Abstract
BACKGROUND
Sleep-disordered breathing (SDB) is associated with repeated intermittent hypoxemia, and it is known as one of the risk factors for cardiovascular diseases. Previous studies assessing the effects of frequency and depth of hypoxemia on cardiovascular diseases have shown conflicting results. The aim of the current study was to clarify what SDB-related parameters most predict endothelial dysfunction to better understand the pathogenesis of endothelial dysfunction in patients with SDB.
METHODS
We conducted polysomnography (PSG) and measured flow-mediated vasodilation response (%FMD) in 50 outpatients suspected of SDB. Evaluated indices included: apnea-hypopnea index (AHI), 3% oxygen desaturation index (3%ODI), averaged arterial oxygen saturation (averaged SpO2), lowest arterial oxygen saturation (lowest SpO2), ratio of arterial oxygen saturation <90% (<SpO2 90%), and averaged time desaturation summation index (TDS: [100%-averaged SpO2] × total sleep time).
RESULTS
Significant differences were observed only in the TDS between the first and third (P = 0.03) and between the first and forth (P = 0.04) quartile groups, stratified by %FMD. The %FMD showed a significant relationship with TDS (β = −0.47, P = 0.001), even after adjusting for confounding factors (β = −0.33, P = 0.02). In contrast, AHI, 3%ODI, averaged SpO2, lowest SpO2, and <SpO2 90% showed no significant relationships.
CONCLUSIONS
This study shows the validity of TDS in predicting endothelial damage in patients with SDB. Cumulative hypoxemia, rather than the frequency of hypoxemic events presented as AHI, may be a greater contributing factor in causing endothelial dysfunction. A simple index like TDS may be a useful and novel indicator of the influence of SDB on the vasculature.
Keywords: blood pressure, cardiovascular diseases, endothelial function, hypertension, hypoxemia, sleep-disordered breathing.
Sleep-disordered breathing (SDB) is associated with recurrent hypoxemia and arousal during sleep. The prevalence of SDB is reported to be 1.2–7.5% in the general population.1 Intermittent hypoxemia by SDB has been reported to produce oxygen-derived free radicals, which can impair endothelial function.2 Previous studies have shown that endothelial dysfunction is strongly related to the pathogenesis of hypertension.3,4 Furthermore, endothelial dysfunction leads to cardiovascular disorders.5 Thus, hypoxemic stress via SDB may play a key role in inducing cardiovascular events in patients with SDB.6–9
However, although it is clear that SDB worsens prognosis and increases cardiovascular events, the previous studies on the relationship between the severity of SDB and cardiovascular events showed conflicting results.10–13 Some studies suggest that the frequency of apnea and hypopnea is weakly related with rates of cardiovascular events.10,12,13 In patients with chronic heart failure, complicated with Cheyne-Stokes respiration, mortality was shown to be related to the existence and severity of SDB, which is represented by the apnea-hypopnea index (AHI).11 Thus far, most previous studies on the influence of SDB have been carried out using AHI, i.e., frequency of apneic/hypopneic episodes as a marker of SDB insult. We speculate that, as for evaluating cardiovascular outcome in patients with SDB, conventional indices of SDB, such as AHI or oxygen desaturation index (ODI), might not adequately indicate the effects of intermittent hypoxemia; as such indices do not contain enough information concerning the accumulation of desaturation. Therefore, we hypothesized that another index, which includes information on the severity and duration of hypoxemia caused by SDB, would be a better predictor of adverse impacts on the cardiovascular system compared with AHI. Thus, the aim of this study was to clarify what factors of SDB most relate to endothelial dysfunction to better understand the pathogenesis of endothelial dysfunction in patients with SDB.
METHODS
Subjects
We recruited 50 outpatients who were referred to our clinic to diagnose SDB using in-hospital polysomnography (PSG). Inclusion criteria for this study were as follows: (i) patients aged 20 years or older and (ii) AHI ≥5/hour by measurements using Type-1 PSG. Exclusion criteria were as follows: (i) patients diagnosed with pulmonary or respiratory diseases, (ii) with overt heart failure, and (iii) that had previously undergone or were currently undergoing treatment for SDB. This study was approved by the Ethics Committee of Kyushu University Graduate School of Medical Sciences. The aims of the study were explained and written informed consent was obtained from all subjects.
Measurements
We acquired data on clinical characteristics from medical records. These included sex, age, body mass index (BMI), history of smoking, underlying diseases (e.g., existence of diagnosed hypertension, diabetes mellitus, and dyslipidemia), use of medications (e.g., angiotensin receptor blocker (ARB), angiotensin-converting enzyme inhibitor (ACE-I), and calcium channel blocker), and overnight PSG data. We developed an index called the time desaturation summation index (TDS) to represent cumulative hypoxemia in a simple fashion. The formula for obtaining TDS is expressed as follows: TDS = (100%-averaged arterial oxygen saturation during sleep [averaged SpO2]) × (multiplied by) total sleep time (hours). The flow-mediated vasodilation response (%FMD) was measured by a single technician blinded to patient information using automated recording, and an analysis system with a cross-sectional 10-MHz linear-array ultrasound probe (UNEX-EF, Unex, Nagoya, Japan) was used to provide the index of endothelial function.5 The protocol of %FMD measurements followed published guidelines,14 and has been described elsewhere.15,16 Briefly, the cuff was placed distal to the probe, and we measured changes in arterial diameter during 2 minutes after cuff release. The arterial measurements were taken with electrocardiography gating. Systolic blood pressure and diastolic blood pressure were measured before %FMD measurements.
Polysomnography
Overnight PSG comprised 4-channel electroencephalography, electrooculogram, submental and leg electromyography, electrocardiography, nasal pressure sensor, thoracic and abdominal bands, oral thermistor, and oxygen saturation with fingertip pulse oximetry (Nihon-Koden, Tokyo, Japan). Respiratory events and sleep stages were scored using criteria as set out by the 2007 guidelines of the American Academy of Sleep Medicine, and Rechtschaffen and Kales, respectively.17,18 Briefly, an apnea event was defined as any reduction in nasal-oral thermistor ≥90% for over 10 seconds. A hypopnea event was defined as any reduction in nasal pressure ≥50% for over 10 seconds associated with 3% desaturation or arousal. AHI and 3%ODI were calculated as the number of respiratory events per hour, and the number of oxygen desaturations ≥3% per hour of sleep, respectively. The ratio of arterial oxygen saturation <90% (SpO2 < 90%) was defined as the percentage of time of arterial oxygen saturation level <90% in total sleeping time.
Statistical analysis
After assessment of the Gaussian distribution of the data using the Kolmogorov–Smirnov test, the data were analyzed using analysis of variance with a Bonferroni post hoc test. The chi-square test was used to analyze binary data. Using general linear model analysis (i.e., single linear analysis and logistic regression analysis), we identified confounders for %FMD. If the significance of a variable was P < 0.1 in the general liner model analysis, we regarded it as a potential confounder. The identified confounders were included multivariate analysis as adjustments. To assess correlations, we used Pearson correlation analysis. All statistics were compiled using SPSS v21.0 (SPSS, Chicago, IL), and 2-sided tests were performed, with P < 0.05 being considered significant. Outcome values are expressed as mean ± SD, correlation coefficient (r), and standardized coefficient (β).
RESULTS
Clinical characteristics
A total of 50 patients with varying severity of SDB were included in this study. The clinical characteristics of these participants are shown in Table 1. The mean age was 58 years, with 86% being male. BMI ranged from 16 to 40kg/m2. With regards to underlying health conditions, 62% of the participants were hypertensive, 32% had diabetes, and 30% had dyslipidemia. A total of 16% of participants used ARB (4%) and ACE-I and calcium channel blocker (12%). The majority of patients were moderate to severe SDB (AHI: 44.6±22.5/hour), and their %FMD was 4.2±2.3%. The mean total sleep time, averaged SpO2, and SpO2 < 90% were 392 minutes, 94.0%, and 8.1%, respectively. The mean value of calculated TDS taken from these values was 38.8%/hour.
Table 1.
Clinical characteristics
| Variable | |
|---|---|
| Subjects (N) | 50 |
| Age (years) | 58±13 |
| Male (N [%]) | 43 [86] |
| BMI (kg/cm2) | 27.0±5.1 |
| Smoking (N [%]) | 16 [32] |
| Hypertension (N [%]) | 31 [62] |
| Diabetes mellitus (N [%)) | 16 [32] |
| Dyslipidemia (N [%]) | 15 [30] |
| ARB (N [%]) | 8 [16] |
| ACE-I (N [%]) | 2 [4] |
| Ca channel blocker (N [%]) | 6 [12] |
| SBP (mm Hg) | 129.6±18.9 |
| DBP (mm Hg) | 80.3±10.4 |
| HR (bpm) | 72.2±10.4 |
| Polysomnography | |
| AHI (/hour) | 44.6±22.5 |
| OAI (/hour) | 16.0±19.4 |
| CAI (/hour) | 1.1±2.9 |
| HI (/hour) | 25.4±15.2 |
| TST (minute) | 392±81 |
| 3%ODI (/hour) | 33.5±19.9 |
| Averaged SpO2 (%) | 94.0±1.8 |
| Lowest SpO2 (%) | 74.9±9.6 |
| SpO2 < 90% (%) | 8.1±9.0 |
| TDS (% × hour) | 38.8±11.5 |
| FMD data | |
| Arterial diameter (mm) | 4.3±0.6 |
| %FMD (%) | 4.2±2.3 |
Abbreviations: N, number; BMI, body mass index; ARB, angiotensin receptor blocker; ACE-I, angiotensin-converting enzyme inhibitor; Ca, calcium; FMD, flow-mediated vasodilation; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; PSG, polysomnography; AHI, apnea-hypopnea index, OAI, obstructive apnea index; CAI, central apnea index; HI, hypopnea index; TST, total sleep time; SpO2, arterial oxygen saturation; ODI, oxygen desaturation index; TDS, time desaturation summation index.
The clinical characteristics among quartile groups stratified by %FMD
Table 2 shows the differences in clinical characteristics among quartile groups stratified by %FMD. Although there were no significant differences in age, BMI, the existence of comorbidities (i.e., hypertension, diabetes mellitus, and dyslipidemia), use of medications, AHI, 3%ODI, averaged SpO2, lowest arterial oxygen saturation (lowest SpO2), and SpO2 < 90% among any of the 4 groups, significant differences were observed in TDS between the 1st and 3rd and between the 1st and 4th quartile groups (F[49, 9] = 3.72, P = 0.02; 1st vs. 3rd quartile, P = 0.03; 1st vs. 4th quartile, P = 0.04).
Table 2.
The clinical characteristics in the patients stratified by flow-mediated vasodilation
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P value | |
|---|---|---|---|---|---|
| Number | 12 | 13 | 12 | 13 | — |
| %FMD (%) | 7.3±1.4 | 4.7±0.3** | 3.3±0.4**,† | 1.5±0.9**,‡,§ | <0.001 |
| Age (years) | 57±15 | 59±12 | 61±10 | 57±16 | 0.87 |
| BMI (kg/cm2) | 25.0±6.2 | 27.0±4.9 | 28.2±4.3 | 27.8±4.7 | 0.43 |
| Smoking (N [%]) | 3 [25] | 6 [46] | 3 [25] | 4 [31] | 0.63 |
| Hypertension (N [%]) | 6 [50] | 5 [39] | 9 [75] | 11 [85] | 0.06 |
| Diabetes mellitus (N [%]) | 3 [25] | 2 [15] | 4 [33] | 7 [54] | 0.19 |
| Dyslipidemia (N [%]) | 2 [17] | 5 [39] | 2 [17] | 6 [46] | 0.25 |
| ARB (N [%]) | 1 [8] | 1 [8] | 1 [8] | 5 [38] | 0.09 |
| ACE-I (N [%]) | 1 [8] | 0 [0] | 0 [0] | 1 [8] | 0.55 |
| Ca channel blocker (N [%]) | 1 [8] | 1 [8] | 0 [0] | 4 [31] | 0.10 |
| AHI (/hour) | 49.3±24.6 | 41.3±21.2 | 50.0±23.9 | 38.7±20.9 | 0.51 |
| OAI (/hour) | 24.6±26.1 | 13.4±10.6 | 18.3±25.2 | 8.4±8.8 | 0.19 |
| CAI (/hour) | 0.6±0.7 | 1.1±1.7 | 2.3±5.4 | 0.4±1.0 | 0.36 |
| HI (/hour) | 22.0±12.7 | 23.7±16.2 | 26.5±16.1 | 29.1±16.4 | 0.67 |
| TST (minute) | 347±80 | 425±103 | 373±55 | 418±60 | 0.04 |
| 3%ODI (/hour) | 33.3±21.0 | 27.2±13.4 | 43.7±21.9 | 30.5±20.9 | 0.19 |
| Averaged SpO2 (%) | 94.5±1.8 | 94.5±1.8 | 93.2±1.6 | 93.9±1.7 | 0.22 |
| Lowest SpO2 (%) | 73.7±11.5 | 77.4±7.7 | 75.5±8.1 | 73.0±11.0 | 0.66 |
| SpO2 < 90% (%) | 7.9±10.2 | 6.7±6.9 | 9.5±10.4 | 8.3±9.1 | 0.90 |
| TDS (% × hour) | 30.6±6.8 | 38.4±11.8 | 43.2±10.7* | 42.8±12.1* | 0.02 |
Abbreviations: FMD, flow-mediated vasodilation; BMI, body mass index; ARB, angiotensin receptor blocker; ACE-I, angiotensin-converting enzyme inhibitor; Ca, calcium; AHI, apnea-hypopnea index; OAI, obstructive apnea index; CAI, central apnea index; HI, hypopnea index; TST, total sleep time; ODI, oxygen desaturation index; SpO2, arterial oxygen saturation; TDS, time desaturation summation index.
*vs. 1st quartile group, P < 0.05. †vs. 2nd quartile group, P < 0.01. **vs. 1st quartile group, P < 0.001. ‡vs. 2nd quartile group, P < 0.001. §vs. 3rd quartile group, P < 0.001.
The relationships between clinical characteristics and flow-mediated vasodilation response
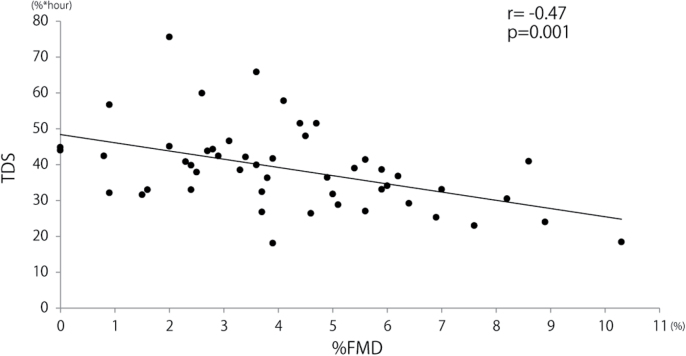
%FMD was marginally related to BMI, the existence of hypertension, and use of ARB in a single regression model (BMI: β = −0.26, P = 0.07; hypertension: β = −0.25, P = 0.07; ARB: β = −0.38, P = 0.08; Table 3). %FMD showed a significant relationship with TDS only (P = 0.001; Table 3, Figure 1). Even in multiple regression analysis after adjusting for BMI, the existence of hypertension, and use of ARB, the relationship between %FMD and TDS remained significant (β = −0.33, P = 0.02).
Table 3.
Unadjusted single regression analysis about the relationships between clinical characteristics and flow-mediated vasodilation
| Variable | β | P value |
|---|---|---|
| Age | 0.01 | 0.94 |
| Sex | 0.12 | 0.51 |
| BMI | −0.26 | 0.07 |
| Smoking | 0.04 | 0.77 |
| Hypertension | −0.25 | 0.07 |
| Diabetes mellitus | −0.20 | 0.17 |
| Dyslipidemia | −0.24 | 0.12 |
| ARB | −0.38 | 0.08 |
| ACE-I | 0.16 | 0.58 |
| Ca channel blocker | −0.35 | 0.14 |
| SBP | −0.15 | 0.30 |
| DBP | 0.17 | 0.25 |
| HR | 0.02 | 0.91 |
| AHI | 0.06 | 0.68 |
| OAI | 0.20 | 0.18 |
| CAI | −0.09 | 0.52 |
| HI | −0.15 | 0.29 |
| 3%ODI | −0.13 | 0.37 |
| Averaged SpO2 | 0.29 | 0.11 |
| Lowest SpO2 | 0.10 | 0.70 |
| SpO2 < 90% | −0.10 | 0.48 |
| Arterial diameter | −0.23 | 0.11 |
| TDS | −0.47 | 0.001 |
Abbreviations: β, standardizing coefficient; BMI, body mass index; ARB, angiotensin receptor blocker; ACE-I, angiotensin-converting enzyme inhibitor; Ca, calcium; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; AHI, apnea-hypopnea index; OAI, obstructive apnea index; CAI, central apnea index; HI, hypopnea index; ODI, oxygen desaturation index; SpO2, arterial oxygen saturation; TDS, time desaturation summation index.
Figure 1.
Correlation between averaged time desaturation summation index and flow-mediated vasodilation. Abbreviations: FMD, flow-mediated vasodilation; TDS, time desaturation summation index.
DISCUSSION
The current study revealed that endothelial function was most strongly and inversely related to cumulative hypoxemia during sleep, as expressed by the TDS index in patients with SDB. In addition, we did not find any significant correlations between %FMD and traditional indices; AHI, 3%ODI, averaged SpO2, or lowest SpO2. This result suggests that cumulative hypoxemia during sleep is likely to be the most influential factor leading to cardiovascular diseases in patients with SDB.
Previous studies reported that hypertension is common in patients with SDB, ranging from 37% to 54%.19 Moreover, a European-based large cohort study showed that the hypoxic state associated with SDB was significantly related to new onset hypertension.20 An etiology of hypertension in patients with SDB is endothelial dysfunction.21 The mechanisms of endothelial dysfunction associated with SDB, caused by hypoxemia during apneic sleep, involve the hypoxic state increasing the amount of inflammatory agents, such as hypoxia-inducible factor and nuclear factor-κB.22,23 Hypoxia-inducible factor upregulation leads to loss of p27 induction, which is a cyclin-dependent kinase that plays a critical role in cell cycle arrest.24 nuclear factor-κB upregulation contributes to the hypoxic state of cells through DNA binding activity, which leads to inflammation, increases in various cytokines, and adhesion of monocytes.25 Finally, the actions of hypoxia-inducible factor and nuclear factor-κB can lead to endothelial cell apoptosis.26,27 Thus, cumulative hypoxemia during sleep may have a strong impact on endothelial function. Specifically, hypoxia-inducible factor and nuclear factor-κB are known to increase according to the lowered oxygen saturation level and sustained hypoxemia.28 Conventional indices of SDB such as AHI and 3%ODI do not directly reflect the degree of hypoxemia. This may be one of the possible reasons for the inconsistent results regarding the relationships between %FMD, AHI, and 3%ODI.29–32 On the other hand, SpO2 < 90% reflects the degree of hypoxemia to a limited extent, because this index measures the duration of atrial oxygen level under 90% only. In contrast, the TDS index contains information concerning oxygen saturation level and duration of hypoxemia during the entire sleep period. Although TDS may not perfectly reflect the actual amount of inflammatory agents, we believe that TDS would approximate actual cumulative hypoxemia more accurately compared with other conventional indices. We set the baseline saturation level as 100% in calculations of TDS, because the fluctuation of “baseline” oxygen level was quite large in some patients even while they were awake. It is likely difficult to set an accurate “baseline” level for these types of patients. Moreover, clinically, it would be easier to measure a total decrease from 100%.
Recently, SDB has been shown to have a strong, negative impact on the cardiovascular system through activation of the sympathetic nervous system, negative intra-thoracic pressure, and hypoxemia.33,34 Since the endothelial layer is involved in critical functions such as preventing blood coagulation, controlling vascular tone, and anti-inflammatory actions,35 its dysfunction can cause hypertension and arteriosclerosis,36,37 leading to heart failure, coronary artery stenosis, and stroke.33,38 Thus, a reliable index that correctly indicates cumulative hypoxemia during sleep is critical for the accurate estimation of the negative impact of SDB on the cardiovascular system.
There are some limitations of the current study. Endothelial function was likely strongly affected by plasma glucose and lipoprotein levels (i.e., hemoglobin A1c, low-density lipoprotein, and high-density lipoprotein).39,40 In this study, we acquired data from a limited number (33 of 50) of patients. However, of these patients, %FMD was associated with BMI, hemoglobin A1c, high-density lipoprotein, hypertension, use of ARB, and TDS in a single regression analysis, but only %FMD was independently associated with TDS after adjusting for BMI, hemoglobin A1c, high-density lipoprotein, hypertension, and use of ARB (data not shown). Furthermore, %FMD was strongly correlated with TDS even in single regression analysis, indicating that TDS may be a powerful predictor.
We did not assess the nitroglycerin-induced vasodilation response. We recognize that this is a limitation that requires further study to clarify our findings. In addition, as this study was retrospective in nature, we were unable to eliminate the possibility of selection bias, though we collected patients as evenly as possible. Finally, we adopted all patients irrespective of total sleep time in this study, but patients with SDB with excessively short sleep times (e.g., 3 hours) during PSG might have significantly confounded the data because of the calculations involved in the TDS index formula. Future, large sample size, prospective studies are needed to address these issues.
In conclusion, cumulative hypoxemia during sleep, expressed by an index such as TDS in this study, may provide important information concerning endothelial function though the causality was not established, and such an index may more properly predict endothelial dysfunction in patients with SDB compared with traditional indices representing SDB severity.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by Grant-in-Aid for Scientific Research B (#15H05104) from the Ministry of Education, Culture, Sports, Science and Technology.
REFERENCES
- 1. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lui MM, Lam DC, Ip MS. Significance of endothelial dysfunction in sleep-related breathing disorder. Respirology 2013; 18:39–46. [DOI] [PubMed] [Google Scholar]
- 3. Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990; 323:22–27. [DOI] [PubMed] [Google Scholar]
- 4. Yoshida M, Imaizumi T, Ando S, Hirooka Y, Harada S, Takeshita A. Impaired forearm vasodilatation by acetylcholine in patients with hypertension. Heart Vessels 1991; 6:218–223. [DOI] [PubMed] [Google Scholar]
- 5. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 6. Meyer B, Mörtl D, Strecker K, Hülsmann M, Kulemann V, Neunteufl T, Pacher R, Berger R. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptide. J Am Coll Cardiol 2005; 46:1011–1018. [DOI] [PubMed] [Google Scholar]
- 7. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 2007; 115:2390–2397. [DOI] [PubMed] [Google Scholar]
- 8. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 2009; 120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015; 147:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005; 127:2076–2084. [DOI] [PubMed] [Google Scholar]
- 11. Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49:2028–2034. [DOI] [PubMed] [Google Scholar]
- 12. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012; 5:720–728. [DOI] [PubMed] [Google Scholar]
- 13. Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010; 122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 15. Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation 2004; 109:2857–2861. [DOI] [PubMed] [Google Scholar]
- 16. Inoue S, Takemoto M, Chishaki A, Ide T, Nishizaka M, Miyazono M, Sawatari H, Sunagawa K. Leg heating using far infra-red radiation in patients with chronic heart failure acutely improves the hemodynamics, vascular endothelial function, and oxidative stress. Intern Med 2012; 51:2263–2270. [DOI] [PubMed] [Google Scholar]
- 17. Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st edn. American Academy of Sleep Medicine: Westchester, IL, 2007. [Google Scholar]
- 18. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. U.S. Government Printing Office: Washington, DC, 1968. [Google Scholar]
- 19. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Brit Med J 2000; 320:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tkacova R, McNicholas WT, Javorsky M, Fietze I, Sliwinski P, Parati G, Grote L, Hedner J. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J 2014; 44:931–941. [DOI] [PubMed] [Google Scholar]
- 21. Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea-induced hypoxemia during sleep. Chest 2001; 119:1085–1091. [DOI] [PubMed] [Google Scholar]
- 22. Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res 1994; 54:1425–1430. [PubMed] [Google Scholar]
- 23. Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 2001; 280:C719–C741. [DOI] [PubMed] [Google Scholar]
- 24. Ortmann B, Druker J, Rocha S. Cell cycle progression in response to oxygen levels. Cell Mol Life Sci 2014; 71:3569–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol 2008; 105:1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iida T, Mine S, Fujimoto H, Suzuki K, Minami Y, Tanaka Y. Hypoxia-inducible factor-1alpha induces cell cycle arrest of endothelial cells. Genes Cells 2002; 7:143–149. [DOI] [PubMed] [Google Scholar]
- 27. Aoki M, Nata T, Morishita R, Matsushita H, Nakagami H, Yamamoto K, Yamazaki K, Nakabayashi M, Ogihara T, Kaneda Y. Endothelial apoptosis induced by oxidative stress through activation of NF-kappaB: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension 2001; 38:48–55. [DOI] [PubMed] [Google Scholar]
- 28. Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 2006; 103:18154–18159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med 2008; 178:984–988. [DOI] [PubMed] [Google Scholar]
- 30. Bayram NA, Ciftci B, Keles T, Durmaz T, Turhan S, Bozkurt E, Peker Y. Endothelial function in normotensive men with obstructive sleep apnea before and 6 months after CPAP treatment. Sleep 2009; 32:1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Del Ben M, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, Brunori M, Albanese F, Augelletti T, Violi F, Angelico F. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med 2012; 12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruno RM, Rossi L, Fabbrini M, Duranti E, Di Coscio E, Maestri M, Guidi P, Frenzilli G, Salvetti A, Taddei S, Bonanni E, Ghiadoni L. Renal vasodilating capacity and endothelial function are impaired in patients with obstructive sleep apnea syndrome and no traditional cardiovascular risk factors. J Hypertens 2013; 31:1456–1464; discussion 1464. [DOI] [PubMed] [Google Scholar]
- 33. Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165. [DOI] [PubMed] [Google Scholar]
- 34. Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation 2003; 107:1671–1678. [DOI] [PubMed] [Google Scholar]
- 35. Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012; 60:1455–1469. [DOI] [PubMed] [Google Scholar]
- 36. McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation 1991; 84:1273–1278. [DOI] [PubMed] [Google Scholar]
- 37. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 38. Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med 2002; 166:159–165. [DOI] [PubMed] [Google Scholar]
- 39. Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 1999; 34:146–154. [DOI] [PubMed] [Google Scholar]
- 40. Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis 2002; 161:1–16. [DOI] [PubMed] [Google Scholar]