Abstract
BACKGROUD
Declining renal function is an independent risk factor for all-cause mortality in cardiovascular disease. Visfatin has been described as a marker of inflammation and endothelial dysfunction, but whether circulating visfatin levels are predictive to a subsequent decline in renal function remains unclear.
METHODS
In total, 200 nondiabetic, non-proteinuric hypertensive outpatients with initial serum creatinine (Scr) ≤1.5mg/dl were enrolled. Plasma visfatin concentration and endothelial function estimated by brachial artery flow-mediated dilatation (FMD) were determined in the study subjects. The primary endpoints were the occurrence of renal events including doubling of Scr, 25% loss of glomerular filtration rate (GFR) from baseline values, and the occurrence of end-stage renal disease during follow-up.
RESULTS
The mean annual rate of GFR decline (ΔGFR/y) was −1.26±2.76ml/min/1.73 m2 per year during follow-up (8.6±2.5 years). At baseline, plasma visfatin was negatively correlated with estimated GFR. In longitudinal analysis, the ΔGFR/y was correlated with visfatin, baseline GFR, FMD, systolic blood pressure, and fasting blood glucose (FBG). Multivariate analysis indicated that increased visfatin (r = −0.331, P <0.001), baseline GFR (r = −0.234, P = 0.001), FMD (r = 0.163, P = 0.015), and FBG (r = −0.160, P = 0.015) are independent predictors of ΔeGFR/y. Cox regression model analysis showed that visfatin (hazard ratio (HR), 1.09; 95% confidence interval (CI), 1.05–1.13, P <0.001), FBG (HR, 1.01; 95% CI, 1.00–1.02, P = 0.020), and FMD (HR, 0.87; 95% CI, 0.76–1.00, P = 0.049) were independently associated with the risk of developing future renal events.
CONCLUSIONS
Increased circulating visfatin are associated with subsequent decline in renal function in nondiabetic hypertensive patients.
Keywords: adipokine, blood pressure, chronic kidney disease, endothelial dysfunction, hypertension, visfatin.
Chronic kidney disease (CKD) is a major public health problem with an increasing incidence and prevalence.1 Because of the high prevalence of early stages of CKD and its high impact on cardiovascular disease, early detection of individuals at risk for the development and progression of CKD is particularly important. The mechanisms for the deterioration of renal function in early CKD are complex, and may take contributions from systemic inflammation, oxidative stress, and endothelial dysfunction.
The adipocytes have pleiotropic functions and secrete a number of bioactive cytokines, called adipokines, which activate a variety of cell signaling pathways that may regulate the pathophysiological process of systemic diseases. Altered levels of the adipokines can decrease the glomerular filtration rate (GFR, which can be estimated by creatinine clearance and is routinely used to assess kidney function) and increase albuminuria, which are pathophysiological changes typical of CKD.2 Clinical reports have also shown that increases in body mass index are associated with greater declines in kidney function in a cohort of young adults with preserved GFR at baseline.3 Visfatin, a 52-kDa molecule, was initially identified as a novel adipokine with insulin-mimetic properties in mice.4 This adipokine was identical to 2 previously described molecules, namely, pre-B cell colony-enhancing factor (PBEF)5 and the enzyme nicotinamide phosphoribosyltransferase (Nampt).6 However, controversy remains in the classification of visfatin, with some researchers regarding it as a marker of inflammation rather than an adipokine, since elevated visfatin levels are associated with increasing inflammatory cytokine production.7 Additionally, visfatin has been proposed as a marker of endothelial dysfunction.8 Our recent study has indicated that hypertensive patients with endothelial dysfunction and reduced vascular repair capacity may experience further deterioration of kidney function.9 Therefore, we designed this study to evaluate prospectively whether increased visfatin level is associated with GFR decline rate and future progression of kidney disease in nondiabetic hypertensive patients with preserved renal function.
METHODS
Study participants
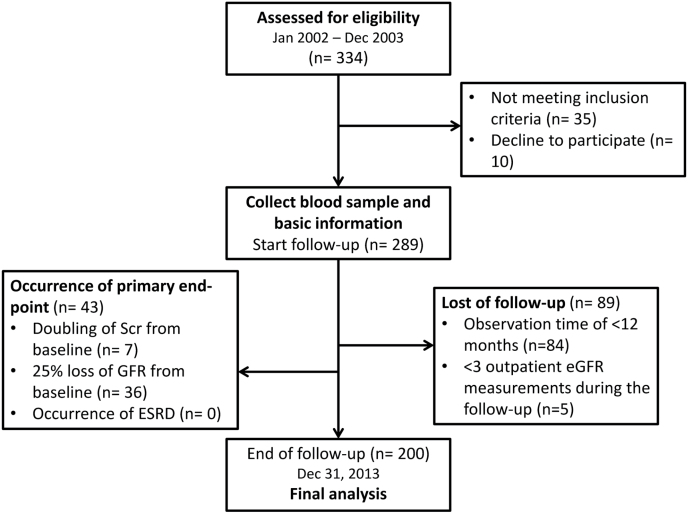
From January 2002 through December 2003, we consecutively recruited 334 nondiabetic outpatients with essential hypertension and a baseline Scr level ≤1.5mg/dl. Hypertension was defined as a systolic blood pressure (BP) ≥140mm Hg, a diastolic BP ≥90mm Hg, or use of antihypertensive drugs. Subjects with history or clinical evidence of angina, myocardial infarction, congestive heart failure, peripheral vascular disease, chronic systemic inflammatory disease, or any disease predisposing to vasculitis were excluded. Causes of secondary hypertension were excluded by appropriate investigations. The study protocol and profile were illustrated in Figure 1. Patients with proteinuria by dipstick were also excluded. At the end of follow-up on 31 December 2013, 200 patients (138 men and 62 women) were available for analysis with a mean follow-up duration of 103±30 months. The study was approved by the research ethics committee of Taipei Veterans General Hospital, and all participants provided their written informed consent.
Figure 1.
Study protocol and profile.
Clinical evaluation
Medical history was obtained during a personal interview and from medical files. All the measurements were made after an overnight fast of at least 8 hours. The impact of antihypertensive drug was considered with treatment during the follow-up. Patients who received a prescription for at least 90 pills of angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, or direct renin inhibitors during follow-up were regarded as renin-angiotensin system (RAS)-blocker users. Endothelium dependent flow-mediated dilatation (FMD) was assessed using a 7.5-MHz linear array transducer (Hewlett-Packard Sonos 5500, Andover, MA) to scan the brachial artery in longitudinal section.10
Measurement of renal function
Creatinine measurements were performed at baseline and at the outpatient follow-up using the Jaffe method implemented in an auto-analyzer. Estimated GFR values (eGFR, ml/min/1.73 m2) were calculated with the equation proposed by investigators in the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration.11,12 The equation has been externally validated in Chinese populations with greater precision and accuracy in terms of eGFR.13 The rate of annual decline in eGFR over the course of the study was determined from the slope of the plot of all outpatient eGFR measurements for each individual. At least 3 eGFR measurements were required to estimate the eGFR slope, which was calculated by linear regression analysis and expressed as ml/min/1.73 m2 per year. The methodology in the current study has been published and validated in our previous works.9,14–16
Laboratory measurements
Venous blood samples were collected from all patients after 8 hours of overnight fasting for measurement. Biochemical parameters including serum total cholesterol, triglyceride, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, fasting blood glucose (FBG), creatinine, and uric acid were determined using commercial kits by a Hitachi 7600 auto-analyzer (Roche Modular; Hitachi, Tokyo, Japan). Plasma visfatin levels were determined by enzyme-linked immunosorbent assay method (Human visfatin ELISA kit, Phoenix Pharmaceuticals, Belmont, CA). The high-sensitivity C-reactive protein levels in plasma were assessed using latex-enhanced immunonephelometric assay (Dade Behring, Marburg, Germany).
Prospective follow-up and study endpoints
After the baseline investigation, patients were followed prospectively until the study endpoints or the observation period ended, on 31 December 2013. The primary endpoint of the study was the occurrence of renal events including doubling of Scr from baseline values, 25% loss of GFR from baseline values, or the occurrence of end-stage renal disease during the follow-up period. The secondary endpoint was the new onset of proteinuria evaluated by a dipstick check-up every 3–6 months during the follow-up observation period. Among all participants, 5 patients were excluded from the study due to follow-up with the observation time of <12 months and/or less than 3 outpatient eGFR measurements during the follow-up.
Statistical analysis
The analysis was performed on the complete data set, and the results were expressed as mean ± SD or as percent frequency. Comparisons between 2 groups were made by Student’s t-test or chi-square test, as appropriate. Comparisons of continuous variables among the groups were performed by analysis of variance and least significant difference test (post hoc test). Variables associated with the annual rate of decline of eGFR were identified using Pearson’s correlation coefficient. Linear regression analysis was used to assess the relationship between annual rate of decline of eGFR and risk factors. We constructed multivariable models using △eGFR as the dependent coefficient. The Kaplan–Meier technique was applied to survival analysis. For the primary and secondary endpoints, observations were censored at the end of the study or the date that patients died, whichever occurred first. Cox proportional hazards model was used to examine the association of baseline variables with progression to renal endpoint and proteinuria. The multivariate Cox regression analysis was performed to evaluate the independent contribution of visfatin levels to the risk of renal events, adjusting for significant variables in univariate analysis. Data were analyzed using SPSS software (version 20, SPSS, Chicago, IL). A P value of <0.05 was considered to indicate statistical significance.
RESULTS
The mean age of the 200 hypertensive patients was 63±14 years. Renal function was determined in all study subjects at baseline and at 103±30 months later (range 32–132 months). The median duration of follow-up for all patients in the trial was 119 months (interquartile range, 89–120). The baseline mean eGFR was 80.6±25.2ml/min/1.73 m2, which decreased to 71.6±26.8ml/min/1.73 m2 by the end of the observation period. In our study cohort, the mean yearly decline in GFR was 1.26±2.76ml/min/1.73 m2 per year, which was similar to the natural history of CKD17 and was not statistically different between males and females (P = 0.254).
Supplementary Table S1 summarizes the correlation analysis between plasma visfatin levels and other baseline parameters. In multiple linear regression analysis, baseline circulating visfatin level was independently associated with baseline eGFR (r = −0.219, P = 0.003) and systolic blood pressure (SBP, r = 0.168, P = 0.021).
Circulating visfatin levels and subsequent renal function deterioration
Based on the visfatin levels, the actual cutoff values for each quartile of the visfatin levels are 9.7ng/ml, 11.0ng/ml and 15.8ng/ml. We classified our patients into 4 groups according to quartiles, including 50 patients in group 1 (Q1) with visfatin level ≤9.7ng/ml, 50 patients in group 2 (Q2) with visfatin level >9.7ng/ml and ≤11.0ng/ml, 50 patients in group 3 (Q3) with visfatin level >11.0ng/ml and ≤15.8ng/ml, and 50 patients in group 4 (Q4) with visfatin level >15.8ng/ml.
The baseline characteristics of the patients in each group are presented in Table 1. There were no significant differences between the 4 groups with respect to sex, body mass index, smoking status, serum levels of total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, triglyceride, and FBG, baseline Scr, SBP, uric acid, Framingham risk score, or high-sensitivity C-reactive protein levels. Patients in group 2 had a higher age, and there was a lower initial eGFR in group 3. There were no significant differences in antihypertensive therapy duration and medication usage among the 4 groups.
Table 1.
Baseline characteristics in 4 groups of hypertensive patients according to plasma visfatin levels
| Quartile groups in plasma visfatin levels | |||||
|---|---|---|---|---|---|
| Characteristic | Q1 (n = 50) | Q2 (n = 50) | Q3 (n = 50) | Q4 (n = 50) | P value |
| Age (years) | 59±12a,b | 67±13c,d | 65±13c | 62±15a | 0.014 |
| Men | 35 (70%) | 34 (68%) | 32 (64%) | 37 (74%) | 0.749 |
| BMI | 25.2±3.7 | 25.1±3.4 | 24.9±3.3 | 25.0±3.3 | 0.978 |
| Current smoker | 6 (12%) | 8 (16%) | 8 (16%) | 8 (16%) | 0.815 |
| Lipid profile | |||||
| Total cholesterol | 202±48 | 198±37 | 196±33 | 196±37 | 0.824 |
| Triglyceride | 190±294 | 144±117 | 128±49 | 143±76 | 0.274 |
| HDL | 45±8 | 48±16 | 49±17 | 47±13 | 0.392 |
| LDL | 120±56 | 121±38 | 121±32 | 120±34 | 0.997 |
| Fasting glucose | 95±29 | 91±18 | 95±28 | 94±32 | 0.874 |
| Serum Cr | 1.02±0.23 | 1.04±0.27 | 1.01±0.38 | 1.03±0.29 | 0.955 |
| Uric acid | 7.0±1.5 | 6.6±1.4 | 6.7±1.5 | 6.6±1.5 | 0.463 |
| Initial eGFR | 87.8±30.3b,d | 84.4±25.4b | 73.7±23.0a,c | 76.3±18.6c | 0.015 |
| Systolic BP | 128±8 | 130±8 | 130±10 | 131±8 | 0.383 |
| FRS | 13.5±6.1 | 14.5±9.3 | 12.9±10.2 | 12.5±9.8 | 0.710 |
| hsCRP | 2.0±3.0 | 2.0±2.6 | 1.9±2.1 | 1.6±2.0 | 0.783 |
| Visfatin level (ng/ml) | 8.2±1.8a,b^ | 10.2±0.3b,c,d | 13.0±1.4a,c,d | 19.8±4.5a,b,c | <0.001 |
| Flow-mediated dilatation (FMD %) | 4.7±3.1 | 4.9±3.0 | 4.4±2.7 | 5.2±2.8 | 0.519 |
| Medications | |||||
| ACE-I/ ARB | 14 (28%) | 11 (22%) | 14 (28%) | 15 (30%) | 0.609 |
| CCB | 21 (42%) | 23 (46%) | 16 (32%) | 17 (34%) | 0.137 |
| Beta-blocker | 9 (18%) | 12 (24%) | 15 (30%) | 12 (24%) | 0.267 |
| Nitrates | 2 (4%) | 6 (12%) | 6 (12%) | 6 (12%) | 0.446 |
| Thiazides | 15 (30%) | 18 (36%) | 11 (22%) | 10 (20%) | 0.245 |
| Statin | 13 (26%) | 10 (20%) | 9 (18%) | 9 (18%) | 0.725 |
Conversion factors for units: Total cholesterol in mg/dl to mmol/l, ×0.02586; triglyceride in mg/dl to mmol/l, ×0.01129; LDL in mg/dl to mmol/l, ×0.02586; HDL in mg/dl to mmol/l, ×0.02586; fasting glucose in mEq/l to mmol/l, ×0.05551; Serum Cr in mg/dl to μmol/l, ×88.4; uric acid in mg/dl to μmol/l, ×59.48. Values are mean ± SD or number (%).
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein-cholesterol (mg/dl); LDL, low-density lipoprotein-cholesterol (mg/dl); Cr, creatinine; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2/year); BP, blood pressure (mm Hg); FRS, Framingham risk score (%); hsCRP, high-sensitivity C-reactive protein (mg/l); ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker.
a P value < 0.05 in comparison to Q2 (post hoc test). b P value < 0.05 in comparison to Q3 (post hoc test). c P value < 0.05 in comparison to Q1 (post hoc test). d P value < 0.05 in comparison to Q4 (post hoc test).
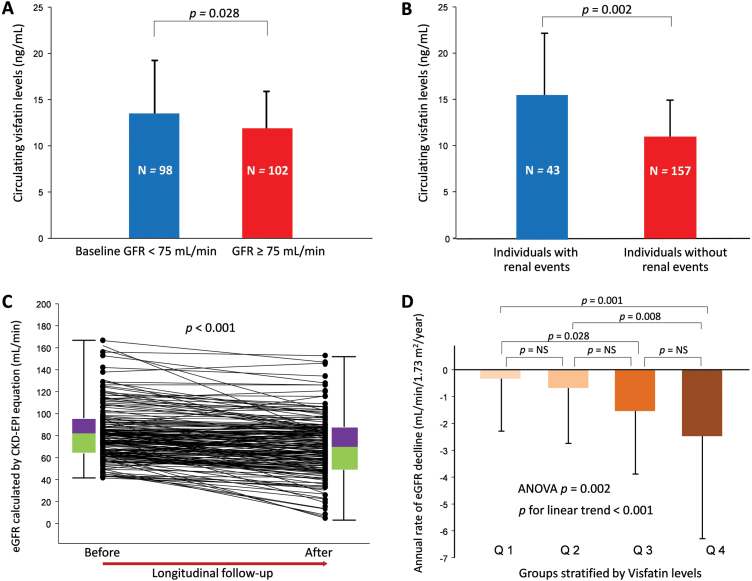
As illustrated in Figure 2A, patients with high GFR (≥75ml/min, n = 102) had significantly lower visfatin levels than those with low GFR (<75ml/min, n = 98) at baseline (P = 0.028). We graphically reported the values of the annual rate of decline of eGFR in the study groups stratified by visfatin level. There were 43 patients reaching the primary endpoint. Among these subjects, none of them developed end-stage renal disease (eGFR <15ml/min), 7 people had doubled their Scr from the baseline, and 36 individuals lost more than 25% of eGFR compared with their baseline levels during follow-up. Serum visfatin levels among these subjects were illustrated in Figure 2B, 15.47±6.57ng/ml vs. 12.04±4.29ng/ml in individuals with (n = 43) and without (n = 157) renal events (P = 0.002). The eGFR change before and after longitudinal follow-up and data distribution were illustrated in Figure 2C (paired t-test P < 0.001). As shown in Figure 2D, there was a significant positive association between the visfatin level and the rate of decline in eGFR (mean △eGFR/y, Q1 vs. Q2 vs. Q3 vs. Q4 = −0.34±1.95 vs. −0.68±2.06 vs. −1.54±2.35 vs. −2.48±3.82ml/min/1.73 m2 P < 0.001).
Figure 2.
The relationship between circulation visfatin levels and the longitudinal changes in renal function among 200 hypertensive patients. (A) Plasma visfatin levels in low (<75ml/min) and high (≥75ml/min) glomerular filtration rate (GFR) groups (independent t-test P = 0.028). The median of the baseline eGFR was 75.8ml/min/1.73 m2 and a convenient eGFR cutoff value set at 75ml/min was used to classify the study cohort into 2 groups (N = 102 and 98 in each group, respectively). (B) Plasma visfatin levels among individuals with and without renal events (independent t-test P = 0.002). (C) The eGFR change before and after longitudinal follow-up (paired t-test P < 0.001) and data distribution. (D) The annual rate of eGFR decline in the study groups stratified by plasma visfatin levels. All patients were divided into 4 groups according to plasma visfatin levels in quartiles: group 1 (Q1), with visfatin level ≤9.7ng/ml; group 2 (Q2), with visfatin level >9.7ng/ml and ≤11.0ng/ml; group 3 (Q3), with visfatin level >11.0ng/ml and ≤15.8ng/ml; group 4 (Q4), with visfatin level >15.8ng/ml.
To further clarify the association between change in eGFR and visfatin level after adjustment for other risk factors, we performed simple and multiple regression analyses that included demographic variables and cardiovascular risk factors. As demonstrated in Table 2, the mean annual decline in eGFR was significantly correlated with serum visfatin level (r = −0.361, P<0.001). Moreover, baseline eGFR (r = −0.179, P = 0.011), FMD (r = 0.175, P = 0.013), SBP (r = −0.162, P = 0.022), and FBG levels (r = −0.149, P = 0.035) were also significantly associated with eGFR decline in univariate analysis. Besides, high-sensitivity C-reactive protein (r = −0.128, P = 0.071) levels showed a marginal association with the annual decline rate in eGFR in univariate analysis.
Table 2.
Univariable and multivariable associations with annual change in eGFR calculated by CKD-EPI equation
| Variables | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Visfatin | −0.361 | <0.001 | −0.331 | <0.001 |
| Baseline GFR | −0.179 | 0.011 | −0.234 | 0.001 |
| Flow-mediated dilatation (FMD) | 0.175 | 0.013 | 0.163 | 0.015 |
| Systolic blood pressure | −0.162 | 0.022 | −0.134 | 0.051 |
| Fasting blood glucose | −0.149 | 0.035 | −0.160 | 0.015 |
| hsCRP level | −0.128 | 0.071 | −0.060 | 0.365 |
| LDL-cholesterol level | −0.103 | 0.143 | ||
| Framingham risk score (%) | −0.093 | 0.191 | ||
| Age | −0.090 | 0.204 | ||
| Triglyceride level | −0.073 | 0.302 | ||
| Uric acid level | −0.034 | 0.626 | ||
| Body mass index | 0.033 | 0.641 | ||
Abbreviations: eGFR, estimated glomerular filtration rate; CKD-EPI equation, Chronic Kidney Disease Epidemiology equation; hsCRP, high-sensitivity C-reactive protein; Framingham risk score included parameters with age, gender, total cholesterol, high-density lipoprotein, smoking, and systolic blood pressure; LDL, low-density lipoprotein.
aThe multivariate regression model included all available variables with P value < 0.100 in the univariate analysis.
By multivariate analysis, serum visfatin (r = −0.331, P<0.001), baseline GFR (r = −0.234, P = 0.001), FMD (r = 0.163, P = 0.015), and FBG levels (r = −0.160, P = 0.015) were significant independent factors to subsequent decline in eGFR. Additionally, a marginal association between SBP and the eGFR decline rate was noted (r = −0.134, P = 0.051).
Visfatin levels predict renal events in nondiabetic hypertensive patients
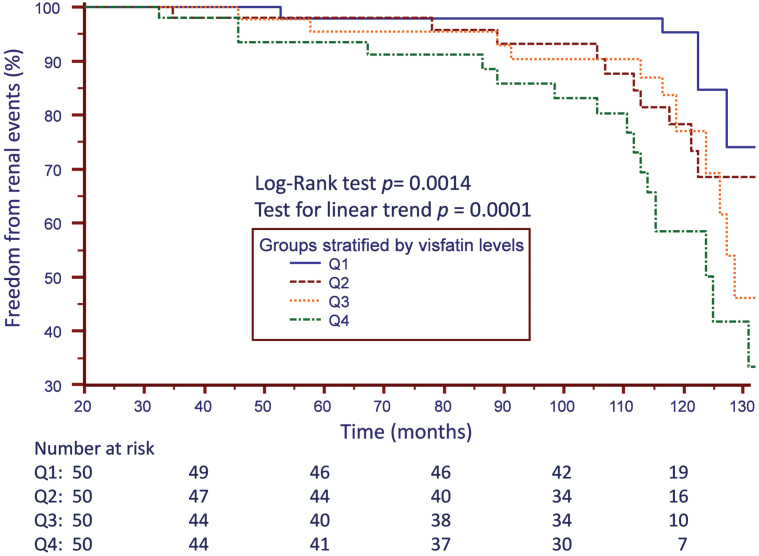
During the mean follow-up period of 8.6±2.5 years, 43 and 69 patients reached the primary and secondary endpoints, respectively. Kaplan–Meier estimates of the primary endpoints (Figure 3) include doubling of Scr from baseline values, 25% loss of GFR from baseline values, and the occurrence of end-stage renal disease in subjects categorized by visfatin level. The event-free survival was significantly different in the Q1 and Q4 groups (log-rank test, P = 0.001).
Figure 3.
Kaplan–Meier estimates of survival free of renal events, including doubling of serum creatinine from baseline values, 25% loss of glomerular filtration rate from baseline values, and the occurrence of end-stage renal disease in subjects categorized by visfatin level.
Univariate Cox regression analysis (Table 3) showed that the predictors of progression to primary endpoint include circulating visfatin, FBG, FMD, SBP, and the use of RAS blockers. In multivariate Cox proportional hazard regression model analysis, only visfatin levels (hazard ratio (HR), 1.08; 95% confidence interval (CI), 1.05–1.13, P < 0.001), FMD (HR, 0.87; 95% CI, 0.76–1.00, P = 0.049), and FBG (HR, 1.01; 95% CI, 1.00–1.02, P = 0.020) were independently associated with the risk of developing future renal events after adjustment.
Table 3.
Factors associated with the primary renal outcome by COX regression analysis
| Variables | Crude HR | Adjusted HRa | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Visfatin: per 1ng/ml | 1.09 (1.05–1.13) | <0.001 | 1.09 (1.05–1.13) | <0.001 |
| Fasting blood glucose: per 1mg/dl | 1.01 (1.00–1.02) | 0.002 | 1.01 (1.00–1.02) | 0.020 |
| Flow-mediated dilatation: per 1% change | 0.85 (0.75–0.96) | 0.010 | 0.87 (0.76–1.00) | 0.049 |
| Systolic blood pressure: per 1mm Hg | 1.05 (1.01–1.10) | 0.019 | 1.02 (0.97–1.07) | 0.453 |
| RAS blockerb use: user vs. nonuser | 0.52 (0.28–0.94) | 0.031 | 0.73 (0.37–1.44) | 0.358 |
| Age: per 1 year | 1.03 (1.00–1.06) | 0.056 | 1.02 (0.98–1.05) | 0.339 |
| Statin use: user vs. nonuser | 0.52 (0.23–1.17) | 0.114 | ||
| Calcium channel blocker use: user vs. nonuser | 0.63 (0.34–1.19) | 0.157 | ||
| hsCRP level: per 1mg/l | 1.08 (0.96–1.21) | 0.193 | ||
| Body mass index: per 1kg/m2 | 0.99 (0.96–1.02) | 0.379 | ||
| Framingham risk score: per 1% | 1.00 (0.99–1.01) | 0.531 | ||
| Uric acid level: per 1mg/dl | 1.05 (0.86–1.27) | 0.634 | ||
| LDL-cholesterol level: per 1mg/dl | 1.00 (0.99–1.01) | 0.743 | ||
| Baseline GFR: per 1ml/min/1.73 m2 | 1.00 (0.99–1.01) | 0.920 | ||
| Triglyceride level: per 1mg/dl | 1.00 (1.00–1.00) | 0.961 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; GFR, glomerular filtration rate; LDL, low-density lipoprotein-cholesterol; hsCRP, high-sensitivity C-reactive protein; RAS, renin-angiotensin system.
aThe adjusted COX regression model included all available variables with crude HR P value < 0.100. bRAS blocker, renin-angiotensin system blocker, including angiotensin converting enzymes (ACE) inhibitors, angiotensin receptor blockers (ARB), and direct renin inhibitors (DRI).
Meanwhile, there were 65 patients reaching the secondary endpoint (new-onset proteinuria). The average time to secondary endpoint was 67±32 months (range 12 to 118 months; median 69 months; interquartile range, 37–95 months). The predictors of new-onset proteinuria were FBG, SBP, Framingham risk score, FMD, age, baseline eGFR, and the use of RAS blockers (Table 4). Regarding the secondary endpoint, FBG (HR, 1.01; 95% CI, 1.01–1.02, P < 0.001) and use of RAS blockers (HR, 0.38; 95% CI, 0.19–0.75, P = 0.005) were independently associated with new-onset proteinuria in hypertensive patients in the multivariate Cox regression model.
Table 4.
Factors associated with new-onset proteinuria by COX regression analysis
| Variables | Crude HR | Adjusted HRa | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Fasting blood glucose: per 1mg/dl | 1.01 (1.00–1.02) | 0.001 | 1.01 (1.01–1.02) | <0.001 |
| Baseline systolic blood pressure: per 1mm Hg | 1.04 (1.01–1.08) | 0.010 | 1.03 (0.99–1.06) | 0.120 |
| Mean of 6-month interval systolic blood pressure during follow-up: per 1mm Hg | 1.04 (1.01–1.07) | 0.014 | 1.02 (0.99–1.05) | 0.207 |
| Framingham risk score: per 1% | 1.03 (1.00–1.07) | 0.027 | 1.01 (0.96–1.05) | 0.770 |
| Flow-mediated dilatation: per 1 % change | 0.90 (0.82–0.99) | 0.030 | 0.96 (0.86–1.08) | 0.510 |
| Age: per 1 year | 1.02 (1.00–1.05) | 0.039 | 1.01 (0.98–1.05) | 0.491 |
| Baseline GFR: per 1ml/min/1.73 m2 | 0.99 (0.98–1.00) | 0.039 | 1.00 (0.99–1.01) | 0.754 |
| RAS blockerb use: user vs. nonuser | 0.52 (0.27–0.98) | 0.044 | 0.38 (0.19–0.75) | 0.005 |
| hsCRP level: per 1mg/l | 1.08 (0.99–1.18) | 0.070 | 1.06 (0.96–1.17) | 0.241 |
| Uric acid level: per 1mg/dl | 1.08 (0.94–1.25) | 0.282 | ||
| Statin use: user vs. nonuser | 0.75 (0.42–1.34) | 0.335 | ||
| Visfatin: per 1ng/ml | 1.02 (0.97–1.07) | 0.381 | ||
| LDL-cholesterol level: per 1mg/dl | 1.00 (0.99–1.00) | 0.421 | ||
| Calcium channel blocker use: user vs. nonuser | 0.89 (0.54–1.46) | 0.651 | ||
| Beta-blocker use: user vs. nonuser | 1.40 (0.87–2.25) | 0.167 | ||
| Diuretics use: user vs. nonuser | 1.45 (0.58–3.61) | 0.423 | ||
| Triglyceride level: per 1mg/dl | 1.00 (1.00–1.00) | 0.844 | ||
| Body mass index: per 1kg/m2 | 1.00 (0.92–1.07) | 0.891 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; GFR, glomerular filtration rate; LDL, low-density lipoprotein-cholesterol; hsCRP, high-sensitivity C-reactive protein; RAS, renin-angiotensin system.
aThe adjusted COX regression model included all available variables with crude HR P value < 0.100. bRAS blocker, renin-angiotensin system blocker, including angiotensin converting enzymes (ACE) inhibitors, angiotensin receptor blockers (ARB), and direct renin inhibitors (DRI).
DISCUSSION
Currently, guidelines for the assessment of the risk of a faster eGFR decline or CKD progression remain focused largely on conventional risk factors,18 such as age, hypertension, insulin resistance, hyperlipidemia, and proteinuria. To the best of our knowledge, this is the first study to show that increased plasma visfatin levels in nondiabetic hypertensive patients are associated with subsequent decline in renal function determined by eGFR and the occurrence of renal events in a long-term prospective study of follow-up data.
Accumulating evidence suggests that the adipose tissue is no longer considered merely a triglyceride-storing depot, but also a real endocrine organ that synthesizes and secretes a wide range of diverse bioactive factors, called adipokines. These adipokines can be secreted and act locally within the adipose tissue, and have been shown to have various roles in vivo, including modulation of lipid metabolism, inflammation, insulin resistance, immune-stress response, and vascular homeostasis.19 Visfatin was initially identified as a novel adipokine with insulin-mimetic properties in mice, and is an adipocytokine whose circulating concentration is found to be elevated in metabolic disorders and obesity.19 Adipocytes are the primary source of visfatin, and the estimated physiologic plasma level of visfatin is 15ng/ml, with plasma concentration of visfatin increasing with adiposity.7,19,20 Several clinical studies have indicated that the circulating visfatin concentrations are significantly elevated in patients with CKD compared to normal controls.8,21 In diabetic patients, it has been reported that there is a significant negative correlation between plasma visfatin concentration and creatinine clearance, or endothelial function estimated by FMD.22 In this prospective cohort, we first show that circulating visfatin was an independent predictor of eGFR decline, together with FMD, systolic BP, FBG, hsCRP, and baseline eGFR. Even when the relationship between the visfatin level and eGFR is expressed by a modest correlation coefficient, our data suggest that the association is as clinically important as that documented by endothelial function and traditional cardiovascular risk factors.
There are several possible mechanisms thought to contribute to the pathogenesis of visfatin in patients with CKD, including insulin resistance,22 oxidative stress,23 inflammation,7,24 endothelial dysfunction,21–23 synthesis of profibrotic molecules in mesangial cells, and activation of intra-renal RAS.25 Notably, although previous published reports showing that antihypertensive drugs targeting the RAS system may have effects on circulating visfatin levels,26 the results were inconsistent.27,28 In our cohort, there was no significant association between baseline visfatin levels and the use of RAS blockers. The result was consistent with the latest report from Kocelak et al.,29 which showed that the plasma visfatin levels were not related to the use of antihypertensive drugs, including ACE inhibitors and angiotensin II receptor blockers.
Some evidence suggests that visfatin is not only an adipokine, but also a marker of inflammation. In human endothelial cells, visfatin promotes the subsequent release of cytokines and chemokines, including interleukin-6 or monocyte chemotactic protein-1.30,31 Romacho et al. indicated that human endothelial cells overproduce visfatin in response to pro-inflammatory stimulus,32 suggesting that visfatin seems to mediate inflammatory responses by induction of pro-inflammatory cytokines.7
There is a growing body of evidence suggesting that the extracellular visfatin can directly promote endothelial dysfunction by exerting a series of deleterious actions on the vascular cells.33,34 In patients with CKD, visfatin levels positively correlate with soluble markers of endothelial dysfunction,35,36 and the improvement of endothelial function after kidney transplantation correlates with a reduction in circulating visfatin levels.37 However, as shown in Supplementary Table S1, circulating visfatin levels were not significantly correlated with FMD in the current study. Although some studies have observed a significant association between the visfatin and FMD values in both CKD8 and diabetic22,26 patients, the relationship between these parameters in nondiabetic hypertensive patients remains uncertain. In addition, using multivariate linear regression model, both circulating visfatin level and FMD were independent predictors to the subsequent eGFR decline in our study cohort. These results suggest that the association between kidney function decline and circulating visfatin levels are more likely to be mediated by other factors (such as oxidative stress, inflammation, renal fibrosis, etc.) rather than a direct impact on the endothelial function. Future studies are warranted to elucidate the causal mechanism of visfatin in the pathogenesis of renal injury in this particular population.
Of note, Villalobos et al. recently demonstrated that visfatin may contribute to vascular aging and its associated pathologies through pro-senescence properties.38 In the latest report from a Polish geriatric study (PolSenior),29 which analyzed totally 2,789 representative subjects with an average eGFR of 70 and consisted of 27.1% individuals whose eGFR values below 60, there was no significant association between plasma visfatin concentration and the presence of eGFR <60 in the multivariable linear regression model. In the present study, our study population was 15 years younger than those in the PolSenior study, with a relatively more preserved eGFR and a lower degree of CKD. The finding from our study may suggest an independent role of visfatin in the pathogenesis of early stage kidney injury in hypertensive patients, beyond the diabetic kidney injury or the aging deterioration of the kidney function.
Some limitations of this study should be mentioned. First, the sample size is rather small, and was assembled from a single center. Therefore, further larger confirmative studies are needed to verify the current result. Second, we cannot exclude the possibility that the visfatin levels increased as the consequence of impaired kidney function and reduced visfatin clearance. However, it is difficult to measure the impact of this clearance effect precisely because, according to the best of our knowledge, the reference data are lacking with regard to visfatin clearance at different stages of CKD. Third, we cannot provide the data of albuminuria from baseline and during the follow-up because of lack of microalbuminuria exam (such as 24-hour urine microalbumin or spot urine albumin-creatinine ratio) from the initial study design.
In summary, this is the first study to show that an increased visfatin level is associated with subsequent declines in eGFR and predicts future renal outcomes independent of the conventional risk factors in nondiabetic hypertensive patients. These findings may partly explain the pathogenetic processes coupling the balance of adipokines in the subsequent progression of hypertensive kidney disease.
DISCLOSURE
Contributions: research idea and study design: P.-H.H., C.-Y.H., and C.-H.C.; data acquisition: P.-H.H., T.-H.C., C.-Y.H., and C.-H.C.; data analysis/interpretation: P.-H.H., C.-Y.H., and C.-H.C.; statistical analysis: H.-B.L. and C.-C.H.; supervision or mentorship: J.-W.C. and S.-J.L. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. P.-H.H. takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Statement of submission: All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
ACKNOWLEDGMENTS
This work was supported, in part, by the following research grants: the National Science Council, NSC 101-2314-B-075–038 and UST-UCSD International Centre of Excellence in Advanced Bio-engineering, NSC-100-2911-I-009-101-A2; VGH-V103A-009, VGH-V102B-016, VGH-V100E2-002, and VGH-V102E2-002 from Taipei Veterans General Hospital; and a grant from the Ministry of Education’s ‘Aim for the Top University’ Plan; and a grant from the Ministry of Health and Welfare (MOHW104-TDU-B-211-113-003). Funding agencies had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139:137–147. [DOI] [PubMed] [Google Scholar]
- 2. Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol 2013; 305:F1629–F1636. [DOI] [PubMed] [Google Scholar]
- 3. Grubbs V, Lin F, Vittinghoff E, Shlipak MG, Peralta CA, Bansal N, Jacobs DR, Siscovick DS, Lewis CE, Bibbins-Domingo K. Body mass index and early kidney function decline in young adults: a longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis 2014; 63:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005; 307:426–430. [DOI] [PubMed] [Google Scholar]
- 5. Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol 1994; 14:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol 2002; 32:3225–3234. [DOI] [PubMed] [Google Scholar]
- 7. Malyszko J, Malyszko JS, Mysliwiec M. Visfatin, a new adipocytokine, is predominantly related to inflammation/endothelial damage in kidney allograft recipients. Transplant Proc 2009; 41:150–153. [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz MI, Saglam M, Carrero JJ, Qureshi AR, Caglar K, Eyileten T, Sonmez A, Cakir E, Yenicesu M, Lindholm B, Stenvinkel P, Axelsson J. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant 2008; 23:959–965. [DOI] [PubMed] [Google Scholar]
- 9. Hsu CY, Huang PH, Chiang CH, Leu HB, Huang CC, Chen JW, Lin SJ. Increased circulating endothelial apoptotic microparticle to endothelial progenitor cell ratio is associated with subsequent decline in glomerular filtration rate in hypertensive patients. PLoS One 2013; 8:e68644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang PH, Chen YH, Chen YL, Wu TC, Chen JW, Lin SJ. Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome X. Heart 2007; 93:1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabanayagam C, Wong TY, Tai ES. The CKD-EPI equation and MDRD study equation find similar prevalence of chronic kidney disease in Asian populations. Ann Intern Med 2009; 151:892–893. [DOI] [PubMed] [Google Scholar]
- 13. Liao Y, Liao W, Liu J, Xu G, Zeng R. Assessment of the CKD-EPI equation to estimate glomerular filtration rate in adults from a Chinese CKD population. J Int Med Res 2011; 39:2273–2280. [DOI] [PubMed] [Google Scholar]
- 14. Hsu TW, Kuo KL, Hung SC, Huang PH, Chen JW, Tarng DC. Progression of kidney disease in non-diabetic patients with coronary artery disease: predictive role of circulating matrix metalloproteinase-2, -3, and -9. PLoS One 2013; 8:e70132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang PH, Huang SS, Chen YH, Lin CP, Chiang KH, Chen JS, Tsai HY, Lin FY, Chen JW, Lin SJ. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens 2010; 28:1655–1665. [DOI] [PubMed] [Google Scholar]
- 16. Chiang CH, Huang PH, Chiu CC, Hsu CY, Leu HB, Huang CC, Chen JW, Lin SJ. Reduction of circulating endothelial progenitor cell level is associated with contrast-induced nephropathy in patients undergoing percutaneous coronary and peripheral interventions. PLoS One 2014; 9:e89942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 1950; 29:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol 2003; 14:S65–S70. [DOI] [PubMed] [Google Scholar]
- 19. Stastny J, Bienertova-Vasku J, Vasku A. Visfatin and its role in obesity development. Diabetes Metab Syndr 2012; 6:120–124. [DOI] [PubMed] [Google Scholar]
- 20. Song HK, Lee MH, Kim BK, Park YG, Ko GJ, Kang YS, Han JY, Han SY, Han KH, Kim HK, Cha DR. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol 2008; 295:F1485–F1494. [DOI] [PubMed] [Google Scholar]
- 21. Axelsson J, Witasp A, Carrero JJ, Qureshi AR, Suliman ME, Heimbürger O, Bárány P, Lindholm B, Alvestrand A, Schalling M, Nordfors L, Stenvinkel P. Circulating levels of visfatin/pre-B-cell colony-enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKD. Am J Kidney Dis 2007; 49:237–244. [DOI] [PubMed] [Google Scholar]
- 22. Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism 2007; 56:451–458. [DOI] [PubMed] [Google Scholar]
- 23. Boini KM, Zhang C, Xia M, Han WQ, Brimson C, Poklis JL, Li PL. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim Biophys Acta 2010; 1801:1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, Michelsen A, Damås JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Frøland SS, Krohg-Sørensen K, Russell D, Aukrust P, Halvorsen B. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 2007; 115:972–980. [DOI] [PubMed] [Google Scholar]
- 25. Huang Q, Guo Y, Zeng H, Xie W, Yan H, Ding H. Visfatin stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Endocr Res 2011; 36:93–100. [DOI] [PubMed] [Google Scholar]
- 26. Eyileten T, Sonmez A, Saglam M, Cakir E, Caglar K, Oguz Y, Vural A, Yenicesu M, Yilmaz MI. Effect of renin-angiotensin-aldosterone system (RAAS) blockade on visfatin levels in diabetic nephropathy. Nephrology (Carlton) 2010; 15:225–229. [DOI] [PubMed] [Google Scholar]
- 27. Derosa G, Maffioli P, Ferrari I, Palumbo I, Randazzo S, Fogari E, D’Angelo A, Cicero AF. Different actions of losartan and ramipril on adipose tissue activity and vascular remodeling biomarkers in hypertensive patients. Hypertens Res 2011; 34:145–151. [DOI] [PubMed] [Google Scholar]
- 28. Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, Palumbo I, Fogari E, D’Angelo A, Cicero AF. Differential effects of candesartan and olmesartan on adipose tissue activity biomarkers in type II diabetic hypertensive patients. Hypertens Res 2010; 33:790–795. [DOI] [PubMed] [Google Scholar]
- 29. Kocelak P, Olszanecka-Glinianowicz M, Owczarek A, Bożentowicz-Wikarek M, Brzozowska A, Mossakowska M, Zdrojewski T, Grodzicki T, Więcek A, Chudek J. Plasma visfatin/nicotinamide phosphoribosyltransferase levels in hypertensive elderly - results from the PolSenior substudy. J Am Soc Hypertens 2015; 9:1–8. [DOI] [PubMed] [Google Scholar]
- 30. Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res 2008; 78:356–365. [DOI] [PubMed] [Google Scholar]
- 31. Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 2007; 178:1748–1758. [DOI] [PubMed] [Google Scholar]
- 32. Romacho T, Villalobos LA, Cercas E, Carraro R, Sánchez-Ferrer CF, Peiró C. Visfatin as a novel mediator released by inflamed human endothelial cells. PLoS One 2013; 8:e78283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun Y, Chen S, Song G, Ren L, Wei L, Liu N, Zhang D, Lv X. Effect of visfatin on the function of endothelial progenitor cells in high-fat-fed obese rats and investigation of its mechanism of action. Int J Mol Med 2012; 30:622–628. [DOI] [PubMed] [Google Scholar]
- 34. Adya R, Tan BK, Chen J, Randeva HS. Nuclear factor-kappaB induction by visfatin in human vascular endothelial cells: its role in MMP-2/9 production and activation. Diabetes Care 2008; 31:758–760. [DOI] [PubMed] [Google Scholar]
- 35. Filippatos TD, Randeva HS, Derdemezis CS, Elisaf MS, Mikhailidis DP. Visfatin/PBEF and atherosclerosis-related diseases. Curr Vasc Pharmacol 2010; 8:12–28. [DOI] [PubMed] [Google Scholar]
- 36. Malyszko J, Malyszko JS, Pawlak K, Mysliwiec M. Visfatin and apelin, new adipocytokines, and their relation to endothelial function in patients with chronic renal failure. Adv Med Sci 2008; 53:32–36. [DOI] [PubMed] [Google Scholar]
- 37. Yilmaz MI, Saglam M, Carrero JJ, Qureshi AR, Caglar K, Eyileten T, Sonmez A, Oguz Y, Aslan I, Vural A, Yenicesu M, Stenvinkel P, Lindholm B, Axelsson J. Normalization of endothelial dysfunction following renal transplantation is accompanied by a reduction of circulating visfatin/NAMPT. A novel marker of endothelial damage? Clin Transplant 2009; 23:241–248. [DOI] [PubMed] [Google Scholar]
- 38. Villalobos LA, Uryga A, Romacho T, Leivas A, Sánchez-Ferrer CF, Erusalimsky JD, Peiró C. Visfatin/Nampt induces telomere damage and senescence in human endothelial cells. Int J Cardiol 2014; 175:573–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.