Abstract
BACKGROUND
Hypertension is associated with the occurrence of cognitive deficits and dementia, probably because hypertension is a major risk factor for the occurrence of brain damage as a result of cerebral small vessel disease (cSVD). Endothelial activation and inflammation have been suggested to play an important role in the pathogenesis of cSVD. We investigated if compound scores of endothelial activation or inflammation, based on several blood markers, are associated with cognitive performance 3 years later in patients with essential hypertension.
METHODS
At baseline, levels of blood markers of endothelial activation (soluble vascular cellular adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), sP-selectin, and sE-selectin) and markers of inflammation (neopterin, C-reactive protein, and sICAM-1) were measured and transformed into compound scores using z-scores. In addition, a brain magnetic resonance imaging (MRI) was performed to determine the presence of cSVD-related MRI markers. Three years later, patients underwent a neuropsychological assessment to determine cognitive performance.
RESULTS
A total of 101 patients with hypertension were included in the present study. In multiple linear regression analyses with correction for demographics and MRI markers, the compound score of endothelial activation (B = −0.19, 95% confidence interval = −0.34 to −0.04, P = 0.014), but not of inflammation (B = −0.09, 95% confidence interval = −0.22 to 0.05, P = 0.198), was associated with worse cognitive performance.
CONCLUSIONS
Our results show that an overall measure of endothelial activation is associated with cognitive performance in patients with essential hypertension. This indicates that a process involving endothelial activation might play a role in the pathogenesis of cognitive problems in patients with hypertension.
Keywords: blood pressure, cerebral small vessel disease, cognition, endothelial activation, hypertension, inflammation.
Hypertension is associated with the occurrence of cognitive deficits and dementia,1 probably because hypertension is a major risk factor for the occurrence of brain damage as a result of cerebral small vessel disease (cSVD).2 Endothelial activation and inflammation have been suggested to play an important role in the pathogenesis of cSVD.3 Studies have shown higher levels of markers of endothelial activation and inflammation in patients with cSVD4,5 or hypertension6 compared with healthy controls. In addition, in Alzheimer’s dementia, in which hypertension is also an important vascular risk factor, endothelial activation and inflammation have been suggested to be involved in the pathogenesis.7,8
Several studies have investigated associations between cognition and markers of endothelial activation or inflammation and have shown inconsistent results.9–15 These studies focused on 1 or more individual markers, attempting to find biomarkers for predicting cognitive functioning. However, conclusions about the pathogenetic role of endothelial activation or inflammation in general are hard to draw, based on these studies testing single blood markers. By considering multiple blood markers of endothelial activation or inflammation and combining them into 1 compound score,16,17 it is possible to draw more general conclusions about the underlying pathogenesis leading to cognitive problems in patients with hypertension.
The aim of the present study is to test the hypothesis that endothelial activation and inflammation, expressed as a compound score of several markers, are associated with cognitive performance 3 years later in patients with essential hypertension. In addition, we investigated if these associations are independent of possible confounders, in particular, the presence of magnetic resonance imaging (MRI) markers of cSVD.
METHODS
Study population
From the hypertension outpatient clinic of the Department of Internal Medicine of Maastricht University Medical Centre, the Netherlands, 218 patients were recruited, for a study on brain damage in patients with essential hypertension (HYBRiD).18 Hypertension was defined as an off-medication, clinically measured conventional blood pressure ≥140mm Hg systolic or ≥90mm Hg diastolic, or both. Details about the HYBRiD study have been described previously.18 Exclusion criteria were a history of symptomatic cardio- or cerebrovascular disease or contraindications for MRI. Two years after inclusion in the HYBRiD study, patients were asked to participate in the present study. Blood samples were taken to determine levels of markers of endothelial activation and inflammation, and a brain MRI was performed. In addition, 3 (on-medication) office blood pressure measurements were taken and the mean arterial pressure and the pulse pressure from the second and third measurement were averaged. At that same time point, information about the vascular risk factors (body mass index, smoking, and the presence of diabetes mellitus or hypercholesterolemia) was obtained. Three years after blood sampling and MRI, patients were asked for a follow-up measurement, during which a neuropsychological assessment was performed. This study was approved by the Medical Ethics Committee of the Maastricht University Medical Centre, and all participants gave written informed consent.
Markers of endothelial activation and inflammation
Blood was sampled in the morning after the blood pressure measurement and after an overnight fast. Medication was taken as usual. Blood sampling was performed without a tourniquet, from an antecubital vein, into 5-ml serum and 4-ml (EDTA) plasma tubes (BD Biosciences, Breda, the Netherlands).19 Markers of endothelial activation included soluble vascular cellular adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), soluble (s)P-selectin and (s)E-selectin. They were all measured using commercially available enzyme-linked immunosorbent assay kits (BioSource Europe, Nivelles, Belgium) according to the manufacturer’s instructions. For sVCAM-1, sICAM-1, sP-selectin, and sE-selectin, intra-assay variability was 3.1%, 4.1%, 2.4%, and 5.4%, respectively, whereas inter-assay variability was 5.2%, 7.7%, 5.2%, and 6.0%, respectively. Markers of inflammation included sICAM-1, neopterin, and high-sensitivity C-reactive protein (hsCRP). sICAM-1 was included in both compound scores, since it is expressed by both monocytes and endothelial cells.20 Neopterin was measured using commercially available enzyme-linked immunosorbent assay kits (IBL, Hamburg, Germany), following manufacturer’s instructions. Intra- and inter-assay variability were 3.6% and 7.2%, respectively. hsCRP was determined with nephelometry, using the BN ProSpec (Siemens, Germany); Intra- and inter-assay variability were 1.4% and 0.9%, respectively.
Levels of individual markers were transformed into standardized values (z-scores), by dividing the difference between the individual score and the sample mean by the sample standard deviation. For each patient, z-scores of sP-selectin, sE-selectin, sICAM-1, and sVCAM-1 were then averaged to an endothelial activation compound score and z-scores of hsCRP, neopterin, and sICAM-1 were averaged to an inflammation compound score. hsCRP levels were missing in 15 patients; inflammation scores of these patients were based on levels of neopterin and sICAM-1 only.
Neuropsychological assessment
Cognitive performance was measured with an extensive neuropsychological assessment, as has been described previously.21 Memory domain was measured with the Rey Auditory Verbal Learning Test22 (immediate recall, delayed recall, and delayed recognition) and the Digit Span Forward (subtest of Wechsler Adult Intelligence Scale-III23). Executive function domain was measured with the Stroop Colour Word Test24 interference score (time of part 3 minus mean time of parts 1 and 2), Trail Making Test25 interference score (time of part 2 minus time of part 1), Category (animals and professions)26 and Letter Fluency,27 Letter-Number Sequencing (subtest of Wechsler Adult Intelligence Scale-III), and Digit Span Backward (subtest of Wechsler Adult Intelligence Scale-III). Information processing speed domain was measured with the Symbol Substitution-Coding (subtest of Wechsler Adult Intelligence Scale-III), Trail Making Test part A, and Stroop Colour Word Test parts 1 and 2. Test scores were transformed into standardized values (z-scores), by dividing the difference between the individual raw score and the sample mean by the sample SD. For each patient, domain scores were calculated by averaging these z-scores of the tests within that domain. Finally, the overall cognition score was calculated by averaging the domain scores of memory, executive function, and information processing speed.
The Dutch Adult Reading Test (DART) was used as a measure of prior cognitive ability at young age.28 The Rotterdam-Cambridge Cognitive Examination (R-CAMCOG) was used to determine the presence of possible dementia, defined as a score <34.29
MRI data
Four recognized MRI features of cSVD (lacunes, cerebral microbleeds, white matter hyperintensities, and perivascular spaces) were measured on baseline brain MRI (standard axial T2-weighted, FLAIR and T2* gradient echo sequences, Intera 1.5-T, Philips Medical Systems, Best, the Netherlands). Two experienced vascular neurologists individually rated the presence of lacunes, cerebral microbleeds, and perivascular spaces in basal ganglia. Definitions and rating method of these lesions have been described previously.21 White matter hyperintensities volume was semiautomatically assessed using the image-processing software package GIANT, version 2V1.26 (General Imaging and Analysis Tools; Department of Psychiatry and Neuropsychology, Maastricht University, Maastricht, The Netherlands).30 A trained observer performed the quantitative assessments after reaching excellent interobserver agreement with an experienced neuroradiologist.
Statistical analysis
IBM SPSS Statistics 20 software was used for all analyses. The associations between endothelial activation or inflammation compound scores (predictor) and overall cognition (dependent variable) were assessed with simple linear regression analyses. Additionally, we corrected these associations for possible confounders. In model 1, the associations were adjusted for demographics (sex, age, and DART score). In model 2, the associations were adjusted for demographics and vascular risk factors (diabetes mellitus, hypercholesterolemia, smoking, body mass index, pulse pressure, and mean arterial pressure). In model 3, the associations were adjusted for demographics and all of the MRI markers of cSVD (white matter hyperintensities volume and the presence of lacunes, cerebral microbleeds, and perivascular spaces). Our sample size precluded a model with all predictors (demographics, vascular risk factors, and MRI markers of cSVD), as this would make the number of predictors in the model too high. Stepwise analyses of the multiple regression analyses models 1, 2, and 3 were performed to investigate how much of the variance in cognitive function was explained by endothelial activation score or inflammation score additional to the covariates. We also tested for interaction effects between MRI markers of cSVD and endothelial activation or inflammation compound scores on overall cognition. Simple and multiple regression analyses were repeated with domain scores of memory, executive function, or information processing speed as dependent variable. Results were considered significant at P < 0.05.
RESULTS
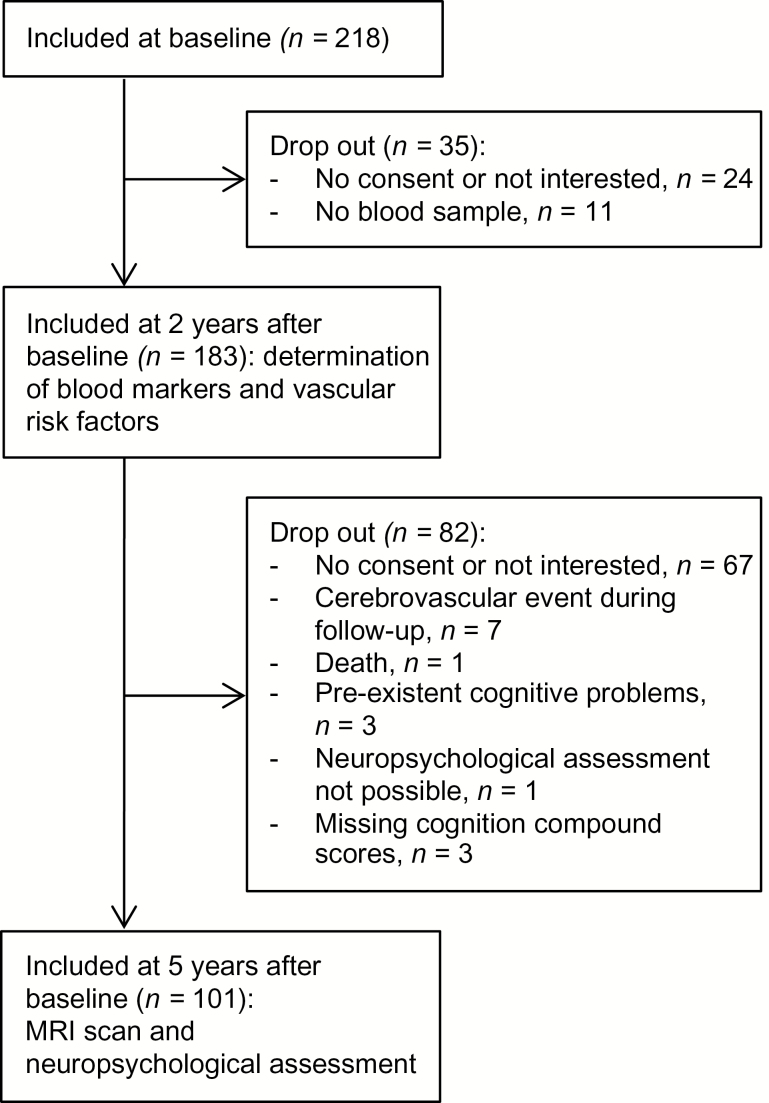
Of the original 218 patients in the HYBRiD study, 183 patients were included in the present study that started 2 years later and 104 patients completed the follow-up 3 years later. Three patients were excluded from analyses because cognition compound scores were missing. Of 1 patient the DART score was missing. Imputation of the mean was used for this missing value to prevent exclusion of this patient. Flow of participants is shown in Figure 1. The included patients (n = 101) did not differ from the excluded patients (n = 117) in age (54.7±11.5 vs. 54.3±13.6, P = 0.819), but included patients were more often male (60.4% vs. 42.5%, P = 0.008). Patients’ characteristics are shown in Table 1. One patient had an R-CAMCOG score <34, which indicates possible dementia.
Figure 1.
Schematic view of flow of participants.
Table 1.
Patients’ characteristics
| Characteristic | All patients (n = 101) |
|---|---|
| Age (years, SD) | 54.7 (11.5) |
| Male sex (%) | 61 (60.4) |
| DART score (SD) | 82.6 (1.4) |
| Median R-CAMCOG (IQR) | 43 (39–47) |
| Diabetes mellitus (%) | 4 (4.0) |
| Hypercholesterolemia (%) | 40 (39.6) |
| Current smoking (%) | 18 (17.8) |
| Body mass index (kg/m2, SD) | 28.0 (4.3) |
| MAP (mm Hg, SD) | 108.3 (11.9) |
| Pulse Pressure (mm Hg, SD) | 54.5 (14.7) |
| Antihypertensive medication use (%) | 90 (89.1) |
| Blood markers | |
| Median of hsCRP (mg/l, IQR)a | 1.8 (0.84–3.8) |
| Median of neopterin (ng/ml, IQR) | 1.5 (1.3–1.8) |
| Median of sP-selectin (ng/ml, IQR) | 35.7 (22.0–61.5) |
| Median of sE-selectin (ng/ml, IQR) | 22.1 (17.2–34.0) |
| Median of sICAM-1 (ng/ml, IQR) | 428.4 (378.1–537.2) |
| Median of sVCAM-1 (ng/ml, IQR) | 677.2 (567.0–760.9) |
| MRI markers | |
| Median of WMH volume (mm3, IQR) | 666.9 (369.3–1,553.8) |
| PVS basal ganglia (%) | 33 (32.7%) |
| Cerebral microbleeds (%) | 12 (11.9%) |
| Lacunes (%) | 6 (6.0%) |
Abbreviations: IQR, interquartile range; MAP, mean arterial pressure; WMH, white matter hyperintensities; PVS, perivascular spaces; DART, Dutch Adult Reading Test; hsCRP, high-sensitivity C-reactive protein.
a n = 86.
In unadjusted regression analyses, overall cognition was significantly associated with compound scores of endothelial activation and inflammation (Table 2). The association between endothelial activation score and overall cognition remained significant after correction for age, sex, and DART score (model 1), as well as after additional correction for vascular risk factors (model 2) or MRI markers (model 3). In model 1, age, sex, and DART score explained 61% of the variance in cognitive performance. In models 1, 2, and 3, respectively, 2.7%, 2.7%, and 2.4% of the variance in cognitive performance was explained by endothelial activation additional to the covariates in these models. The association between inflammation score and overall cognition was no longer significant after adjustment for age, sex, and DART score (model 1). In models 1, 2, and 3 of the multiple regression analyses, respectively, 0.8%, 1.0% and 0.7% of the variance in cognitive performance was explained by inflammation. No interaction effects were found between MRI markers of cSVD and endothelial activation or inflammation score on overall cognition (results not shown).
Table 2.
Association between endothelial activation and inflammation scores and overall cognition score
| Endothelial activation | Inflammation B (95% CI) | ||||
|---|---|---|---|---|---|
| B (95% CI) | P | P | |||
| Simple linear regression | −0.39 (−0.60 to −0.19) | <0.001 | −0.22 (−0.42 to −0.01) | 0.038 | |
| Multiple linear regression | Model 1 | −0.19 (−0.34 to −0.05) | 0.008 | −0.10 (−0.23 to 0.04) | 0.155 |
| Model 2 | −0.20 (−0.35 to −0.05) | 0.008 | −0.11 (−0.25 to 0.03) | 0.116 | |
| Model 3 | −0.19 (−0.34 to −0.04) | 0.014 | −0.09 (−0.22 to 0.05) | 0.198 | |
Abbreviations: B, unstandardized regression coefficient; CI, confidence interval.
Model 1: adjusted for age, sex, and DART score; Model 2: adjusted for age, sex, DART score, and vascular risk factors; Model 3: adjusted for age, sex, DART score, and MRI markers.
Associations between endothelial activation score and the domain scores of executive function and information processing speed were significant in the simple regression analyses as well as in the adjusted regression analyses (Table 3), except for the association between endothelial activation and information processing speed additionally corrected for MRI markers. Memory was not associated with endothelial activation in the adjusted regression analyses. Associations between inflammation score and memory, executive function, and information processing speed were not significant in the adjusted regression analyses (Table 3).
Table 3.
Association between endothelial activation and inflammation scores and cognitive domains
| Endothelial activation | Inflammation | ||||
|---|---|---|---|---|---|
| B (95% CI) | P | B (95% CI) | P | ||
| Memory | |||||
| Simple linear regression | −0.33 (−0.56 to −0.11) | 0.004 | −0.17 (−0.39 to 0.05) | 0.124 | |
| Multiple linear regression | Model 1 | −0.15 (−0.34 to 0.05) | 0.145 | −0.08 (−0.27 to 0.10) | 0.369 |
| Model 2 | −0.16 (−0.36 to 0.04) | 0.124 | −0.09 (−0.28 to 0.10) | 0.339 | |
| Model 3 | −0.15 (−0.35 to 0.06) | 0.159 | −0.08 (−0.26 to 0.11) | 0.395 | |
| Executive function | |||||
| Simple linear regression | −0.41 (−0.63 to −0.19) | <0.001 | −0.26 (−0.48 to −0.04) | 0.020 | |
| Multiple linear regression | Model 1 | −0.23 (−0.40 to −0.07) | 0.008 | −0.13 (−0.29 to 0.03) | 0.104 |
| Model 2 | −0.24 (−0.42 to −0.07) | 0.008 | −0.16 (−0.32 to 0.01) | 0.071 | |
| Model 3 | −0.22 (−0.40 to −0.04) | 0.015 | −0.12 (−0.28 to 0.04) | 0.137 | |
| Information processing speed | |||||
| Simple linear regression | −0.43 (−0.69 to −0.16) | 0.002 | −0.22 (−0.48 to 0.04) | 0.101 | |
| Multiple linear regression | Model 1 | −0.21 (−0.40 to −0.02) | 0.035 | −0.08 (−0.25 to 0.10) | 0.390 |
| Model 2 | −0.20 (−0.39 to −0.01) | 0.042 | −0.09 (−0.27 to 0.09) | 0.324 | |
| Model 3 | −0.19 (−0.39 to 0.01) | 0.061 | −0.06 (−0.24 to 0.12) | 0.480 | |
Abbreviations: B, unstandardized regression coefficient; CI, confidence interval.
Model 1: adjusted for age, sex, and DART score; Model 2: adjusted for age, sex, DART score, and vascular risk factors; Model 3: adjusted for age, sex, DART score, and MRI markers.
Associations between overall cognition and all individual blood markers can be found in Supplementary Table S1.
DISCUSSION
We showed that endothelial activation, represented by a compound score of several blood markers for endothelial function, is associated with cognitive performance 3 years later in patients with hypertension, also after controlling for MRI markers of cSVD or vascular risk factors. In addition, results showed that 2.7% of the variance in cognitive performance was explained by endothelial activation after controlling for demographics and vascular risk factors. In contrast, we could not find an independent association between inflammation score and cognitive performance.
Previous studies have reported associations between cognitive performance and levels of several individual markers, such as CRP, haptoglobin, plasma fibrinogen, interleukin-6, and sICAM-1,10–12 whereas other studies failed to show associations with these and other markers.12,13 We examined the association with a compound score of markers, which enables us to draw conclusions about the overall effect of endothelial activation and inflammation instead of a diversity of conclusions based on individual markers. Furthermore, the use of compound scores reduces the effect of biological variability of each blood marker and reduces the number of investigated associations and thereby minimizes problems of multiple testing.
We could find only one other recent study16 that investigated this association between cognitive performance and inflammation or endothelial activation, in a sample of elderly people using compound scores of blood markers. In this study, an association of both inflammation and endothelial dysfunction with worse cognitive performance on the information processing speed domain and the attention and executive function domain was shown.16 We only showed an association between cognition and endothelial activation, which suggests that endothelial activation is primary involved in the pathogenesis leading to cognitive problems in patients with hypertension. Disruption of the blood–brain barrier might be such an endothelium mediated process.31 The results of our study also support the vascular hypothesis of cognitive decline and are in agreement with other studies showing elevated levels of markers of endothelial activation in patients with Alzheimer’s disease.32,33 The partly contradicting results of our study and the previous one16 involving inflammation could possibly be explained by the different populations that were investigated or by the small sample size of our study (101 patients) in contrast to the 363 patients included in the aforementioned study. The post hoc calculated power of the observed small effect size for the association between inflammation and cognitive performance (effect size f 2 = 0.021, in multiple regression with correction for age, sex, and DART score) was only 30%. In contrast, our multiple regression analysis investigating the association between endothelial activation and cognitive performance showed an effect size (f 2) of 0.074, which established a power of 77.4%
Strengths of our study include the extensive neuropsychological assessment and accounting for the presence of MRI markers of cSVD. These MRI markers might be the causal intermediates in the association between endothelial activation or inflammation and cognitive performance. However, we have shown that the association between endothelial activation and cognition still exists after correction for MRI markers, and the regression coefficient of endothelial activation or inflammation even hardly changed in the model including the MRI markers. Therefore, we can conclude that this association is not dependent on the presence of macrostructural brain damage. There must be other pathways present between endothelial activation and cognitive performance. For example, microstructural damage, not visible on the used MRI sequences, or other processes than cSVD might have considerable effect on cognitive function. Similarly, endothelial activation was associated with cognitive performance, independently of vascular risk factors. Although the influence of vascular risk factors on endothelial activation is considered important, our results implicate that others factors, besides vascular risk factors, also influence endothelial activation.
A limitation of the study is the lack of neuropsychological assessment at baseline, which withheld us from determining cognitive decline. However, since we used the DART to control for prior cognitive performance, results actually reflect associations with a lifetime change in cognitive performance. Secondly, information about the hypertension status or treatment at the time of the neuropsychological assessment was not available. Thirdly, since we included only 101 patients of the 218 patients of the previous study, this might have caused a certain selection bias. Last, all markers were equally weighted in the compound scores of endothelial activation and inflammation, but it is unsure whether they really all are equally important in the process of endothelial activation or inflammation. Furthermore, there might be other blood markers of interest that we did not measure. However, the circulating adhesion molecules that we used are believed to serve as markers for the detection of general endothelial activation34 and therefore give an overall insight into endothelial activation. We used hsCRP, neopterin, and sICAM-1 to assess inflammation; these markers globally encompass the process of inflammation (leukocyte adhesion and activation). In addition, inflammation and endothelial activation markers were measured only once and intra-individual fluctuations cannot be excluded. However, markers were measured in a non-active phase of a chronic condition, so levels are expected to remain relatively stable.
In conclusion, our study shows that a compound score of endothelial activation is associated with cognitive performance in patients with hypertension. These results suggest that a process of endothelial activation might be involved in the pathogenesis of cognitive problems in patients with hypertension. Future research is necessary to further investigate the role of endothelial activation in processes causing cognitive problems in these patients.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the “Stichting De Weijerhorst.” We thank E. Gronenschild for assistance with the assessment of the white matter hyperintensities volume.
REFERENCES
- 1. Iadecola C. Hypertension and dementia. Hypertension 2014; 64:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veglio F, Paglieri C, Rabbia F, Bisbocci D, Bergui M, Cerrato P. Hypertension and cerebrovascular damage. Atherosclerosis 2009; 205:331–341. [DOI] [PubMed] [Google Scholar]
- 3. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 2005; 112:900–905. [DOI] [PubMed] [Google Scholar]
- 5. Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, Thomas DJ, Markus HS. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003; 126:424–432. [DOI] [PubMed] [Google Scholar]
- 6. Boulbou MS, Koukoulis GN, Makri ED, Petinaki EA, Gourgoulianis KI, Germenis AE. Circulating adhesion molecules levels in type 2 diabetes mellitus and hypertension. Int J Cardiol 2005; 98:39–44. [DOI] [PubMed] [Google Scholar]
- 7. Kelleher RJ, Soiza RL. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am J Cardiovasc Dis 2013; 3:197–226. [PMC free article] [PubMed] [Google Scholar]
- 8. Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 2014; 6. Article no. 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umemura T, Kawamura T, Umegaki H, Mashita S, Kanai A, Sakakibara T, Hotta N, Sobue G. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2011; 82:1186–1194. [DOI] [PubMed] [Google Scholar]
- 10. Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, Wauters A, Maes M, Jolles J, Steinbusch HW, de Vente J. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol 2003; 134:142–150. [DOI] [PubMed] [Google Scholar]
- 11. Gunstad J, Bausserman L, Paul RH, Tate DF, Hoth K, Poppas A, Jefferson AL, Cohen RA. C-reactive protein, but not homocysteine, is related to cognitive dysfunction in older adults with cardiovascular disease. J Clin Neurosci 2006; 13:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc 2007; 55:700–707. [DOI] [PubMed] [Google Scholar]
- 13. Miralbell J, Soriano JJ, Spulber G, López-Cancio E, Arenillas JF, Bargalló N, Galán A, Barrios MT, Cáceres C, Alzamora MT, Pera G, Kivipelto M, Wahlund LO, Dávalos A, Mataró M. Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiol Aging 2012; 33:1003.e9–1003.17. [DOI] [PubMed] [Google Scholar]
- 14. Quinn TJ, Gallacher J, Deary IJ, Lowe GD, Fenton C, Stott DJ. Association between circulating hemostatic measures and dementia or cognitive impairment: systematic review and meta-analyzes. J Thromb Haemost 2011; 9:1475–1482. [DOI] [PubMed] [Google Scholar]
- 15. Obasi CN, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Nieto FJ, Shankar A, Fischer ME, Tsai MY, Chappell R. Association of biomarkers for inflammation, endothelial dysfunction and oxidative stress with cognitive impairment. The Epidemiology of Hearing Loss Study (EHLS). Oxid Antioxid Med Sci 2012; 1:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, Teerlink T, Scheffer PG, van den Hurk K, Kappelle LJ, Dekker JM, Biessels GJ. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population - the Hoorn Study. Psychoneuroendocrinology 2014; 40:108–118. [DOI] [PubMed] [Google Scholar]
- 17. Aribisala BS, Wiseman S, Morris Z, Valdés-Hernández MC, Royle NA, Maniega SM, Gow AJ, Corley J, Bastin ME, Starr J, Deary IJ, Wardlaw JM. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 2014; 45:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henskens LH, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Lodder J. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension 2008; 51:62–68. [DOI] [PubMed] [Google Scholar]
- 19. Rouhl RP, Damoiseaux JG, Lodder J, Theunissen RO, Knottnerus IL, Staals J, Henskens LH, Kroon AA, de Leeuw PW, Tervaert JW, van Oostenbrugge RJ. Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 2012; 33:1800–1806. [DOI] [PubMed] [Google Scholar]
- 20. Schram MT, Stehouwer CD. Endothelial dysfunction, cellular adhesion molecules and the metabolic syndrome. Horm Metab Res 2005; 37(Suppl 1):49–55. [DOI] [PubMed] [Google Scholar]
- 21. Uiterwijk R, Huijts M, Staals J, Duits A, Gronenschild E, Kroon AA, de Leeuw PW, van Oostenbrugge RJ. Subjective cognitive failures in patients with hypertension are related to cognitive performance and cerebral microbleeds. Hypertension 2014; 64:653–657. [DOI] [PubMed] [Google Scholar]
- 22. Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol 1985; 112:201–210. [DOI] [PubMed] [Google Scholar]
- 23. Wechsler D. WAIS III, Nederlandstalige Bewerking: Technische Handleiding. Swets Test Publishers: Lisse, 2001. [Google Scholar]
- 24. Golden CJ. Stroop Colour and Word Test. Stoelting: Chicago, IL, 1978. [Google Scholar]
- 25. Reitan R. Trail Making Test: Manual for Administration, Scoring and Interpretation. Indiana University: Bloomington, 1956. [Google Scholar]
- 26. Luteyn F. Een nieuwe verkorte GIT. Dutch J Psychol 1966; 2:675–682. [Google Scholar]
- 27. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press: New York, 2004. [Google Scholar]
- 28. Schmand B, Bakker D, Saan R, Louman J. The Dutch Reading Test for Adults: a measure of premorbid intelligence level. Tijdschr Gerontol Geriatr 1991; 22:15–19. [PubMed] [Google Scholar]
- 29. de Koning I, van Kooten F, Koudstaal PJ, Dippel DW. Diagnostic value of the Rotterdam-CAMCOG in post-stroke dementia. J Neurol Neurosurg Psychiatry 2005; 76:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Hofman PA, Lodder J, de Leeuw PW. Associations of ambulatory blood pressure levels with white matter hyperintensity volumes in hypertensive patients. J Hypertens 2009; 27:1446–1452. [DOI] [PubMed] [Google Scholar]
- 31. Oakley R, Tharakan B. Vascular hyperpermeability and aging. Aging Dis 2014; 5:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuliani G, Cavalieri M, Galvani M, Passaro A, Munari MR, Bosi C, Zurlo A, Fellin R. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci 2008; 272:164–170. [DOI] [PubMed] [Google Scholar]
- 33. Borroni B, Volpi R, Martini G, Del Bono R, Archetti S, Colciaghi F, Akkawi NM, Di Luca M, Romanelli G, Caimi L, Padovani A. Peripheral blood abnormalities in Alzheimer disease: evidence for early endothelial dysfunction. Alzheimer Dis Assoc Disord 2002; 16:150–155. [DOI] [PubMed] [Google Scholar]
- 34. Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers 2011; 16(Suppl 1):S11–S21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.