Abstract
AIMS
Observational studies report inconsistent associations between moderate alcohol intake and blood pressure (BP). In a sub-study of a larger randomized controlled trial, we assessed the effect of initiating moderate red wine consumption on 24-h BP recordings and the effect of a common genetic variant of alcohol dehydrogenases (ADH) among patients with type 2 diabetes.
METHODS
Fifty-four type 2 diabetes, alcohol abstainers were randomized to consume 150ml/dinner dry red wine or mineral water. Both groups were guided to adhere to a Mediterranean diet, without caloric restriction. We measured 24-h ambulatory BP monitoring (ABPM) at baseline and after 6 months.
RESULTS
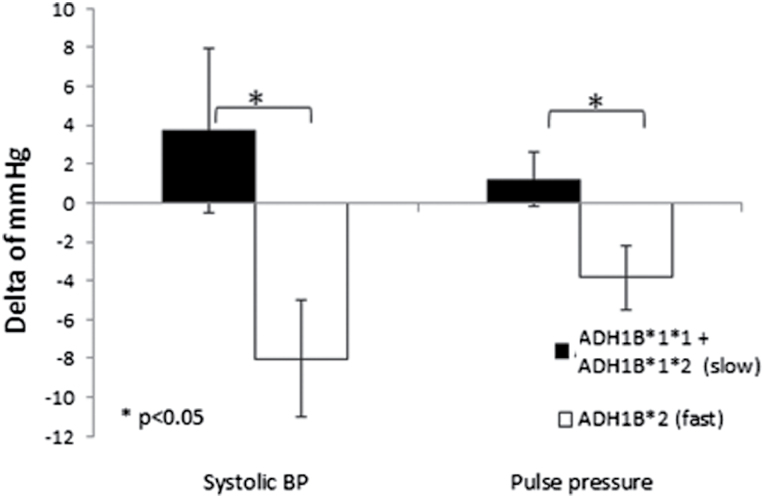
Participants (age = 57 years; 85% men; mean 24-h BP = 129/77mm Hg) had 92% 6-month retention. After 6 months of intervention, the average 24-h BP did not differ between the wine and water groups. A transient decrease in BP was observed in the red wine group at midnight (3–4 hours after wine intake: systolic BP: red wine = −10.6mm Hg vs. mineral water = +2.3mm Hg; P = 0.031) and the following morning at 7–9 am (red wine: −6.2mm Hg vs. mineral water: +5.6mm Hg; P = 0.014). In a second post hoc sub-analysis among the red wine consumers, individuals who were homozygous for the gene encoding ADH1B*2 variant (Arg48His; rs1229984, TT, fast ethanol metabolizers), exhibited a reduction in mean 24-h systolic BP (−8.0mm Hg vs. +3.7mm Hg; P = 0.002) and pulse pressure (−3.8mm Hg vs. +1.2mm Hg; P = 0.032) compared to heterozygotes and those homozygous for the ADH1B*1 variant (CC, slow metabolizers).
CONCLUSIONS
Initiating moderate red wine consumption at dinner among type 2 diabetes patients does not have a discernable effect on mean 24-h BP. Yet, a modest temporal BP reduction could be documented, and a more pronounced BP-lowering effect is suggested among fast ethanol metabolizers.
CLINICAL TRIALS REGISTRATION
ClinicalTrials.gov Identifier: NCT00784433.
Keywords: ambulatory measurement, blood pressure, hypertension, moderate alcohol, pulse pressure, randomized controlled trial, type 2 diabetes.
Elevated blood pressure (BP) is prevalent in about one-quarter of adults in industrialized countries and contributes to considerable morbidity and mortality attributed to stroke, renal failure, heart failure, and coronary heart disease.1 Observational studies suggest an association between moderate alcohol intake and reduced cardiovascular risk in healthy populations,2,3 which appears to be even more pronounced among patients with type 2 diabetes.4,5 While there is clear evidence that heavy alcohol intake is positively associated with hypertension,6–8 the association of moderate alcohol intake and BP remains unclear in observational studies. Some studies suggest a trend toward higher BP in moderate alcohol consumers, especially men,8–10 while others suggest a beneficial association.11,12 Some short-term randomized controlled trials of red wine consumption did not report significant effects on BP among hypertensive or normotensive participants13,14 or in patients with type 2 diabetes.15 Yet, a recent clinical trial found beneficial effects of red wine polyphenols on BP.16 Alcohol consumption patterns, as well as the timing of BP measurements, likely modulate the observed associations between alcohol consumption and BP.17
Most of the available studies are based on office BP readings, which can be affected by variable measuring techniques, the “white coat effect,” and the diurnal variability of BP. Short-term studies using ambulatory BP monitoring (ABPM) show no significant effect of moderate alcohol consumption on the average 24-h BP, but suggest a reduction in BP several hours after alcohol intake (apparently due to its direct vasodilatory effect) in 1 study18 and higher values of systolic and diastolic BP during the morning and during waking hours among moderate and heavy alcohol drinkers in another study.19
In humans, ethanol is oxidized to acetaldehyde, mainly (~70%) via hepatic class I alcohol dehydrogenase (ADH1B).20,21 A common genetic variant of its beta polypeptide, ADH1B rs1229984, encodes an amino acid substitution from Arg to His at position 48 (Arg48His), greatly enhancing the enzyme’s reaction rate.21,22 Observational studies suggest that the slow metabolizing variant is associated with elevated BP in the Japanese population23 compared to fast metabolizers (ALDH*2*2), but such an association was not observed in a Caucasian population.24
In our preparatory 3-month randomized controlled trial of wine consumption in patients with type 2 diabetes,15 we did not detect a red wine-mediated effect on office BP measurements. Hence, here we evaluate the effect of 6 months of moderate red wine consumption on BP dynamics by ABPM in type 2 diabetic, alcohol abstainers. This allows specific detection of more transient BP effects after wine consumption, substantiating our analysis of safety issues. Furthermore, given the high prevalence and impact on enzyme activity of the rs1229984 polymorphism of ADH1B, the effect of this polymorphism on ABPM was assessed.
METHODS
This trial is a predefined sub-study of the 2-year CaArdiovaSCulAr Diabetes & Ethanol (CASCADE) randomized clinical trial (ClinicalTrials.gov Identifier: NCT00784433). The large CASCADE study conducted in 2 sites: the Nuclear Research Center Negev, Israel and at Ben-Gurion University-Soroka Medical Center, Israel; 224 type 2 diabetes patients were randomized to mineral water, white wine, or red wine (150ml/dinner) consumption for 2 years in a 1:1:1 mode. In this sub-study we randomize participants only from the Nuclear Research Center Negev, and limited intervention to “water” and “red wine” with randomization ratio 1:1 (performed on SAS 9.2 software using procedure PROC PLAN) to enhance statistical power to compare these groups. We measured ABPM at baseline and after 6 months of intervention. Wine and water were provided. Both groups were guided to adhere to a Mediterranean diet. In the present analysis, the predefined outcome measure is BP safety, a cardiovascular disease status component. The trial commenced in 1-phase design in June 2010. Inclusion criteria for the CASCADE trial were: (i) age between 40 and 75 years; (ii) diagnosis of type 2 diabetes according to the American Diabetes Association criteria25; (iii) alcohol abstainers (≤1 drink/week); (iv) nonsmokers; (v) clinically stable; and (vi) willingness to drink wine if so assigned by randomization, as part of a Mediterranean diet intervention. Exclusion criteria included: (i) hemoglobin A1c < 6.4% (46 mmol/mol) or >10% (86 mmol/mol); (ii) insulin >2 injections/day or use of an insulin pump; (iii) fasting serum triglyceride ≥400mg/dl; (iv) serum creatinine >2mg/dl; (v) liver dysfunction (≥3-fold increase in serum alanine aminotransferase and/or aspartate aminotransferase); (vi) evidence of severe diabetic complications (such as proliferative retinopathy or diabetic nephropathy); (vii) evidence of autonomic neuropathy manifesting as postural hypotension and/or hypoglycemia unawareness; (viii) use of medications that might interact with moderate alcohol consumption; (ix) presence of active cancer and/or chemotherapy treatment in the last 3 years; (x) presence of a major illness that might require hospitalization; (xi) clinically assessed as having high potential of addictive behavior and/or personal or family history of addiction or alcohol abuse; (xii) women with first degree relatives with breast cancer; (xiii) pregnant or lactating women; and (xiv) participation in another interventional trial.
Based on a systematic review,18 the current investigation has been powered to detect a minimal difference of 2.7mm Hg in the average change of systolic BP between the groups, assuming an SD of 2.5mm Hg, α = 0.05, and 80% power. The actual differences between the intervention arms were of a larger magnitude. After randomization, the main parameters were evenly distributed between the 2 groups (Table 1). The main study as well as the ABPM sub-study were approved and monitored by the human subjects’ ethics committee of Soroka Medical Center and Ben-Gurion University; the participants received no financial compensation or gifts.
Table 1.
Baseline characteristics of the study population across intervention groups
| Variables | Assigned intervention group | ||
|---|---|---|---|
| Red wine (n = 27) |
Water (n = 27) |
Entire group (n = 54) |
|
| Age years | 56.5±7.2 | 57.7±5.7 | 57.1±6.5 |
| Male sex, no. (%) | 25 (92.6) | 21 (77.8) | 46 (85.2) |
| BMI, kg/m2 | 30±4.4 | 28.6±3.6 | 29.3±4 |
| Hypertensive (%) | 63 | 59.3 | 61.1 |
| HbA1c%, mmol/mol | 6.6±0.9, 49 | 6.9±1.4, 52 | 6.7±1.1, 50 |
| 24-h ambulatory blood pressure | |||
| Systolic | |||
| Mean 24h, mm Hg | 130.1±10.9 | 128.3±14.6 | 129.2±12.8 |
| Daytime (6 am to 11 pm) mean, mm Hg | 133.2±11.2 | 130.4±14.9 | 131.8±13.1 |
| Nighttime (11 pm to 6 am) mean, mm Hg | 117.7±12.6 | 110.1±35.2 | 113.9.3±26.4 |
| Diastolic | |||
| Mean 24h, mm Hg | 78.6±7.4 | 74.6±8.8 | 76.6±8.3 |
| Daytime (6 am to 11 pm) mean, mm Hg | 81.1±7.5 | 76.4±9.1 | 78.7±8.6 |
| Nighttime (11 pm to 6 am) mean, mm Hg | 68.1±9.4 | 61±19.8 | 64.5±15.7 |
| Pulse pressure a | |||
| Mean 24h mm Hg | 52.2±6.9 | 53±9.1 | 52.6±8 |
| 24-h Blood pressure variability | |||
| Systolic (SD) | 14±2.4 | 13.2±2.7 | 13.6±2.6 |
| Diastolic (SD) | 11.3±2.4 | 10.7±3.4 | 11±2.9 |
| Medication use | |||
| Antihypertensive drugs, no. (%) No. of antihypertensive drugs |
8 (29.6) 0.3±0.4 |
11 (40.7) 0.4±0.5 |
19 (35.2) 0.35±0.5 |
| Lipid-lowering therapy, no. (%) | 10 (37) | 12 (44.4) | 22 (40.7) |
| Anti-platelet therapy, no. (%) | 7 (25.9) | 10 (37) | 17 (31.5) |
| Oral glycemic-control medications, no. (%) | 13 (48.1) | 17 (63) | 30 (55.6) |
| Insulin treatment, no. (%) | 2 (7.4) | 0 | 2 (3.7) |
Data presented as mean ± SD unless otherwise indicated. All differences between groups were insignificant.
Abbreviations: BMI, body mass index; HbA1c%, hemoglobin A1c%.
aThe average of the differences between the systolic and diastolic blood pressure over 24 hours.
Intervention
The participants were instructed to consume 150ml (5 ounces) of the assigned beverage during dinner for 6 months, using a provided standard 150-ml measuring glass. The randomized groups were: dry red wine ((16.9g alcohol; (14.2% by volume); 270.1mg gallic acid equivalents (GAE) total phenols; 120 kcal/150ml) or mineral water (0g alcohol, 0 kcal). The water group received 18.9 l each month and the wine group received 14 bottles of 325ml a month. Patients assigned to consume alcohol were instructed to start drinking gradually at dinner over the first weeks and to avoid driving after drinking. All the beverages were provided at no cost during the monthly visits after returning the empty bottles, to facilitate private usage only. The participants in the study met a physician at baseline, after 3 and 6 months, and were treated regardless of the intervention arm to which they were randomized.
Mediterranean dietary guidelines
All participants received counseling for a Mediterranean diet, without caloric restriction. The monthly dietary guidance was based on quality rather than quantity of foods as meals rich in vegetables and low in red meat and no more than 35% of calories from fat (main source of olive oil and nuts). Wine issues were not discussed at these meetings.
Data collection
Ambulatory blood pressure monitoring (ABPM).
We measured 24-h ABPM (Oscar 2 system, SunTech Medical, Morrisville, NC) at baseline and after 6 months of intervention among all 54 participants. The participants were invited to the clinic at their workplace at 9 am to fit the cuff size by arm circumference26 and for a detailed explanation about the use of the ABPM, including avoidance of vigorous movements during the increase in cuff pressure, fitting the cuff’s proper position, keeping the cuff dry, and adhering as much as possible to their usual daily routine. In case of shift workers, ABPM was performed only during a morning shift day. The participants were asked to record the time they drank the beverage and the time they went to sleep and woke up on the day of recording. ABPM recorded systolic BP and diastolic BP from the morning it was fitted for the next 24 hours, every 30 minutes during the day (6 am to 11 pm) and every 60 minutes at night (11 pm to 6 am). Only ABPM studies with at least 70% of expected measurements were included.
Anthropometric measurements.
Participants were weighed without shoes to the nearest 0.1kg with the use of a wall-mounted stadiometer. Height was measured to the nearest millimeter at baseline for determination of body mass index. Waist circumference was measured halfway between the last rib and the iliac crest. Participants were measured at baseline and at 6 months.
Genetic analysis of alcohol dehydrogenase
ADH1B*1 and ADH1B*2 genotyping was determined using a 7300 Real Time PCR system (Applied Biosystems, Foster City, CA) using AccuStart Genotyping ToughMix Rox (Biosearch Technologies, Novato, CA) and designated primers for the specific single nucleotide polymorphism according to the manufacturer’s protocol.
Statistical analysis
For intention-to-treat analyses, we included all 54 participants. The primary outcome was the 24-h BP changes from baseline between the assigned groups, as assessed by ABPM. We calculated mean BP measurements from all 24-h readings and separately for day (6 am to 11 pm) and night time (11 pm to 6 am), as well as means from the values at each hour. The delta between means of 6 months to baseline was analyzed without imputation of missing data. Nonparametric test analyses were performed as the BP parameters were not normally distributed and the sample size was small; we assessed the within-person changes from baseline in each beverage group by Wilcoxon test and compared the effect between groups by Mann–Whitney test. Stratification by hemoglobin A1c% groups, body mass index groups, and use of antihypertensive groups was used for sensitivity analysis. The tests were 2-sided, performed on SPSS software, version 20.
RESULTS
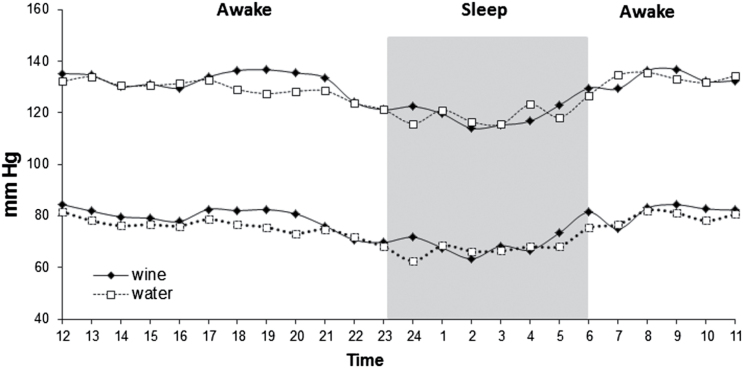
The overall retention rate was 92% after 6 months of intervention (Supplementary Appendix 1). Four participants dropped out during the study; 1 withdrew from the mineral water intervention group (lack of motivation) and 3 from the wine group (2 were unable to drink wine and 1 lacked motivation). According to data from the food frequency questionnaire, the adherence to daily consumption of the supplied beverages was 76.0% in the wine group and 80.9% in the water group (P = 0.71 between groups). The baseline characteristics of the participants are shown in Table 1. The 2 study groups demonstrated similar distribution. The average age was 57 years, 85% were men with a mean body mass index of 29.2±4kg/m2, and mean hemoglobin A1c 6.7±1.1% (50 mmol/mol). Hypertension was diagnosed in 61% of the participants and 35% were on chronic antihypertensive therapy (average number of antihypertensive medications was 1.5 among consumers); no significant changes were found between groups and compared to baseline measurement. Mean baseline 24-h ABPM was 129/77mm Hg and mean pulse pressure was 52.6±8mm Hg. Mean baseline intake of alcohol was 2.6g/day or about 1 drink per week. The baseline 24-h ABPM (Figure 1) did not reveal a substantial difference of BP levels between day and night among the type 2 diabetes participants, though such difference would be expected in non-type 2 diabetes population.
Figure 1.
Baseline average systolic and diastolic ambulatory blood pressure monitoring over 24 hours of the red wine and mineral water groups. Red wine (n = 27), mineral water (n = 27). Black (wine group) and white (water group) points present means of ABPM in each hour.
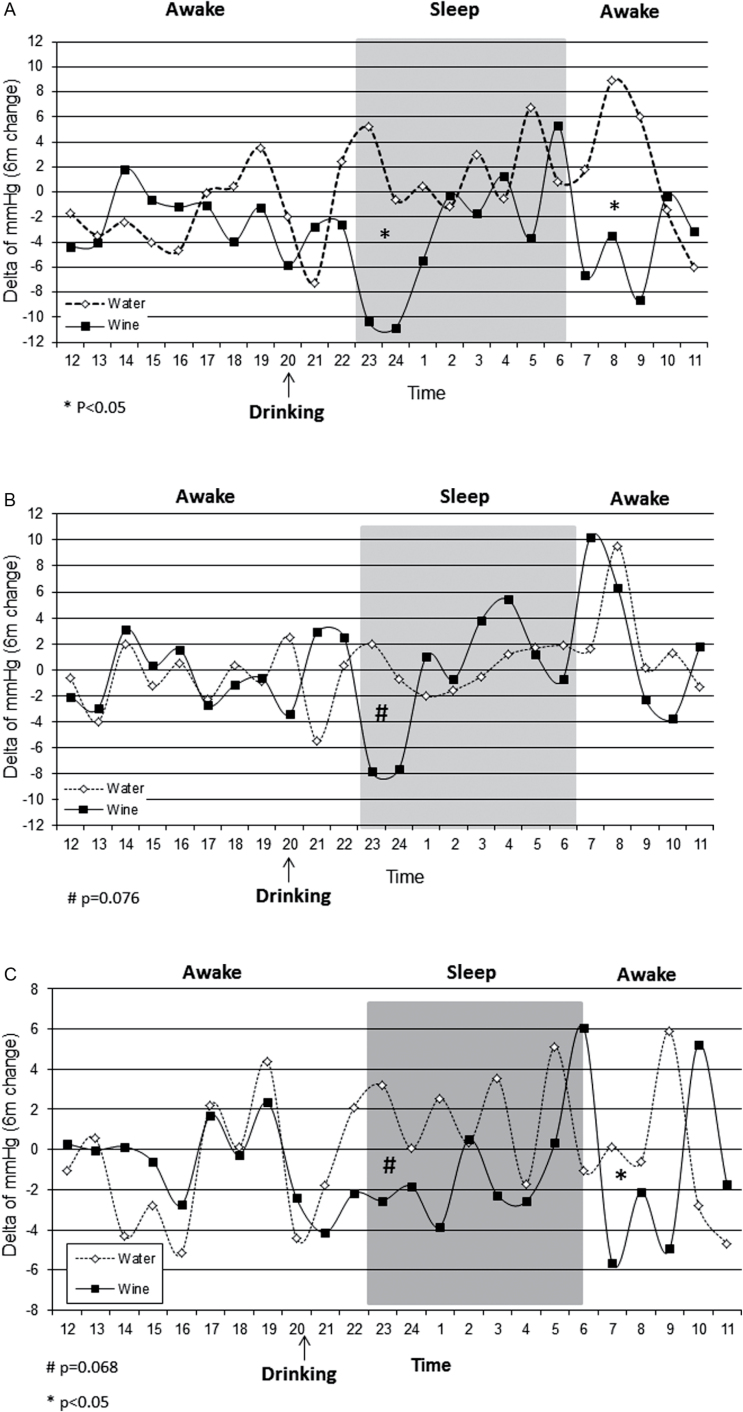
After 6 months of intervention the participants achieved a minor weight loss of −1.3kg ± 3.7, similarly between groups, those changes were not correlated with systolic or diastolic BP changes. Six-month differences in mean 24-h BP were negligible (systolic −2.1mm Hg in the wine group vs. −2.0mm Hg in the mineral water group (P = 0.66; confidence interval (CI): −3.8 to 3.7); diastolic: +0.5mm Hg in wine group vs. −0.6mm Hg in the water group (P = 0.71; CI: −2.7 to 3.8)), as well as minor differences in mean daytime or nighttime and mean 24-h changes in pulse pressure (−0.3mm Hg in the wine group vs. −0.6mm Hg in the water group (P = 0.9; CI: −1.1 to 1.4)). However, when comparing the 6-month changes between the individual hours across the 24-h trajectory (Figure 2a–c), reductions in BP were observed in the red wine group at midnight (3–4 hours after ingestion): systolic: red wine: −10.6mm Hg vs. mineral water: +2.3mm Hg (P = 0.03; CI: −14.1 to −0.6), diastolic: red wine: −7.7mm Hg vs. mineral water:+0.7mm Hg (P = 0.076; CI: −11.8 to 0.9); and at 7–9 am: systolic: red wine: −6.2mm Hg vs. mineral water: +5.6mm Hg (P = 0.014; CI: −17.3 to −0.8). These effects were accordingly reflected in a pulse pressure reduction (Figure 2c) in the early morning hours (red wine: −4.2mm Hg vs. mineral water: +1.8mm Hg (P = 0.032; CI: −9.9 to −0.2)) and a trend toward a decrease at midnight (red wine: −2.7mm Hg vs. mineral water: +1.9mm Hg (P = 0.068; CI: −12.3 to 1.6). The findings remained similar in additional stratified analyses across median hemoglobin A1c% and median body mass index (data not shown). We followed medication usage and could not identify specific interactions. However, the wine hypotensive effect, for systolic BP, at the 2 time points was more pronounced among users of antihypertensive medications, compared to nonuser of antihypertensive medications (Supplementary Appendix 3).
Figure 2.
The effect of 6-month wine intervention on changes of systolic BP (a), diastolic BP (b) and pulse pressure (c) from baseline; results from 24-h ambulatory blood pressure monitoring. Black (wine group, n = 24) and white (water group, n = 26) points present delta of ABPM in each hour after 6 months.
The association between alcohol and ADH1B genetic variants is presented in Figure 3. Among those assigned to the wine group, participants who were homozygous for the gene variant encoding fast ethanol metabolism, ADH1B*2 (TT), had a significant decrease in mean 24-h systolic BP (−8 vs. +3.7mm Hg (P = 0.002, CI: −5.7 to −3.2)) and pulse pressure (−3.8 vs. +1.2mm Hg (P = 0.032; CI: −9.8 to −0.20)) as compared to the non-fast metabolizers (i.e., those either homozygote to the slow, wild-type, ethanol metabolism variant, or heterozygotes). Nonsignificant differences were found in the polymorphisms groups through beverages intervention. Moreover, no genetic association was observed for the water group in the different polymorphisms (Supplementary Appendix 2).
Figure 3.
The effect of ethanol metabolism genotypes on mean 24-h systolic blood pressure and pulse pressure; changes over 6 months of intervention among the wine group. Slow (black bar; n = 12) and fast (white bar; n = 9) ethanol metabolizers.
DISCUSSION
We found that the daily consumption of moderate wine intake (150ml of red wine, equivalent to approximately 17g alcohol) within an interventional randomized control trial setting does not affect 24-h BP, with potential hints toward a transient hypotensive response at specific time intervals. We also found a differential association of wine and BP between fast and slow ethanol metabolizers, as defined by a common ADH1B variant. Amongst fast alcohol metabolizers we found a significant reduction of average 24-h BP, which was not apparent among the slow metabolizers.
Our trial has several limitations, including being a sub-group analysis with a relatively small sample size, particularly in the genetic analysis, and the under-representation of women (reflecting their proportion in this specific workplace). Thus, our results should not be interpreted as strong evidence of a wine effect, and can only be used to inspire hypotheses that require further verification. Although only participants recruited from the Nuclear Research Center Negev were recruited to this sub-study, we did not find significant clinical or demographic differences between this sub-group and the entire CASCADE study group, other than the smaller proportion of women. Second, although the retention rate of the participants was high, as assessed by their monthly attendance to follow-up visits, return of empty bottles, and questionnaires (data not shown), blood or urine levels of alcohol or its metabolites were not available.
The strengths of the study include the enrollment of alcohol abstainers in a 1-phase study design (in which all participants started the intervention simultaneously), and the relatively long duration of intervention compared to previous randomized alcohol studies. The fact that all the participants work in the same workplace under similar conditions, and commute to work by the same transport at identical times, provided a relatively uniform background to the study in both groups and minimized confounding effects that could be introduced by variable waking hours activity and work place conditions.
After 6 months of intervention we did not find a wine effect on mean 24-h BP in the entire group. Previous studies showed a decrease in BP approximately 4 hours after alcohol consumption.18 We also found evidence for such BP-lowering effect in our study without long-term effects on mean BP throughout the day. We also did not observe an elevation in systolic BP 10–15 hours after alcohol ingestion, as was described in a previous study.18 On the contrary, we demonstrated that wine intervention modestly restrained increasing BP in the morning that is often seen in ABPM.27,28 This discrepancy between our and previous studies may reflect differences between short-term and long-term alcohol consumption, difference in the populations studied, and/or the background Mediterranean diet. Whether the transient decrease in BP around midnight and in the early morning hours would translate to a long-term clinical benefit remains to be determined. The analysis with resolution to an hour or other time interval was of an exploratory nature and suggests that the wine effect is plausible, without need for further multiple comparisons.
Modest reductions in mean 24-h systolic BP were found in a sub-group, post hoc analysis, in participants homozygote to the ADH1B*2 (TT) gene variant encoding for fast ethanol metabolism. Some observational studies have shown that alcohol consumers who are slow ethanol metabolizers (genotype CC) had a higher risk of hypertension than fast ethanol metabolizers (genotype CT or TT)24,29 and had higher levels of office BP,30 while other studies found no difference between the genotypes.31,32 Although the results of our post hoc analysis cannot be taken as definite proof of a genetic interaction and should only be regarded as hypothesis generating, some support to our findings can be found in a recent study of Mendelian randomization analysis based on individual participant data of 261,991 individuals of European descent from 56 studies showed that individuals with the A-allele of the ADH1B rs1229984 gene (“fast metabolizers”), who also consumed less alcohol, had lower mean systolic BP.33 However, the explanation proposed in that study was that the gene likely exerts its influence through increased propensity to drink alcohol,34 expressed by greater quantity of alcohol consumption among individuals with the ALDH *1*1 (wild-type) genotype, compared to ALDH*2*2 genotype. Such factors are eliminated in a randomized intervention study design. The fact that fast ethanol metabolizers had a greater reduction in systolic BP suggests that ethanol itself may have little effect on BP, and the fast degradation to one of its metabolites may cause the hypotensive effect. One possible candidate is acetaldehyde, which has been shown to cause vasodilation and thus to reduce BP in experimental settings.35–37
Our dry red wine (from “Golan Heights” winery) included 14.2% ethanol by volume and ≃270mg gallic acid equivalents (GAE) total phenols. As red wine is the main alcoholic beverage source of total phenols and, in particular, of resveratrol, we assume that those wine phenols had collateral effects on BP. However, we could not dissect the specific effect of each red wine component.
While it has been hypothesized that the phenolic compound content of wine also contributes to its BP-lowering effect,16 the neutral effect of wine in slow metabolizers does not particularly support this possibility but does not eliminate it completely. Observational studies, which found BP-raising effects of moderate alcohol consumption, described no relationship to the phenol content in the alcoholic beverages9; while other observational studies11,12 found a beneficial effect of red wine on BP. We cannot exclude the possibility that in slow alcohol metabolizers the phenols in red wine have the potential to counter-balance a BP-raising effect of ethanol. Furthermore, the hypotensive effect 3–4 hours post-ingestion might be caused by rapid metabolism of ethanol that unmasks the favorable effect of polyphenol activity.
In summary, our findings suggest that moderate consumption of red wine exerts no discernable effect on the mean daily BP in the entire group, though hints for a potential hypotensive effect at specific time intervals. These effects appear to be prominent in diabetic patients who are homozygous for the fast ethanol metabolizing variant, in whom BP parameters are significantly reduced. Whether similar effects also occur in nondiabetic hypertensive patients remains to be evaluated. It is also pertinent to evaluate other metabolic effects of ethanol, which may show differential effects in relation to the rate of ethanol metabolism.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
All the 3 conditions below were met by all authors:
1. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data.
2. Drafting the article or revising it critically for important intellectual content.
3. Final approval of the version to be published.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participants in the CASCADE randomized controlled trial for their consistent cooperation. We deeply thank Harel Segal for his significant contribution. We thank the “Golan Heights” winery and the “Mey-Eden” mineral water company for providing the beverages to the study participants. These companies were not involved in any stage of the design, conduct, or analysis of the study and had no access to the study results before publication. We thank the consultants and health care providers: Drs Arie Moran and Amos Katz from Ben-Gurion University of the Negev, Israel; Dr Tatiana Shuster, Sagit Kachlon, Malka Kaminsky, Yasmin Asuly, Tamara Lipovski, and Roman Tsirkin from the Soroka University Medical Center, Israel; Eyal Goshen, Meir Aviv, Hassia Krakauer, Haim Strasler, Drs Ziva Schwartz, Einat Sheiner, and Dov Brickner, Rachel Marko, Esther Katorza, Ilanit Asulin, and Tzvika Tzur from the Nuclear Research Center Negev, Israel; and Drs Eran Leitersdorf and Shai Blag from Hadassah Hebrew University Medical Center, Israel.
The work was funded by a grant from the European Foundation for the Study of Diabetes (EFSD) of the European Association for the Study of Diabetes (EASD).
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
REFERENCES
- 1. Guidelines Subcommittee. World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens 1999; 17:151–183 [PubMed] [Google Scholar]
- 2. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011; 342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tolstrup J, Jensen MK, Tjønneland A, Overvad K, Mukamal KJ, Grønbaek M. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ 2006; 332:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanasescu M, Hu FB, Willett WC, Stampfer MJ, Rimm EB. Alcohol consumption and risk of coronary heart disease among men with type 2 diabetes mellitus. J Am Coll Cardiol 2001; 38:1836–1842. [DOI] [PubMed] [Google Scholar]
- 5. Solomon CG, Hu FB, Stampfer MJ, Colditz GA, Speizer FE, Rimm EB, Willett WC, Manson JE. Moderate alcohol consumption and risk of coronary heart disease among women with type 2 diabetes mellitus. Circulation 2000; 102:494–499. [DOI] [PubMed] [Google Scholar]
- 6. Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension 2001; 37:1242–1250. [DOI] [PubMed] [Google Scholar]
- 7. Ascherio A, Rimm EB, Giovannucci EL, Colditz GA, Rosner B, Willett WC, Sacks F, Stampfer MJ. A prospective study of nutritional factors and hypertension among US men. Circulation 1992; 86:1475–1484. [DOI] [PubMed] [Google Scholar]
- 8. Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2012; 14:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaubert MP, Jin Z, Russo C, Schwartz JE, Homma S, Elkind MS, Rundek T, Sacco RL, Di Tullio MR. Alcohol consumption and ambulatory blood pressure: a community-based study in an elderly cohort. Am J Hypertens 2014; 27:688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wakabayashi I. Influence of gender on the association of alcohol drinking with blood pressure. Am J Hypertens 2008; 21:1310–1317. [DOI] [PubMed] [Google Scholar]
- 11. Chiva-Blanch G, Urpi-Sarda M, Ros E, Arranz S, Valderas-Martínez P, Casas R, Sacanella E, Llorach R, Lamuela-Raventos RM, Andres-Lacueva C, Estruch R. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: short communication. Circ Res 2012; 111:1065–1068. [DOI] [PubMed] [Google Scholar]
- 12. Thadhani R, Camargo CA, Jr, Stampfer MJ, Curhan GC, Willett WC, Rimm EB. Prospective study of moderate alcohol consumption and risk of hypertension in young women. Arch Intern Med 2002; 162:569–574. [DOI] [PubMed] [Google Scholar]
- 13. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 14. Botden IP, Draijer R, Westerhof BE, Rutten JH, Langendonk JG, Sijbrands EJ, Danser AH, Zock PL, van den Meiracker AH. Red wine polyphenols do not lower peripheral or central blood pressure in high normal blood pressure and hypertension. Am J Hypertens 2012; 25:718–723. [DOI] [PubMed] [Google Scholar]
- 15. Shai I, Wainstein J, Harman-Boehm I, Raz I, Fraser D, Rudich A, Stampfer MJ. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care 2007; 30:3011–3016. [DOI] [PubMed] [Google Scholar]
- 16. Chiva-Blanch G, Urpi-Sarda M, Ros E, Arranz S, Valderas-Martínez P, Casas R, Sacanella E, Llorach R, Lamuela-Raventos RM, Andres-Lacueva C, Estruch R. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: short communication. Circ Res 2012; 111:1065–1068. [DOI] [PubMed] [Google Scholar]
- 17. Wallace RB, Lynch CF, Pomrehn PR, Criqui MH, Heiss G. Alcohol and hypertension: epidemiologic and experimental considerations. The Lipid Research Clinics Program. Circulation 1981; 64:III 41–III 47. [PubMed] [Google Scholar]
- 18. McFadden CB, Brensinger CM, Berlin JA, Townsend RR. Systematic review of the effect of daily alcohol intake on blood pressure. Am J Hypertens 2005; 18:276–286. [DOI] [PubMed] [Google Scholar]
- 19. Ohira T, Tanigawa T, Tabata M, Imano H, Kitamura A, Kiyama M, Sato S, Okamura T, Cui R, Koike KA, Shimamoto T, Iso H. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension 2009; 53:13–19. [DOI] [PubMed] [Google Scholar]
- 20. Edenberg HJ, Xuei X, Chen HJ, Tian H, Wetherill LF, Dick DM, Almasy L, Bierut L, Bucholz KK, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Porjesz B, Rice J, Schuckit M, Tischfield J, Begleiter H, Foroud T. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet 2006; 15:1539–1549. [DOI] [PubMed] [Google Scholar]
- 21. Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 2007; 30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 22. Linneberg A, Gonzalez-Quintela A, Vidal C, Jørgensen T, Fenger M, Hansen T, Pedersen O, Husemoen LL. Genetic determinants of both ethanol and acetaldehyde metabolism influence alcohol hypersensitivity and drinking behaviour among Scandinavians. Clin Exp Allergy 2010; 40:123–130. [DOI] [PubMed] [Google Scholar]
- 23. Hashimoto Y, Nakayama T, Futamura A, Omura M, Nakarai H, Nakahara K. Relationship between genetic polymorphisms of alcohol-metabolizing enzymes and changes in risk factors for coronary heart disease associated with alcohol consumption. Clin Chem 2002; 48:1043–1048. [PubMed] [Google Scholar]
- 24. Husemoen LL, Jørgensen T, Borch-Johnsen K, Hansen T, Pedersen O, Linneberg A. The association of alcohol and alcohol metabolizing gene variants with diabetes and coronary heart disease risk factors in a white population. PLoS One 2010; 5:e11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997) Diabetes Care 20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 26. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161. [DOI] [PubMed] [Google Scholar]
- 27. Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 28. Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 29. Zhang WS, Xu L, Schooling CM, Jiang CQ, Cheng KK, Liu B, Lam TH. Effect of alcohol and aldehyde dehydrogenase gene polymorphisms on alcohol-associated hypertension: the Guangzhou Biobank Cohort Study. Hypertens Res 2012; 36:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saito K, Yokoyama T, Yoshiike N, Date C, Yamamoto A, Muramatsu M, Tanaka H. Do the ethanol metabolizing enzymes modify the relationship between alcohol consumption and blood pressure? J Hypertens 2003; 21:1097–1105. [DOI] [PubMed] [Google Scholar]
- 31. Yamada Y, Sun F, Tsuritani I, Honda R. Genetic differences in ethanol metabolizing enzymes and blood pressure in Japanese alcohol consumers. J Hum Hypertens 2002; 16:479–486. [DOI] [PubMed] [Google Scholar]
- 32. Amamoto K, Okamura T, Tamaki S, Kita Y, Tsujita Y, Kadowaki T, Nakamura Y, Ueshima H. Epidemiologic study of the association of low-Km mitochondrial acetaldehyde dehydrogenase genotypes with blood pressure level and the prevalence of hypertension in a general population. Hypertens Res 2002; 25:857–864. [DOI] [PubMed] [Google Scholar]
- 33. Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, Prieto-Merino D, Dehghan A, Trompet S, Wong A, Cavadino A, Drogan D, Padmanabhan S, Li S, Yesupriya A, Leusink M, Sundstrom J, Hubacek JA, Pikhart H, Swerdlow DI, Panayiotou AG, Borinskaya SA, Finan C, Shah S, Kuchenbaecker KB, Shah T, Engmann J, Folkersen L, Eriksson P, Ricceri F, Melander O, Sacerdote C, Gamble DM, Rayaprolu S, Ross OA, McLachlan S, Vikhireva O, Sluijs I, Scott RA, Adamkova V, Flicker L, Bockxmeer FM, Power C, Marques-Vidal P, Meade T, Marmot MG, Ferro JM, Paulos-Pinheiro S, Humphries SE, Talmud PJ, Mateo Leach I, Verweij N, Linneberg A, Skaaby T, Doevendans PA, Cramer MJ, van der Harst P, Klungel OH, Dowling NF, Dominiczak AF, Kumari M, Nicolaides AN, Weikert C, Boeing H, Ebrahim S, Gaunt TR, Price JF, Lannfelt L, Peasey A, Kubinova R, Pajak A, Malyutina S, Voevoda MI, Tamosiunas A, Maitland-van der Zee AH, Norman PE, Hankey GJ, Bergmann MM, Hofman A, Franco OH, Cooper J, Palmen J, Spiering W, de Jong PA, Kuh D, Hardy R, Uitterlinden AG, Ikram MA, Ford I, Hyppönen E, Almeida OP, Wareham NJ, Khaw KT, Hamsten A, Husemoen LL, Tjønneland A, Tolstrup JS, Rimm E, Beulens JW, Verschuren WM, Onland-Moret NC, Hofker MH, Wannamethee SG, Whincup PH, Morris R, Vicente AM, Watkins H, Farrall M, Jukema JW, Meschia J, Cupples LA, Sharp SJ, Fornage M, Kooperberg C, LaCroix AZ, Dai JY, Lanktree MB, Siscovick DS, Jorgenson E, Spring B, Coresh J, Li YR, Buxbaum SG, Schreiner PJ, Ellison RC, Tsai MY, Patel SR, Redline S, Johnson AD, Hoogeveen RC, Hakonarson H, Rotter JI, Boerwinkle E, de Bakker PI, Kivimaki M, Asselbergs FW, Sattar N, Lawlor DA, Whittaker J, Davey Smith G, Mukamal K, Psaty BM, Wilson JG, Lange LA, Hamidovic A, Hingorani AD, Nordestgaard BG, Bobak M, Leon DA, Langenberg C, Palmer TM, Reiner AP, Keating BJ, Dudbridge F, Casas JP; InterAct Consortium. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014; 349:g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L, Smith GD, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med 2008; 5:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tottmar O, Hellström E. Blood pressure response to ethanol in relation to acetaldehyde levels and dopamine-beta-hydroxylase activity in rats pretreated with disulfiram, cyanamide and coprine. Acta Pharmacol Toxicol (Copenh) 1979; 45:272–281. [DOI] [PubMed] [Google Scholar]
- 36. Mizoi Y, Ijiri I, Tatsuno Y, Kijima T, Fujiwara S, Adachi J, Hishida S. Relationship between facial flushing and blood acetaldehyde levels after alcohol intake. Pharmacol Biochem Behav 1979; 10:303–311. [DOI] [PubMed] [Google Scholar]
- 37. Altura BM, Carella A, Altura BT. Acetaldehyde on vascular smooth muscle: possible role in vasodilator action of ethanol. Eur J Pharmacol 1978; 52:73–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.