Abstract
BACKGROUND
Genome-wide association studies have linked CYP17A1 coding for the steroid hormone synthesizing enzyme 17α-hydroxylase (CYP17A1) to blood pressure (BP). We hypothesized that the genetic signal may translate into a correlation of ambulatory BP (ABP) with apparent CYP17A1 activity in a family-based population study and estimated the heritability of CYP17A1 activity.
METHODS
In the Swiss Kidney Project on Genes in Hypertension, day and night urinary excretions of steroid hormone metabolites were measured in 518 participants (220 men, 298 women), randomly selected from the general population. CYP17A1 activity was assessed by 2 ratios of urinary steroid metabolites: one estimating the combined 17α-hydroxylase/17,20-lyase activity (ratio 1) and the other predominantly 17α-hydroxylase activity (ratio 2). A mixed linear model was used to investigate the association of ABP with log-transformed CYP17A1 activities exploring effect modification by urinary sodium excretion.
RESULTS
Daytime ABP was positively associated with ratio 1 under conditions of high, but not low urinary sodium excretion (P interaction <0.05). Ratio 2 was not associated with ABP. Heritability estimates (SE) for day and night CYP17A1 activities were 0.39 (0.10) and 0.40 (0.09) for ratio 1, and 0.71 (0.09) and 0.55 (0.09) for ratio 2 (P values <0.001). CYP17A1 activities, assessed with ratio 1, were lower in older participants.
CONCLUSIONS
Low apparent CYP17A1 activity (assessed with ratio 1) is associated with elevated daytime ABP when salt intake is high. CYP17A1 activity is heritable and diminished in the elderly. These observations highlight the modifying effect of salt intake on the association of CYP17A1 with BP.
Keywords: aging, blood pressure, CYP17A1, heritability, hypertension, salt, steroids.
Arterial hypertension is a major public health threat due to its high prevalence and associated increased risk for cardiovascular disease.1 Primary hypertension, also known as essential or idiopathic hypertension, accounts for about 95% of all cases of hypertension. As a complex trait, it is influenced by environmental (e.g., high salt intake) and genetic factors.2,3 From genome-wide association studies, CYP17A1 was identified as a sensitive locus, linked to blood pressure (BP) or arterial hypertension in the general population.4–6 Functionally, this gene is translated into the cytochrome P450 (CYP) type II enzyme, 17α-hydroxylase/17,20-lyase (CYP17A1). CYP17A1 is required for the production of cortisol in the adrenal cortex and androgen precursors of sex hormones in both the adrenal glands and gonads.7 Therefore, it would be of interest to investigate the association of BP and apparent CYP17A1 activity, by assessing precursor-to-product hormone metabolites ratios in the general population.
Until highlighted by genome-wide association studies, the association of CYP17A1 activity and BP was mainly investigated in patients with a 17α-hydroxylase deficiency (OMIM #202110), in whom a loss-of-function mutant of CYP17A1 leads to elevation of adrenocorticotropic hormone, with consecutive overproduction of mineralocorticoid active hormones,8 leading to salt sensitive hypertension.9 As a consequence, arterial hypertension in 17α-hydroxylase-deficient patients is responsive to supplementation of glucocorticoids.7 Further information about the development of hypertension by an altered CYP17A1 activity came recently from men with advanced prostate cancer who were treated with an inhibitor of CYP17A1 (abiraterone) to stop androgen production in adrenal glands. As a side effect, some developed hypertension due to mineralcorticoid excess.10 To prevent the overproduction by the stimulation with adrenocorticotropic hormone, patients were treated in further studies with glucocorticoids.11 However in 2 large trials, hypertension was more frequently reported in the abiraterone plus prednisone than in the prednisone alone group, suggesting that increased BP may be unresponsive to exogenous glucocorticoid supplementation in such patients.12,13
CYP17A1 activity catalyzes 2 enzymatic reactions: first, hydroxylation of pregnenolone and progesterone to 17α-hydroxypregnenolone and progesterone (17α-hydroxylase activity), respectively, and second, enhancement of side-chain cleavage of 17-hydroxylated steroids to generate dehydroepiandrosterone (DHEA) and androstenedione, the precursors of testosterone (17,20-lyase activity).14 Comparing the precursor-to-product ratios of the steroid metabolites, CYP17A1 activity in humans can be assessed by calculating the ratios of the total urinary excretion of 17-hydroxylated steroids with or without side-chain cleavage (i.e., androsterone (An) and etiocholanolone (Et)) and the metabolites of cortisol (tetrahydrocortisone (THE), tetrahydrocortisol (THF) and 5α-tetrahydrocortisol (5α-THF)), with C-21 steroids without 17-hydroxyl groups (i.e., total urinary metabolites of corticosterone (tetrahydro-11-dehydrocorticosterone (THA), tetrahydrocorticosterone (THB), and 5α-tetrahydrocorticosterone (5α-THB)).15
The availability of steroid hormone profiles in a large cohort of participants with ambulatory BP (ABP) measurement of European descent offered us the possibility to explore the distribution of CYP17A1 activities in a contemporary population and investigate its association with ABP, while urinary sodium excretion served as proxies for dietary salt intake.16 The family-based study design allowed assessment of the heritability of apparent CYP17A1 activities.
METHODS
Study population
Swiss Kidney Project on Genes in Hypertension (SKIPOGH) is a family-based cross-sectional study exploring the role of genes and kidney hemodynamics in BP regulation and kidney function in the general population. A detailed description of the methods is provided elsewhere17,18 and will be briefly described here. From December 2009 to March 2013, adult participants were recruited in 2 regions (Bern and Geneva) and in 1 city (Lausanne) of Switzerland. A random sample of the inhabitants were drawn using different strategies. Inclusion criteria were: (i) having a minimum age of 18 years; (ii) being of European ancestry; (iii) having at least 1 first degree family member willing to participate; and (iv) providing written, informed consent. Pregnant or breastfeeding women were not included. The general participation rate was 25.6%.
The SKIPOGH study has been carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association, and has been approved by the Ethics Committees of each participating university hospital.
Measurements and definitions
The study visit was performed in the morning after an overnight fast. Body weight was measured in kilograms to the nearest 100g using electronic scales (Seca, Hamburg, Germany). Height was measured to the nearest 05mm using a Seca height gauge. Body mass index was calculated as weight (kilograms) divided by the height squared (square meters). BP was measured with a validated non-mercury manual auscultatory sphygmomanometer (A&D UM-101, A&D Company, Toshima Ku, Tokyo, Japan).19 Each subjects conventional office BP was the mean of the 5 consecutive readings, and hypertension was defined as a mean office BP ≥140/90mm Hg.20 ABP was measured using Diasys Integra devices (Novacor, Rueil-Malmaison, France). Measurements were taken every 15 minutes during the day, and every 30 minutes during the night (from 10 pm to 7 am). Participants were included in the analyses if they had at least 14 systolic BP (SBP) and diastolic BP (DBP) measurements during the day and at least 7 readings during the night in accordance with European Society of Hypertension recommendations.20 During the measurement, a urine sample was saved separately for day- and nighttime covering 24 hours. To take potential incomplete urine collection into account, we excluded participants with a 24-hour urine volume below 300ml and added urinary creatinine excretion per kilogram body weight as covariate in the analyses.21 Renal function tests, as well as serum and urinary electrolytes, were analyzed by standard clinical laboratory methods in each center. Creatinine was measured using isotope dilution mass spectrometry-traceable methods. The Chronic Kidney Disease Epidemiology Collaboration formula was used to calculate the estimated glomerular filtration rate.22
Gas chromatography-mass spectrometry of steroid metabolites
Urinary steroid metabolites were extracted and analyzed by gas chromatography-mass spectrometry according to the method described by Shackleton.23 Measured steroid metabolites were divided by urinary creatinine excretion. To assess the apparent CYP17A1 activity, the following precursor-to-product metabolite ratios were derived from the steroid measurements: ratio 1 (THA + THB + 5α-THB)/(An + Et) and ratio 2 (THA + THB + 5α-THB)/(THE + THF + 5α-THF)24 (Supplementary Figure 1). To more specifically target 17,20-lyase activity, we explored the distribution of the ratios of the total cortisol precursors to the androgen precursors and metabolites (pregnanediol (PD) + 17-hydroxyprogesterone (17HP) + pregnanetriol (PT)/(DHEA + An + Et)) and its relationship to ABP.
Statistical analyses
All the continuous variables with normal distribution (assessed graphically) are expressed as mean and ±SD and as median and 25th to 75th interquartile ranges whenever distribution was skewed. Categorical variables are expressed as numbers and frequencies. Student’s t-tests or Mann-Whitney U-tests, whenever appropriate, and chi-square tests were performed to compare baseline characteristics for continuous and categorical variables, respectively.
Association analyses
Ratios 1 and 2 were log-transformed for statistical analysis. Univariate analyses were performed to examine the associations between either log-transformed ratio 1 or 2 with systolic and diastolic ABP during night and day. Pearson tests with P values were performed to obtain correlations for continuous variables. Statistical significance was considered for a 2-sided P < 0.05. For multivariable analyses, we used mixed linear models to analyze the association of systolic and diastolic ABP with log-transformed CYP17A1 ratios 1 and 2, taken one at a time, using separate models for day and night, while taking family correlations into account by way of a random family effect. We included age, sex, center, body mass index, urine flow rate, urinary potassium excretion, urinary creatinine excretion (24 hour per kilogram body weight), antihypertensive treatment, and estimated glomerular filtration rate as covariates in the models. We explored whether urinary sodium excretion modified the association of ABP with log-transformed CYP17A1 ratios by adding the appropriate interaction term in the model. For graphical illustration, we performed separate analyses for participants with urinary sodium excretion above median vs. those below the median. Statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX).
Heritability analyses
We estimated heritability of CYP17A1 activity using the ASSOC program in the Statistical Analysis for Genetic Epidemiology (S.A.G.E.) package, version 6.3, as previously described.25 To estimate heritability, ASSOC uses a linear regression, allowing for covariates to be entered in the model. Heritability estimates are expressed as h2 values with SE. The main model included age and sex as covariates. Another model additionally included body mass index, 24-hour urinary sodium excretion, antihypertensive treatment, and estrogen covariate. The estrogen covariate (0/1) was coded as 1 for women having regular periods or taking oral contraceptive pill or for postmenopausal women taking hormonal replacement therapy.
RESULTS
From December 2009 to March 2013, 1,128 participants from 271 nuclear families were included in the SKIPOGH study. Participants with missing or insufficient data for serum or urinary values, steroid metabolites, and 24-hour ABP were excluded, leaving 518 participants coming from 193 families (median size (interquartile range) = 3 (2;4), maximum size of 8) for the purpose of this analysis. The characteristics of the 298 women and 220 men are presented in Table 1. Urinary excretion of sodium, potassium, creatinine, and steroid hormone metabolites corrected for creatinine were higher in men than in women during both day and night (P < 0.001, except for pregnanediol and tetrahydroaldosterone, Table 2). However, urine flow rate was similar in men and women.
Table 1.
Characteristics of participants
| Variables | Men | Women |
|---|---|---|
| Numbers | 220 | 298 |
| Age, years | 48 (17.7) | 48.5 (17.4) |
| Use of contraceptive pill, numbers (%) | 61 (23) | |
| Menopause, numbers (%) | 150 (54) | |
| On antihypertensive treatment, numbers (%) | 42 (19) | 37 (12) |
| BMI, kg/m2 | 25.7 (3.9) | 23.7 (4) |
| Serum Na, mmol/l | 141 (3) | 141 (2) |
| Serum K, mmol/l | 4.2 (0.3) | 4.1 (0.4) |
| eGFR, ml/min/1.73 m2 | 97.3 (18.9) | 93.9 (16.6) |
| Number of day measures | 52 (8) | 52 (10) |
| Day SBP, mm Hg | 127.8 (13.7) | 120.1 (14.7) |
| Day DBP, mm Hg | 83.3 (9.9) | 78.7 (9.1) |
| Number of night measures | 17 (4) | 17 (5) |
| Night SBP, mm Hg | 111.1 (14.7) | 103.9 (13.3) |
| Night DBP, mm Hg | 71.1 (8.3) | 65.7 (7.1) |
Data are mean and SD unless otherwise specified.
Abbreviations: BMI, body mass index; Na, sodium; K, potassium; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
Table 2.
Descriptive data for day and night urine for sodium, potassium, urinary volume, and creatinine excretion as well as steroid hormone metabolites (corrected for creatinine)
| Variables | Men | Women | P-value | |
|---|---|---|---|---|
| Creatinine excretion, mg/kg BW/24 h | 22.3 (19;24.6) | 18 (14.8;20.5) | <0.001 | |
| Day | Urine duration, min | 960 (900;1005) | 945 (870;990) | <0.01 |
| Urine volume, ml | 1,171 (836;1657) | 1191 (843;1596) | 0.51 | |
| Urine flow rate, ml/min | 1.22 (0.88;1.75) | 1.29 (0.91;1.73) | 0.38 | |
| Sodium excretion, mmol | 109 (81;146) | 83 (60;111) | <0.001 | |
| Potassium excretion, mmol | 54.4 (41.3;67.6) | 42 (32.1;54.2) | <0.001 | |
| Tetrahydrodehydrocorticosterone, µg/creat | 4.83 (3.17;6.91) | 3.39 (2.11;5.45) | <0.001 | |
| Tetrahydrocorticosterone, µg/creat | 100.5 (70;137.7) | 70.2 (48.2;97.7) | <0.001 | |
| 5α-Tetrahydrocorticosterone, µg/creat | 241 (172;328) | 114 (80;169) | <0.001 | |
| Tetrahydro-11-dehydrocorticosterone, µg/creat | 71.5 (49.9;95.4) | 50.1 (35;68.6) | <0.001 | |
| Tetrahydroaldosterone, µg/creat | 13.1 (7.8;21.7) | 12 (7.3;19.7) | 0.48 | |
| Pregnanediol, µg/creat | 132 (97;201) | 126 (73;232) | 0.29 | |
| 17-Hydroxypregnanolone, µg/creat | 115.1 (76.4;170.7) | 31 (20.6;59.8) | <0.001 | |
| Pregnanetriol, µg/creat | 442 (333;601) | 203 (122;297) | <0.001 | |
| Tetrahydrosubstance S, µg/creat | 47.9 (34.3;63.6) | 35.8 (24.7;47.2) | <0.001 | |
| Tetrahydrocortisol, µg/creat | 1351 (1051;1722) | 827 (579;1054) | <0.001 | |
| 5α-Tetrahydrocortisol, µg/creat | 1,076 (774;1466) | 383 (247;585) | <0.001 | |
| Tetrahydrocortisone, µg/creat | 2,262 (1810;2864) | 1253 (956;1863) | <0.001 | |
| Dehydroepiandrosterone, µg/creat | 101.4 (37.2;400.2) | 32.8 (14.8;95) | <0.001 | |
| Androsterone, µg/creat | 1,185 (811;1736) | 402 (229;734) | <0.001 | |
| Etiocholanolone, µg/creat | 1,093 (659;1530) | 561 (306;869) | <0.001 | |
| Night | Urine duration, min | 480 (435;515) | 492.5 (450;540) | <0.05 |
| Urine volume, ml | 500 (357;700) | 500 (300;683) | 0.5 | |
| Urine flow rate, ml/min | 1.06 (0.72;1.49) | 1.02 (0.63;1.39) | 0.48 | |
| Sodium excretion, mmol | 50.1 (34.2;66.5) | 34.3 (23.2;50.7) | <0.001 | |
| Potassium excretion, mmol | 15.1 (10.6;21.3) | 11.3 (8.4;16.1) | <0.001 | |
| Tetrahydrodehydrocorticosterone, µg/creat | 1.94 (1.4;2.96) | 1.37 (0.89;2.44) | <0.001 | |
| Tetrahydrocorticosterone, µg/creat | 39.7 (27.7;63.5) | 29.4 (19.4;41.7) | <0.001 | |
| 5α-Tetrahydrocorticosterone, µg/creat | 72.4 (51.9;101) | 33.9 (21.7;54) | <0.001 | |
| Tetrahydro-11-dehydrocorticosterone, µg/creat | 25 (17.4;36) | 18.1 (12.2;26.5) | <0.001 | |
| Tetrahydroaldosterone, µg/creat | 5.25 (3.29;8.95) | 4.8 (2.77;8.51) | 0.33 | |
| Pregnanediol, µg/creat | 65.3 (45.2;93.3) | 58.9 (36.7;98) | 0.29 | |
| 17-Hydroxypregnanolone, µg/creat | 55.9 (33.2;83.2) | 12.3 (8;25.1) | <0.001 | |
| Pregnanetriol, µg/creat | 228 (159;307) | 101 (61;150) | <0.001 | |
| Tetrahydrosubstance S, µg/creat | 18 (12.9;25.1) | 14 (9.6;19.6) | <0.001 | |
| Tetrahydrocortisol, µg/creat | 423 (297;554) | 248 (179;355) | <0.001 | |
| 5α-Tetrahydrocortisol, µg/creat | 344 (236;482) | 120 (79;185) | <0.001 | |
| Tetrahydrocortisone, µg/creat | 734 (540;995) | 440 (296;618) | <0.001 | |
| Dehydroepiandrosterone, µg/creat | 38.5 (13.1;131.8) | 13.8 (6.4;32) | <0.001 | |
| Androsterone, µg/creat | 577 (366;864) | 210 (105;339) | <0.001 | |
| Etiocholanolone, µg/creat | 533 (345;780) | 281 (163;449) | <0.001 |
Data are median and interquartile range (IQR).
Abbreviation: BW, body weight.
Distribution of CYP17A1 activities in the general adult population
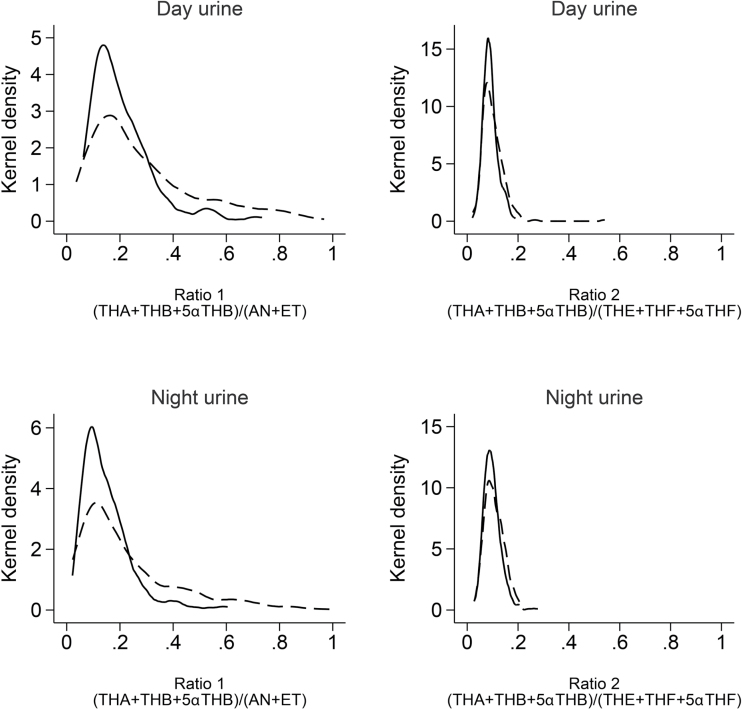
The distribution of CYP17A1 activities based upon 2 different ratios of urinary steroid hormone metabolites are shown in Figure 1. Higher precursor-to-product metabolites ratios indicate a lower activity of the enzyme, while lower ratios denote higher enzyme activity. Both ratios showed a unimodal distribution. Ratio 1 had a second peak at higher values, which indicates participants with lower apparent CYP17A1 activities.
Figure 1.
Apparent CYP17A1 activities separated by gender, day- or nighttime and ratio 1 or 2. Dashed lines represent women, black lines represent men. Abbreviations: THA, tetrahydro-11-dehydrocorticosterone; THB, tetrahydrocorticosterone; 5α-THB, 5α-tetrahydrocorticosterone; THE, tetrahydrocortisone; THF, tetrahydrocortisol; 5α-THF, 5α-tetrahydrocortisol; An, androsterone; Et, etiocholanolone.
Association of CYP17A1 ratios with ambulatory blood pressure
Due to their asymmetric unimodal distribution, the CYP17A1 ratios were log-transformed for association analyses with ABP. In univariable mixed linear regression analyses, including all participants (n = 518), SBP and DBP tended to be higher during day and night in participants with lower apparent CPY17A1 activity specified by ratio 1 (Supplementary Figure 2), although not reaching statistical significance. There was no association of ABP with ratio 2 (Supplementary Figure 3) nor with estimated 17,20-lyase activity (data not shown). As CYP17A1 inhibition is associated with an excessive mineralocorticoid signaling, the impact of salt intake—using urinary sodium excretion as a surrogate marker—on ABP in relation to CYP17A1 activity was addressed. To graphically illustrate the effect modification, men and women were separated into low and high sodium excretion subgroups. Day SBP and DBP were associated positively with ratio 1 (hence negatively with CYP17A1 activity) in participants with high sodium excretion (P for interaction between log-CYP17A1 ratio 1 and salt strata = 0.033 for day SBP and =0.004 for day DBP), whereas no such positive association was found in participants with low sodium excretion (Figure 2). We also used sex-specific tertiles of sodium excretion, instead of low and high sodium excretion strata, which leads to similar observations and illustrates the dose-response modifying effect of sodium excretion (Supplementary Figure 4). Nighttime SBP showed the same pattern; however, the corresponding interaction was not statistically significant. We found no significant effect modification of sex for its effect on the association of ABP with CYP17A1 activity (data not shown). There was no association of ABP with ratio 2 (Supplementary Figure 5) nor with estimated 17,20-lyase activity (data not shown) upon dichotomizing the participants based on urinary sodium excretion.
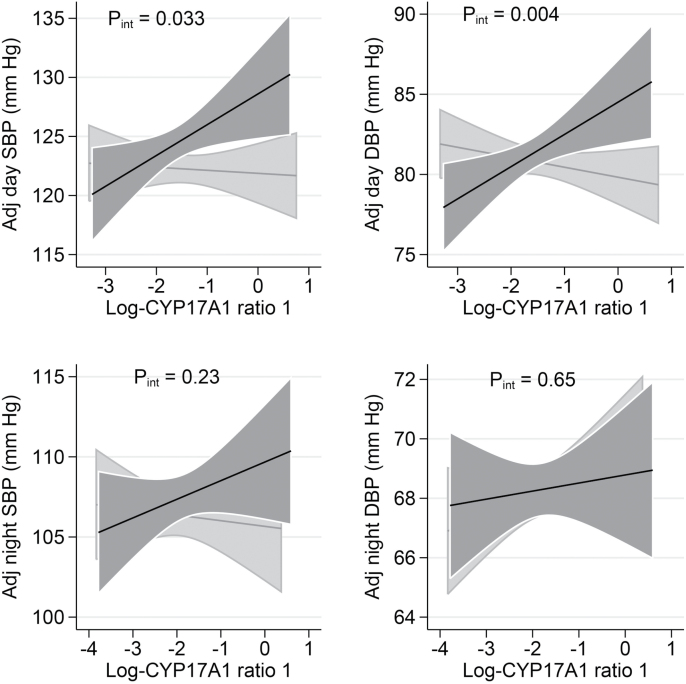
Figure 2.
Association of day and night ABP with log transformed ratio 1 by salt intake strata. Sex-specific medians for daytime and nighttime urinary sodium excretion were used to separate low (below median) vs. high (above median) salt intake. The black lines indicate the high sodium excretion group, the gray lines the low sodium excretion group. For daytime, the median urinary Na excretion was 146 mmol in men and 107 mmol in women for high intakes and 80 mmol in men and 58 mmol in women for low intakes. For nighttime, the median urinary Na excretion was 65 mmol for men and 51 mmol for women for high intakes and 32 mmol in men and 23 mmol in women for low intakes. Data are adjusted for age, sex, center, body mass index, urine flow rate (day or night), urinary potassium excretion (day or night), 24-hour urinary creatinine excretion per kilogram body weight, antihypertensive treatment, and estimated glomerular filtration rate (GFR) based on the Chronic Kidney Disease Epidemiology Collaboration equation (systolic blood pressure (SBP), diastolic blood pressure (DBP), P for interaction (P int)).
Heritability estimates of CYP17A1 ratios
Unadjusted heritability estimates showed only a significant heritability for ratio 2 (Table 3). Adjustment for age and sex showed a significant adjusted heritability for ratio 1 (0.36 for day and 0.38 for night, P < 0.001) and for ratio 2 (0.58 for day and 0.52 for night, P < 0.001). Further adjustment for body mass index, 24-hour urinary sodium excretion, antihypertensive treatment, and estrogen status slightly modified heritability estimates, which remained approximately 0.40 for ratio 1 and between 0.55–0.71 for ratio 2 (all P < 0.001).
Table 3.
Heritability estimates of log-transformed CYP17A1 activity ratios 1 and 2 and day and night, respectively
| Variables | Model | h2 | P-value |
|---|---|---|---|
| CYP17A1 ratio 1, day | Unadjusted | 0.10 (0.09) | 0.133 |
| Model 1 | 0.36 (0.10) | <0.001 | |
| Model 2 | 0.39 (0.10) | <0.001 | |
| CYP17A1 ratio 1, night | Unadjusted | 0.11 (0.09) | 0.102 |
| Model 1 | 0.38 (0.09) | <0.001 | |
| Model 2 | 0.40 (0.09) | <0.001 | |
| CYP17A1 ratio 2, day | Unadjusted | 0.58 (0.10) | <0.001 |
| Model 1 | 0.75 (0.09) | <0.001 | |
| Model 2 | 0.71 (0.09) | <0.001 | |
| CYP17A1 ratio 2, night | Unadjusted | 0.52 (0.09) | <0.001 |
| Model 1 | 0.63 (0.09) | <0.001 | |
| Model 2 | 0.55 (0.09) | <0.001 |
Data are narrow sense h2 estimates in percentage ± SE.
Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, study center, body mass index, 24-hour urinary sodium excretion, antihypertensive treatment, and estrogen status.
Association of CYP17A1 ratios with age
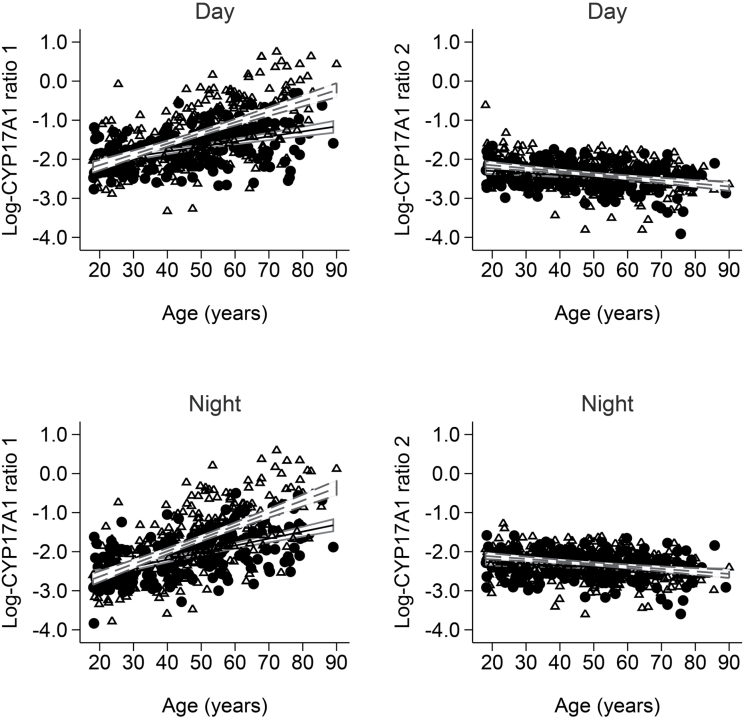
We observed a significant positive association of day and night CYP17A1 ratio 1 with age (P < 0.001) and a significant negative association of day and night CYP17A1 ratio 2 with age (P < 0.001) (Figure 3 ).
Figure 3.
Apparent CYP17A1 activities—assessed with ratios 1 and 2—separated by gender and day- and nighttime. Open triangles represent women and black circles represent men. Dashed lines are the regression lines of participating women and solid lines the ones of men.
Different urinary steroid profile patterns for ratios 1 and 2
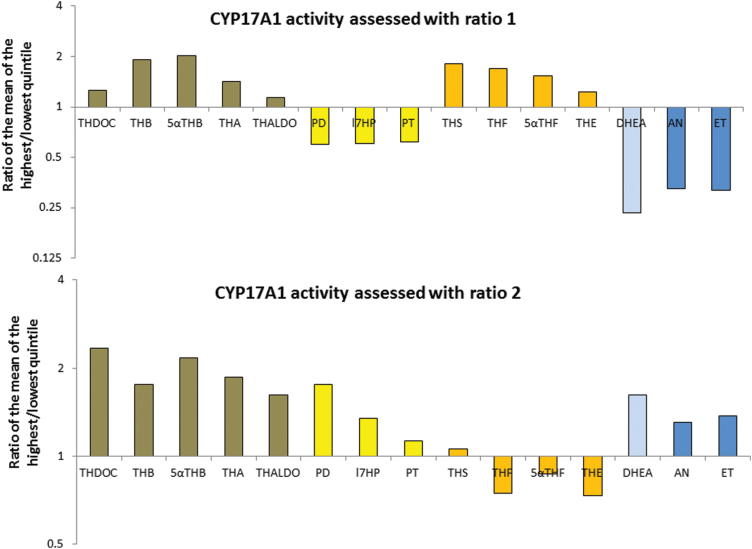
Because ratios 1 and 2 showed different results, the pattern of the ratios of urinary steroid metabolites between participants in the highest and those in the lowest quintiles of ratio 1 or ratio 2 during the day are presented (Figure 4). This figure indicates which urinary metabolites predominate in participants with a lower CYP17A1 activity assessed with ratio 1 or ratio 2, respectively. During the day, participants in the highest quintile of ratio 1 or 2 showed a higher excretion of mineralocorticoid active hormones than the participants in the lowest quintile, but the pattern for the other steroid metabolites was different. Participants with a higher CYP17A1 ratio 1 had a trend to a higher excretion of glucocorticoids, a lower excretion of 17α-hydroxylated glucocorticoid precursors and a much lower excretion of androgen precursors and androgen metabolites. Therefore, in the SKIPOGH population, ratio 1 seems to indicate a higher mineralocorticoid production with a maintained cortisol availability. This first pattern of diminished apparent CYP17A1 activity was associated with higher BP under high sodium intake. In contrast, participants with a higher CYP17A1 ratio 2 had a trend to a lower excretion of glucocorticoids and a higher excretion of 17α-hydroxylated glucocorticoid precursors, androgen precursors and androgen metabolites, thus indicating an increased production of mineralocorticoid hormones with a decreased glucocorticoid availability. This pattern was not associated with higher BP.
Figure 4.
Pattern of the urinary steroid metabolites between participants in the highest and the lowest quintiles of ratio 1 or ratio 2 during the day. Abbreviations: THDOC, tetrahydrodeoxycorticosterone; THA, tetrahydro-11-dehydrocorticosterone; THB, tetrahydrocorticosterone; 5α-THB, 5α-tetrahydrocorticosterone; THALDO, tetrahydroaldosterone; PD, pregnanediol; 17HP, 17-hydroxyprogesterone metabolites; PT, pregnanetriol; THF, tetrahydrocortisol; 5α-THF, 5α-tetrahydrocortisol; THE, tetrahydrocortisone; DHEA, dehydroepiandrosterone; An, androsterone; Et, etiocholanolone. THDOC, THB, α-THB, THA, and THALDO are mineralocorticoid active hormones, PD, 17HP, and PT are glucocorticoid precursors, THS, THF, 5α-THF and THE are metabolites of glucocorticoids, DHEA is an androgen precursors, and An and Et are androgen metabolites.
DISCUSSION
We provide evidence that a decreased CYP17A1 activity (assessed with ratio 1) is associated with increased systolic and diastolic daytime ABP, when salt intake is high. Our data support the heritability of CYP17A1 activity and highlight the genetic role of CYP17A1 in BP control in the adult population. To our knowledge, this is also the first study to report the distribution of CYP17A1 activities—assessed with 2 different ratios—in the general adult population.
The association of ratios 1 and 2 to ABP is different, however. In patients affected with 17α-hydroxylase deficiency, lack of CYP17A1 activity is characterized by hypertension and elevated CYP17A1 ratios 1 and 2, whereas in our study ABP was associated with CYP17A1 ratio 1, but not with ratio 2. This suggests that the assessment of apparent CYP17A1 activity with 2 different ratios is not the same in patients with 17α-hydroxylase deficiency, compared to the general population. In 17α-hydroxylase-deficient patients, glucocorticoid deficiency leads to an increased secretion of adrenocorticotropic hormone, which stimulates the synthesis of mineralocorticoid hormones. In contrast, participants with an elevated CYP17A1 ratio 1, in our study, had a preserved production of glucocorticoid hormones, with a decreased secretion of 17α-hydroxylated glucocorticoid precursors and side-chain cleaved androgens. Therefore, participants with a diminished CYP17A1 activity (assessed with ratio 1) seem to have a diminished 17α-hydroxylase and 17,20-lyase activity with an increased secretion of mineralocorticoid hormone that is not triggered by glucocorticoid deficiency. When our participants were assessed with ratio 2, participants with an elevated CYP17A1 ratio 2 had a decreased excretion of glucocorticoid hormones, but a preserved excretion of 17α-hydroxylated glucocorticoid precursors and androgen metabolites, indicating a decrease in glucocorticoid production without a concomitantly decrease in 17α-hydroxylated glucocorticoid precursors and side-chain cleaved androgens. From this point of view, it seems likely that a higher CYP17A1 ratio 2 reflects a deficiency of glucocorticoid hormones that triggers adrenocorticotropic hormone secretion. However, this pattern of increased secretion of mineralocorticoid hormones had no effect on ABP in our population.
Our observational results are supported by results from interventional studies in men with advanced prostate cancer. In these patients, decreased androgen synthesis and increased mineralcorticoid hormone excretion was observed upon treatment with abiraterone, a CYP17A1-inhibitor. Under abiraterone treatment, a proportion of these men developed hypertension.10 To overcome this side effect, these patients were additionally treated with prednisone.11–13 However, in 2 large prospective randomized studies, mineralocorticoid side effects were more commonly reported in the abiraterone and prednisone treated groups, rather than the prednisone therapy groups, suggesting that prednisone only partially prevents the symptoms of mineralocorticoid excess induced by abiraterone.12,13 This latter situation is in line with our observation of combined mineralocorticoid excess and maintained glucocorticoid availability in the presence of low CYP17A1 activity (assessed with a high ratio 1).
Our novel findings of the close association of BP control with CYP17A1 activity are in line with recent associations of the CYP17A1 gene locus with BP in the adult population.4–6 In the International Consortium for Blood Pressure analysis, including data on 200,000 individuals of European descent, the single nucleotide polymorphism located within the CYP17A1 gene had the strongest effect size of all genome-wide signals for BP.6 This locus was further associated with BP in East-Asians,26 Japanese,27 Han Chinese,28 and She Chinese.29 The size of the effect we observe sharply contrasts with that observed in the genetic association studies (i.e., 1mm Hg per allele).6 We found a systolic and diastolic daytime ABP difference of 10 and 7mm Hg between extremes of CYP17A1 activity, when assessed with ratio 1, under conditions of high sodium intake. If confirmed in other studies and in experimental settings, these results may have public health relevance.
We observed CYP17A1 activities to be substantially heritable in the general population, which is compatible with a genetic continuum between rare monogenic arterial hypertension and essential hypertension. Even in the rare monogenic form of hypertension with loss-of function mutations in the CYP17A1 gene, BP is highly sensitive to salt intake,9 which highlights the importance of environmental factors. The lower heritability estimate and larger variance of ratio 1 as compared to ratio 2 suggest that environmental factors are more susceptible to impact on ratio 1. Furthermore, the lower heritability of ratio 1 may result, in part, from the fact that it captures a more complex enzymatic activity than ratio 2. Similarly, the substantial heritability of CYP17A1 activities could participate in the clinical observation that a family history of high BP predisposes other family members to arterial hypertension.2,3
This study also revealed a lower CYP17A1 activity—assessed with ratio 1—in older participants. It is known that DHEA and androstenedione levels decrease with age in men and women between the age groups of 20- to 30 years old and 50- to 60 years old, with smaller changes observed after the age of 60 years.30 The decreased androgen levels are most likely due to a decreased CYP17A1 activity. Given this observation, it seems obvious, but until now not formally shown, that the decline in androgen synthesis leads also to an increased secretion of steroid precursors with mineralocorticoid properties exposing older individuals to a higher risk of increases in BP in the presence of excess salt intake.31
In summary, we identified individuals of European ancestry with a diminished CYP17A1 activity who might profit from reduced salt intake, such as currently recommended.32,33 Further interventional trials should investigate the extent blood pressure could be lowered in participants with lower estimated CYP17A1 activity.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
D.A., M.P., B.P., I.G., G.E., and the study were supported by a grant from the Swiss National Science Foundation (FN 33CM30-124087, Schweizerischer Nationalfonds (SNF), Wildhainweg 3, Postfach 8232, 3001 Bern, Switzerland).
We thank the study nurses Marie-Odile Levy, Guler Gök-Sogüt, Ulla Schüpbach, Dominique Siminski, and Sandrine Estoppey for the data collection and Hiten Mistry for English proofreading.
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotchen TA, Kotchen JM, Grim CE, George V, Kaldunski ML, Cowley AW, Hamet P, Chelius TH. Genetic determinants of hypertension: identification of candidate phenotypes. Hypertension 2000; 36:7–13. [DOI] [PubMed] [Google Scholar]
- 3. Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJ. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 2005; 45:80–85. [DOI] [PubMed] [Google Scholar]
- 4. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB; Wellcome Trust Case Control Consortium. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009; 41:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kahonen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Kottgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grassler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stancakova A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikainen LP, Soininen P, Tukiainen T, Wurtz P, Ong RT, Dorr M, Kroemer HK, Volker U, Volzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Jarvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanase T, Simpson ER, Waterman MR. 17 alpha-hydroxylase/17,20-lyase deficiency: from clinical investigation to molecular definition. Endocr Rev 1991; 12:91–108. [DOI] [PubMed] [Google Scholar]
- 8. Kater CE, Biglieri EG, Brust N, Chang B, Hirai J. The unique patterns of plasma aldosterone and 18-hydroxycorticosterone concentrations in the 17 alpha-hydroxylase deficiency syndrome. J Clin Endocrinol Metab 1982; 55:295–302. [DOI] [PubMed] [Google Scholar]
- 9. Nagase M, Ando K, Fujita T. Salt sensitivity of blood pressure in patients with 17 alpha-hydroxylase deficiency. Am J Hypertens 1994; 7:1005–1011. [DOI] [PubMed] [Google Scholar]
- 10. Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, Dearnaley D, Swennenhuis JF, Terstappen LW, Lee G, Kheoh T, Molina A, Ryan CJ, Small E, Scher HI, de Bono JS. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol 2010; 28:1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Attard G, Reid AH, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W, de Bono JS. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab 2012; 97:507–516. [DOI] [PubMed] [Google Scholar]
- 12. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biglieri EG, Herron MA, Brust N. 17-hydroxylation deficiency in man. J Clin Invest 1966; 45:1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol 2010; 121:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Intersalt: An International Study of Electrolyte Excretion and Blood Pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 1988; 297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponte B, Pruijm M, Ackermann D, Vuistiner P, Eisenberger U, Guessous I, Rousson V, Mohaupt MG, Alwan H, Ehret G, Pechere-Bertschi A, Paccaud F, Staessen JA, Vogt B, Burnier M, Martin PY, Bochud M. Reference values and factors associated with renal resistive index in a family-based population study. Hypertension 2014; 63:136–142. [DOI] [PubMed] [Google Scholar]
- 18. Guessous I, Pruijm M, Ponte B, Ackermann D, Ehret G, Ansermot N, Vuistiner P, Staessen J, Gu Y, Paccaud F, Mohaupt M, Vogt B, Pechère-Berstchi A, Martin PY, Burnier M, Eap CB, Bochud M. Associations of ambulatory blood pressure with urinary caffeine and caffeine metabolite excretions. Hypertension 2015; 65:691–696. [DOI] [PubMed] [Google Scholar]
- 19. Pruijm MT, Wuerzner G, Glatz N, Alwan H, Ponte B, Ackermann D, Burnier M, Bochud M. A new technique for simultaneous validation of two manual nonmercury auscultatory sphygmomanometers (A&D UM-101 and Accoson Greenlight 300) based on the International protocol. Blood Press Monit 2010; 15:322–325. [DOI] [PubMed] [Google Scholar]
- 20. O’Brien E Asmar R Beilin L Imai Y Mallion JM Mancia G Mengden T Myers M Padfield P Palatini P Parati G Pickering T Redon J Staessen J Stergiou G and Verdecchia P. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003; 21:821–848. [DOI] [PubMed] [Google Scholar]
- 21. Ponte B, Pruijm M, Ackermann D, Vuistiner P, Guessous I, Ehret G, Alwan H, Youhanna S, Paccaud F, Mohaupt M, Pechere-Bertschi A, Vogt B, Burnier M, Martin PY, Devuyst O, Bochud M. Copeptin is associated with kidney length, renal function, and prevalence of simple cysts in a population-based study. J Am Soc Nephrol 2015; 26:1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shackleton CH. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J Steroid Biochem Mol Biol 1993; 45:127–140. [DOI] [PubMed] [Google Scholar]
- 24. D'Armiento M, Reda G, Kater C, Shackleton CH, Biglieri EG. 17 alpha-hydroxylase deficiency: mineralocorticoid hormone profiles in an affected family. J Clin Endocrinol Metab 1983; 56:697–701. [DOI] [PubMed] [Google Scholar]
- 25. Bochud M. Estimating heritability from nuclear family and pedigree data. Methods Mol Biol 2012; 850:171–186. [DOI] [PubMed] [Google Scholar]
- 26. Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 2011; 43:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyaki K, Htun NC, Song Y, Ikeda S, Muramatsu M, Shimbo T. The combined impact of 12 common variants on hypertension in Japanese men, considering GWAS results. J Hum Hypertens 2012; 26:430–436. [DOI] [PubMed] [Google Scholar]
- 28. Li X, Ling Y, Lu D, Lu Z, Liu Y, Chen H, Gao X. Common polymorphism rs11191548 near the CYP17A1 gene is associated with hypertension and systolic blood pressure in the Han Chinese population. Am J Hypertens 2013; 26:465–472. [DOI] [PubMed] [Google Scholar]
- 29. Lin Y, Lai X, Chen B, Xu Y, Huang B, Chen Z, Zhu S, Yao J, Jiang Q, Huang H, Wen J, Chen G. Genetic variations in CYP17A1, CACNB2 and PLEKHA7 are associated with blood pressure and/or hypertension in She ethnic minority of China. Atherosclerosis 2011; 219:709–714. [DOI] [PubMed] [Google Scholar]
- 30. Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 1997; 82:2396–2402. [DOI] [PubMed] [Google Scholar]
- 31. Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ 1996; 312:1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mozaffarian D Fahimi S Singh GM Micha R Khatibzadeh S Engell RE Lim S Danaei G Ezzati M and Powles J. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014; 371:624–634. [DOI] [PubMed] [Google Scholar]
- 33. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S76–S99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.