT-3′ and reverse primer Ssob287R, 5′-CCAGCCTGAGATTCAGCTTGT-3′) were used to generate a 88-bp amplicon [21]; the positive control for the qPCR was S. sobrinus OMZ65. DNA samples of control strains were serially diluted ten-fold (the range was 107 to 101 fg/μl) to generate standard curves as an external standard for absolute quantification. For each qPCR, the tube contained 25 μl of reaction mixture, including 1× PCR Master Mix (QuantiTect SYBR Green PCR Kits, Qiagen Inc.), 10 ng of DNA sample, and 10 μM of each primer. Amplification and detection were performed with the following cycle profiles: 15 min at 95 °C for activation of HotStarTaq DNA Polymerase, 44 cycles of 15 sec at 94 °C for denaturation, 30 sec at annealing temperature of each primer (56 °C for Sm479F/Sm479R and 58 °C for Ssob287F/Ssob287R), and 30 sec at 72 °C for extension, followed by a melting curve analysis of the PCR product.
All qPCRs for the standards and the bacterial samples were performed in duplicate to eliminate variation between tubes with the same templates. The final analysis was based on the mean of the two reactions. The specificity of the primer sets was detected with melting curve analysis. All qPCR output data were analyzed using MyiQ software (Bio-Rad iQ5 optical system software, version 2.1, Hercules, CA). The correct amplicon size was confirmed for the correct molecular size by electrophoresis in a 1.5 % agarose gel. The gel was stained with ethidium bromide (1 ug/ml) for 15 min and washed with water for 5 min. The gel images were photographed and recorded with the AlphaImager 3300 imaging system (Alpha Innotech Corp., San Leandro, CA, USA).
Statistical analyses
All statistical evaluations were performed using SPSS software, version 21.0 (IBM, Armonk, New York). Pearson's correlation, the chi-square test, and the t test were used to evaluate the correlation between the age, gender, caries status, and the microbiological findings. A two-sided P<0.05 was considered to be significant for all analyses.
Results
As illustrated in Fig. 1, qPCR standard curves presented an excellent linear correlation between the quantity of bacterial-type strain DNA in serial dilutions and the CT values for S. mutans (Fig. 1a) and S. sobrinus (Fig. 1b). The qPCR approach could detect S. mutans and S. sobrinus DNA with a high sensitivity at a concentration of 10 fg/μl (Fig. 1c, d). A high degree of specificity was demonstrated as a single band on the agarose gels (Fig. 1e, f).
Fig. 1.
Quantitative real-time PCR validation. The standard curve of qPCR assay for a S. mutans UA159 (ATCC 700610) and b S. sobrinus (OMZ65) generated from a plot of threshold cycle (CT) values against log value for the tenfold serial dilutions of a known concentration (107–101 fg/μl). Amplification plot and the threshold level obtained using SYBR Green assay for c S. mutans UA159 (ATCC 700610) and d S. sobrinus (OMZ65) quantification. The confirmation of qPCR amplification and the specificity detected by agarose gel electrophoresis for e S. mutans and f S. sobrinus. M molecular marker; lanes 1–7 tenfold serial dilutions of bacterial standard DNA from 107 to 101 fg/μl; blank qPCR negative control. As expected, the sizes of S. mutans and S. sobrinus amplicons were 479 bp and 88 bp, respectively
Based on the qPCR results, S. mutans was detected in 84.5 % of the Thai children. The percentage of children colonized by S. mutans was significantly correlated with an order age, birth weight (normal versus low birth weight), bedtime sleep with a bottle, and frequency of soft drink and candy consumption (Table 1). Our findings indicate that more 3-year-old children born by vaginally delivery were S. mutans positive compared to that of C-section. However, the overall correlation between the presence of S. mutans and the mode of delivery was not statistically significant (Table 1).
Table 1.
Colonization of S. mutans or S. sobrinus in 3- and 5-year-old Thai children
| Group | N |
S. mutans (%) |
S. sobrinus (%) |
||||
|---|---|---|---|---|---|---|---|
| + | OR (CI 95 %) | P value | + | OR (CI 95 %) | P value | ||
| Age | 343 | 290 (84.5) | 174 (50.9) | ||||
| 3 years | 180 | 138 (76.7) | 3.5 (1.8~6.5) | <0.001 | 84 (46.7) | 1.2(1.0~1.1) | N.S. |
| 5 years | 163 | 152 (93.3) | 90 (55.6) | ||||
| Gender | |||||||
| Male | 188 | 161 (85.6) | 1.2 (0.7~2.2) | N.S. | 98 (52.1) | 1.1 (0.7~1.7) | N.S. |
| Female | 155 | 129 (83.2) | 76 (49.4) | ||||
| Delivery mode | |||||||
| Vaginal | 181 | 159 (87.8) | 1.7 (0.9~3.1) | N.S. | 97 (53.6) | 1.3 (0.8~1.9) | N.S. |
| C-section | 162 | 131 (80.9) | 77 (47.8) | ||||
| 3 years old | |||||||
| Vaginal | 97 | 81 (83.5) | 2.3 (1.1~4.7) | 0.019 | 46 (47.4) | 1.1 (0.6~1.9) | N.S. |
| C-section | 83 | 57 (68.7) | 38 (45.8) | ||||
| 5 years old | |||||||
| Vaginal | 84 | 78 (92.9) | 0.9 (0.3~3.0) | N.S. | 51 (60.7) | 1.5 (0.8~2.9) | N.S. |
| C-section | 79 | 74 (93.7) | 39 (50.0) | ||||
| Birth weight | |||||||
| <2,500 g | 13 | 8 (61.5) | 2.6 (1.3~5.5) | 0.019 | 4 (30.8) | 1.4 (1.0~2.1) | N.S. |
| ≥2,500 g | 330 | 282 (85.5) | 170 (51.7) | ||||
| Primary caregiver | |||||||
| Mother | 172 | 141 (82.0) | 0.7 (0.4~1.2) | N.S. | 79 (46.2) | 0.7 (0.4~1.1) | N.S. |
| Othersa | 171 | 149 (87.1) | 95 (55.9) | ||||
| Sleep with bottle | |||||||
| Yes | 158 | 141 (89.2) | 2.1 (1.1~3.9) | 0.020 | 92 (58.6) | 1.7 (1.1~2.7) | 0.013 |
| No | 180 | 144 (80.0) | 81 (45.0) | ||||
| Mother's premastication | |||||||
| Yes | 109 | 93 (85.3) | 1.1 (0.5~2.1) | N.S. | 65 (59.6) | 1.7 (1.1~2.7) | 0.024 |
| No | 233 | 196 (84.1) | 108 (46.6) | ||||
| Soft drink | |||||||
| ≥1 per day | 183 | 162 (88.5) | <0.001 | 97 (53.3) | N.S. | ||
| Sometimes | 80 | 74 (92.5) | 41 (51.3) | ||||
| Never | 80 | 54 (67.5) | 36 (45.0) | ||||
| Gum | |||||||
| ≥1 per day | 139 | 127 (91.4) | 2.7 (1.4~5.3) | 0.003 | 98 (48.8) | 1.2 (0.8~1.9) | N.S. |
| Never/rare | 202 | 161 (79.7) | 75 (54.0) | ||||
| Lollipop candy | |||||||
| ≥1 per day | 152 | 138 (90.8) | 2.5 (1.3~4.9) | 0.004 | 78 (51.3) | 1.0 (0.7~1.6) | N.S. |
| Never/rare | 190 | 151 (79.5) | 96 (50.8) | ||||
The “Others” category includes grandmother (2.9 %), grandfather (1.1 %), babysitter (10.3 %), or other relatives of the family (35.6 %).
In comparison, S. sobrinus was detected in 50.9 % of the children. Interestingly, most factors associated with increased S. mutans colonization were insignificant for S. sobrinus colonization, except for the practice of sleeping with a bottle. In addition to that habit, the mother's practice of premastication before feeding, particularly for the 3-year-old group significantly correlated with the colonization of S. sobrinus (Table 1).
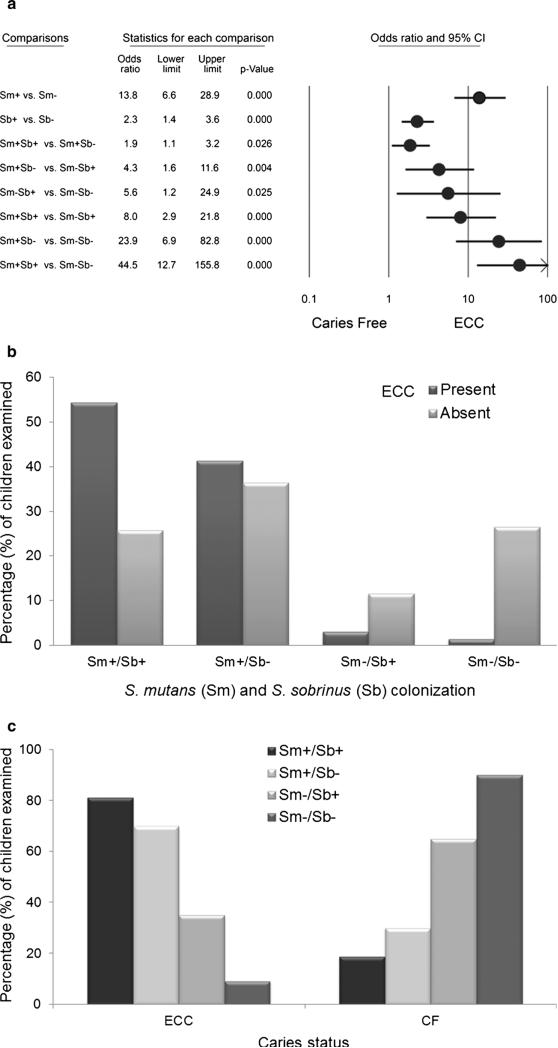
While comparing the presence of S. mutans and S. sobrinus colonization simultaneously, we found that the percentage of children positive for both S. mutans and S. sobrinus (Sm+/Sb+) was significantly correlated with higher ECC prevalence, more severe ECC (mean dmft score), the practice of premastication before feeding to 3-year-old group, and higher frequency of soft drink and candy consumption (Table 2). More specifically, for children colonized by either S. mutans or S. sobrinus alone, the odds ratio (OR) for developing ECC was 13.8 or 2.3, respectively. Children who were positive for both S. mutans and S. sobrinus (Sm+/Sb+) were 8 times more likely to develop ECC than children who were Sm−/Sb+(P<0.001), and 44 times more likely to develop ECC than those who were Sm−/Sb− (P<0.001; Fig. 2a). The distribution of S. mutans and S. sobrinus colonization was also significantly different between children with or without ECC (Fig. 2b) and within the ECC or the caries-free (CF) group (Fig. 2c).
Table 2.
Effect of the colonization of S. mutans together with S. sobrinus on 3- and 5-year-old Thai children
| N | Sm+/Sb+ | Sm+/Sb– | Sm–/Sb+ | Sm–/Sb– | P value | |
|---|---|---|---|---|---|---|
| Caries | ||||||
| Yes | 230 | 125 (54.3) | 95 (41.3) | 7 (3.0) | 3 (1.3) | <0.001 |
| No | 113 | 29 (25.7) | 41 (36.3) | 13 (11.5) | 30 (26.5) | |
| Mean±SD | ||||||
| dmft | 230 | 6.7±4.5 | 5.5±4.0 | 4.3±4.2 | 2.0±1.7 | 0.046 |
| dmfs | 230 | 15.2±15.8 | 11.3±12.3 | 7.8±11.6 | 2.7±2.1 | |
| Age | ||||||
| 3 years | 180 | 68 (37.8) | 70 (38.9) | 16 (8.9) | 26 (14.4) | <0.001 |
| 5 years | 163 | 86 (52.8) | 66 (40.5) | 4 (2.5) | 7 (4.3) | |
| Gender | ||||||
| Male | 188 | 89 (47.3) | 72 (38.3) | 9 (4.8) | 18 (9.6) | N.S. |
| Female | 155 | 65 (41.9) | 64 (41.3) | 11 (7.1) | 15 (9.7) | |
| Delivery mode | ||||||
| Vaginal | 181 | 87 (48.1) | 72 (39.8) | 10 (5.5) | 12 (6.6) | N.S. |
| C-section | 162 | 67 (41.4) | 64 (39.5) | 10 (6.2) | 21 (13.0) | |
| Birth weight | ||||||
| <2,500 g | 13 | 3 (23.1) | 5 (38.5) | 1 (7.7) | 4 (30.8) | 0.050 |
| ≥2,500 g | 330 | 151 (45.8) | 131 (39.7) | 19 (5.8) | 29 (8.8) | |
| Mother's premastication | ||||||
| 3-year-old | ||||||
| Yes | 57 | 29 (50.9) | 17 (29.8) | 6 (10.5) | 5 (8.8) | 0.042 |
| No | 123 | 39 (31.7) | 53 (43.1) | 10 (8.1) | 21 (17.1) | |
| Sleep with bottle | ||||||
| Yes | 158 | 84 (53.2) | 57 (36.1) | 8 (5.1) | 9 (5.7) | 0.017 |
| No | 180 | 69 (38.3) | 75 (41.7) | 12 (6.7) | 24 (13.3) | |
| Soft drink | ||||||
| ≥1 per day | 183 | 88 (48.1) | 74 (40.4) | 9 (4.9) | 12 (6.6) | <0.001 |
| Never | 80 | 26 (32.5) | 28 (35.0) | 10 (12.5) | 16 (20.0) | |
| Sometimes | 80 | 40 (50.0) | 34 (42.5) | 1 (1.3) | 5 (6.3) | |
| Gum | ||||||
| ≥1 per day | 139 | 70 (50.4) | 57 (41.0) | 5 (3.6) | 7 (5.0) | 0.030 |
| Never/rare | 202 | 83 (41.1) | 78 (38.6) | 15 (7.4) | 26 (12.9) | |
| Lollipop candy | ||||||
| ≥1 per day | 152 | 71 (46.7) | 67 (44.1) | 7 (4.6) | 7 (4.6) | 0.023 |
| Never/rare | 190 | 83 (43.7) | 68 (35.8) | 13 (6.8) | 26 (13.7) | |
Fig. 2.
Comparison of S. mutans and S. sobrinus colonization and caries status of children. a Odds ratio (OR) and 95 % confidence interval (CI) were listed for each paired comparison between S. mutans and/or S. sobrinus qPCR results as positive (+) or negative (−) and the risk for developing ECC. b The distribution of children was grouped by S. mutans and S. sobrinus positive (+) or negative (−). c The distribution of children was grouped by ECC status. The distributions were statistically significant (Chi-square test, P<0.001). ECC was defined as “the presence of one or more decayed (noncavitated or cavitated lesions), missing (due to caries), or filled tooth surfaces in any primary tooth in a child under the age of six.” (The American Academy of Pediatric Dentistry. http://www.aapd.org/media/Policies_Guidelines/P_ECCClassifications.pdf)
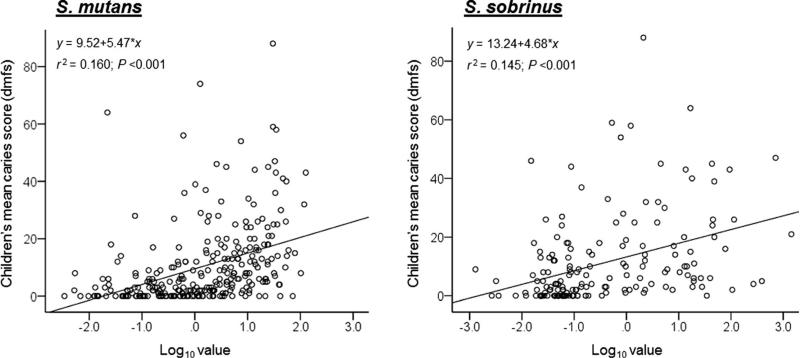
The present study further revealed a positive correlation between ECC status and the DNA levels of S. mutans and S. sobrinus in the plaque samples. Each qPCR result was converted to DNA concentration based on CT values using the standard curves generated in each qPCR experiment. Overall, there was a positive correlation between the mean ECC score (dmfs) and DNA levels (ng/μl; log10 value) of S. mutans (r=0.399, P<0.001) and S. sobrinus (r=0.382, P<0.001; Fig. 3). Increases in caries severity were significantly correlated with increases in the bacterial DNA level in the dental plaques.
Fig. 3.
Scatterplots for the associations of ECC scores (dmfs) and qPCR detected DNA levels of S. mutans and S. sobrinus in supra-gingival plaque samples of the children. The encircled dots represent actual data points. The black line represents the best-fit line of the correlation coefficient. Overall, there was a positive correlation between the mean ECC score (dmfs) and the bacterial DNA levels (Pearson's correlation analysis)
Discussion
Data presented in this study were consecutive findings based on a cross-sectional epidemiological study conducted in a socio-economically homogenous group of Thai preschool children who came to the local health center for their regular immunization or annual physical checkup. Previously, based on the chairside Dentocult® SM Strip Mutans method, we reported that 76.5 % of children colonized by mutans streptococci and a correction of MS scores to caries status, delivery mode, and dietary behavior [3]. However, the Dentocult approach could not adequately differentiates S. mutans from S. sobrinus nor provides quantitative analysis for either bacterium.
In this study, we used the molecular-based qPCR method to further examine the two best known cariogenic bacteria, S. mutans and S. sobrinus, in the oral cavity, as well as to determine potential risk factors associated with the bacterial colonization. Our results showed that 84.5 and 50.9 % of the 3- and 5-year-old Thai children studied were colonized by S. mutans and S. sobrinus, respectively. It was evident that the qPCR method is more sensitive in detecting MS compared to the Dentocult method. The study demonstrated a strong association among ECC prevalence, ECC disease severity, and colonization of S. mutans and S. sobrinus in the plaque samples. Several ECC-associated risk factors proved to be positively correlated with S. mutans and S. sobrinus colonization in these 3- and 5-year-old Thai preschool children. Importantly, children colonized by both S. mutans and S. sobrinus were at much greater risk of developing ECC than those who were free of S. mutans and S. sobrinus or who were colonized by either S. mutans or S. sobrinus in their oral cavities. These results suggest that S. sobrinus may play a synergistic role in the disease development and progression.
S. mutans and S. sobrinus are known as key oral pathogens for dental decay [22]. For decades, many studies have focused mainly on the cariogenicity of S. mutans using various advanced methodologies and tools, but there are only a few clinical studies of S. sobrinus and its correlation with ECC. The lack of phenotypic distinguishing characteristics and specific selective mediums for S. sobrinus could be the main reasons. Although a number of laboratory-based tests are currently available, such as cultivation and colony morphology differentiation [22–24], biochemical testing [9, 25], the antisera test [26], and monoclonal antibodies [27], for identification and quantification of S. sobrinus, the implementation of those tests for epidemiology studies or population-based clinical trials has always been challenging. Moreover, most lab-based tests, including in vitro cultures, require viable samples, making impractical their application in field epidemio-logical studies and high-throughput research.
Recently, a number of studies have used conventional PCR, direct PCR or nested PCR to distinguish S. mutans from S. sobrinus based on gene conservation in bacteria (16S rRNA gene) [28–30]. To achieve high fidelity and precision, qPCR methods were developed and validated to distinguish between S. mutans and S. sobrinus based on amplification of glucosyltransferase genes (gtfB and gtfT genes), which encode a glucosyltransferase that synthesizes water-insoluble glucan from sucrose [21, 29]. In the present study, we selected a set of primers specifically targeting gtfT genes of S. sobrinus [21] and a set of S. mutans-specific primers targeting an intergenic locus of S. mutans genome [20] as our main study approaches. All of the results showed high specificity and accuracy for the detection of the presence or absence, as well as the quantification of S. mutans and S. sobrinus DNA levels in clinical samples. The positive correlation with ECC found in the our study is comparable to that observed in several previous reports using similar qPCR methods, although the detection rate of S. mutans and S. sobrinus in our study might be different from those of other studies [13, 15–17, 31].
The main variation was observed in the caries-free group. Previously, S. sobrinus was almost undetectable in Choi's study [15], but it was positive in a significant proportion of children examined in other studies [17]. In our study, at least 37 % of the caries-free children were either S. sobrinus-positive (11.5 %) or S. mutans- and S. sobrinus-positive (25.7 %). Although S. sobrinus showed less frequency than S. mutans, it has been suggested that evidence of S. sobrinus infection found in saliva represents an important additional risk factor for caries by its acidogenicity and aciduricity characteristics. De Soet et al. showed that S. sobrinus-type strains were more acidogenic than S. mutans-type strains in vitro [32]. Using human isolates, Kohler et al. demonstrated that S. sobrinus presented higher acid production activity than S. mutans [33]. Elevated S. sobrinus colonization was associated with aggressive caries activity in young children, as shown in several cross-sectional clinical studies [15, 17], as well as a longitudinal study, which indicated that children harboring both species had a significantly higher incidence of dental caries than those who were positive for S. mutans alone [13].
Our study revealed that 91 % of the Thai children who were negative for S. mutans and S. sobrinus were caries free. In contrast, children who were colonized by both S. mutans and S. sobrinus were 44.5 times more likely to develop ECC than those who were negative for both S. mutans and S. sobrinus. Meanwhile, for children who were positive for S. sobrinus alone, the OR for ECC was only 2.3 compared to the OR of 13.8 for the children who were positive for S. mutans alone. Being S. sobrinus-positive appears to accelerate the OR for ECC formation. In addition, the study found a positive correlation between ECC scores and S. sobrinus DNA levels in the plaque samples. These findings provide new quantifiable evidence to demonstrate that S. sobrinus plays a significant role in ECC formation and more importantly, the two microorganisms collectively undertake a cariogenic role in ECC development. As ECC is a bacterial-dependent disease, it is plausible that the pathogenic capacity of these two microorganisms may be far greater than that of either S. mutans or S. sobrinus acting alone. More research is warranted to elucidate the synergistic mechanisms of the two microorganisms, particularly in a mixed microbial environment. In a recent prospective study, Hughes et al. demonstrated that baseline counts of S. sobrinus, but not S. mutans, were significantly higher in children (2–6 years) with recurrent caries compared with no recurrent caries [34]. The findings from our study suggest that besides S. mutans, S. sobrinus should be considered as a risk determinant of caries, especially for high-risk children.
In summary, the use of qPCR to detect and quantify S. mutans and S. sobrinus colonization offers valuable advantages for conducting epidemiological and clinical studies of risk assessment for ECC. A positive correlation between ECC status and S. mutans and S. sobrinus colonization in the plaque samples was determined. The study further suggests that children colonized by both S. mutans and S. sobrinus are at a higher risk for ECC. In addition to S. mutans and S. sobrinus, recent discoveries have shown that other microorganisms in saliva and dental plaque may also contribute to the pathogenicity of ECC [35, 36]. Thus, a better understanding of the composition and the interaction of the oral pathogens associated with ECC is critical for determining disease progression and prevention. The study also demonstrated that the approach using molecular-based qPCR with bacterial species-specific primers could become more accessible since immediate bacterial cultivation procedure is not required, therefore, it can be used to evaluate S. mutans and S. sobrinus colonization in field study and to assess children's risk for ECC.
Acknowledgments
This study was supported by research funds from the Faculty of Dentistry of Chiang Mai University, Thailand, the New York University Research Challenge Fund Award, the New York University College of Dentistry Dean's Award for Student Summer Research, and the National Institute of Dental and Craniofacial Research (R03 DE015706, R01 DE013937).
Footnotes
Conflict of interest The authors declare no conflicts of interest.
Contributor Information
P. Saraithong, Department of Basic Science and Craniofacial Biology, New York University College of Dentistry, 345 E. 24th Street, New York, NY 10010-4086, USA
K. Pattanaporn, Department of Oral Health Sciences, Faculty of Dentistry, University of British Columbia, Vancouver, BC, Canada
Z. Chen, Department of Basic Science and Craniofacial Biology, New York University College of Dentistry, 345 E. 24th Street, New York, NY 10010-4086, USA
S. Khongkhunthian, Department of Restorative Dentistry and Periodontology, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand
P. Laohapensang, Dental Department, Health Promoting Hospital, Chiang Mai, Thailand
N. Chhun, New York University College of Nursing Global, 726 Broadway, New York, NY 10003, USA
W. Pattanaporn, Dental Department, Health Promoting Hospital, Chiang Mai, Thailand
H. Y. Gaw, Department of Basic Science and Craniofacial Biology, New York University College of Dentistry, 345 E. 24th Street, New York, NY 10010-4086, USA
Y. Li, Department of Basic Science and Craniofacial Biology, New York University College of Dentistry, 345 E. 24th Street, New York, NY 10010-4086, USA
References
- 1.Abanto J, Carvalho TS, Mendes FM, Wanderley MT, Bonecker M, Raggio DP. Impact of oral diseases and disorders on oral health-related quality of life of preschool children. Community Dent Oral Epidemiol. 2011;39:105–114. doi: 10.1111/j.1600-0528.2010.00580.x. doi: 10.1111/j.1600-0528.2010.00580.x. [DOI] [PubMed] [Google Scholar]
- 2.Sutthavong S, Cae-Ngow S, Rangsin R. Oral health survey of military personnel in the Phramongkutklao Hospital, Thailand. J Med Assoc Thai. 2009;92(Suppl 1):S84–90. [PubMed] [Google Scholar]
- 3.Pattanaporn K, Saraithong P, Khongkhunthian S, Aleksejuniene J, Laohapensang P, Chhun N, Chen Z, Li Y. Mode of delivery, mutans streptococci colonization, and early childhood caries in three-to five-year-old Thai children. Community Dent Oral Epidemiol. 2013;41:212–223. doi: 10.1111/cdoe.12013. doi:10.1111/cdoe.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darojn K, Adunyanon S, Thinkamrop B, Bhumat N. Effectiveness of fluoride varnish application by health officers in early childhood caries prevention. Journal of the Dental Association of Thailand. 2005;55(1-2):1–13. [Google Scholar]
- 5.Vachirarojpisan T, Shinada K, Kawaguchi Y, Laungwechakan P, Somkote T, Detsomboonrat P. Early childhood caries in children aged 6–19 months. Community Dent Oral Epidemiol. 2004;32:133–142. doi: 10.1111/j.0301-5661.2004.00145.x. doi:10.1111/j.0301-5661.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 6.Peltzer K, Mongkolchati A, Satchaiyan G, Rajchagool S, Pimpak T. Sociobehavioral factors associated with caries increment: a longitudinal study from 24 to 36 months old children in Thailand. Int J Environ Res Public Health. 2014;11:10838–10850. doi: 10.3390/ijerph111010838. doi:10.3390/ijerph111010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dental Health Division . Report on the 6th national oral health survey in Thailand (2006–2007) Department of Health, Ministry of Public Health; Nonthaburi, Thailand: 2008. [Google Scholar]
- 8.Beighton D, Manji F, Baelum V, Fejerskov O, Johnson NW, Wilton JM. Associations between salivary levels of Streptococcus mutans, Streptococcus sobrinus, lactobacilli, and caries experience in Kenyan adolescents. J Dent Res. 1989;68:1242–1246. doi: 10.1177/00220345890680080601. [DOI] [PubMed] [Google Scholar]
- 9.Hirose H, Hirose K, Isogai E, Miura H, Ueda I. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 1993;27:292–297. doi: 10.1159/000261553. [DOI] [PubMed] [Google Scholar]
- 10.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler B, Andreen I, Jonsson B. The effect of caries-preventive measures in mothers on dental caries and the oral presence of the bacteria Streptococcus mutans and lactobacilli in their children. Arch Oral Biol. 1984;29:879–883. doi: 10.1016/0003-9969(84)90086-4. [DOI] [PubMed] [Google Scholar]
- 12.Tankkunnasombut S, Youcharoen K, Wisuttisak W, Vichayanrat S, Tiranathanagul S. Early colonization of mutans streptococci in 2- to 36-month-old Thai children. Pediatr Dent. 2009;31:47–51. [PubMed] [Google Scholar]
- 13.Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, Kozai K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol. 2005;54:661–665. doi: 10.1099/jmm.0.46069-0. [DOI] [PubMed] [Google Scholar]
- 14.Seki M, Yamashita Y, Shibata Y, Torigoe H, Tsuda H, Maeno M. Effect of mixed mutans streptococci colonization on caries development. Oral Microbiol Immunol. 2006;21:47–52. doi: 10.1111/j.1399-302X.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi EJ, Lee SH, Kim YJ. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediatr Dent. 2009;19:141–147. doi: 10.1111/j.1365-263X.2008.00942.x. doi:10.1111/j.1365-263X.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- 16.Kishi M, Abe A, Kishi K, Ohara-Nemoto Y, Kimura S, Yonemitsu M. Relationship of quantitative salivary levels of Streptococcus mutans and Streptococcus sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Community Dent Oral Epidemiol. 2009;37:241–249. doi: 10.1111/j.1600-0528.2009.00472.x. doi:10.1111/j.1600-0528.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 17.Nurelhuda NM, Al-Haroni M, Trovik TA, Bakken V. Caries experience and quantification of Streptococcus mutans and Streptococcus sobrinus in saliva of Sudanese schoolchildren. Caries Res. 2010;44:402–407. doi: 10.1159/000316664. doi:10.1159/000316664. [DOI] [PubMed] [Google Scholar]
- 18.WHO . Oral health surveys: basic methods. World Health Organization; France: 2013. [Google Scholar]
- 19.Li Y, Ge Y, Saxena D, Caufield PW. Genetic profiling of the oral microbiota associated with severe early-childhood caries. J Clin Microbiol. 2007;45:81–87. doi: 10.1128/JCM.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Saxena D, Caufield PW, Ge Y, Wang M, Li Y. Development of species-specific primers for detection of Streptococcus mutans in mixed bacterial samples. FEMS Microbiol Lett. 2007;272:154–162. doi: 10.1111/j.1574-6968.2007.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T. Development of a 5' nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J Clin Microbiol. 2003;41:4438–4441. doi: 10.1128/JCM.41.9.4438-4441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loesche WJ, Eklund S, Earnest R, Burt B. Longitudinal investigation of bacteriology of human fissure decay: epidemiological studies in molars shortly after eruption. Infect Immun. 1984;46:765–772. doi: 10.1128/iai.46.3.765-772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emilson CG. Prevalence of Streptococcus mutans with different colonial morphologies in human plaque and saliva. Scand J Dent Res. 1983;91:26–32. doi: 10.1111/j.1600-0722.1983.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 24.Gold OG, Jordan HV, Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;18:1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 25.Beighton D, Russell RR, Whiley RA. A simple biochemical scheme for the differentiation of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1991;25:174–178. doi: 10.1159/000261363. [DOI] [PubMed] [Google Scholar]
- 26.Bratthall D. Immunofluorescent identification of Streptococcus mutans. Odontol Revy. 1972;23:181–196. [PubMed] [Google Scholar]
- 27.de Soet JJ, van Dalen PJ, Appelmelk BJ, de Graaff J. Identification of Streptococcus sobrinus with monoclonal antibodies. J Clin Microbiol. 1987;25:2285–2288. doi: 10.1128/jcm.25.12.2285-2288.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunnion DT, Spiro A, 3rd, Jones JA, Rich SE, Papageorgiou CP, Tate A, Casamassimo P, Hayes C, Garcia RI. Pediatric oral health-related quality of life improvement after treatment of early childhood caries: a prospective multisite study. J Dent Child (Chic) 2010;77:4–11. [PMC free article] [PubMed] [Google Scholar]
- 29.Oho T, Yamashita Y, Shimazaki Y, Kushiyama M, Koga T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol Immunol. 2000;15:258–262. doi: 10.1034/j.1399-302x.2000.150408.x. [DOI] [PubMed] [Google Scholar]
- 30.Sato T, Matsuyama J, Kumagai T, Mayanagi G, Yamaura M, Washio J, Takahashi N. Nested PCR for detection of mutans streptococci in dental plaque. Lett Appl Microbiol. 2003;37:66–69. doi: 10.1046/j.1472-765x.2003.01359.x. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Acedo M, Montiel-Company JM, Dasi-Fernandez F, Almerich-Silla JM. Streptococcus mutans and Streptococcus sobrinus detection by polymerase chain reaction and their relation to dental caries in 12 and 15 year-old schoolchildren in Valencia (Spain). Med Oral Patol Oral Cir Bucal. 2013;18:e839–845. doi: 10.4317/medoral.18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Soet JJ, Toors FA, de Graaff J. Acidogenesis by oral streptococci at different pH values. Caries Res. 1989;23:14–17. doi: 10.1159/000261148. [DOI] [PubMed] [Google Scholar]
- 33.Kohler B, Birkhed D, Olsson S. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1995;29:402–406. doi: 10.1159/000262099. [DOI] [PubMed] [Google Scholar]
- 34.Hughes CV, Dahlan M, Papadopolou E, Loo CY, Pradhan NS, Lu SC, Mathney JM, Bravoco A, Kent RL, Jr, Tanner AC. Aciduric microbiota and mutans streptococci in severe and recurrent severe early childhood caries. Pediatr Dent. 2012;34:e16–23. [PMC free article] [PubMed] [Google Scholar]
- 35.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, Dewhirst FE. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–1474. doi: 10.1128/JCM.02427-10. doi:10.1128/JCM. 02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]