Abstract
To better understand the binding interaction between antigen and antibody we need to distinguish protein residues critical to the binding energy and mechanism from residues merely localized in the interface. By analyzing the binding of monoclonal antibodies to recombinant wild-type and mutant myohemerythrin (MHr) proteins, we were able to test the role of individual critical residues at the highly antigenic site MHr-(79-84), within the context of the folded protein. The results directly show the existence of antigenically critical residues, whose mutations significantly reduce antibody binding to the folded protein, thus verifying peptide-based assignments of these critical residues and demonstrating the ability of buried side chains to influence antigenicity. Taken together, these results (i) distinguish the antigenic surface from the solvent-exposed protein surface before binding, (ii) support a two-stage interaction mechanism allowing inducible changes in protein antigens by antibody binding, and (iii) show that protein antigenicity can be significantly reduced by alteration of single critical residues without destroying biological activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989 Feb 23;337(6209):709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
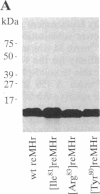
- Alexander H., Alexander S., Heffron F., Fieser T. M., Hay B. N., Getzoff E. D., Tainer J. A., Lerner R. A. Synthesis and characterization of a recombinant myohemerythrin protein encoded by a synthetic gene. Gene. 1991 Mar 15;99(2):151–156. doi: 10.1016/0378-1119(91)90121-q. [DOI] [PubMed] [Google Scholar]
- Appel J. R., Pinilla C., Niman H., Houghten R. Elucidation of discontinuous linear determinants in peptides. J Immunol. 1990 Feb 1;144(3):976–983. [PubMed] [Google Scholar]
- Colman P. M. Structure of antibody-antigen complexes: implications for immune recognition. Adv Immunol. 1988;43:99–132. doi: 10.1016/s0065-2776(08)60364-8. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Sheriff S., Padlan E. A. Antibody-antigen complexes. J Biol Chem. 1988 Aug 5;263(22):10541–10544. [PubMed] [Google Scholar]
- Fieser T. M., Tainer J. A., Geysen H. M., Houghten R. A., Lerner R. A. Influence of protein flexibility and peptide conformation on reactivity of monoclonal anti-peptide antibodies with a protein alpha-helix. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8568–8572. doi: 10.1073/pnas.84.23.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzoff E. D., Geysen H. M., Rodda S. J., Alexander H., Tainer J. A., Lerner R. A. Mechanisms of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1191–1196. doi: 10.1126/science.3823879. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Lerner R. A., Geysen H. M. The chemistry and mechanism of antibody binding to protein antigens. Adv Immunol. 1988;43:1–98. doi: 10.1016/s0065-2776(08)60363-6. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Klippenstein G. L., Van Riper D. A., Oosterom E. A. A comparative study of the oxygen transport proteins of Dendrostomum pyroides. Isolation and characterization of hemerythrins from muscle, the vascular system, and the coelom. J Biol Chem. 1972 Sep 25;247(18):5959–5963. [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Posthumus W. P., Lenstra J. A., Schaaper W. M., van Nieuwstadt A. P., Enjuanes L., Meloen R. H. Analysis and simulation of a neutralizing epitope of transmissible gastroenteritis virus. J Virol. 1990 Jul;64(7):3304–3309. doi: 10.1128/jvi.64.7.3304-3309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthumus W. P., Lenstra J. A., van Nieuwstadt A. P., Schaaper W. M., van der Zeijst B. A., Meloen R. H. Immunogenicity of peptides simulating a neutralization epitope of transmissible gastroenteritis virus. Virology. 1991 May;182(1):371–375. doi: 10.1016/0042-6822(91)90684-4. [DOI] [PubMed] [Google Scholar]
- Redlich P. N., Hoeprich P. D., Jr, Colby C. B., Grossberg S. E. Antibodies that neutralize human beta interferon biologic activity recognize a linear epitope: analysis by synthetic peptide mapping. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4040–4044. doi: 10.1073/pnas.88.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca R., Schwab C., Bosshard H. R. Epitope mapping employing immobilized synthetic peptides. How specific is the reactivity of these peptides with antiserum raised against the parent protein? J Immunol Methods. 1991 Aug 9;141(2):245–252. doi: 10.1016/0022-1759(91)90151-5. [DOI] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Smith J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987 Sep 20;197(2):273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Tribbick G., Triantafyllou B., Lauricella R., Rodda S. J., Mason T. J., Geysen H. M. Systematic fractionation of serum antibodies using multiple antigen homologous peptides as affinity ligands. J Immunol Methods. 1991 Jun 3;139(2):155–166. doi: 10.1016/0022-1759(91)90185-i. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel M. H. Structural and functional approaches to the study of protein antigenicity. Immunol Today. 1989 Aug;10(8):266–272. doi: 10.1016/0167-5699(89)90140-0. [DOI] [PubMed] [Google Scholar]