Abstract
The “Workshop on Standards & Measurements for Tissue Engineering Scaffolds” was held on May 21, 2013 in Indianapolis, IN and was sponsored by the ASTM International (ASTM). The purpose of the workshop was to identify the highest priority items for future standards work for scaffolds used in the development and manufacture of tissue engineered medical products (TEMPs). Eighteen speakers and 78 attendees met to assess current scaffold standards and to prioritize needs for future standards. A key finding was that the ASTM TEMPs subcommittees (F04.41-46) have many active “guide” documents for educational purposes, but that few standard “test methods” or “practices” have been published. Overwhelmingly, the most clearly identified need was standards for measuring the structure of scaffolds, followed by standards for biological characterization, including in vitro testing, animal models and cell-material interactions. The third most pressing need was to develop standards for assessing the mechanical properties of scaffolds. Additional needs included standards for assessing scaffold degradation, clinical outcomes with scaffolds, effects of sterilization on scaffolds, scaffold composition and drug release from scaffolds. Discussions also highlighted the need for additional scaffold reference materials and the need to use them for measurement traceability. Finally, dialogue emphasized the needs to promote the use of standards in scaffold fabrication, characterization, and commercialization and to assess the use and impact of standards in the TEMPs community. Many scaffold standard needs have been identified and focus should now turn to generating these standards to support the use of scaffolds in TEMPs.
Keywords: ASTM International, documentary standards, reference materials, tissue scaffold, workshop report
1. Introduction
Benefits of standards
Standards are critical for the advancement of the science and engineering involved in the tissue engineering and regenerative medicine (TERM) enterprises, and add value to a broad array of activities ranging from simple lab procedures to control of complex biological processes. Standards make it possible to compare data collected in different labs,1–7 which is essential for progress in TERM, because it is not possible for a single lab to complete the comprehensive, large-scale studies that are necessary for tissue-engineered therapies to move forward. Comparability over time enables batch to batch comparison of device properties to assure consistent performance. Standards contribute to the reliability of materials, products, systems and services, by establishing dependable measurements and guidelines. Standards also facilitate international commerce by creating a level playing field for trade in terms of product quality and can accelerate the regulatory process by establishing well-characterized measurements for demonstrating safety and efficacy. Standards are created by consensus by scientists and clinicians from academia, government, and industry, creating confidence in measurements that are made in accordance with the procedures that they define. Standards bodies, such as ASTM and ISO, have proven infrastructures for the development of consensus standards and are committed to generating standards for tissue engineered medical products (TEMPs).
What are standards
There are many types of standards that are used to improve measurement assurance. There are standards for defining units, such as the meter, which is defined as the distance that light travels in 1/299 792 458 of a second. There are standard test methods which provide detailed procedures for conducting a measurement, including acceptance criteria and tolerances. There are standard reference data, such as spectra of purified molecules that can be used for chemical identification. Standard codes of practice highlight procedures that should be followed when performing a particular task, such as how to sterilize a device. When a research community begins to pursue standards, the first order of business is usually standard terminology, since clear definitions and a common language are essential for advancement. Standards can also come in the form of material artifacts, or reference materials, that have well-defined properties and serve as a reference point during a measurement. For example, from 1889 to 1960, the length of a meter was defined by an “International Metre” bar made of an alloy of platinum and iridium that was housed near Paris. The many different types of standards can come in the form of written documents available from standards organizations, physical artifacts that can be purchased from a standards body or as data tables that can be downloaded from the web. It is important to note that standards are not static, but are highly dynamic. They are frequently reviewed, every 5 years for ASTM documentary standards, and may be withdrawn or modified to reflect the state of the art in scientific measurements.
The time for standards is now
Recently, extensive discussion about the inability to reproduce research findings, especially in biological investigations, has taken place.8–16 Data simulations of common research study designs predict that many published research conclusions may be incorrect.8 Scientists from Bayer reported that they could only reproduce published research findings regarding drug targets between 21% and 32% of the time in the 67 papers that they examined.12 Another team found that they could confirm results from only 11% (6 out of 53) of “landmark” oncology studies that were assessed for drug leads.13 A recent survey of 16 big pharma companies conducted by the Alliance for Regenerative Medicine found that “product consistency and lack of standards is possibly the single greatest challenge facing the field” of regenerative medicine.17 A number of efforts are taking shape to improve the reliability of research findings, including a renewed appreciation of how standards and reference materials can provide measurement assurance. The Science Exchange has launched the “Reproducibility Initiative” which partners scientists with contractors to validate their key experiments.18 The U.S. National Institutes of Health (NIH) is discussing requirements for experimental validation in grant applications, especially for work that motivates a clinical trial.16 Leaders in biological research have formed the Global Biological Standards Institute to promote and facilitate the use of standards in biological research.14 The Alliance for Regenerative Medicine, a tissue engineering advocacy group, has formed a Science and Technology Committee whose primary focus is development of research standards that could be adopted by industry. Thus, the biomedical research climate is evolving and the time is right to embrace and develop standards for TERM.
Tissue engineering scaffolds
A tissue engineering scaffold is defined as “a support, delivery vehicle or matrix for facilitating the migration, binding, or transport of cells or bioactive molecules used to replace, repair, or regenerate tissues”.19 Scaffolds are a critical component of TEMPs and many designs have been studied to determine the optimal scaffold physical properties for enhancing tissue regeneration. Strategically, the use of scaffolds to induce regeneration is an attractive opportunity because, relative to cells and growth factors, scaffold physical properties can be more precisely controlled and have a lower regulatory burden.20 Standards for scaffolds, both reference materials and documentary standards, are necessary due to the many properties available for exploration, such as scaffold chemistry, structure, mechanics and biological interactions.
The scaffolds workshop
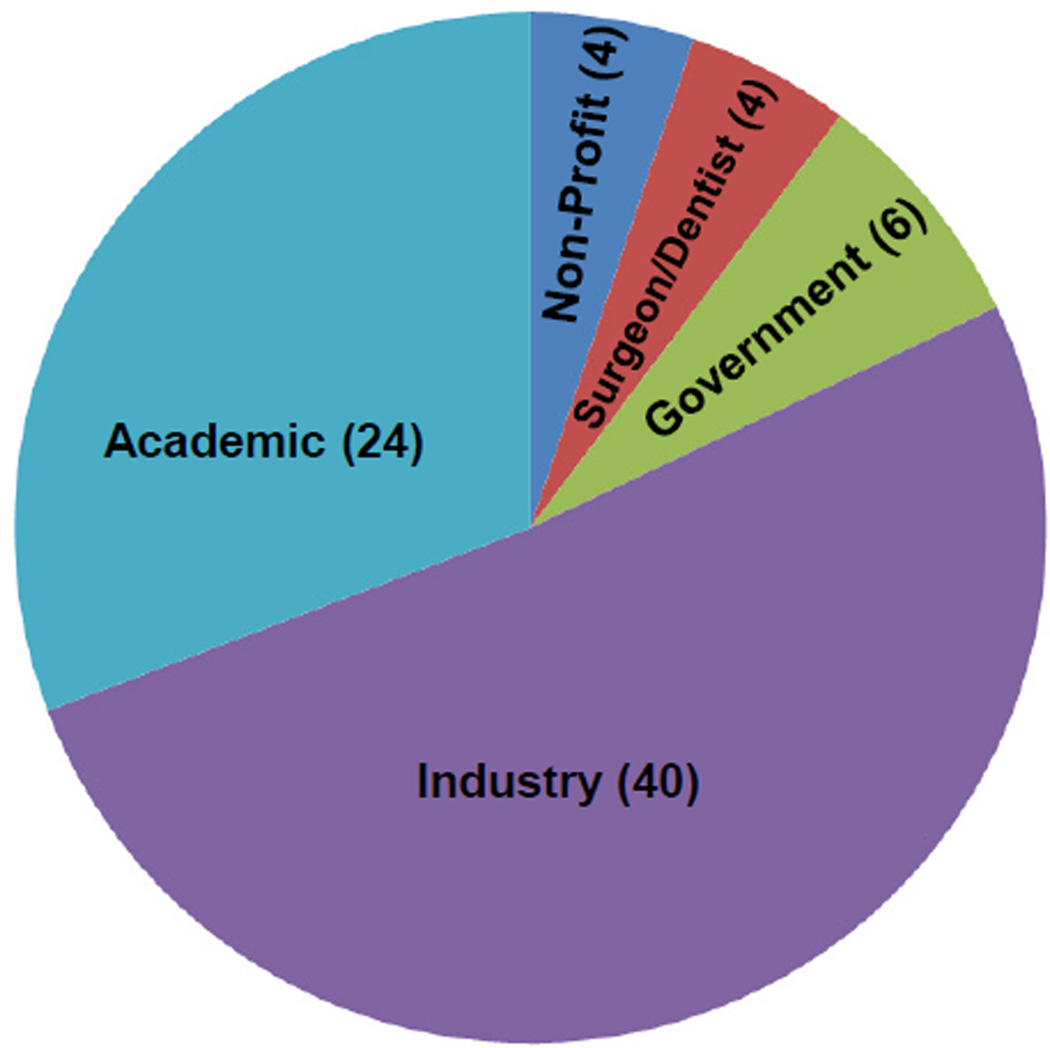
In order to assess needs for new scaffold standards, the “Workshop on Standards & Measurements for Tissue Engineering Scaffolds” was held on May 21, 2013 in Indianapolis, IN. The workshop was sponsored by the ASTM International (ASTM), Committee F04.04 Division 4 (Tissue Engineered Medical Products (TEMPs)) and Subcommittee F04.42 (Biomaterials and Biomolecules for TEMPs). The workshop hosted 18 speakers (Supplemental File 1) and 78 participants, comprising a diverse group of stakeholders (Fig. 1), including industry (51%), academics (31%), government (8%), end users (5%, surgeons and dentists) and non-profit organizations (5%). A Workshop Report was prepared to summarize the findings and is included in Supplemental File 2, the highlights of which are covered in this paper.
Fig. 1.
Pie chart indicating the number of workshop participants according to affiliation.
2. Scaffold standards needs
Current portfolio of TEMPs standards
ASTM has 30 existing TEMPs standards and 19 that have been proposed, while ISO has 1 existing and 1 proposed (tabulated in Table 1, listed in Supplemental File 3). Of these, there are 10 standards that focus on tissue engineering scaffolds (7 existing and 3 proposed, see Table 2).21–27 Of the 7 existing scaffold standards, all are from ASTM and all are “Standard Guides.” Standard Guides are one of the 6 types of ASTM standards and they provide advice on making a measurement or characterizing a material without recommending “a specific course of action”. See Table 3 for an overview of the 6 types of ASTM standards and a breakdown of where the TEMPs standards fall.
Table 1.
ASTM International & ISO Standards Documents for TEMPs*
| Active | Proposed | |
|---|---|---|
| ASTM | 30 | 19 |
| ISO | 1 | 1 |
| Total | 31 | 20 |
Data in table were generated on Dec. 12, 2013
Table 2.
Published ASTM International and ISO Standards Documents with a Focus on Scaffolds*
| Active or Proposed |
Primary Application | Source | Document Title |
|---|---|---|---|
| Active | Scaffolds in General | ASTM F04.42 |
F2150-13, Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products |
| Active | Scaffolds in General (Ceramic Scaffolds) |
ASTM F04.42 |
F2883-11, Standard Guide for Characterization of Ceramic & Mineral Based Scaffolds used for TEMPs & as Devices for Surgical Implant Applications |
| Active | Scaffolds in General (Hydrogel Scaffolds) |
ASTM F04.42 |
F2900-11, Standard Guide for Characterization of Hydrogels used in Regenerative Medicine |
| Active | Physical (Scaffold Structure) |
ASTM F04.42 |
F2450-10, Standard Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue Engineered Medical Products |
| Active | Physical Scaffold Structure) |
ASTM F04.42 |
F2603-06(2012), Standard Guide for Interpreting Images of Polymeric Tissue Scaffolds |
| Active | Biological | ASTM F04.43 |
F2315-11, Standard Guide for Immobilization or Encapsulation of Living Cells or Tissue in Alginate Gels |
| Active | Biological | ASTM F04.43 |
F2739-08, Standard Guide for Quantitating Cell Viability Within Biomaterial Scaffolds |
| Proposed | Physical (Scaffold Structure) |
ASTM F04.42 |
WK24374, New Guide for Determining Darcy Permeability Coefficients for Porous Tissue Scaffolds |
| Proposed | Biological | ASTM F04.42 |
WK39698, New Test Method for Using NIST Tissue Engineering Reference Scaffolds for Cell Culture Tests |
| Proposed | Biological | ISO TC150/SC7 |
New Work Item Proposal N80, Cell Migration Ability Test for Porous Body |
Data in table was generated on December 12, 2013
Table 3.
The Six Types of ASTM International Standards Documents and the ASTM TEMPs Inventory for Each Type
| Type of Standard |
Definition | Active | Proposed |
|---|---|---|---|
| Guide | An organized collection of information or series of options that does not recommend a specific course of action |
23 | 11 |
| Test Method | A definitive procedure that produces a test result | 7 | 3 |
| Practice | A set of instructions for performing one or more specific operations that does not produce a test result |
0 | 2 |
| Specification | An explicit set of requirements to be satisfied by a material, product, system or service |
0 | 2 |
| Terminology | A document composed of terms, definitions of terms, descriptions of terms, nomenclature, and explanations of abbreviations, acronyms & symbols |
0 | 1 |
| Classification | Systematic arrangement or division of materials, products, systems, or services into groups based on similar characteristics such as origin, composition, properties, or use |
0 | 0 |
| Totals | 30 | 19 | |
Standard practices and test methods
A primary discussion point and conclusion from the scaffolds workshop was that there are many standard “guides” for scaffolds but not enough “practices” or “test methods” (Table 4). Twenty three of the 30 existing ASTM TEMPs are Standard Guides (77%), while only 7 are Test Methods (23%) (Table 3). Practices provide instructions for performing specific tasks or measurements while Test Methods provide “a definitive procedure that produces a test result.” Although Standard Guides are useful in providing overviews and guidance, Practices and Test Methods deliver more rigorous instruction on making standardized measurements that can be broadly compared among labs. The workshop participants advocated that the development of new Practices and Test Methods for scaffolds was needed to advance scaffold science and therapeutic benefit.
Table 4.
Scaffold Standards Needs Identified by the Workshop
| 1st | Need more “Test Methods” & “Practices” (there are many “Standard Guides”) |
| 2nd | Measuring scaffold structure |
| 3rd | Biological characterization: in vitro testing, animal models & cell-material interactions |
| 4th | Measuring mechanical properties |
| Others | Standards for measuring scaffold degradation |
| Assessing clinical outcomes of scaffold-based devices | |
| Assessing effect of sterilization on scaffold properties | |
| Measuring scaffold composition | |
| Assessing drug release from scaffolds | |
| Reporting scaffold research results |
Standards for measuring scaffold structure
Second on the list of scaffold standards needs was the most discussed topic at the workshop; the need for standards for measuring scaffold structure (Table 4). Of the 7 existing scaffolds-focused standards, 3 are generalized guides for scaffold characterization,24,25,27 two are for biological measurements21,23 and two are focused on scaffold structural characterization (Table 2).22,26
Important structural features of scaffolds for standards include porosity, pore size, fraction of open cells, pore uniformity, pore size distribution and pore connectivity. Structural characterization of scaffolds is challenging since relevant nanoscale features, to which cells respond, are difficult to quantify in 3D (three-dimensions). Confocal microscopy and X-ray tomography are the most promising approaches for quantitative 3D structural characterization.
Standards for biological characterization
Third on the list of scaffold standards needs was biological characterization, including in vitro testing, animal models and cell-material interactions. Workshop discussions indicated that standards for biological measurements of scaffolds are needed for toxicity, biocompatibility and cell-material interactions. Many variables influence biological measurements including cell type, passage number, material properties, animal variability, donor/patient variability and batch-to-batch variability in materials. Therefore, standards for measuring cell seeding, morphology, viability, adhesion, proliferation, migration, differentiation and distribution in 3D scaffolds are needed. The discussion noted that new standards for scaffolds should avoid overlap with the highly effective ISO 10993 series of documents for assessing the biocompatibility of materials.28
Of particular need are new standards for measuring cell-scaffold interactions and the effects of scaffold chemical, mechanical and structural properties on cell response.29 These advanced measurement standards will be critical for optimizing 3D stem cell niches. In addition, stakeholders expressed needs for standards for measuring deposition of extracellular matrix (ECM) in scaffolds and standardized in vitro models of tissue regeneration that can be used for screening new materials. Standards for measuring tissue growth in vivo and standard models for specific clinical applications are desired, such as the goat models under development for cartilage repair and articular cartilage fixation.30
Standards for measuring scaffold mechanics
Fourth on the list of scaffold standards needs was mechanical characterization. It is difficult to reliably measure the mechanical properties of porous, soft materials and large variability in results is observed between labs. Parameters that contribute to this large variability include environmental conditions, measurement length-scales, measurement kinetics, mounting scheme, material stiffness, specimen size, viscoelastic properties, instrument differences and different mechanical measurement methods. Workshop participants identified needs for measuring the mechanical properties of all types of scaffolds, including hydrogels, solid scaffolds, self-assembled scaffolds and scaffolds made from different materials, especially natural materials.
Standard methods for hydrogels are especially vital, since they are needed for measuring polymerization kinetics, swelling ratios and device uniformity. Standards are needed for measuring the biomechanical properties of tissues, such as articular cartilage, and the sclera of the eye, since wide variability in the mechanical measurements of these tissues have been reported. Mechanical measurements are used for lot release of biologic scaffolds, such as decellularized extracellular matrix, and for assessing batch-to-batch variability of scaffold devices. Standards would make these processes more effective, informative and reliable.
Additional standards needs
Aside from the most highly discussed standard needs (1st to 4th in Table 4), several additional scaffold standards needs were discussed. Standards for measuring scaffold degradation were identified by industrial participants as a major concern, because this is one of the most common measurements required of companies that make resorbable scaffold products. It is well recognized that degradation measurements are highly dependent on assay variables such as specimen geometry, porosity, temperature, medium composition and pH. Assessment of clinical outcomes of scaffold-based devices will benefit from standardization. For example, patient-reported outcomes of function do not correlate with intermediate clinical (biomechanical) measures such as knee range of motion31 and surgeon-reported outcomes and patient-reported outcomes can be significantly different.32
Standards for assessing the effect of sterilization on scaffolds, for measuring scaffold composition and for measuring drug release from scaffolds are needed by the device community, as there was agreement that a variety of approaches are used which prohibit comparability. Participants admitted frustration with how scaffold studies are reported in the literature, whereby critical properties, sometimes even composition, are not adequately described. This can be improved by standards for the minimal data set required for describing scaffolds, which journal editors and granting agencies could require. This underscores the need for biologists, clinicians and materials scientists to collaborate to optimize material, molecular and cellular characterization.
Need for reference materials
A need for TEMPs reference materials development was recognized. Reference materials (RMs) are material artifacts that have well-characterized properties that can be used during a measurement and give a known response. They enable instrument calibration and can be used to normalize results obtained in different labs. NIST deployed the first reference materials for TEMPs in 2009; scaffolds with well-defined structure and porosity that are intended for use during scaffold structural characterization (Fig. 2).33–35 In 2012 NIST deployed a second generation reference scaffold that has been characterized for cell adhesion and cell proliferation.36
Fig. 2.
NIST reference material scaffolds. (a) RM8395, RM8396 and RM8397 are freeform fabricated scaffolds with 3 different strut spacings, 200 µm, 300 µm and 450 µm, respectively. They are characterized for structure and porosity. (b) RM8394 is a set of 24 freeform fabricated scaffolds in a 96-well plate and are characterized for cell adhesion and proliferation.
It is important to emphasize that the NIST reference material scaffolds are not meant to be the best scaffolds in the world, but are meant to serve as a benchmark.37 Two new scaffold formulations prepared in two different labs can be compared to one another, if each is compared to the reference material scaffold. Aluminum wedges have been developed as reference materials for measuring bone mass via radiography. Yet, no one would argue that aluminum wedges can serve as bone. Similarly, reference material scaffolds provided by NIST can be used as a reference point for comparing other scaffolds, without being the best scaffold for a particular clinical indication.
NIST has a number of additional reference materials that are relevant to TEMPs. For example, SRM2910 calcium hydroxyapatite can be used to evaluate the physical and chemical properties of calcium apatites.38 RM8011, RM8012 and RM8013 are a series of gold nanoparticles with narrow size distributions.39–41 RM2372 is a DNA reference material used for calibrating DNA measurements.42 RM8456 and RM8457 are ultra-high molecular weight polyethylene reference materials designed for measuring mechanical properties and cross-link density after sterilization by gamma irradiation.43,44 SRM2374 is a library of 96 RNA “spike-in” controls that provide quantitative assessment of a gene expression measurement.45
Conversation at the workshop focused on the NIST RM8394 “Reference Scaffolds for Cell Culture”,36 acknowledging their value in enabling interlab comparisons when testing new scaffold designs. Participants felt that pricing was an issue, with RM8394 costing $250 per unit. However, this is only $50 greater than the retail price ($200 per unit), suggesting that high cost is a barrier to entry into 3D culture, especially for routine culture or drug screening. Discussion also indicated a need for a 2D tissue culture polystyrene (TCPS) reference material that is inexpensive and widely available, since users have experienced vendor and batch-to-batch variability.
Need to promote the use of standards for TEMPs
The use of standards must be promoted in order to increase their use by the TEMPs community. Workshop participants felt that education regarding the benefits of voluntary consensus standards should occur at both the undergraduate and graduate levels in the engineering, business, and science curricula of our nation’s colleges and universities. Joint symposia should be held with relevant societies (Society for Biomaterials, Tissue Engineering and Regenerative Medicine International Society (TERMIS), Orthopedic Research Society, American Academy of Orthopaedic Surgeons). The TERM community would benefit from education that strengthens the concept that standards are applicable in all aspects of research and development, not just to meet regulatory and industrial requirements.
An increase in funding for standards research is required to motivate their use and development. The main funding sources for university-based bioengineers and biomaterialists in the U.S. are the National Institutes of Health (NIH), National Science Foundation (NSF) and U.S. Department of Defense (DOD). Currently, it is difficult to obtain support for standards research via these mechanisms since review panels and study sections place a high value on innovation and discovery. A multicenter proposal to validate a bioassay via “round robin”, an activity that is typical of standards work and critical for translation of TEMPs to the market, may not be viewed as innovative. However, innovation without validation will lead to inefficient use of resources.46 The U.S. Food and Drug Administration (FDA) has funded several Centers of Excellence in Regulatory Science and Innovation (CERSI), one of which, in recognition of the importance of standards for TEMPs, has a project on standardizing the characterization of scaffold properties.47
Another deterrent to becoming involved with standards work is that standards documents do not have an author list. Hiring committees, promotion committees and graduate thesis committees should encourage young scientists, graduate students and postdoctoral fellows to participate in standards work. It must be made clear by academic leaders that volunteer consensus standards work is valued as highly as is research that leads to peer-reviewed publications. In some respects, developing a consensus standard is more difficult than publishing a peer-reviewed manuscript, since there are many strong opinions on how measurements should be made, all dissenting votes and opinions must be formally addressed and the consensus must be unanimous. Building the consensus required for a standard is challenging and it requires stamina to get an entire community to agree on something, especially if it has commercial value. The consensus process is a rigorous peer-review that requires acceptance by the voting members of the committee prior to publication.
Need to assess the use and impact of TEMPs standards
The workshop recognized a need to assess the use of standards by the TEMPS community in order to evaluate their impact. Although measuring impact is difficult, it is critical for recruiting the best technical experts to develop standards. Measuring impact could be done in part by searching literature and patent databases. A Scopus literature search revealed 46474 citations with “tissue engineering” in the “title, abstract or keywords”.48 Of these, 148 (0.3%) had “ASTM” in the “references”. These results suggest that ASTM standards are not being widely used by TEMPs researchers. This might be explained by the fact that the majority of TEMPs standards are standard “guides” (Table 3). “Guides” are unlikely to be cited since they are intentionally broad and do not recommend a specific course of action. As mentioned earlier, this observation calls for the development of standard “test methods” and “practices.”
Impact could also be demonstrated by tracking the use of standards in device premarket applications to the FDA. Although this data is proprietary and not publicly accessible, their use could be assessed through discussions with device manufacturers. A member of the workshop organizing committee, Anthony Ratcliffe, Ph.D., leads the company that brought X-Repair rotator cuff surgical mesh to market in 2009 via the FDA’s 510k mechanism. Dr. Ratcliffe shared with the working group that his company, Synthasome, Inc., used and cited 10 standards documents in its application (Table 5),19,27,28,49–55 three of which are TEMPs standards listed in Supplemental File 3.19,27,53
Table 5.
Standards Used to Gain 510k Clearance for X-Repair*
| ASTM D3786 Standard Test Method for Bursting Strength of Textile Fabrics - Diaphragm Bursting Strength Tester Method |
| ASTM D5035 Standard Test Method for Breaking Force & Elongation of Textile Fabrics (Strip Method) |
| ASTM D5587 Standard Test Method for Tearing Strength of Fabrics by Trapezoid Procedure |
| ASTM F1635-11 Standard Test Method for in vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants |
| ASTM F2211 Standard Classification for Tissue Engineered Medical Products (TEMPs) |
| ASTM F2312 Standard Terminology Relating to TEMPs |
| ASTM F2027 Standard Guide for Characterization and Testing of Raw or Starting Biomaterials for Tissue- Engineered Medical Products |
| ASTM F2150 Standard Guide for Characterization of Biomaterial Scaffolds Used in TEMPs |
| ISO 10993 Biological Evaluation of Medical Devices |
| ISO 11135 Sterilization of Health Care Products |
X-Repair is a degradable surgical mesh indicated for augmentation of rotator cuff repair that is marketed by Synthasome and received 510k clearance from FDA in 2009.
Another TEMPs standards success story is “ISO 10993: Biological Evaluation of Medical Devices”.28 In 1982, ASTM published F748,56 which was later adopted and broadened by ISO as ISO 10993.29 ISO 10993 addresses measurements such as cell culture toxicity testing [57], quantification of polymeric degradation products,58 tests for genotoxicity and carcinogenicity,59 and animal models for local inflammation60 and skin irritation.61. This comprehensive, 20-part series of consensus standards is widely used by industry and regulatory agencies, providing a common language that substantially accelerates applications for new devices. The prevalence of ISO 10993 use by companies that are working through the FDA device approval process has produced a parallel industry of companies that perform contract ISO 10993 testing, enabling device manufacturing enterprises to outsource these activities and streamline their operations.
Although the 10993 standards have been highly effective for basic biological testing, a new generation of standards is required for the more complex devices, biologics, drugs and combination products that are in the TEMPs pipeline. This is especially true for scaffold devices which present unique measurement challenges. The 3D structure of scaffolds makes it difficult to quantify their structure and commonly used bioassays become challenging. It is difficult to seed cells evenly throughout a 3D scaffold, to extract and measure biomolecules from scaffolds and to image cells within scaffolds. Additionally, many relevant scaffold properties, such as modulus, density, degradation rate and biomolecule release profile, are highly dependent on scaffold material type and geometry.
Standards for reporting research results
Russell urged the editorial board of Tissue Engineering to negotiate minimal data standards and methodologies for authors in order to facilitate reproducibility and comparability.2 Guidelines for describing the scaffolds would help others to interpret research results and to replicate the experiments. If a defined set of experiments was required to test cell adhesion to a new material, then global data sets could emerge to accelerate the pace of materials development. The ASTM Scaffolds Workshop participants were in accord with Russell and believe that standards for reporting scaffold research results are needed.
3. Workshop discussion points
Simple and low cost
In addition to identifying standards needs, discussion at the workshop touched on many additional points. The workshop attendees agreed that standards and reference materials must be kept as simple and low cost as possible (Table 6). Affordability is key, since research budgets are constrained. Profit margins on devices are smaller than those for pharmaceuticals and current TEMPs companies are typically smaller in both size and budget.20,62,63 TEMPs manufacturing costs are high and require many materials and processes that are hard to control, such as cell and growth factor incorporation into and delivery from scaffolds. Reimbursement can take years to reach a level which covers the true cost of the devices. Also, complex protocols are harder to standardize and make reproducible. Multi-center “round robin” studies are informative because they typically identify complexity and uncertainty surround ing operations that appear straightforward.64 Something as innocuous as a wash step, if it is performed differently in different labs, can introduce variability into an assay’s result. Procedural details such as water purity, ionic strength, pH, type of agitation, length of wash, shear forces during liquid removal and the temperature at which the assay is performed can affect results. Thus, the simpler and better-defined an assay is, the more likely it can be effectively reproduced in different labs by different operators.
Table 6.
Main Discussion Points from the Workshop
| Discussion | Point Comments |
|---|---|
| Simple & low cost | Methods for scaffold characterization methods must be simple & low cost; complex measurements are difficult to standardize; TEMPs costs are becoming prohibitive |
| Need scaffold reference materials |
To enable measurement comparisons between different labs |
| Need to promote the use of standards |
|
| Need to assess use of & need for scaffold standards |
|
| Specific application | Each scaffold has to be tailored for a specific unmet need, there can be no universal cell or universal scaffold |
| CLINICAL is most important |
BIOmaterials & bioMATERIALs are both incorrect |
| Measurements provide information about a specimen’s state |
A measurement assesses the state of a specimen and does not predict efficacy; meeting a standard indicates that it is same as before |
| Length scales | What are the important scaffold-based metrics at different length scales (nano/micro/macro) that influence clinical outcomes? |
Specificity versus universality
Specificity versus universality a common debate regarding standards. Something so general that it applies to everything tends to be nearly useless, while something that is very specific may only be useful to such a small subset of stakeholders that it also may be nearly useless. Thus, it is always a struggle to find the correct balance of specificity versus universality. ASTM has several types of documents that span this spectrum (Table 3). ASTM F2150, “Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products,” is quite broad, comprising a shopping list of measurements and issues to consider when characterizing scaffolds. Though an expert in scaffolds might find this document to be elementary, F2150 can serve as a starting point for neophytes and as a checklist for the experienced. In contrast, ASTM F2131, “Standard Test Method for In Vitro Biological Activity of rhBMP-2,” is highly detailed and is most useful for those working specifically with BMP-2.65 Despite its specificity to BMP-2, F2131 is of value to those using other growth factors because it provides a vetted template for a growth factor bioactivity assay. This is important as it is unlikely that an ASTM standard will be developed for each growth factor with therapeutic value. In this way, highly specific standards can also have broader value.
Voluntary, not mandatory
Standards can cause anxiety due to the perception that they restrict creativity.2 However, this interpretation should not be a deterrent to the development of standards. Standards are voluntary and represent a consensus of the best way to make a measurement. Standards lie at the heart of measurement technology and are essential for making repeatable measurements, comparison of results between laboratories and reporting experimental procedures. Standards enable results to be externally validated and save researchers the effort of having to develop their own analytical measurement protocols through literature searching and consulting with technical experts. The important, relevant issues for a particular measurement will be covered by the standard enabling newcomers to efficiently learn the best practices. While standards should be followed whenever possible, there will be cases where the existing standards are not appropriate. Standards do not and should not squelch innovation and new and improved measurement technologies should be vigorously pursued. In these ways, standards expedite the regulatory process and build confidence in innovative products.
Measurements provide information about a specimen’s state
An important TEMPs standards discussion is “measuring clinical relevance”. Remember that measurements provide information about the state of a specimen. Although the ideal is a simple in vitro assay that predicts clinical outcomes, this is difficult or impossible to achieve.66 What can be done is to choose measurements of a specimen’s state that one believes are relevant to the ability of a device to have therapeutic value in a patient. This goal is best served when the device has been rationally designed with a hypothesized mechanism of action. In this way, measurements can be selected based on their ability to provide information about the device’s influence on the mechanism of action. The measurement provides information about the state of the device and will not predict efficacy. The role of a standards document is to provide information for making the measurements as reproducible, accurate, precise and reliable as possible. Good measurements confirm that the state of the specimen is the same as it was previously, that one batch of product is similar to another batch or that your materials are the same as someone else’s.
A standard test method does not define the best measurement
A common misconception of a standard for a measurement test method is that it represents the “best method for measuring a particular state of a material.” This is not true. A standard test method does not have to address the best measurement method, but does address the best way to practice a particular measurement method. There may be many measurement methods that can be used to determine a particular material state. For instance, there are many ways to measure the mechanical properties of a material. Thus, a standard test method presents detailed advice on how to effectively perform a particular measurement method according to a consensus of technical experts in the community.
Focus on “CLINICAL”
Interactions between bioengineers, biologists, clinicians and material scientists naturally stir dialogues as to whether biology (“BIOmaterials”) or material issues (“bioMATERIALS”) are of greater concern. At the workshop, however, the end-users, surgeons and dentists, rightfully emphasized that “CLINICAL” should be the greatest concern – the goal is to treat patients. This serves as a reminder that interdisciplinary TEMPs teams should strive to include physicians, even if only as consultants, to help maintain focus on clinical endpoints.
4. Conclusions and the future
The workshop was a great opportunity to reflect on the current portfolio of TEMPs standards and to identify the highest priority items for future scaffold standards. The four highest priority items identified were as follows: 1) standard test methods and standard practices (we have many standard guides); 2) standards for measuring scaffold structure; 3) standards for biological characterization of scaffolds; and 4) standards for measuring scaffold mechanical properties. The next steps are identifying and recruiting additional technical experts from academia, clinics and industry to advance these efforts. Several new ASTM work items have already been initiated, including standards for measuring stem cell-catheter interactions, advanced characterization of collagen-based products, measuring decellularization of extracellular matrices and characterizing bioglasses. Many scaffold standard needs have been identified and the real challenge of writing those standards is underway. Please contact Dr. Carl Simon (301-975-8574, carl.simonnist.gov) if you would like to become involved in the development of the medical device consensus standards discussed within.
Supplementary Material
Acknowledgments
The authors thank all the speakers and attendees for their participation and in making the workshop a success. The authors also thank ASTM for hosting the workshop. We thank both ASTM and ISO for providing efficient and well-organized forums for the development of consensus standards.
References
- 1.Plant AL, Horowitz E. Symposium on metrology and standards for cell signaling: impact on tissue engineering, National Institute for Standards and Technology, October 14–15, 2003. Tissue Eng. 2005;11:985–990. doi: 10.1089/ten.2005.11.985. [DOI] [PubMed] [Google Scholar]
- 2.Russell AJ. Standardized experimental procedures in tissue engineering: cure or curse. Tissue Eng. 2005;11:vii–vix. doi: 10.1089/ten.2005.11.vii. [DOI] [PubMed] [Google Scholar]
- 3.U.S.Food and Drug Administration. White Oak, MD: FDA; 2007. Guidance for Industry and FDA Staff: Frequently Asked Questions on Recognition of Consensus Standards. Available from URL: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm074973.htm. [Google Scholar]
- 4.Messenger MP, Tomlins PE. Regenerative medicine: a snapshot of the current regulatory environment and standards. Adv Healthcare Mater. 2011;23:H10–H17. doi: 10.1002/adma.201100254. [DOI] [PubMed] [Google Scholar]
- 5.Leitner E, Bischoff P. setting standards for technologies in regenerative medicine. Biomed Tech (Berl) 2012;57:1051–1054. [Google Scholar]
- 6.American Academy of Orthopaedic Surgeons. Position Statement: Consensus Standards for Medical Devices. Rosemont, IL: AAOS; 2013. Available from URL: http://www.aaos.org/about/papers/position/1169.asp. [Google Scholar]
- 7.Tomlins P. Chapter 6: Standards in cell and tissue engineering. In: Salih V, editor. Standardisation in cell and tissue engineering. Cambridge, UK: Woodhead International Limited; 2013. pp. 107–123. [Google Scholar]
- 8.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:696–701. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes KR, Wang J, Baggerly KA. Microarrays: retracing steps. Nat Med. 2007;13:1276–1277. doi: 10.1038/nm1107-1276b. [DOI] [PubMed] [Google Scholar]
- 10.Freedman DH. Lies, damned lies and medical science. The Atlantic. 2010 Nov 4; [Google Scholar]
- 11.Lehrer J. The truth wears off: is there something wrong with the scientific method? The New Yorker. 2010 Dec 13; [Google Scholar]
- 12.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712–713. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 13.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 14.Global Biological Standards Institute. The Case for Standards in Life Science Research: Seizing Opportunities at a Time of Critical Need. Washington, DC: GBSI; 2013. Available from URL: http://gbsi.org/CaseForStandards. [Google Scholar]
- 15.Plant AL, Parker GC. Translating stem cell research from the bench to the clinic: a need for better quality data. Stem Cells Dev. 2013;22:2457–2458. doi: 10.1089/scd.2013.0188. [DOI] [PubMed] [Google Scholar]
- 16.Wadman M. NIH mulls rules for validating key results. Nature. 2013;500:14–16. doi: 10.1038/500014a. [DOI] [PubMed] [Google Scholar]
- 17.Alliance for Regenerative Medicine. Pharma and Biotech Survey. Washington, DC: ARM; 2014. Available from URL: http://alliancerm.org/sites/default/files/ARM_Pharma_SurveyRept_Mar2014_e.pdf. [Google Scholar]
- 18.The Science Exchange. The Reproducibility Initiative. 2013 Available from URL: https://www.scienceexchange.com/reproducibility. [Google Scholar]
- 19.ASTM Standard F2312-11. Standard Terminology Relating to Tissue Engineered Medical Products. West Conshocken, PA: ASTM International; 2011. [Google Scholar]
- 20.Makower J, Meer A, Denend L. In: FDA Impact on U.S. medical technology innovation: A survey of over 200 medical technology companies. PricewaterhouseCoopers LLP, editor. 2010. Available from URL: http://nvcaccess.nvca.org/index.php/topics/public-policy/155-fda-impact-on-innovation-study-out-today.html. [Google Scholar]
- 21.ASTM Standard F2739-08. Standard Guide for Quantitating Cell Viability within Biomaterial Scaffolds. West Conshocken, PA: ASTM International; 2008. [Google Scholar]
- 22.ASTM Standard F2450-10. Standard Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue-Engineered Medical Products. West Conshocken, PA: ASTM International; 2010. [Google Scholar]
- 23.ASTM Standard F2315-11. Standard Guide for Immobilization or Encapsulation of Living Cells or Tissue in Alginate Gels. West Conshocken, PA: ASTM International; 2011. [Google Scholar]
- 24.ASTM Standard F2883-11. Standard Guide for Characterization of Ceramic and Mineral Based Scaffolds used for Tissue-Engineered Medical Products (TEMPs) and as Devices for Surgical Implant Applications. West Conshocken, PA: ASTM International; 2011. [Google Scholar]
- 25.ASTM Standard F2900-11. Standard Guide for Characterization of Hydrogels used in Regenerative Medicine. West Conshocken, PA: ASTM International; 2011. [Google Scholar]
- 26.ASTM Standard F2630-06(2012) Standard Guide for Interpreting Images of Polymeric Tissue Scaffolds. West Conshocken, PA: ASTM International; 2012. [Google Scholar]
- 27.ASTM Standard F2150-13. Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products. West Conshocken, PA: ASTM International; 2013. [Google Scholar]
- 28.ISO Standards 10993 Series. Biological evaluation of medical devices -- Parts 1–20. Geneva, Switzerland: International Organization for Standardization; 2012. [Google Scholar]
- 29.Bruinink A, Luginbuehl R. Evaluation of biocompatibility using in vitro methods: interpretation and limitations. Adv Biochem Eng Biotechnol. 2012;126:117–152. doi: 10.1007/10_2011_111. [DOI] [PubMed] [Google Scholar]
- 30.Lane JG, Massie JB, Ball ST, Amiel ME, Chen AC, Bae WC, et al. Follow-up of osteochondral plug transfers in a goat model: a 6-month study. Am J Sports Med. 2004;32:1440–1450. doi: 10.1177/0363546504263945. [DOI] [PubMed] [Google Scholar]
- 31.Naylor JM, Ko V, Rougellis S, Green N, Hackett D, Magrath A, et al. Is discharge knee range of motion a useful and relevant clinical indicator after total knee replacement? Part 2. J Eval Clin Pract. 2012;18:652–658. doi: 10.1111/j.1365-2753.2011.01656.x. [DOI] [PubMed] [Google Scholar]
- 32.Khanna G, Singh JA, Pomeroy DL, Gioe TJ. Comparison of patient-reported and clinician-assessed outcomes following total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1–7. doi: 10.2106/JBJS.J.00850. [DOI] [PubMed] [Google Scholar]
- 33.NIST Reference Material 8395. Tissue Engineering Reference Scaffold, 200 Micrometer Strut Spacing, Report of Investigation. Gaithersburg, MD: NIST; 2009. [Google Scholar]
- 34.NIST Reference Material 8396. Tissue Engineering Reference Scaffold, 300 Micrometer Strut Spacing, Report of Investigation. Gaithersburg, MD: NIST; 2009. [Google Scholar]
- 35.NIST Reference Material 8397. Tissue Engineering Reference Scaffold, 450 Micrometer Strut Spacing, Report of Investigation. Gaithersburg, MD: NIST; 2009. [Google Scholar]
- 36.NIST Reference Material 8394. Tissue Engineering Reference Scaffolds for Cell Culture, Report of Investigation. Gaithersburg, MD: NIST; 2012. [Google Scholar]
- 37.Viswanathan S, Keating A, Deans R, Hematti P, Prockop D, Stroncek DF, et al. Soliciting strategies for developing cell-based reference materials to advance MSC research and clinical translation. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0591. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NIST Standard Reference Material 2910. Calcium Hydroxyapatite, Report of Investigation. Gaithersburg, MD: NIST; 2008. [Google Scholar]
- 39.NIST Reference Material 8011. Gold Nanoparticles, Nominal 10nm Diameter, Report of Investigation. Gaithersburg, MD: NIST; 2012. [Google Scholar]
- 40.NIST Reference Material 8012. Gold Nanoparticles, Nominal 30nm Diameter, Report of Investigation. Gaithersburg, MD: NIST; 2012. [Google Scholar]
- 41.NIST Reference Material 8013. Gold Nanoparticles, Nominal 60nm Diameter, Report of Investigation. Gaithersburg, MD: NIST; 2012. [Google Scholar]
- 42.NIST Standard Reference Material 2372. Human DNA Quantitation Standard, Report of Investigation. Gaithersburg, MD: NIST; 2013. [Google Scholar]
- 43.NIST Reference Material 8456. Ultra High Molecular Weight Polyethylene, Report of Investigation. Gaithersburg, MD: NIST; 2011. [Google Scholar]
- 44.NIST Reference Material 8457. Ultra High Molecular Weight Polyethylene, Report of Investigation. Gaithersburg, MD: NIST; 2012. [Google Scholar]
- 45.NIST Standard Reference Material 2374. DNA Sequence Library for External RNA Controls, Report of Investigatio. Gaithersburg, MD: NIST; 2013. [Google Scholar]
- 46.Luginbuehl R. Do's and don'ts in characterizing scaffolds for tissue engineering. Dübendorf, Switzerland: Fifth World Materials Research Institutes Forum:"Materials Meet Life"; 2013. [Google Scholar]
- 47.University of Maryland Center for Excellence in Regulatory Science and Innovation. College Park, MD: University of Maryland; 2013. Available from URL: http://www.cersi.umd.edu/ [Google Scholar]
- 48.Scopus. Elsevier Inc.; 2014. Available from URL: http://www.scopus.com/ [Google Scholar]
- 49.ASTM Standard F1635-11. ASTM F1635-11 Standard Test Method for in vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants. West Conshocken, PA: ASTM International; 2011. [Google Scholar]
- 50.ASTM Standard D3786-13. Standard Test Method for Bursting Strength of Textile Fabrics-Diaphragm Bursting Strength Tester Method. West Conshocken, PA: ASTM International; 2013. [Google Scholar]
- 51.ASTM Standard D5035-11. Standard Test Method for Breaking Force and Elongation of Textile Fabrics (Strip Method) West Conshocken, PA: ASTM International; 2011. [Google Scholar]
- 52.ASTM Standard D5587-08. Standard Test Method for Tearing Strength of Fabrics by Trapezoid Procedure. West Conshocken, PA: ASTM International; 2008. [Google Scholar]
- 53.ASTM Standard F2027-08. Standard Guide for Characterization and Testing of Raw or Starting Biomaterials for Tissue-Engineered Medical Products. West Conshocken, PA: ASTM International; 2008. [Google Scholar]
- 54.ASTM Standard F2211-13. Standard Classification for Tissue Engineered Medical Products (TEMPs) West Conshocken, PA: ASTM International; 2013. [Google Scholar]
- 55.ISO Standards 11135 Series. Sterilization of health care products -- Ethylene oxide -- Parts 1–2. Geneva, Switzerland: International Organization for Standardization; 2008. [Google Scholar]
- 56.ASTM Standard F748-06. Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices. West Conshocken, PA: ASTM International; 2010. [Google Scholar]
- 57.ISO Standard 10993-5. Biological evaluation of medical devices -- Part 5: Tests for in vitro cytotoxicity. Geneva, Switzerland: International Organization for Standardization; 2009. [Google Scholar]
- 58.ISO Standard 10993-13. Biological evaluation of medical devices -- Part 13: Identification and quantification of degradation products from polymeric medical devices. Geneva, Switzerland: International Organization for Standardization; 2010. [Google Scholar]
- 59.ISO Standard 10993-3. Biological evaluation of medical devices -- Part 3: Tests for genotoxicity, carcinogenicity and reproductive toxicity. Geneva, Switzerland: International Organization for Standardization; 2003. [Google Scholar]
- 60.ISO Standard 10993-6. Biological evaluation of medical devices -- Part 6: Tests for local effects after implantation. Geneva, Switzerland: International Organization for Standardization; 2007. [Google Scholar]
- 61.ISO Standard 10993-10. Biological evaluation of medical devices -- Part 10: Tests for irritation and skin sensitization. Geneva, Switzerland: International Organization for Standardization; 2010. [Google Scholar]
- 62.Jaklenec A, Stamp A, Deweerd E, Sherwin A, Langer R. Progress in the tissue engineering and stem cell industry" are we there yet? Tissue Eng Part B Rev. 2012;18:155–166. doi: 10.1089/ten.TEB.2011.0553. [DOI] [PubMed] [Google Scholar]
- 63.Williams D, O'Donnell P. The Xi'an Papers, World Summit on Regenerative Medicine. Xi'an, China: Book Conference Solution Co; 2013. Available from URL: http://northheadcomms.com/cn/wp-content/uploads/2013/11/200131031-Xian-Papers-RM-summit.pdf. [Google Scholar]
- 64.Yokoi M, Hattori K, Narikawa K, Ohgushi H, Tadokoro M, Hoshi K, et al. Feasibility and limitations of the round robin test for assessment of in vitro chondrogenesis evaluation protocol in a tissue-engineered medical product. J Tissue Eng Regen Med. 2012;6:550–558. doi: 10.1002/term.460. [DOI] [PubMed] [Google Scholar]
- 65.ASTM Standard F2131-02. Standard Test Method for In Vitro Biological Activity of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Using the W-20 Mouse Stromal Cell Line. West Conshocken, PA: ASTM International; 2007. [Google Scholar]
- 66.Bravery CA, Carmen J, Fong T, Oprea W, Hoogendoorn KH, Woda J, et al. Potency assay development for cellular therapy products: an ISCT review of the requirements and experiences in the industry. Cytotherapy. 2013;15:9–19. doi: 10.1016/j.jcyt.2012.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.