Abstract
BACKGROUND
Structural atherosclerotic damage, arterial stiffness, pulse pressure (PP), and renal hemodynamics may interact and influence each other. Renal resistance index (RRI) appears as a good indicator of systemic vascular changes. The aim of our study was to assess the independent relationships of carotid intima-media thickness (cIMT), aortic pulse wave velocity (aPWV), and peripheral PP with RRI in hypertensives with various degrees of renal function.
METHODS
We enrolled 463 hypertensive patients (30–70 years) with normal renal function (group 0; n = 280) and with chronic kidney disease (groups I–V; n = 183). All subjects underwent ultrasonographic examination of intrarenal and carotid vasculature, as well as a 24-h ambulatory blood pressure monitoring.
RESULTS
A statistically significant difference in RRI, cIMT, aPWV, and clinic PP was observed in the different 6 groups (all P < 0.001), even after adjustment for age. RRI correlated with cIMT (r = 0.460, P < 0.001), aPWV (r = 0.386, P < 0.001), clinic PP (r = 0.279, P < 0.001), and 24-h PP (r = 0.229, P < 0.001) in the entire study population. These correlations were similar in subjects with and without renal dysfunction. In the overall study population, the association between RRI, cIMT, and clinic PP remained statistically significant even after adjustment for various confounding factors, whereas the relationship between RRI and aPWV was lost in multivariate analysis.
CONCLUSIONS
cIMT and clinic PP rather than directly aPWV are associated with intrarenal hemodynamics. Our results confirm that in hypertensives RRI not only detects derangement of intrarenal circulation but may also be considered as a sensor of systemic vascular changes, independently of level of renal function.
Keywords: arterial stiffness, atherosclerosis, blood pressure, chronic kidney disease; hypertension, pulse pressure, renal hemodynamics, renal resistance index.
Renal resistance index (RRI), noninvasively measured by Doppler ultrasonography, was classically used to assess renal hemodynamics for kidney disease detection and prognosis.1,2 However, RRI does not merely reflect renal parenchymal resistance, mainly depending from upstream factors 3,4, and it appears a good indicator of vascular morphofunctional changes in different populations.5–10
The link between renal hemodynamics and systemic vascular changes was widely investigated, especially in hypertensive and chronic kidney disease (CKD) patients. Several studies reported a close association between RRI and carotid intima-media thickness (cIMT),5–7,9 expression of structural carotid vascular damage and mirror of generalized atherosclerosis, supporting the role of RRI as marker of systemic vascular impairment.10 Likewise, arterial stiffness, epiphenomenon of functional, and structural alterations of the vessel wall and well-documented marker of target organ damage in hypertensive subjects, was associated with renal hemodynamic changes, particularly with RRI.11 However, more recent reports highlighted that pulse pressure (PP), rather than directly arterial stiffness, correlated with impaired renal hemodynamics, and progressive decline of renal function.12,13
Structural atherosclerotic damage, arterial stiffness, pulse pressure, and renal hemodynamics may interact and influence each other through mechanisms that remain largely to be clarified. The aim of our study was to assess the independent relationships of cIMT, aPWV, and peripheral PP with renal resistive index in hypertensive patients with various degrees of renal function.
MATERIALS AND METHODS
Subjects
The population of this cross-sectional study was selected from 584 Caucasian hypertensive patients consecutively attending our unit of Nephrology and Hypertension.
The exclusion criteria were: age < 30 years and >70 years; renovascular, malignant, or endocrine hypertension; severe obesity, defined as a body mass index (BMI) ≥ 40kg/m2; renal replacement therapy (transplanted or dialyzed patients); rapid deterioration of renal function, defined as a reduction in estimated glomerular filtration rate (eGFR)>25% within 7 days; hydronephrosis of grade 2 or higher; patients with significant difference in size or morphology between kidneys (difference in renal length > 1.5cm between kidneys, solitary or supernumerary kidneys, congenital renal abnormalities); permanent atrial fibrillation; heart failure; heart rate>100 bpm or <50 bpm; moderate to severe aortic/mitral valve disease; aortic aneurysm; major noncardiovascular diseases; carotid percutaneous angioplasty or endoarterectomy.
Written informed consent was obtained from each subject. The study protocol, that conforms to the ethical guidelines of Helsinki declaration, was approved by local review board.
In all subjects, careful clinical history and physical examination were performed. Persons who reported smoking cigarettes regularly during the past year were considered as current smokers.
Clinic blood pressure (BP) was recorded by a doctor, following the recommendations of the 2013 European Society of Hypertension/European Society of Cardiology guidelines.14 It was considered as mean of 3 consecutive measurements obtained, at 2min intervals, by an electronic oscillometric validated device (Microlife Watch BP Office)15, after 5min of rest in sitting position. Furthermore, a portable, noninvasive SpaceLabs 90207 recorder (Redmond, WA) was used to perform 24-h ABPM. BP was recorded automatically at 15min intervals during the day and at 20min intervals during night-time resting.
Fasting blood samples were drawn to perform routine blood chemistry and a 24-h urine sample was collected to evaluate the levels of albumin excretion. Definition and classification of CKD followed the K/DIGO guidelines16, and eGFR was estimated by using Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation.17
Moreover, a B-mode and Duplex-Doppler ultrasonographic examination of intrarenal and carotid vasculature was performed through a GE Logiq P5-PRO instrument (General Electric Company, Milan, Italy).
Renal ultrasonography
The intrarenal color duplex ultrasonography was performed through a 4 MHz transducer operating at 2.5 MHz for Doppler analysis. Doppler signal was obtained from the interlobar arteries by placing the sample volume at the level of the cortico-medullary junction. Peak systolic velocity (PSV) and telediastolic velocity (TDV) were measured with a Doppler angle < 60°, and RRI was calculated by the formula: RRI = (PSV − TDV)/PSV. The values were computed as the average of 6 measurements (3 from each kidney).9
Carotid ultrasonography
The carotid ultrasonographic investigation was performed through a 10 MHz linear-array transducer for the measurements, operating at 5 MHz for Doppler analysis.
The executing technique was described previously.9 Carotid IMT was defined as the distance between the vascular lumen-intima interface and the media-adventitia transition, and it was obtained on the common carotid artery through the examination of a freezed longitudinal 10mm section at a distance of 1cm from bifurcation, at end-diastolic state, in the proximal and far wall, by using lateral, anterior and posterior projections. A total of 6 measurements were obtained for each side, and we used the overall average cIMT value. In correspondence of a carotid plaque, cIMT measurement was not obtained and was shifted proximally on the plaque-free site.
We considered plaques those focal structures encroaching into the arterial lumen of at least 0.5mm or 50% of the surrounding cIMT value, or demonstrating a cIMT > 1.5mm.18
Pulse wave velocity
All measurements were performed in a supine position after 15min rest in a quiet, temperature-controlled room. Arterial stiffness was assessed using the operator-independent, non-invasive Arteriograph system (Tensiomed Kft., Budapest, Hungary), which was validated against invasive and non-invasive techniques.19,20
Aortic pulse wave velocity (aPWV) measurements was performed through an upper arm BP cuff when the pressure exceeds systolic BP by 35–40 mm Hg, with a completely occluded brachial artery. As described previously11, aPWV was calculated with the formula: aPWV = (distance Jug/Sy(m))/(RT/2(s)), where distance Jug/Sy(m) is the distance between the jugulum (sternal notch) and the pubic symphysis, and RT is the sum of the forward and the backward transit time.19,20
Statistical analysis
A total of 121 subjects met the exclusion criteria. Therefore, final analysis involved 463 patients.
Statistical analysis was initially performed in the whole study population, in subjects with normal renal function (n = 280) and in CKD patients (n = 183). Subsequently, it was carried out in 6 groups: (i) hypertensive subjects without CKD (group 0); (ii) hypertensive subjects with different CKD stages (groups I–V).
Continuous variables were given as mean ± SD. Albuminuria and triglycerides (expressed as median and interquartile range because of their skewed distribution) were log-transformed to better satisfy distributional assumptions before parametric tests were used. Categorical variables were expressed as percentage values.
Student’s t test for independent samples was used to compare continuous variables between 2 groups, whereas χ2 test (or Fisher exact test when appropriate) was used to compare categorical variables. We also used 1-way analysis of variance (ANOVA) and the Holm–Sidak post hoc test to evaluate differences in means among different subgroups. Proportional differences between groups were assessed by the χ2 test, with Yates’ correction (or Fisher exact test when appropriate).
Comparison between continuous variables was adjusted for age by analysis of covariance (ANCOVA).
The univariate and multivariate relationships between RRI, cIMT, aPWV, PP, and other variables were tested by simple and multiple linear regression analyses. The strength of the associations between the variables was expressed respectively by the Pearson correlation coefficients (r) and the unstandardized (B) and standardized (β) multiple regression coefficients.
The stepwise multiple regression models were built considering RRI as outcome variable, and including into the models as potential explanatory parameters: cIMT, aPWV, clinic PP, age, sex (0 = females; 1 = males), eGFR, (Log)albuminuria, serum uric acid, smoking habit, serum glucose levels (or diabetes as dichotomous variable), BMI, (Log)triglycerides, antiplatelet therapy (0 = no treatment, 1 = treatment).
Collinearity was assessed by calculating the variance inflation factor (VIF). Variables with VIF ≥ 2 were excluded from the model.
The models were run again replacing clinic PP with 24-h PP or clinic or 24-h systolic or mean BP.
Moreover, in the patients with normal renal function and in the whole group with CKD, we calculated partial correlation coefficients relating RRI with both cIMT and clinic PP, after correction for potential confounders (age and, respectively, clinic pulse pressure or cIMT).
In order to further assess the influence of renal function, regarded as continuous variable (eGFR), on the relationship between RRI and clinic pulse pressure, another multivariate model was built including the multiplicative 2-way interaction terms “Clinic pulse pressure x eGFR” along with the variables associated with RRI in previous linear multiple regression analysis.
Multivariate analysis was lastly repeated replacing diabetes, in order to avoid problems of multicollinearity, with CKD etiology (coded as follows: 1 = hypertensive nephropathy; 2 = diabetic nephropathy; 3 = unknown nephropathy; 4 = chronic glomerulonephritis).
In all multiple regression analyses, a backward stepwise procedure was used with α = 0.15 as the cutoff for entry or removal of variables.
The independent effects on RRI of clinic PP and cIMT from each other were also assessed by a 2-way analysis of variance. Interaction between PP and cIMT was formally tested by assessing the significance of the multiplicative 2-way interaction term “PP and cIMT” along with the main effects of PP and cIMT.
The null hypothesis was rejected at a 2-tailed P ≤ 0.05.
The statistical analyses were performed using the SYSTAT DATA software package, version 13 (Systat, Chicago, IL).
RESULTS
A total of 463 patients were enrolled. Mean age of the overall study population was 54±16 years, 56.9% were males and mean BMI was 28.0±5.0kg/m2. The average values of RRI, cIMT, aPWV, and clinic PP were 0.64±0.07, 0.91±0.24mm, 11.3±2.5 m/s and 55±14mm Hg, respectively. Diabetes mellitus was present in 25.7% of the subjects, and 30.5% were former or current smokers. Diabetic patients had significant higher values of RRI, cIMT, aPWV, and clinic PP, respectively (0.67±0.07, 1.03±0.22 mm, 12.3±2.3 m/s, 61±18 mm Hg) than nondiabetic subjects (0.63±0.07, 0.92±0.27 mm, 11.1±2.3 m/s, 53±12 mm Hg) (all P < 0.001), as well as greater cIMT, aPWV, and clinic PP were observed in smokers (0.99±0.33 mm, 12.2±2.5 m/s, 59±15 mm Hg) when compared with nonsmokers (0.92±0.24 mm, 10.9±2.3 m/s, 54±14 mm Hg) (P = 0.036, P < 0.001, P = 0.002, respectively).
Subjects with CKD were 39.5% (n = 183). Among them, 44% had hypertensive nephropathy, 30% diabetic nephropathy, 17% unknown nephropathy, 8% chronic glomerulonephritis, and less than 1% cryoglobulinemia. RRI, cIMT, aPWV, and clinic PP was significantly higher in CKD patients (0.66±0.07, 1.00±0.23mm, 11.9±2.4 m/s, 59±16mm Hg, respectively) than hypertensive subjects with normal renal function (0.62±0.06, 0.89±0.24mm, 10.8±2.4 m/s, 53±12mm Hg, respectively), with all P values < 0.001.
Clinical characteristics of the study population divided into hypertensive subjects without CKD (group 0) and hypertensive subjects with different CKD stages (groups I–V) are listed in Table 1. A statistically significant difference in RRI, cIMT, aPWV, and clinic PP was observed in the different 6 groups (all P < 0.001). The difference regarding these parameters among groups with progressive renal impairment held even after adjustment for age.
Table 1.
Demographic and clinical data of hypertensive patients
| No CKD | Stage I | Stage II | Stage III | Stage IV | Stage V | P value | ||
|---|---|---|---|---|---|---|---|---|
| (n = 280) | (n = 34) | (n = 42) | (n = 61) | (n = 32) | (n = 14) | P value | (°) | |
| Age (years) | 50±15 | 40±11*** | 57±13* | 65±13*** | 67±13*** | 67±8*** | <0.001 | NA |
| Men (%) | 54.6 | 55.9 | 54.8 | 62.3 | 46.9 | 64.3 | NS | NA |
| Smokers (%) | 30.2 | 23.3 | 18.9 | 35.2 | 28.6 | 58.3 | NS | NA |
| Diabetic (%) | 20.8 | 7.1 | 28.2 | 34.5 | 56.3 | 61.5 | <0.001 | NA |
| BMI (kg/m2) | 27.9±5.3 | 27.3±4.8 | 28.3±5.4 | 28.0±4.0 | 29.8±5.5 | 28.0±4.4 | NS | NS |
| Serum glucose (mg/dl) | 96±36 | 96±18NS | 107±45 NS | 107±34NS | 116±43* | 103±19NS | 0.019 | NS |
| Serum uric acid (mg/dl) | 5.4±1.6 | 5.6±1.5NS | 5.8±1.7 NS | 6.9±1.9*** | 8.0±2.0*** | 7.1±1.0** | <0.001 | 0.015 |
| Total cholesterol (mg/dl) | 191±40 | 201±41NS | 195±42NS | 179±45NS | 176±52NS | 157±56NS | 0.023 | NS |
| HDLc (mg/dl) | 50±16 | 46±9 | 52±17 | 44±13 | 47±19 | 40±12 | NS | NS |
| LDLc (mg/dl) | 122±37 | 126±37NS | 119±39NS | 113±37NS | 98±36NS | 87±44NS | 0.008 | NS |
| Triglycerides (mg/dl) | 112.0 (78.0–149.5) | 116.0 (91.0–165.3) | 126.0 (79.0–153.5) | 119.0 (98.0–165.0) | 127.5 (83.5–168.3) | 127.5 (95.5–173.8) | NS | NS |
| Albuminuria (mg/die) | 12.6 (4.9–18.9) | 181.4 (50.5–246.4)*** | 218.2 (44.9–569.4)*** | 354.0 (37.2–745.8)*** | 630.1 (135.5–900.4)*** | 769.8 (173.3–1515.4)*** | <0.001 | <0.001 |
| Serum creatinine (mg/dl) | 0,86±0.16 | 0.82±0.14NS | 0.98±0.15NS | 1.50±0.36*** | 2.47±0.52*** | 5.06±1.00*** | <0.001 | <0.001 |
| eGFR (ml/min/1.73 m2) | 91.8±16.9 | 103.7±10.7*** | 76.4±10.2*** | 45.9±9.2*** | 23.3±4.0*** | 10.2±1.8*** | <0.001 | <0.001 |
| Clinic systolic BP (mm Hg) | 135±14 | 139±20NS | 138±18NS | 139±18NS | 142±15NS | 146±18NS | 0.012 | 0.031 |
| Clinic diastolic BP(mm Hg) | 82±10 | 85±12 | 82±11 | 80±13 | 78±11 | 78±10 | NS | NS |
| Clinic pulse pressure (mm Hg) | 53±12 | 54±15NS | 56±18NS | 59±12* | 64±17*** | 68±20*** | <0.001 | 0.007 |
| Clinic heart rate (beats/min) | 74±11 | 78±8NS | 71±7NS | 67±11NS | 68±6NS | 62±7NS | 0.026 | NS |
| 24-h systolic BP (mm Hg) | 128±13 | 134±14NS | 129±15NS | 134±14NS | 136±18NS | 131±7NS | 0.011 | 0.003 |
| 24-h diastolic BP (mm Hg) | 79±10 | 83±12 | 78±9 | 78±11 | 79±11 | 75±7 | NS | NS |
| 24-h pulse pressure (mm Hg) | 48±9 | 50±9NS | 51±11NS | 55±11** | 54±11NS | 56±8NS | 0.002 | 0.040 |
| RRI | 0.62±0.06 | 0.60±0.06NS | 0.65±0.06NS | 0.68±0.07*** | 0.69±0.03*** | 0.71±0.05*** | <0.001 | <0.001 |
| cIMT (mm) | 0.89±0.24 | 0.80±0.29NS | 0.97±0.21NS | 1.05±0.20*** | 1.09±0.15*** | 1.13±0.17* | <0.001 | <0.001 |
| Plaques (%) | 18.2 | 3.8 | 23.7 | 46.5 | 24 | 45.5 | <0.001 | / |
| aPWV (m/s) | 10.8±2.4 | 9.8±2.4NS | 11.9±2.0NS | 12.5±1.9*** | 12.8±2.5*** | 12.8±1.9NS | <0.001 | 0.012 |
Abbreviations: aPWV: aortic pulse wave velocity; BMI, body mass index; CKD, chronic kidney disease; cIMT, carotid intima-media thickness; eGFR, glomerular filtration rate; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; NA, not applicable; PP, pulse pressure; RRI, renal resistive index.
NS P > 0.05; *P ≤ 0.05; **P ≤ 0.01; *** P ≤ 0.001. (°) P value adjusted for age.
In Table 2, the percentages of patients treated with cardiovascular drugs in the overall study population and in the patients with and without CKD are presented. Subjects using antiplatelet drugs showed a significantly higher mean cIMT and clinic PP (1.03±0.22mm, 58±16mm Hg, respectively) than subjects not using these agents (0.93±0.27mm, 54±14mm Hg; all P < 0.05), whereas subjects treated with other cardiovascular drugs did not differ regarding the abovementioned parameters when compared with those not treated.
Table 2.
Cardiovascular drugs in the study population
| Overall study population | Patients without CKD | Patients with CKD | P value | |
|---|---|---|---|---|
| (n = 463) | (n = 280) | (n = 183) | ||
| Angiotensin II receptor antagonists (%) | 33.3 | 34.3 | 33.1 | NS |
| ACE inhibitors (%) | 38.3 | 34.4 | 43.9 | 0.049 |
| Diuretics (%) | 38.8 | 37.7 | 42.7 | NS |
| Calcium antagonists | 39.4 | 42.5 | 37.2 | NS |
| β-blockers (%) | 15.3 | 16.7 | 12.3 | NS |
| αβ-blockers (%) | 10.7 | 12.3 | 9.4 | NS |
| α-blockers (%) | 32.4 | 24.6 | 46.8 | <0.001 |
| Centrally acting antiadrenergic drugs (%) | 6.8 | 5.6 | 9.4 | NS |
| Statins (%) | 16.4 | 17.1 | 16.3 | NS |
| Antiplatelet drugs (%) | 27.1 | 26.1 | 30.4 | NS |
| Allopurinol (%) | 8.2 | 4 | 11.7 | NS |
NS P > 0.05.
Abbreviations: CKD: chronic kidney disease.
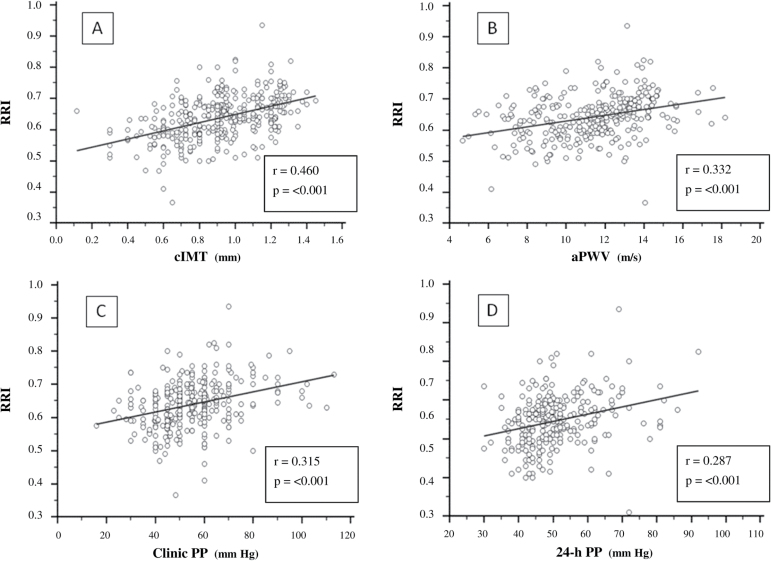
Table 3 shows the correlations between RRI, cIMT, aPWV, clinic PP, and 24-h PP and other variables in the whole study population. As shown in Figure 1, RRI correlated with cIMT, aPWV, clinic PP, and 24-h PP in the entire study population. These relationships did not significantly differ between subjects with normal renal function and CKD patients.
Table 3.
Main correlations in the study population
| Overall study population (n = 463) | |||||
|---|---|---|---|---|---|
| RRI | cIMT | aPWV | 24-h PP | Clinic PP | |
| r | r | r | r | r | |
| RRI | NA | 0.460*** | 0.332*** | 0.287*** | 0.315*** |
| cIMT | 0.460*** | NA | 0.386*** | 0.229*** | 0.279*** |
| aPWV | 0.332*** | 0.386*** | NA | 0.324*** | 0.369*** |
| Clinic systolic BP | 0.175** | 0.234*** | 0.365*** | 0.524*** | 0.737*** |
| Clinic diastolic BP | −0.177** | NS | NS | NS | −0.205*** |
| Clinic PP | 0.315*** | 0.279*** | 0.369*** | 0.617*** | NA |
| Clinic Heart rate | −0.285*** | NS | −0.331*** | −0.334*** | −0.329*** |
| 24- h systolic BP | NS | 0.203*** | 0.233*** | 0.728*** | 0.387*** |
| 24-h diastolic BP | −0.211*** | NS | NS | NS | −0.112* |
| 24-h PP | 0.287*** | 0.229*** | 0.324*** | / | 0.617*** |
| Serum creatinine | 0.330*** | 0.266*** | 0.222*** | 0.196** | 0.222*** |
| eGFR | −0.439*** | −0.416*** | −0.400*** | −0.233*** | −0.303*** |
| Age | 0.473*** | 0.556*** | 0.497*** | 0.182** | 0.311*** |
| BMI | 0.147** | 0.261*** | 0.112* | 0.136* | NS |
| Serum glucose | 0.221*** | 0.168** | 0.207*** | 0.206** | 0.129** |
| HDLc | NS | −0.200** | NS | NS | NS |
| LDLc | −0.133* | NS | −0.159* | −0.162* | NS |
| (Log) Triglycerides | 0.144* | 0.200** | NS | NS | NS |
| Serum uric acid | 0.299*** | 0.328*** | 0.213** | NS | 0.123* |
| (Log) Albuminuria | 0.328*** | 0.203*** | 0.343*** | 0.259*** | 0.218*** |
Abbreviations: aPWV, aortic pulse wave velocity; cIMT, carotid intima-media thickness; NA, not applicable; PP, pulse pressure; RRI, renal resistive index; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol.
NS P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Figure 1.
Relationships between renal resistive index (RRI) with (A) carotid intima-media thickness (cIMT), (B) aortic pulse wave velocity (aPWV), (C) clinic pulse pressure (PP), and (D) 24-h pulse pressure (24-h PP) in the overall study population. Regression lines, correlation coefficients, and P values are reported.
In the overall study population, the association between RRI, cIMT, and clinic PP remained statistically significant even after adjustment for various confounding factors in stepwise multiple linear regression analyses (Table 4(A)).
Table 4.
Independent multivariate correlates of renal resistive index in the overall study population
| Outcome variable: Renal resistive index |
Regression coefficients | ||||
|---|---|---|---|---|---|
| Not standardized | Standardized | ||||
| B | Standard error | β | t | P | |
| (A) Model (R 2 = 0.33) | |||||
| (Constant) | 0.496 | 0.025 | / | 19.834 | <0.001 |
| Carotid IMT | 0.648 | 0.109 | 0.258 | 5.936 | <0.001 |
| Clinic PP | 0.001 | <0.001 | 0.117 | 2.747 | 0.006 |
| Age | 0.001 | <0.001 | 0.151 | 2.768 | 0.006 |
| Log (albuminuria) | 0.010 | 0.004 | 0.115 | 2.568 | 0.011 |
| eGFR | <0.001 | <0.001 | −0.097 | −1.739 | 0.083 |
| (B) Model (R 2 = 0.34) | |||||
| (Constant) | 0.500 | 0.023 | <0.001 | 22.181 | <0.001 |
| Carotid IMT | 0.649 | 0.177 | 0.229 | 3.669 | <0.001 |
| Clinic PP | 0.001 | <0.001 | 0.193 | 3.061 | 0.002 |
| Age | 0.001 | <0.001 | 0.172 | 2.406 | 0.017 |
| Log (albuminuria) | 0.010 | 0.004 | 0.133 | 2.237 | 0.026 |
| Clinic PP × eGFR | <0.001 | <0.001 | −0.153 | −2.211 | 0.028 |
See text for further explanation about the multivariate models. Variables shown in italics came close to statistical significance. [A] aPWV did not attain statistical significance (β = 0.072; P = 0.272). [B] aPWV did not attain statistical significance (β = 0.043; P = 0.394).
Abbreviations: eGFR, glomerular filtration rate; PP, pulse pressure; IMT, intima-media thickness.
Subsequently, we calculated partial correlation coefficients relating RRI with cIMT, after correction for age and clinic PP, in overall study population (r = 0.348; P < 0.001) and in both patients with normal renal function (r = 0.178; P = 0.026) and in the whole group with CKD (r = 0.223; P = 0.009). Likewise, partial correlation coefficients relating RRI with clinic PP, adjusted for age and cIMT, were 0.231, 0.120, and 0.176, respectively (P < 0.001, P = 0.136, and P = 0.041, respectively) in all patients, in subjects without CKD and in the group with CKD.
Thus, a further multivariate model was built including the multiplicative 2-way interaction term “Clinic pulse pressure × eGFR”, along with the variables associated with RRI in previous linear multiple regression analysis, and similar conclusions were attained (Table 4(B)). The standardized regression coefficient relating this interaction term with RRI was statistically significant.
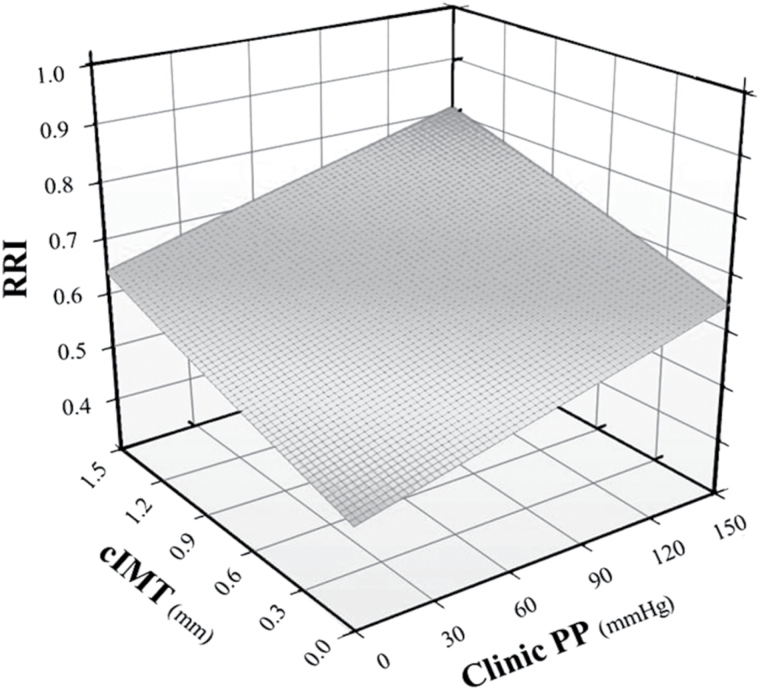
The multivariate relationship of RRI (dependent variable) with cIMT and with clinic PP is shown in Figure 2.
Figure 2.
Three-dimensional graph depicting the best-fit regression surface describing the multivariate relation between renal resistance index (RRI) (dependent variable) and carotid intima-media thickness (cIMT) and clinic pulse pressure (PP) in the overall study population.
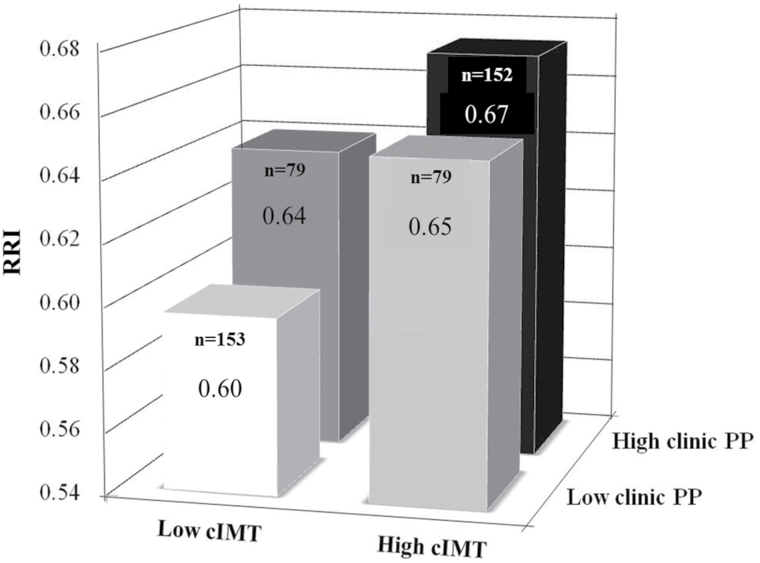
As presented in Figure 3, when participants were categorized into 4 subgroups based on the presence of cIMT and clinic PP values below (low) or above (high) the corresponding medians, RRI progressively increased from the subgroup with low cIMT and clinic PP to the subgroup with high cIMT and PP (P < 0.001).
Figure 3.
Renal resistance index (RRI) in subjects categorized into 4 subgroups based on the presence of carotid intima-media thickness (cIMT) and clinic pulse pressure (PP) values below (low) or above (high) the corresponding medians. ANOVA: P < 0.001. No significant interactive effect of cIMT and PP on RRI was observed (P = 0.130).
No significant interactive effect of cIMT and PP on RRI was observed (P = 0.130).
DISCUSSION
To the best of our knowledge, this is the first study exploring the simultaneous influences of cIMT, aPWV, and PP on renal hemodynamics in hypertensive patients with and without CKD. We found that RRI values, as well as cIMT, aPWV, and clinic PP, were higher in CKD patients than in hypertensive subjects without renal dysfunction, and they progressively increased with advancing CKD stages. Moreover, a close significant correlation between RRI, carotid atherosclerosis, arterial stiffness, and clinic PP was observed in the overall study population.
Many investigations showed that RRI, cIMT, aPWV, and PP increased with progressive decline of renal function.9,11,13,21–29 Moreover, we previously showed that RRI is associated with cIMT and severity of carotid atherosclerotic disease in hypertensive patients,9 and Hashimoto and Ito similarly observed a statistically significant univariate correlation between RRI, carotid-femoral PWV, and PP.12
However, the independent relationships of all these parameters and the mechanisms by which they affect renal vasculature remain largely to be clarified. The main finding of our work is that the association between RRI, cIMT, and clinic PP remained statistically significant after multivariate adjustment. Interestingly, the relationship between RRI and aPWV lost significance when adjusted for cIMT and clinic PP, whereas the correlation between RRI and clinic PP persisted even taking into account aPWV and cIMT.
The cross-sectional design of our study does not allow us to establish neither causal links between RRI and markers of vascular damage, nor the direction of these relationships. However, it seems reasonable to suggest some hypotheses to explain these findings.
Increased RRI might reflect in situ structural damage of intrarenal vessels, conceivable mirror of systemic structural vascular impairment. Histological studies showed that hypertensive patients with and without CKD had hyaline changes, intimal thickening and increased intima/media ratio of renal small arteries.2,30–34 Moreover, autopsy studies showed rarefaction and strictures of renal vascular tree, emphasizing the importance of these findings in increasing vascular resistance in hypertensive subjects.33,34 In different populations, some authors found that RRI was closely related to severe renal arteriosclerosis and intrarenal interstitial fibrosis 2,32, in part explaining higher RRI values with advancing CKD stages.
Systemic and renal structural vascular damage could be connected in different ways. Cardiovascular risk factors might concur to development of both renal and generalized vessel alterations. Moreover, small renal arteries could represent the reflection site of pulse waves, and their structural changes might generate an increased central pulse pressure and exert a load on nonmuscular components of the aortic wall, so contributing to systemic atherosclerotic damage.35 Finally, atherosclerosis per se could increase PP36, thus stimulating myogenic response of renal afferent arterioles, vascular remodeling, and increased renal vascular resistance.11,12
It is noteworthy that RRI was independently associated with PP in overall population and in patients with CKD, but not in those with normal renal function. Previous data demonstrated a greater systemic and renal vascular damage and a worse renal hemodynamics in CKD patients than in subjects without renal dysfunction.9–11,21–28 All these findings lead to hypothesize a gate-control like mechanism involved in the transmission of pulsatile flow from large vessels to renal microcirculation. According to this interpretation, an increased PP could adversely affect intrarenal hemodynamics only in subjects with kidney structural damage and impaired glomerular blood flow autoregulation: when autoregulation is disturbed, the glomerular vascular bed might become more vulnerable to the mechanical insults deriving from wide BP fluctuations.
However, in our work clinic PP was independently related to RRI, even in the absence of systemic structural vascular damage as detected by cIMT, and this is in agreement with animal and human studies supporting PP as one of the major factors influencing RRI.4,12,37 The exposure of small renal vessels to highly pulsatile pressure and flow might stimulate vascular remodeling and determine increased renal resistance.38,39 Moreover, in agreement with our data, some authors recently showed that PP, more than aPWV, affect renal vasculature.12,13 Pulsatile pressure, being determined by the interaction of intermittent ventricular ejection and viscoelastic properties of large arteries, might per se increase renal resistance. However, the design of our study does not allow to exclude the possibility that heightened PP might be the last mediator of the deleterious effect of increased arterial stiffness on intrarenal hemodynamics.38,40 In other words, although aPWV was not statistically related in multivariate analysis with RRI, it is conceivable that, in the pathophysiological sequence of events that leads to increased renal vascular resistance, large artery stiffness might antedate and determine PP widening which in turn unfavorably affects intrarenal hemodynamics.38,40 Similarly, the influence on RRI of factors, such as diabetes or hyperuricemia, associated in univariate analyses with arterial stiffness and cIMT in our study, as well as in previous ones, may be lost when the effect of PP and cIMT were taken into account. Moreover, increase of arterial stiffness is only one of many variables able to influence PP, and for these reasons its effect on renal vasculature might be statistically masked by pulse pressure.
In conclusion, the association we found of cIMT and clinic PP with intrarenal hemodynamics corroborates the role of kidney as sensor of systemic cardiovascular damage.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension 2002; 39:699–703. [DOI] [PubMed] [Google Scholar]
- 2. Bigé N, Lévy PP, Callard P, Faintuch JM, Chigot V, Jousselin V, Ronco P, Boffa JJ. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol 2012; 13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology 1999; 211:411–417. [DOI] [PubMed] [Google Scholar]
- 4. Heine GH, Gerhart MK, Ulrich C, Köhler H, Girndt M. Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int 2005; 68:878–885. [DOI] [PubMed] [Google Scholar]
- 5. Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, Berruti V, Leoncini G, Ruello N, Zagami P, Bezante GP, Derchi LE, Deferrari G. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant 1999; 14:360–365. [DOI] [PubMed] [Google Scholar]
- 6. Doi Y, Iwashima Y, Yoshihara F, Kamide K, Takata H, Fujii T, Kubota Y, Nakamura S, Horio T, Kawano Y. Association of renal resistive index with target organ damage in essential hypertension. Am J Hypertens 2012; 25:1292–1298. [DOI] [PubMed] [Google Scholar]
- 7. Tedesco MA, Natale F, Mocerino R, Tassinario G, Calabrò R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J Hum Hypertens 2007; 21:291–296. [DOI] [PubMed] [Google Scholar]
- 8. Ghadirpour A, Tarzamni MK, Naghavi-Behzad M, Abedi-Azar S, Koushavar H, Nezami N. Renal vascular Doppler ultrasonographic indices and carotid artery intima-media thickness in diabetic nephropathy. Med Ultrason 2014; 16: 95–99. [DOI] [PubMed] [Google Scholar]
- 9. Geraci G, Mulè G, Mogavero M, Geraci C, D’Ignoti D, Guglielmo C, Cottone S. Renal haemodynamics and severity of carotid atherosclerosis in hypertensive patients with and without impaired renal function. Nutr Metab Cardiovasc Dis. 2015; 25:160–166. [DOI] [PubMed] [Google Scholar]
- 10. Heine GH, Reichart B, Ulrich C, Köhler H, Girndt M. Do ultrasound renal resistance indices reflect systemic rather than renal vascular damage in chronic kidney disease? Nephrol Dial Transplant 2007; 22:163–170. [DOI] [PubMed] [Google Scholar]
- 11. Geraci G, Mulè G, Geraci C, Mogavero M, D’Ignoto F, Morreale M, Foraci AC, Cottone S. Association of renal resistive index with aortic pulse wave velocity in hypertensive patients. Eur J Prev Cardiol. 2015; 22:415–422. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 2011; 58:839–846. [DOI] [PubMed] [Google Scholar]
- 13. Kim CS, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kim SW. Association of Pulse Wave Velocity and Pulse Pressure With Decline in Kidney Function. J Clin Hypertens 2014; 16:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 15. Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303. [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3(Suppl):1–150 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012; 34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajzer MW, Wojciechowska W, Klocek M, Palka I, Brzozowska-Kiszka M, Kawecka-Jaszcz K. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens 2008; 26:2001–2007. [DOI] [PubMed] [Google Scholar]
- 20. Horváth IG, Németh A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziráki A. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens 2010; 28:2068–2075. [DOI] [PubMed] [Google Scholar]
- 21. Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol 2012;2012:139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawai T, Kamide K, Onishi M, Yamamoto-Hanasaki H, Baba Y, Hongyo K, Shimaoka I, Tatara Y, Takeya Y, Ohishi M, Rakugi H. Usefulness of the resistive index in renal Doppler ultrasonography as an indicator of vascular damage in patients with risks of atherosclerosis. Nephrol Dial Transplant 2011; 26:3256–3262. [DOI] [PubMed] [Google Scholar]
- 23. Ito H, Komatsu Y, Mifune M, Antoku S, Ishida H, Takeuchi Y, Togane M. The estimated GFR, but not the stage of diabetic nephropathy graded by the urinary albumin excretion, is associated with the carotid intima-media thickness in patients with type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol 2010; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawamoto R, Ohtsuka N, Kusunoki T, Yorimitsu N. An association between the estimated glomerular filtration rate and carotid atherosclerosis. Intern Med 2008; 47:391–398. [DOI] [PubMed] [Google Scholar]
- 25. Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension 2004; 43:163–168. [DOI] [PubMed] [Google Scholar]
- 26. Mulè G, Cottone S, Cusimano P, Palermo A, Geraci C, Nardi E, Castiglia A, Costanzo M, Cerasola G. Unfavourable interaction of microalbuminuria and mildly reduced creatinine clearance on aortic stiffness in essential hypertension. Int J Cardiol 2010; 145:372–375. [DOI] [PubMed] [Google Scholar]
- 27. Matsuda N, Takei T, Fujiu A, Ogawa T, Nitta K. Arterial stiffness in patients with non-diabetic chronic kidney disease (CKD). J Atheroscler Thromb 2009; 16:57–62. [DOI] [PubMed] [Google Scholar]
- 28. Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 2005; 45:494–501. [DOI] [PubMed] [Google Scholar]
- 29. Safar ME, Plante GE, Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens 2015; 28:561–569. [DOI] [PubMed] [Google Scholar]
- 30. Muiesan ML, Salvetti M, Rizzoni D, Paini A, Agabiti-Rosei C, Aggiusti C, Bertacchini F, Stassaldi D, Gavazzi A, Porteri E, De Ciuceis C, Agabiti-Rosei E. Pulsatile hemodynamics and microcirculation: evidence for a close relationship in hypertensive patients. Hypertension 2013; 61:130–136. [DOI] [PubMed] [Google Scholar]
- 31. Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension 1999; 33:998–1001. [DOI] [PubMed] [Google Scholar]
- 32. Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, Suzuki S, Miura S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 2005; 46:603–609. [DOI] [PubMed] [Google Scholar]
- 33. Tracy RE. The heterogeneity of vascular findings in the kidneys of patients with benign essential hypertension. Nephrol Dial Transplant 1999; 14:1634–1639. [DOI] [PubMed] [Google Scholar]
- 34. Katafuchi R, Takebayashi S. Morphometrical and functional correlations in benign nephrosclerosis. Clin Nephrol 1987; 28:238–243. [PubMed] [Google Scholar]
- 35. Safar ME, Rizzoni D, Blacher J, Muiesan ML, Agabiti-Rosei E. Macro and microvasculature in hypertension: therapeutic aspects. J Hum Hypertens 2008; 22:590–595. [DOI] [PubMed] [Google Scholar]
- 36. van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 2001; 32:454–460. [DOI] [PubMed] [Google Scholar]
- 37. Claudon M, Barnewolt CE, Taylor GA, Dunning PS, Gobet R, Badawy AB. Renal blood flow in pigs: changes depicted with contrast-enhanced harmonic US imaging during acute urinary obstruction. Radiology 1999; 212:725–731. [DOI] [PubMed] [Google Scholar]
- 38. Salvi P, Parati G. Chronic kidney disease: Arterial stiffness and renal function–a complex relationship. Nat Rev Nephrol 2015; 11:11–13. [DOI] [PubMed] [Google Scholar]
- 39. Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 2009; 54:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 2015; 26:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]