Abstract
Obese subjects often have hypertension and related cardiovascular and renal diseases, and this has become a serious worldwide health problem. In obese subjects, impaired renal-pressure natriuresis causes sodium retention, leading to the development of salt-sensitive hypertension. Physical compression of the kidneys by visceral fat and activation of the sympathetic nervous system, renin–angiotensin systems (RAS), and aldosterone/mineralocorticoid receptor (MR) system are involved in this mechanism. Obese subjects often exhibit hyperaldosteronism, with increased salt sensitivity of blood pressure (BP). Adipose tissue excretes aldosterone-releasing factors, thereby stimulating aldosterone secretion independently of the systemic RAS, and aldosterone/MR activation plays a key role in the development of hypertension and organ damage in obesity. In obese subjects, both salt sensitivity of BP, enhanced by obesity-related metabolic disorders including aldosterone excess, and increased dietary sodium intake are closely related to the incidence of hypertension. Some salt sensitivity-related gene variants affect the risk of obesity, and together with salt intake, its combination is possibly associated with the development of hypertension in obese subjects. With high salt levels common in modern diets, salt restriction and weight control are undoubtedly important. However, not only MR blockade but also new diagnostic modalities and therapies targeting and modifying genes that are related to salt sensitivity, obesity, or RAS regulation are expected to prevent obesity and obesity-related hypertension.
Keywords: aldosterone, blood pressure, hypertension, obesity, salt, salt sensitivity of blood pressure.
The prevalence of obesity and its associated cardiovascular, metabolic, and renal diseases has increased markedly and this has become a serious worldwide health problem over the past decade. The Obesity and Overweight Fact Sheet published by the World Health Organization revealed that more than 1.9 billion adults were overweight in 2014, of whom more than 600 million were obese, accounting for 39% and 13% of adults, respectively. Moreover, the medical costs of obese individuals were approximately 30% higher than those of normal weight peers.1 Obesity appears to be responsible for a substantial economic burden in many countries.2
The risk of hypertension significantly increases in proportion with the amount of excess weight.3 Moreover, obesity is associated with the incidence of cardiovascular disease (CVD),4 type 2 diabetes mellitus, stroke, and dyslipidemia.5 Obesity is related to a combination of metabolic and cardiovascular disorders and is strongly linked to the mortality risk.6
On the other hand, growing evidence recently suggested that both plasma and urinary aldosterone concentrations are increased in obese individuals,7,8 and aldosterone is involved in cardiorenal and metabolic disease.9 Classically, aldosterone has long been considered to play a central role in the regulation of electrolyte and fluid volume and the maintenance of blood pressure (BP) homeostasis.10 However, the aldosterone/mineralocorticoid receptor (MR) system plays a key role in cardiovascular and renal damage.11,12 These nonclassical actions of aldosterone and the involvement of MR activation in CVD were proven by 3 large-scale randomized clinical trials, the Randomized Aldactone Evaluation Study (RALES),13 the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),14 and the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF).15 Moreover, the recent clinical trials of the Eplerenone Combination versus Conventional Agents to Lower Blood Pressure on Urinary Antialbuminuric Treatment Effect (EVALUATE) study16 and the Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN)17 demonstrated the pathogenetic role of aldosterone/MR activation in chronic kidney disease (CKD). In this review, we focus on the role of the aldosterone/MR system in obesity-related hypertension.
OBESITY AND HYPERTENSION
Obesity, especially visceral obesity, is closely related to hypertension.18,19 The relationship between human obesity and hypertension was first described by Vague in 1956.20 He divided obese patients into “android” type (upper body obesity) and “gynoid” type (lower body obesity) and reported that cardiovascular and metabolic complications of obesity were more prevalent in the former. However, such complications do not develop in all obese individuals, and some normal weight adults exhibit metabolic syndrome and its comorbidities.21,22 This is because abnormal body fat distribution, such as excess visceral adipose tissue, is a more important factor than body mass index for morbidity.23,24 Visceral adiposity reportedly plays a major role in the occurrence of hypertension, diabetes mellitus, hyperlipidemia, and atherosclerosis in obese humans and in animal models.24 Visceral and subcutaneous adipocytes may play a role in different ways. Visceral adipose tissue is a source of numerous adipokines, such as tumor necrosis factor-α, interleukin-6, plasminogen activator inhibitor-1, angiotensinogen (Agt), C-reactive protein, and leptin, most of which are deemed to be proinflammatory.25 Unlike these adipokines, serum levels of adiponectin, an anti-inflammatory and vasculoprotective adipokine, are decreased in obesity. Furthermore, the plasma aldosterone concentration (PAC) is positively correlated with the amount of visceral adipose tissue, independent of plasma renin activity (PRA).8 Several studies suggest that adipose tissue secretes adipokines that stimulate aldosterone release from adrenal cells,26–29 so-called aldosterone-releasing factors (ARFs).
Aside from this, genetic studies of humans suggest the association of obesity-related hypertension with the variants of several genes, such as tumor necrosis factor-α,30 glucocorticoid receptor,31 CYP11B2,32 and serum and glucocorticoid-regulated kinase 1 (SGK1).33 Most of these are closely related to aldosterone secretion and signaling. For example, SGK1, a downstream effector of aldosterone, has gene variants, which confer predisposition to hypertension, stroke, obesity, and type 2 diabetes mellitus.33 A high-fructose or high-fat diet, leading to hyperinsulinism and obesity, sensitizes BP to high salt intake in wild-type mice, but not in SGK1-knockout mice.33,34 In this mechanism, activation of SGK1 by insulin presumably stimulates renal tubular salt reabsorption and contributes to the development of hypertension. In obese subjects, increased visceral adiposity enhances an imbalance of pro- and anti-inflammatory adipokines, hyperaldosteronemia, and metabolic disorders including hyperinsulinemia, and aldosterone and its related factors are deeply involved in the development of hypertension.
MECHANISM UNDERLYING OBESITY-RELATED HYPERTENSION
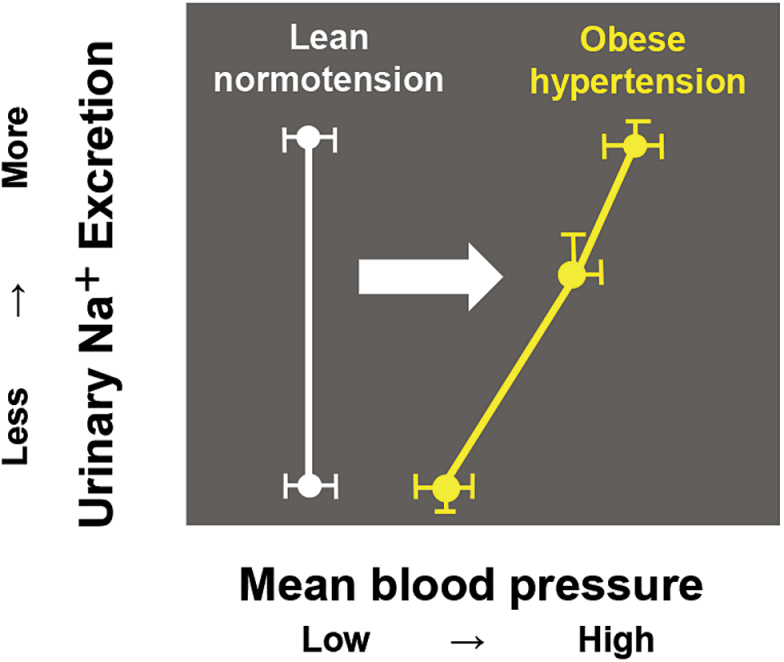
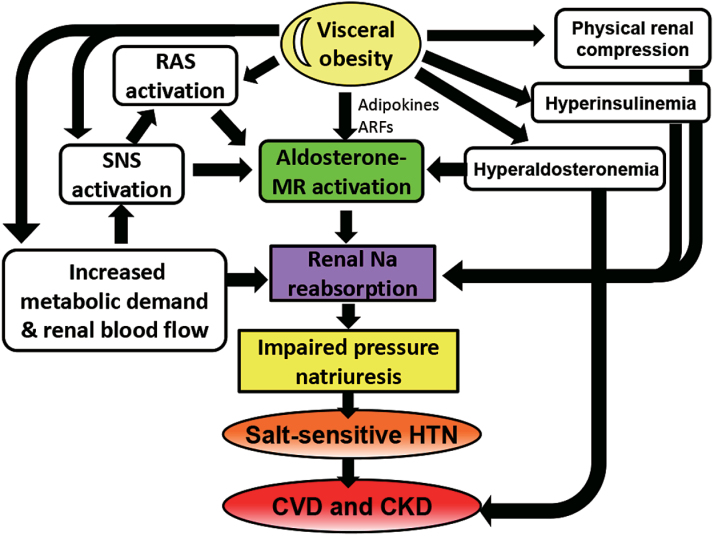
Obese subjects exhibit extracellular fluid volume expansion and increased blood flow in many tissues and have increased venous return and cardiac output.35 Cardiac output increases in parallel with weight gain because blood flow should supply the increased adipose tissue and other tissues to meet metabolic demands. In the early stage of obesity, the increased glomerular filtration rate and renal blood flow induce an increase in renal sodium absorption. With prolonged hypertension, renal vasodilation, glomerular hyperfiltration, and neurohumoral activation lead to severe hypertension, glomerular injury, and an impaired renal capacity for sodium excretion, resulting in the gradual loss of nephron and kidney function. In this way, obese subjects require a higher BP than lean subjects to maintain the sodium balance, indicating impaired renal-pressure natriuresis,36 and thus obese subjects can exhibit salt-sensitive hypertension (Figure 1). Important factors that influence the renal capacity for sodium excretion in obese subjects include physical compression of the kidneys by increased visceral fat, and activation of the sympathetic nervous system (SNS), renin–angiotensin systems (RAS), and aldosterone/MR system.37 Moreover, obese subjects are characterized by metabolic disorders, such as hyperinsulinemia, glucose intolerance, dyslipidemia, and inflammation, and most of these accelerate renal sodium reabsorption and renal injury. These factors influence each other, and aldosterone plays a key role in the pathophysiology of obesity-related hypertension (Figure 2).
Figure 1.
The blood pressure (BP)–natriuresis curve of obese subjects. In obese subjects, the increased glomerular filtration rate and renal blood flow induce increased renal sodium absorption, which in turn initiates impaired renal-pressure natriuresis, resulting in hypertension. Because obese hypertensive subjects require a higher BP than lean normotensive subjects to maintain the sodium balance, the BP–natriuresis curve is slanted and shifted to the right, indicating impaired renal-pressure natriuresis. As a result, obese patients exhibit salt-sensitive hypertension.
Figure 2.
The mechanism underlying obesity-related hypertension and kidney impairment. Obesity induces functional vasodilation to fulfill the increased metabolic demand of the tissue. In the early stage of obesity, the increased renal blood flow induces an increase in renal sodium reabsorption, which in turn initiates impaired renal-pressure natriuresis. Physical compression of the kidneys by increased visceral fat, hyperinsulinemia, and hyperaldosteronemia and activation of the SNS, RAS, and aldosterone/MR system also promote renal tubular sodium absorption. Prolonged hypertension, glomerular hyperfiltration, and neurohumoral activation cause further severe glomerular injury, increased impairment of renal-pressure natriuresis, and HTN and finally result in gradual nephron loss and kidney disease. Abbreviations: ARF, aldosterone-releasing factor; CKD, chronic kidney disease; CVD, cardiovascular disease; HTN, hypertension; MR, mineralocorticoid receptor; RAS, renin–angiotensin systems; SNS, sympathetic nervous system.
Sympathetic nervous system
Increased sympathetic nerve activity has been demonstrated in many animal models of obesity38,39 and human obese subjects.40,41 Multiple lines of evidence indicate that increased SNS activity contributes to obesity-related hypertension.35 Moreover, administration of α/β-adrenergic blockers reduces SNS activity and prevents obesity-related hypertension.42 In addition, obese hypertensive patients and animals often exhibit both salt sensitivity of BP and increased SNS activity, specifically in the kidney.43,44 These reports also suggested that the renal SNS is an important factor influencing the salt sensitivity of BP. The anti-natriuretic effect of increased renal SNS activity is considered to be mainly mediated by increased renin secretion, reduced renal blood flow, and increased renal tubular reabsorption.45 According to norepinephrine-induced tubular sodium reabsorption, stimulation of β2-adrenergic receptors leads to activation of the Na–Cl cotransporter through suppression of the serine-threonine protein kinase WNK4 in the distal tubule.46 WNK kinases are modulated by changes in dietary sodium through effects on the circulating RAS and SNS and thus influence Na–Cl cotransporter activity.46–48 On a low-salt diet, increased angiotensin II (AngII) is involved in Na–Cl cotransporter activation in an STE20/SPS-1-related proline/alanine-rich kinase-dependent manner.49,50 Aldosterone also promotes the dietary salt-mediated increase in Na–Cl cotransporter protein levels through the WNK4-extracellular signal-regulated kinase 1/2 signaling pathway.48 In this way, SNS overactivity in obesity is indicated to be responsible for salt-sensitive hypertension in cooperation with AngII or aldosterone.
Several candidates may activate the SNS in obesity, including impaired baroreceptor reflexes, activation of chemoreceptor-mediated reflexes associated with sleep apnea, intermittent hypoxia, hyperinsulinemia, AngII, adipokines (such as leptin, tumor necrosis factor-α, and interleukin-6), and the pro-opiomelanocortin pathway of the central nervous system. In particular, leptin and the pro-opiomelanocortin pathway are important in obesity-induced SNS activation and hypertension.51 The plasma leptin concentration is increased in obese hypertensive subjects.52 Leptin influences leptin-sensitive neurons in the central nervous system and is involved in the regulation of appetite, energy expenditure, and the appropriate balance of automatic nerve activities.37
Renin–angiotensin systems
RAS activation is deeply related to the development of obesity-induced hypertension.37,53 Obese subjects, especially those with visceral obesity, often have mild-to-moderate increases in PRA, Agt, angiotensin-converting enzyme (ACE) activity, AngII, and aldosterone.54 Despite sodium retention, RAS activation occurs in obese subjects. RAS activation in obesity is induced by multiple factors, such as compression of the kidneys, increased SNS activation, and possibly the local RAS in adipose tissue.55 Adipose tissue contains all the components of the RAS, including Agt, renin, ACE, AngII, and AngII receptor type 1 (AT1R) and type 2, and can produce AngII.56 In general, Agt is produced mainly in the liver; however, Agt secretion by adipose tissue is substantially augmented in obese subjects.57 Yiannikouris et al. suggested the importance of adipocyte-derived AngII in the development of obesity-related hypertension by using adipocyte Agt-deficient mice (AgtaP2).58 They found that a high-fat diet induced an increase in BP only in wild-type littermate, while there was no increase in AgtaP2, although both gained weight similarly and the amounts of fat mass were almost the same. Plasma Agt protein levels were similar in these 2 types of mice; however, the plasma AngII level was only increased in wild-type littermate on a high-fat diet, not in AgtaP2. This study suggested that adipose tissue serves as a major source of AngII in the development of obesity hypertension. However, it is yet to be determined whether adipocyte-derived Agt or AngII has a major influence on BP regulation in obesity. The adipose tissue RAS not only has the classic pathway of AngII generation catalyzed by ACE but also by cathepsins and chymase.59 It has not been established whether this system is associated with hypertension and obesity and this needs to be studied.
ALDOSTERONE AND OBESITY-RELATED HYPERTENSION
Several studies show that aldosterone excess is often present in obesity and suggest its involvement in the pathogenesis of obesity-related hypertension.60,61 Tuck et al. demonstrated that weight loss is accompanied by reductions in PRA and aldosterone, irrespective of sodium intake, and this affects the decline in BP in obese patients.60 On the other hand, Rocchini et al. showed that obese adolescents had a significantly higher PAC than nonobese adolescents, and weight loss elicited a significant reduction in both the PAC and BP without a decrease in PRA.61 In addition, the decrease in the PAC significantly correlated with the decrease in BP. Similarly, weight loss linked to a reduction in the PAC and BP in menopausal obese woman was reported.62 Interestingly, high levels of PRA, ACE, aldosterone, and insulin with sodium retention and potassium loss were found in patients with visceral obesity, but all of these tended to disappear upon weight reduction and were not found in patients with peripheral obesity.63 On the other hand, Goodfriend et al. demonstrated that the PAC is positively correlated with the amount of visceral adipose tissue and is inversely correlated with insulin sensitivity, independent of the PRA level.8 This suggested that a fat derived-substance contributes to aldosterone excess in patients with visceral obesity. Furthermore, they reported that a candidate ARF is 12,13-epoxy-9-keto-10(trans)-octadecenoic acid, an oxidized fatty acid derivative.28 Ehrhart-Bornstein et al. validated that adipocyte secretory products directly stimulate aldosterone secretion in human adrenocortical cells (NCI-H295R) and suggested that unknown ARFs exist and might explain a direct link between obesity and hypertension.27 Moreover, Nagase et al. investigated aldosterone secretagogue activity in conditioned medium of rat visceral adipocytes and found it was significantly higher in adipocytes from obese rats than in the adipocytes from nonobese rats.26 The aldosterone-releasing activity of these cells was not inhibited by candesartan. These data suggest that ARFs are not regulated by the systemic RAS and contribute to the aldosterone excess in obese rats. Complement-C1q tumor necrosis factor-related protein 1 (CTRP1) is another candidate ARF.29 CTRP1 is expressed at high levels in adipose tissues of obese Zucker diabetic fatty (fa/fa) rats, specifically in the zona glomerulosa of the adrenal cortex, where aldosterone is produced.29,64 CTRP1 dose dependently promoted aldosterone production and expression of the aldosterone synthase CYP11B2 in the human adrenal cortical cell line H295R. AngII-induced aldosterone production is mediated, at least in part, by stimulation of CTRP1, and levels of CTRP1 were significantly upregulated in the serum of hypertensive patients.29 Recently, Huby et al. newly reported that the adipokine leptin is a direct transcriptional upregulator of aldosterone synthase CYP11B2 in adrenal glomerulosa cell and enhances aldosterone production via calcium-dependent mechanisms.65 They also implied that leptin-mediated hyperaldosteronemia contributes to CVD in obese subjects by promoting endothelial dysfunction and increasing profibrotic markers in the heart. Aldosterone excess is closely associated with visceral adipose tissue and contributes to obesity-related hypertension and organ damages.
To maintain a normal BP in normotensive lean subjects, there is a negative correlation between dietary salt intake and the PAC. On a high-salt diet, the level of plasma aldosterone is appropriately decreased through suppression of the RAS, leading to natriuresis. By contrast, in obese hypertensive subjects, ARFs lack negative feedback regulation by salt, and consequently plasma aldosterone is inappropriately secreted on a high-salt diet despite the decreased PRA, resulting in MR activation. Supporting this, salt loading did not only aggravate podocyte injury and albuminuria66 but also induced diastolic dysfunction with severe perivascular fibrosis67 in obese hypertensive rats. However, treatment with an MR antagonist apparently inhibited salt-induced abnormalities in the heart and kidneys of these rats. Of note, continuous infusion of aldosterone in Sprague Dawley rats fed a high-salt diet causes a marked elevation in BP and albuminuria, whereas aldosterone-induced elevation of BP and albuminuria disappear on a low-salt diet.68 These findings clearly suggest that dietary salt is needed for the effects of aldosterone. Taken together, excessive salt intake and aldosterone excess due to ARFs synergistically activate the MR in the heart and kidneys of obese hypertensive humans and animals, leading to BP elevation, CKD, and CVD.
THE PATHOGENETIC ROLE OF ALDOSTERONE/MR ACTIVATION IN OBESITY-RELATED ORGAN DAMAGE
Classically, aldosterone has long been considered to play a central role in regulation of the electrolyte and fluid volume and maintenance of BP homeostasis.10 Moreover, the aldosterone/MR system plays a key role in cardiovascular and renal damage.11,12 In mineralocorticoid target epithelial tissues, such as the kidney and colon, the ligand selectivity of the MR is guaranteed by the presence of HSD11β2, which converts cortisol in humans and corticosterone in rodents to their inactive 11-keto analogues.69 Although the concentration of glucocorticoids in the circulation is 100–1,000-fold greater than that of aldosterone, aldosterone acts as the ligand for the MR in the kidney.69 HSD11β2 activity plays a role in hypertension.70,71 Indeed, decreased activity of HSD11β2, inducing overstimulation of the MR by intrarenal cortisol excess, was detected in hypertensive obese children in comparison to normotensive obese and nonobese children.72 In the heart, MRs are occupied by glucocorticoids because cardiomyocytes almost completely lack HSD11β2, but the glucocorticoid-MR complex does not activate the MR under physiological conditions. Some investigators postulated that this complex becomes active under conditions of increased oxidative stress in pathological states, such as myocardial infarction.73 However, this remains to be explored. On the other hand, aldosterone acts directly on kidney, nonepithelial cells in the heart, vasculature, and brain to cause tissue remodeling, inflammation, fibrosis, and endothelial dysfunction through induction of oxidative stress.74 Supporting this, endothelial-specific, but not cardiomyocyte-specific, MR-knockout prevents the heart from pressure overload. Clinically, the pathological influence of aldosterone in left ventricular hypertrophy75 and myocardial perfusion76 was established by studies of patients with primary hyperaldosteronism. As mentioned earlier, the involvement of the aldosterone/MR system in CVD was proven by 3 large-scale randomized clinical trials, RALES, EPHESUS, and EMPHASIS-HF.13–15 Addition of MR antagonists to standard therapy with ACE inhibitors, AngII receptor blockers, diuretics, and beta-blockers significantly improved the outcomes of patients with severe congestive heart failure or left ventricular systolic dysfunction after myocardial infarction.
In the 1950s, Selye and others realized that aldosterone acts on nonepithelial tissues through the induction of inflammatory processes, collagen formation, fibrosis, and necrosis.77 In the early 1990s, interest in the nonclassical aspect of aldosterone actions resurfaced. Weber and colleagues studied the effects of aldosterone in cardiac remodeling and reported that chronic aldosterone excess in the presence of salt loading caused cardiac fibrosis in experimental rodents,78 which was ameliorated by a MR blocker.79 This implied the involvement of MR activation in salt-induced cardiac fibrosis.79 Rocha et al. also showed that a MR blocker significantly reduces vascular injury in saline-drinking stroke-prone spontaneously hypertensive rats (SHRs).80 Clinically, the pathological influence of aldosterone in left ventricular hypertrophy75 and myocardial perfusion76 was established by studies of patients with primary hyperaldosteronism.
In the kidney, aldosterone/MR activation causes podocyte injury, which leads to proteinuria and glomerulosclerosis,81 and proinflammatory responses, mediating perivascular and interstitial fibrosis.82,83 Indeed, numerous clinical studies have shown that MR antagonists effectively ameliorate proteinuria in patients with hypertension,84,85 diabetes mellitus,86 and CKD.87 Furthermore, MR antagonists had a renoprotective effect in patients who experienced aldosterone breakthrough after they received ACE inhibitors or AngII receptor blockers, associated with residual albuminuria.88,89 Results of the 2 recent double-blind, randomized, placebo-controlled trials named EVALUATE16 and ARTS-DN17 showed that addition of a selective MR antagonist to RAS inhibitors markedly decreased residual albuminuria in both nondiabetic16 and diabetic CKD17 hypertensive patients without serious safety concerns. Nagase et al. examined the role of aldosterone/MR signaling in obesity-associated CKD using obese SHRs, which are models of metabolic syndrome.26 As these rats got older, they developed marked proteinuria with podocyte injury accompanied by an elevated PAC and renal MR activation, while lean SHRs did not show these complications, despite having a similar BP. However, this was effectively suppressed by the selective MR antagonist eplerenone. These results suggested that aldosterone/MR signaling is deeply involved not only in hypertension but also in kidney injury in obese subjects.
Moreover, obese/insulin-resistant subjects are characterized by endothelial dysfunction and endothelial resistance to the effect of insulin on endothelium-dependent vasodilation enhancement, contributing to atherosclerosis.90 Endothelial-specific MR deletion in mice prevented obesity-induced endothelial dysfunction.91 MR antagonism improved insulin resistance in patients with primary aldosteronism.92 Garg et al. demonstrated that spironolactone did not change insulin resistance, despite a reduced systolic BP, in normotensive obese individuals with insulin resistance.93 However, a randomized, double-blind, placebo-controlled, cross-over study by Hwang et al. showed that eplerenone-dependent improvements in vascular endothelial function were positively associated with changes in total and abdominal adiposity and baseline fasting glucose in healthy older adults taking 100mg eplerenone for 1 month.94 A MR antagonist did not improve insulin resistance in obese patients, but the MR influences vascular function in an adiposity-dependent manner; therefore, further studies are needed to elucidate the association of MR signaling with obesity-related vascular dysfunction.
Several studies have implicated MR signaling in adipocyte biology.95 Importantly, given the lack of significant 11HSD2 expression and activity in adipocytes, physiological glucocorticoid is the main endogenous ligand of the MR. Selective MR stimulation of white adipocytes with aldosterone, within the physiologic range, promoted the expression of proinflammatory genes.96 By contrast, adipose tissue-specific amplification of active cortisol in transgenic mice resulted in the typical phenotype of full metabolic syndrome, including central obesity.97 In white adipose tissue, although both aldosterone and corticoids are ligands of the MR, the MR plays a key role in the differentiation and proliferation of adipocytes by upregulating peroxisome proliferator-activated receptor γ and CCAAT-enhancer-binding protein α and in inflammation by recruiting macrophages with proinflammatory cytokines.98 MR antagonism in animals with metabolic syndrome improved insulin sensitivity, suppressed inflammation, and reduced hypertrophic adipocytes.98
In this way, aldosterone/MR activation is involved in various obesity-related organ injuries. However, there is no large clinical study of MR blockade in obese patients; therefore, further studies are expected.
SALT INTAKE, OBESITY, AND ALDOSTERONE
Epidemiological studies demonstrate that the amount of daily salt intake, as estimated by urinary sodium excretion over 24 hours, is proportionally increased with the incidence of high BP and obesity.99 Ma et al. showed that salt intake was higher in overweight and obese individuals and that a 1g/d increase in salt intake was associated with an increase in the risk of obesity by 28% in children and 26% in adults.100 In their study, higher salt intake was also significantly related to higher body fat mass in both children and adults after adjusting for age, sex, ethnic group, and energy intake.100 This direct association of salt intake and body fat mass was also observed in another epidemiological study.101 Previously, salt intake was considered to be associated with obesity through energy intake such as the consumption of sugar-sweetened beverages or salty energy-dense food; however, Ma et al. showed that salt intake is a potential risk factor for obesity independent of energy intake.
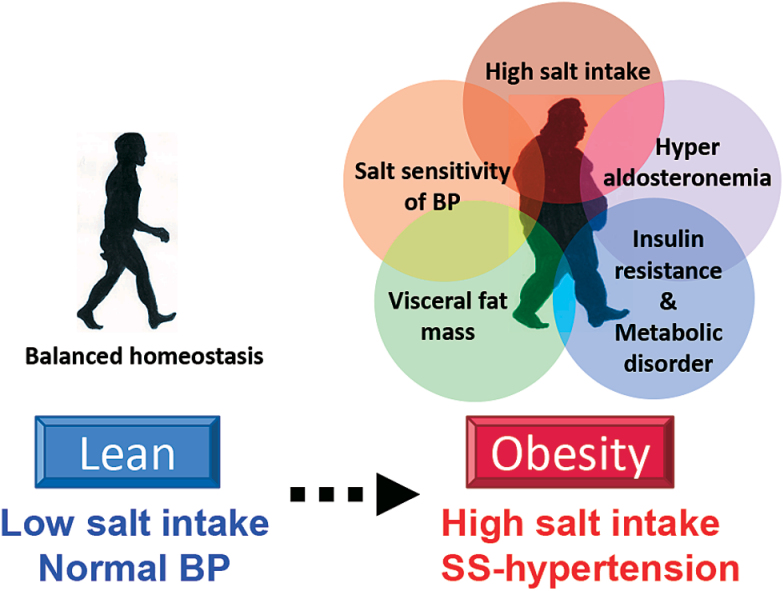
In addition to the deep association with salt intake, obese people show higher salt sensitivity of BP than nonobese people.102 Rocchini et al. reported that obese adolescents showed a significantly larger BP change than nonobese adolescents when they were changed from 2-week periods of a high-salt diet to a low-salt diet. After a 20-week weight loss program, weight loss of more than 1kg was accompanied by a reduced salt sensitivity of BP. Chen et al. showed increased salt sensitivity of BP in patients with metabolic syndrome and a positive relationship between the salt sensitivity of BP and risk factors of metabolic syndrome, including waist circumference, hypertriglyceridemia, low high-density lipoprotein concentration, hypertension, and hyperglycemia. These studies suggested that the salt sensitivity of BP in obese subjects is affected by obesity-related metabolic disorders, including hyperinsulinemia, aldosterone excess, proinflammatory adipokines from the large amount of adipose tissue, and increased activity of the SNS, which are all characteristics of obesity. As previously mentioned, salt-sensitive hypertension in obese subjects is solely due to impaired renal-pressure natriuresis (Figure 1). The progression of obesity is associated with increased visceral fat mass, hyperaldosteronemia, and metabolic disorders and results in the increased salt sensitivity of BP and cardiovascular injury in obese subjects who consume a large amount of salt (Figure 3).
Figure 3.
High salt intake increases the risk of obesity. The level of salt intake proportionally increases with the incidence of hypertension and obesity. Obese subjects consume more salt and exhibit a higher salt sensitivity of BP than lean subjects, and this is synergistically enhanced by obesity-induced homeostatic environmental changes, including a large amount of visceral fat, hyperaldosteronemia, and metabolic disorders. Abbreviations: BP, blood pressure; SS, salt-sensitive.
Moreover, Lee et al. examined single nucleotide polymorphisms related to salt-sensitive genes and sodium intake in children.103 BP, insulin resistance, and serum cholesterol and triglyceride levels are positively correlated with an increased body mass index. Regardless of sex, as dietary sodium intake increased, the risk of obesity significantly increased, especially in those with G-protein–coupled receptor kinase type 4 (GRK4) A486V, and CYP11β-2 variants among girls and with GRK4 A486V, ACE, and SLC12A3 variants among boys. This showed there is a gender-based difference in the interaction between sodium intake and obesity. Dopamine produced by the kidney is natriuretic and antihypertensive and disruption of any of the dopamine receptor genes in mice increases BP.104 GRK4 suppresses the renal dopaminergic signal through desensitizing D1 and D3 dopamine receptors. GRK4 gene variants are associated with salt-sensitive or low-renin hypertension, decreased sodium excretion in hypertensive subjects,104,105 and insulin resistance in obesity.106 Moreover, GRK4 plays a key role in counter-regulation between the renal dopamine system and RAS in the renal tubular sodium reabsorption. Yatabe et al. indicated the interaction between GRK4 and AT1R may be important in the overall regulation of sodium balance and BP.107 When the renal expressions of AT1R were inhibited in SHR, BP increased because the interruption of the renin–angiotensin negative feedback loop resulted in increased circulating renin and AngII. But the inhibition of both GRK4 and AT1R in the kidney decreased BP in SHR to a greater extent than when only GRK4 was inhibited. These studies imply that combinations of salt sensitivity-related gene variants, which are also involved in the pathogenesis of obesity or regulation of the RAS, affect the interaction of obesity and sodium intake, causing hypertension. Further investigations of this may be useful to realize personalized diagnosis and therapy in obesity-related hypertension and salt sensitivity.
SUMMARY AND PERSPECTIVES
Obesity, especially visceral obesity, is closely related to salt-sensitive hypertension. A hyperfiltration-induced increase in renal sodium reabsorption is a trait of obese subjects, and obese hypertensive subjects have impaired renal-pressure natriuresis and require a higher BP than lean normotensive subjects to maintain the sodium balance. Physical compression of the kidneys and activation of the SNS, RAS, and aldosterone/MR system are involved in the development of obesity-related hypertension. As described previously, aldosterone excess, presumably prolonged by adipose-derived ARFs independently of the systemic RAS, is often present in obese subjects, and aldosterone/MR activation in the kidney and cardiovascular system induces organ injuries. Given the inappropriate secretion of aldosterone from adrenal glands by ARFs during the salt load, both the increased salt sensitivity of BP and salt-induced cardiorenal injury appear in obese hypertensive animals through MR activation. Moreover, increased dietary sodium intake is closely associated with the incidence of hypertension and obesity. Some salt sensitivity-related gene variants affect the risk of obesity, and together with salt intake, its combination is possibly associated with the development of hypertension in obese subjects. People often consume a large amount of salt, mainly as a food preservative and taste enhancer, with a high-calorie diet. Both high-salt and high-calorie diets are closely linked and synergistically promote obesity and hypertension. Salt restriction and weight control is important; however, not only MR blockade but also new diagnostic modalities and therapies targeting and modifying genes that are related to salt sensitivity, obesity, or RAS regulation are expected to prevent obesity and obesity-related hypertension.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant number 15K19443).
REFERENCES
- 1. Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 2011; 12:131–141. [DOI] [PubMed] [Google Scholar]
- 2. Müller-Riemenschneider F, Reinhold T, Berghöfer A, Willich SN. Health-economic burden of obesity in Europe. Eur J Epidemiol 2008; 23:499–509. [DOI] [PubMed] [Google Scholar]
- 3. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999; 282:1523–1529. [DOI] [PubMed] [Google Scholar]
- 4. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983; 67:968–977. [DOI] [PubMed] [Google Scholar]
- 5. Burton BT, Foster WR, Hirsch J, Van Itallie TB. Health implications of obesity: an NIH Consensus Development Conference. Int J Obes 1985; 9:155–170. [PubMed] [Google Scholar]
- 6. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab 2007; 92:4472–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res 1999; 7:355–362. [DOI] [PubMed] [Google Scholar]
- 9. Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, Rodeheffer RJ, Dessì-Fulgheri P, Sarzani R, Burnett JC., Jr Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension 2015; 65:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams JS, Williams GH. 50th anniversary of aldosterone. J Clin Endocrinol Metab 2003; 88:2364–2372. [DOI] [PubMed] [Google Scholar]
- 11. Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med 2006; 119:912–919. [DOI] [PubMed] [Google Scholar]
- 12. Hostetter TH, Ibrahim HN. Aldosterone in chronic kidney and cardiac disease. J Am Soc Nephrol 2003; 14:2395–2401. [DOI] [PubMed] [Google Scholar]
- 13. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Investigators EP-AMIHFEaSS Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 15. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E-HS Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.21073363 [Google Scholar]
- 16. Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T; EVALUATE Study Group Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2:944–953. [DOI] [PubMed] [Google Scholar]
- 17. Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM, Group MRATSDNA-DS Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015; 314:884–894. [DOI] [PubMed] [Google Scholar]
- 18. Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet 2001; 358:1682–1686. [DOI] [PubMed] [Google Scholar]
- 19. Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med 2004; 140:992–1000. [DOI] [PubMed] [Google Scholar]
- 20. VAGUE J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956; 4:20–34. [DOI] [PubMed] [Google Scholar]
- 21. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008; 168:1617–1624. [DOI] [PubMed] [Google Scholar]
- 22. St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 2004; 27:2222–2228. [DOI] [PubMed] [Google Scholar]
- 23. Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes Metab Rev 1997; 13:3–13. [DOI] [PubMed] [Google Scholar]
- 24. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 2011; 18:629–639. [DOI] [PubMed] [Google Scholar]
- 25. Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol 2015; 31:177–183. [DOI] [PubMed] [Google Scholar]
- 26. Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol 2006; 17:3438–3446. [DOI] [PubMed] [Google Scholar]
- 27. Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 2003; 100:14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension 2004; 43:358–363. [DOI] [PubMed] [Google Scholar]
- 29. Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH, Kim JW, Kim D, Han SH, Lim JS, Kim KI, Yoon do Y, Kim SH, Oh GT, Kim E, Yang Y. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 2008; 22:1502–1511. [DOI] [PubMed] [Google Scholar]
- 30. Sookoian SC, González C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res 2005; 13:2122–2131. [DOI] [PubMed] [Google Scholar]
- 31. Di Blasio AM, van Rossum EF, Maestrini S, Berselli ME, Tagliaferri M, Podestà F, Koper JW, Liuzzi A, Lamberts SW. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 2003; 59:68–74. [DOI] [PubMed] [Google Scholar]
- 32. Bellili NM, Foucan L, Fumeron F, Mohammedi K, Travert F, Roussel R, Balkau B, Tichet J, Marre M. Associations of the -344 T>C and the 3097 G>A polymorphisms of CYP11B2 gene with hypertension, type 2 diabetes, and metabolic syndrome in a French population. Am J Hypertens 2010; 23:660–667. [DOI] [PubMed] [Google Scholar]
- 33. Lang F, Stournaras C. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Hormones (Athens) 2013; 12:160–171. [DOI] [PubMed] [Google Scholar]
- 34. Huang DY, Boini KM, Friedrich B, Metzger M, Just L, Osswald H, Wulff P, Kuhl D, Vallon V, Lang F. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am J Physiol Regul Integr Comp Physiol 2006; 290:R935–R944. [DOI] [PubMed] [Google Scholar]
- 35. Hall JE. The kidney, hypertension, and obesity. Hypertension 2003; 41:625–633. [DOI] [PubMed] [Google Scholar]
- 36. Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens 1997; 10:49S–55S. [PubMed] [Google Scholar]
- 37. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015; 116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995; 25:893–897. [DOI] [PubMed] [Google Scholar]
- 39. Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 2010; 55:862–868. [DOI] [PubMed] [Google Scholar]
- 40. Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 1995; 25:560–563. [DOI] [PubMed] [Google Scholar]
- 41. Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 1997; 96:3423–3429. [DOI] [PubMed] [Google Scholar]
- 42. Wofford MR, Anderson DC, Jr, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens 2001; 14:694–698. [DOI] [PubMed] [Google Scholar]
- 43. Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012; 59:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 2009; 119:978–986. [DOI] [PubMed] [Google Scholar]
- 45. DiBona GF. Physiology in perspective: The Wisdom of the Body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol 2005; 289:R633–R641. [DOI] [PubMed] [Google Scholar]
- 46. Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 2011; 17:573–580. [DOI] [PubMed] [Google Scholar]
- 47. O’Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 2006; 17:2402–2413. [DOI] [PubMed] [Google Scholar]
- 48. Lai L, Feng X, Liu D, Chen J, Zhang Y, Niu B, Gu Y, Cai H. Dietary salt modulates the sodium chloride cotransporter expression likely through an aldosterone-mediated WNK4-ERK1/2 signaling pathway. Pflugers Arch 2012; 463:477–485. [DOI] [PubMed] [Google Scholar]
- 49. Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 2012; 109:7929–7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 2008; 74:1403–1409. [DOI] [PubMed] [Google Scholar]
- 51. Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010; 285:17271–17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334:292–295. [DOI] [PubMed] [Google Scholar]
- 53. Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol 2012; 302:H1219–H1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 2001; 79:21–29. [DOI] [PubMed] [Google Scholar]
- 55. Marcus Y, Shefer G, Stern N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol 2013; 378:1–14. [DOI] [PubMed] [Google Scholar]
- 56. Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep 2008; 10:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, Fujikura J, Ebihara K, Hosoda K, Katsurada A, Ohashi N, Urushihara M, Kobori H, Morimoto N, Kawazoe T, Naitoh M, Okada M, Sakaue H, Suzuki S, Nakao K. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens 2010; 23:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension 2012; 60:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karlsson C, Lindell K, Ottosson M, Sjöström L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab 1998; 83:3925–3929. [DOI] [PubMed] [Google Scholar]
- 60. Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 1981; 304:930–933. [DOI] [PubMed] [Google Scholar]
- 61. Rocchini AP, Katch VL, Grekin R, Moorehead C, Anderson J. Role for aldosterone in blood pressure regulation of obese adolescents. Am J Cardiol 1986; 57:613–618. [DOI] [PubMed] [Google Scholar]
- 62. Engeli S, Böhnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 2005; 45:356–362. [DOI] [PubMed] [Google Scholar]
- 63. Ruano M, Silvestre V, Castro R, García-Lescún MC, Rodríguez A, Marco A, García-Blanch G. Morbid obesity, hypertensive disease and the renin-angiotensin-aldosterone axis. Obes Surg 2005; 15:670–676. [DOI] [PubMed] [Google Scholar]
- 64. Kim KY, Kim HY, Kim JH, Lee CH, Kim DH, Lee YH, Han SH, Lim JS, Cho DH, Lee MS, Yoon S, Kim KI, Yoon DY, Yang Y. Tumor necrosis factor-alpha and interleukin-1beta increases CTRP1 expression in adipose tissue. FEBS Lett 2006; 580:3953–3960. [DOI] [PubMed] [Google Scholar]
- 65. Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation 2015; 132:2134–2145. [DOI] [PubMed] [Google Scholar]
- 66. Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension 2007; 50:877–883. [DOI] [PubMed] [Google Scholar]
- 67. Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, Nagase M, Fujita T. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension 2008; 52:287–294. [DOI] [PubMed] [Google Scholar]
- 68. Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 2011; 121:3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 1988; 242:583–585. [DOI] [PubMed] [Google Scholar]
- 70. Ferrari P, Krozowski Z. Role of the 11beta-hydroxysteroid dehydrogenase type 2 in blood pressure regulation. Kidney Int 2000; 57:1374–1381. [DOI] [PubMed] [Google Scholar]
- 71. Ferrari P, Sansonnens A, Dick B, Frey FJ. In vivo 11beta-HSD-2 activity: variability, salt-sensitivity, and effect of licorice. Hypertension 2001; 38:1330–1336. [DOI] [PubMed] [Google Scholar]
- 72. Csábi GY, Juricskay S, Molnár D. Urinary cortisol to cortisone metabolites in hypertensive obese children. J Endocrinol Invest 2000; 23:435–439. [DOI] [PubMed] [Google Scholar]
- 73. Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension 2009; 54:1306–1312. [DOI] [PubMed] [Google Scholar]
- 74. Stier CT, Jr, Rocha R, Chander PN. Effect of aldosterone and MR blockade on the brain and the kidney. Heart Fail Rev 2005; 10:53–62. [DOI] [PubMed] [Google Scholar]
- 75. Rossi GP, Sacchetto A, Pavan E, Scognamiglio R, Pietra M, Pessina AC. Left ventricular systolic function in primary aldosteronism and hypertension. J Hypertens 1998; 16:2075–2077. [DOI] [PubMed] [Google Scholar]
- 76. Napoli C, Di Gregorio F, Leccese M, Abete P, Ambrosio G, Giusti R, Casini A, Ferrara N, De Matteis C, Sibilio G, Donzelli R, Montemarano A, Mazzeo C, Rengo F, Mansi L, Liguori A. Evidence of exercise-induced myocardial ischemia in patients with primary aldosteronism: the Cross-sectional Primary Aldosteronism and Heart Italian Multicenter Study. J Investig Med 1999; 47:212–221. [PubMed] [Google Scholar]
- 77. Selye H. Protection by a steroid-spirolactone against certain types of cardiac necroses. Exp Biol Med 1960;104:212–213. [DOI] [PubMed] [Google Scholar]
- 78. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 1990; 67:1355–1364. [DOI] [PubMed] [Google Scholar]
- 79. Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 1993; 25:563–575. [DOI] [PubMed] [Google Scholar]
- 80. Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT., Jr Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 1998; 31:451–458. [DOI] [PubMed] [Google Scholar]
- 81. Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 2007; 49:355–364. [DOI] [PubMed] [Google Scholar]
- 82. Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 2003; 63:1791–1800. [DOI] [PubMed] [Google Scholar]
- 83. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 2004; 43:841–848. [DOI] [PubMed] [Google Scholar]
- 84. White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J, Weber MA. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension 2003; 41:1021–1026. [DOI] [PubMed] [Google Scholar]
- 85. Williams GH, Burgess E, Kolloch RE, Ruilope LM, Niegowska J, Kipnes MS, Roniker B, Patrick JL, Krause SL. Efficacy of eplerenone versus enalapril as monotherapy in systemic hypertension. Am J Cardiol 2004; 93:990–996. [DOI] [PubMed] [Google Scholar]
- 86. Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006; 1:940–951. [DOI] [PubMed] [Google Scholar]
- 87. Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int 2006; 70:2116–2123. [DOI] [PubMed] [Google Scholar]
- 88. Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003; 41:64–68. [DOI] [PubMed] [Google Scholar]
- 89. Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1:256–262. [DOI] [PubMed] [Google Scholar]
- 90. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996; 97:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F, Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 2013; 34:3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, Favret G, Melis A, Cavarape A, Sechi LA. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 2006; 91:3457–3463. [DOI] [PubMed] [Google Scholar]
- 93. Garg R, Kneen L, Williams GH, Adler GK. Effect of mineralocorticoid receptor antagonist on insulin resistance and endothelial function in obese subjects. Diabetes Obes Metab 2014; 16:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, Segal MS, Christou DD. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond) 2013; 125:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Caprio M, Fève B, Claës A, Viengchareun S, Lombès M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J 2007; 21:2185–2194. [DOI] [PubMed] [Google Scholar]
- 96. Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H, Klein J. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J Endocrinol 2010; 204:153–164. [DOI] [PubMed] [Google Scholar]
- 97. Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294:2166–2170. [DOI] [PubMed] [Google Scholar]
- 98. Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GM, Caprio M. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol 2012; 350:281–288. [DOI] [PubMed] [Google Scholar]
- 99. Aparicio A, Rodríguez-Rodríguez E, Cuadrado-Soto E, Navia B, López-Sobaler AM, Ortega RM. Estimation of salt intake assessed by urinary excretion of sodium over 24h in Spanish subjects aged 7–11 years. Eur J Nutr 2015; e-pub ahead of print 19 October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension 2015; 66:843–849. [DOI] [PubMed] [Google Scholar]
- 101. Zhu H, Pollock NK, Kotak I, Gutin B, Wang X, Bhagatwala J, Parikh S, Harshfield GA, Dong Y. Dietary sodium, adiposity, and inflammation in healthy adolescents. Pediatrics 2014; 133:e635–e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 1989; 321:580–585. [DOI] [PubMed] [Google Scholar]
- 103. Lee M, Kim MK, Kim SM, Park H, Park CG, Park HK. Gender-based differences on the association between salt-sensitive genes and obesity in Korean children aged between 8 and 9 years. PLoS One 2015; 10:e0120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 2010; 1802:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, Eisner GM, Jose PA, Felder RA. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem 2006; 52:352–360. [DOI] [PubMed] [Google Scholar]
- 106. Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol 2005; 289:F298–F304. [DOI] [PubMed] [Google Scholar]
- 107. Yatabe J, Sanada H, Midorikawa S, Hashimoto S, Watanabe T, Andrews PM, Armando I, Wang X, Felder RA, Jose PA. Effects of decreased renal cortical expression of G protein-coupled receptor kinase 4 and angiotensin type 1 receptors in rats. Hypertens Res 2008; 31:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]