SUMMARY
OBJECTIVE
To evaluate the cost-effectiveness of the Three I’s for HW/TB (human immunodeficiency virus/tuberculosis): antiretroviral therapy (ART), intensified TB case finding (ICF), isoniazid preventive treatment (IPT), and TB infection control (IC).
METHODS
Using a 3-year decision-analytic model, we estimated the cost-effectiveness of a base scenario (55% ART coverage at CD4 count ≤350 cells/mm3) and 19 strategies that included one or more of the following: 1) 90% ART coverage, 2) IC and 3) ICF using four-symptom screening and 6- or 36-month IPT. The TB diagnostic algorithm included 1) sputum smear microscopy with chest X-ray, and 2) Xpert® MTB/RIF.
RESULTS
In resource-constrained settings with a high burden of HIV and TB, the most cost-effective strategies under both diagnostic algorithms included 1) 55% ART coverage and IC, 2) 55% ART coverage, IC and 36-month IPT, and 3) expanded ART at 90% coverage with IC and 36-month IPT. The latter averted more TB cases than other scenarios with increased ART coverage, IC, 6-month IPT and/or IPT for tuberculin skin test positive individuals. The cost-effectiveness results did not change significantly under the sensitivity analyses.
CONCLUSION
Expanded ART to 90% coverage, IC and a 36-month IPT strategy averted most TB cases and is among the cost-effective strategies.
Keywords: isoniazid, Xpert®, MTB/RIF, intensified TB case finding, economic analysis
RESUME
CONTEXTE
Nous évaluons le rapport coût-efficacité de l’approche des « 3 I » dans la lutte contre le virus de l’immunodéficience humaine (VIH) et la TB : traitement antirétroviral (ART) et intensification de la recherche de cas de TB (ICF), traitement préventif par isoniazide (IPT) et lutte contre l’infection tuberculeuse (IC).
MÉTHODES
Nous avons estimé, grâce à un modèle de décision analytique de 3 ans, le rapport coût-efficacité d’un scénario de base (55% de couverture du traitement ART quand la numération des CD4 est ≤350 cellules/mm3) et 19 stratégies qui incluaient une ou plusieurs des stratégies suivantes : 1) 90% de couverture par ART, 2) IC et 3) ICF grâce à un dépistage basé sur quatre symptômes et un IPT pendant 6 ou 36 mois. L’algorithme de diagnostic de la TB incluait 1) microscopie des frottis de crachats et radio pulmonaire et 2) Xpert® MTB/RIF.
RÉSULTATS
Dans les contextes ressources limitées confrontés à un lourd fardeau de VIH et de TB, les stratégies les plus rentables en termes d’algorithmes de diagnostic incluaient 1) 55% de couverture par ART et IC; 2) 55 % de couverture par ART, IC et 36 mois d’IPT; et 3) expansion de l’ART à une couverture de 90% avec IC et IPT de 36 mois. Cette dernière stratégie a évité davantage de cas de TB que les autres scénarios avec augmentation de la couverture par ART, IC, 6 mois d’IPT et/ou IPT pour les cas positifs au test cutané tuberculinique. Les résultats en termes de coût-efficacité n’ont pas changé significativement avec les analyses de sensibilité.
CONCLUSION
La stratégie d’expansion de la couverture par ART à 90%, IC et 36 mois d’IPT a évité le plus de cas de TB et elle est parmi les stratégies les plus rentables.
RESUMEN
MARCO DE REFERENClA
Se llevó a cabo una evaluación de la rentabilidad de las intervenciones de prevención de la tuberculosis (TB), el tratamiento antirretrovírico (ART) y la estrategia de las ‘Tres íes’ (que comporta la intensificación de la búsqueda de casos [ICF] de coinfección por el virus de la inmunodeficiencia humana [VIH] y TB, el tratamiento preventive con isoniazida [IPT] y el control de la infección [IC] tuberculosa).
MÉTODOS
Se construyó unmodelo analítico decisional destinado a evaluar en una población positiva frente al VIH durante un período de 3 años, la rentabilidad de la prevención de la TB en un contexto hipotético de base (cobertura del 55% con el ART en pacientes con recuentos de linfocitos CD4 ≤350 células/µl) y en 19 estrategias comparativas que comportaban una o varias de las siguientes condiciones: 1) una cobertura del 90% con el ART, 2) medidas de IC tuberculosa y 3) la ICF mediante un sistema de detección por cuatro síntomas y el IPT durante 6 meses o 36 meses en los casos negatives. Se compararon todas las estrategias al usar dos algoritmos diagnósticos diferentes: 1) la baciloscopia del esputo con radiografía de tórax y 2) la prueba Xpert® MTB/RIF.
RESULTADOS
En los entornos con recursos limitados y una alta carga de morbilidad por TB e infección por el VIH, las estrategias más rentables con ambos algoritmos diagnósticos fueron: 1) una cobertura del 55% con el ART y las medidas de IC tuberculosa; 2) una cobertura del 55% con el ART y 36 meses de IPT; y 3) la ampliación de la cobertura con el ART al 90%, con medidas de IC tuberculosa y 36 meses de IPT. Esta última estrategia evitó más casos de TB que otras dos hipótesis con ampliación de la cobertura a 90%, IC y 6 meses de IPT o con tratamiento preventive en los casos de reacción tuberculínica positiva. Los resultados de rentabilidad no se modificaron de manera significativa en los análisis de sensibilidad.
CONCLUSION
La estrategia que comporta la ampliación de la cobertura con el ART a 90%, las medidas de IC tuberculosa y el IPT durante 36 meses evitó el mayor número de casos de TB y es rentable.
The human immunodeficiency virus (HIV) infection and tuberculosis (TB) epidemics are major threats to global public health. About one third of the 35.3 million people living with HIV are latently infected with Mycobacterium tuberculosis, and are more likely to develop active TB disease than people who are not infected with HIV1,2 The World Health Organization (WHO) recommends the Three I’s for HIV/TB and early initiation of antiretroviral therapy (ART) to reduce the burden of TB in HIV-positive people.3 They include 1) intensified TB case finding (ICF), 2) TB prevention with isoniazid preventive treatment (IPT) and early ART, and 3) TB infection control (IC) in health care facilities and congregate settings.
A recent systematic review confirmed that ART reduces the risk of developing TB by 65% across all CD4 count strata.4 The expansion of ART coverage also reduces TB incidence at the community and population levels in settings with a large burden of HIV-associated TB.5,6 The 2010 WHO ART guidelines recommend ART initiation at CD4 count ≤350 cells/mm3 for all asymptomatic people living with HIV, and irrespective of CD4 cell count for those with active TB.7 More recently, the WHO recognised the benefits of earlier treatment and recommended ART at CD4 count ≤500 cells/mm3 and irrespective of CD4 count for serodiscordant couples, pregnant women, children aged <5 years and persons with TB or hepatitis B.8 The 2011 WHO ICF/IPT guidelines recommend four-symptom screening (current cough, fever, weight loss or night sweats) to identify HIV-infected persons eligible for either IPT or further diagnostic work-up for TB and other conditions.9 The tuberculin skin test (TST) is not a requirement for the administration of IPT, but may be used where feasible.9 The 2009 WHO policy on IC proposes managerial, administrative and environmental controls and personal protection measures in health facilities to reduce the risk of nosocomial TB transmission.10
Most countries currently recommend and implement different TB prevention strategies to reduce morbidity and mortality among people living with HIV This study evaluates the cost-effectiveness of different TB prevention strategies in a setting with a generalised HIV epidemic and high TB burden among people living with HIV to determine the optimal mix of interventions that will maximise health benefits with the given resources.
METHODS
We developed a decision-analytic model (Appendix Figure A.1)* to estimate the cost-effectiveness of TB prevention strategies in a hypothetical cohort of 10 000 HIV-positive people with a prevalence of active TB of 5%. TB prevention interventions were provided at time zero, and the cohort was followed up for a period of 3 years. Ethical approval was not required for the study. The model for Year 1 was constructed using TreeAge 2012 (TreeAge Software, Inc, Williamstown, MA, USA). TB cases among people living with HIV and costs in subsequent years were calculated using Microsoft Excel 2011 (Microsoft, Redmond, WA, USA).
The base scenario was defined as one with ART coverage at the 2011 global ART coverage rate of 55% (at CD4 count ≤350 cells/mm3).11 The TB symptom considered for screening was current cough. The 19 comparative strategies included one or more of the following TB prevention interventions: 1) ART coverage increased to 90% at CD4 count ≤350 cells/mm3; 2) IC measures in health care facilities, which included administrative measures such as triage, personal protective measures such as respirators for nurses and surgical masks for patients with cough and natural ventilation through construction and maintenance of fans and windows; and 3) ICF using a four-symptom screening algorithm and 6-month or 36-month IPT (life-long IPT) for those screening negative (Table 1). The costs and number of TB cases were calculated for all strategies under two separate TB diagnostic algorithms: 1) sputum smear microscopy with chest X-ray (CXR); and 2) Xpert® MTB/RIF assay for diagnosis of Mycobacterium tuberculosis and resistance to rifampicin (Cepheid, Sunnyvale, CA, USA).
Table 1.
Description of TB prevention interventions included in the 20 scenarios*
| Scenario | ART coverage % |
TB screening | TST | Duration of IPT | IC | |
|---|---|---|---|---|---|---|
| A | Base | 55 | Cough | No | No | |
| B | ART IC | 55 | Cough | No | Yes | |
| C | ART ICF IPT 6 | 55 | Four symptom | No | 6 months | No |
| D | ART ICF IPT 36 | 55 | Four symptom | No | 36 months | No |
| E | ART ICF TST IPT 36 | 55 | Four symptom | Yes | 36 months for TST+ | No |
| F | ART ICF IPT 6 TST IPT 36 | 55 | Four symptom | Yes | 36 months for TST+; 6 months for TST+ | No |
| G | ART IC ICF IPT 6 | 55 | Four symptom | No | 6 months | Yes |
| H | ART IC ICF IPT 36 | 55 | Four symptom | No | 36 months | Yes |
| ART IC ICF TST IPT 36 | 55 | Four symptom | Yes | 36 months for TST+ | Yes | |
| J | ART IC ICF IPT 6 TST IPT 36 | 55 | Four symptom | Yes | 36 months for TST+; 6 months for TST- | Yes |
| K | ARTexp | 90 | Cough | No | No | |
| L | ARTexp IC | 90 | Cough | No | Yes | |
| M | ARTexp ICF IPT 6 | 90 | Four symptom | No | 6 months | No |
| N | ARTexp ICF IPT 36 | 90 | Four symptom | No | 36 months | No |
| 0 | ARTexp ICF TST IPT 36 | 90 | Four symptom | Yes | 36 months for TST+ | No |
| P | ARTexp ICF IPT 6 TST IPT 36 | 90 | Four symptom | Yes | 36 months for TST+; 6 months for TST- | No |
| Q | ARTexp IC ICF IPT 6 | 90 | Four symptom | No | 6 months | Yes |
| R | ARTexp IC ICF IPT 36 | 90 | Four symptom | No | 36 months | Yes |
| S | ARTexp IC ICF TST IPT 36 | 90 | Four symptom | Yes | 36 months for TST+ | Yes |
| T | ARTexp IC ICF IPT 6 TST IPT 36 | 90 | Four symptom | Yes | 36 months for TST+; 6 months for TST- | Yes |
TB cases and costs under all the strategies were calculated for two different TB diagnostic algorithms: 1) sputum smear microscopy and chest radiography; and 2) Xpert® MTB/RIF assay for Mycobacterium tuberculosis and resistance to rifampicin.
TB=tuberculosis; ART =antiretroviral therapy; TST=tuberculin skin test; IPT = isoniazid preventive therapy; IC = infection control; ICF = intensified case finding; += positive; — = negative; ARTexp = expanded ART (90% coverage).
Model parameters and assumptions
The parameter values for TB prevalence, TB incidence, sensitivity and specificity of TB screening and diagnostic tests and the efficacy of the TB prevention interventions were derived from the published literature (Table 2).11–24 Outcomes and costs related to drug-resistant TB were not considered in the analysis.
Table 2.
Model parameter inputs and their range
| Variables | Base value | Range | Source (author, year, reference) |
|---|---|---|---|
| TB prevalence among HIV-positive people | 0.05 | 0.004–0.26 | Getahun, 201113 |
| Proportion of PLHIV eligible for ART at CD4 count ≤350 cells/mm3, % |
45 | 43–49 | UNAIDS report, 201211 |
| ART coverage, % | 55 | 53–60 | |
| Expanded ART coverage, % | 90 | 80–100 | Assumption |
| Annual incidence of TB in PLHIV | |||
| All | 0.0395* | 0.034–0.1 | Shrestha, 200714 |
| TST-positive | 0.047 | 0.034–0.1 | |
| TST-negative | 0.037 | 0.034–0.1 | |
| Relative risk of TB | |||
| ART | 0.33 | 0.27–0.39 | Lawn 201115 |
| 6-month IPT | 0.89† | 0.84–0.96 | Akolo 201016 |
| 36-month IPT (all) | 0.51 | 0.27–0.93 | Samandari, 201117 |
| 36-month IPT in TST-positive | 0.21 | 0.04–0.75 | |
| TB infection control | 0.87 | 0.63–1.00 | Harries, 200212, 18 |
| Sensitivity of screening and diagnostic test | |||
| Cough | 0.385 | 0.19–0.62 | Getahun, 201113 |
| Four-symptom | 0.789 | 0.58–0.91 | |
| Sputum smear | 0.36 | 0.09–0.50 | Monkongdee, 200919 |
| Chest radiography | 0.65 | 0.60–0.83 | Cain, 201020 |
| Xpert | 0.82 | 0.60–0.87 | Lawn, 2011,21 Boehme, 201122 |
| Specificity of screening and diagnostic test | |||
| Cough | 0.818 | 0.65–0.92 | Getahun, 201113 |
| Four-symptom | 0.496 | 0.29–0.70 | |
| Sputum smear | 0.99 | 0.98–1.00 | Cain, 201020 |
| Chest radiography | 0.85 | 0.35–1.00 | Cain, 201020 |
| Xpert | 0.99 | 0.98–1.00 | Lawn, 2011,21 Boehme, 201122 |
| Expected proportion of PLHIV testing | |||
| TST-positive | |||
| Active TB | 0.65 | 0.50–0.74 | Rangaka, 200723 |
| No active TB | 0.26 | 0.19–0.35 | Kerkhoff, 201224 |
Assuming 25% of HIV-positive people are TST-positive,24 we calculated the annual incidence of TB in PLHIV
Relative risk of TB from 6-month IPT is 0.67. Assuming 6-month IPT will only be effective in year 1, the average relative risk was calculated as 0.89.
TB = tuberculosis; HIV = human immunodeficiency virus; PLHIV = people living with HIV; ART=antiretroviral therapy; UNAIDS =Joint United Nations Programme on HIV/AIDS; TST = tuberculin skin test; IPT = isoniazid preventive therapy
We chose a 3-year analytic horizon to be able to compare the preventive effect of long-term IPT use (i.e., 36 months) against the use of short-term IPT (i.e., 6 months). We assumed that the 6 months of IPT would be effective for a period of 1 year. We assumed that the effectiveness of the IC package was similar to that of a study investigating the effects of IC measures in the epidemic trajectory of a setting with high TB-HIV co-infection.12
Costs
The analysis took a health system perspective and considered health care utilisation costs from South Africa (Table 3).9,25–30 Development and maintenance costs of diagnostic capacity, productivity loss and out-of-pocket costs incurred by individuals to seek care were not included. Costs for TB diagnostic tests and drugs were derived from published studies; in-patient and out-patient costs came from WHO-CHOICE (CHOosing Interventions that are Cost Effective).28 Cost data for IC interventions were taken from a primary health care facility in South Africa, and building cost indices from the Bureau of Economic Research, Stellenbosch University, Cape Town, South Africa.
Table 3.
Cost inputs (in 2010 USD)
| Parameter | Cost/unit USD |
Units | Cost/patient USD |
Source (author, year, reference) |
|---|---|---|---|---|
| Diagnostic | ||||
| Sputum smear | 1.60 | 2 tests | 3.20 | Vassall, 201125 |
| Chest radiography | 7.80 | 1 test | 7.80 | Fairall, 201026 |
| Tuberculin skin test | 6.20 | 1 test | 6.20 | 2011 WHO ICF/IPT Guidelines9 |
| Xpert | 22.00 | 1 test | 22.00 | Vassall, 201125 |
| Anti-tuberculosis treatment | ||||
| Drugs and DOT visits | 437.20 | DOTS course and DOTS visits | 437.20 | Dowdy, 200827 |
| TB hospitalisation | 62 | 21 days average stay | 1300.00 | WHO-CHOICE28 |
| TB infection control | ||||
| Respirator | 0.60 | 1 per nurse per week* | 0.29 | Data from a primary hospital, and prices and building cost indices from the Bureau of Economic Research, Stellenbosch University, Cape Town, South Africa |
| Surgical masks | 0.17 | 1 per patient with cough per visit† |
2.50 | |
| Windows: construction | 3 years capital cost | 0.35 | ||
| Windows: maintenance | 3 years maintenance cost | 0.12 | ||
| Fan | Cost of capital and maintenance for 3 years |
0.13 | ||
| INH | ||||
| 6-month course | 0.92 | One course of 300 mg INH daily | 16.40 | International drug price indicator§ |
| 36-month course | 3-year cost | 103.50 | ||
| INH-related hepatitis† | ||||
| LFT | 4.50# | 1 test | Arnold, 201129 | |
| Out-patient cost | 3 LFT and 3 clinical visits | 50 | WHO-CHOICE28 | |
| In-patient cost | 1 LFT and 7 days hospital stay | 438 | ||
| Antiretroviral therapy | ||||
| Antiretroviral drugs | 274.50 | 3 years drug cost | 867.20 | Granich, 201230 |
| Monitoring visit (first) | 34.30 | 1 visit | 34.30 | |
| Subsequent visits | 25.70 | 2 visits (once every year) | 55.50 |
Assuming 2 nurses per consulting room, the cost of respirators (life of 1 week) was calculated at US$2944 (in 2010 USD)
The cost calculation assumed annual out-patient visit/patient/year to be approximately 2.5
We assumed that 10% of hepatitis cases were hospitalised for an average of 7 days after an LFT; all other cases were managed on an out-patient basis requiring three clinic visits and three LFTs.29
Using an exchange rate of US$1 = R8.8 and £1 = US$1.6.
USD = US dollars; WHO = World Health Organization; ICF = intensified case finding; IPT = INH preventive therapy; DOT = directly observed therapy; TB = tuberculosis; INH = isoniazid; LFT = liver function test; R = South African rand; £ = Great British pound.
Using the South African consumer price index for medical goods and services from 2006 to 2012 (Statistics South Africa, South Africa Consumer Price Index, Pretoria, South Africa, http://www.statssa.gov.za), all the costs were converted into 2010 USD and adjusted for inflation for 2011 and 2012.
Cost-effectiveness analysis
The primary outcome of the analysis was cases of TB averted, calculated as the difference between the number of TB cases under two different strategies. The incremental cost-effectiveness ratio (ICER) was calculated as the ratio of the difference between total costs under alternative TB prevention strategies to the TB cases averted. This ratio was expressed in terms of US dollars spent in health care costs per TB case averted. Strategies that prevented fewer TB cases and at greater cost compared to other strategies (i.e., strongly dominated), and strategies with higher ICER than the next most effective alternative (i.e., weakly dominated) were excluded from the incremental analysis.
Sensitivity analysis
For strategies under the two TB diagnostic algorithms, univariate sensitivity analyses were performed using TreeAge for Year 1 using calculations of net monetary benefit. For different willingness-to-pay (WTP) thresholds, we generated tornado diagrams for all parameters except for those that were common across all policy alternatives. The ranges for relative risk (RR) of TB from different interventions, and the sensitivity and specificity of TB screening and diagnostic tests came from the published literature. Cost inputs were varied by 50–200% of their base values. We considered two WTP thresholds: 1) US$1000 (approximate cost of diagnosis and treatment of active TB) and 2) US$35 000 (equivalent to highest ICER value generated when only TB prevention benefits of ART are taken into account). We further conducted threshold analysis for parameters with the greatest influence on outcomes to determine the value at which other alternatives become more effective. We also conducted Monte Carlo simulation probabilistic sensitivity analysis (PSA) and compared the two most comprehensive strategies: ‘ART IC ICF IPT 36′ and ‘ARTexp IC ICF IPT 36′ (Appendix Figure A.2).
RESULTS
TB diagnostic algorithm: sputum smear and chest radiography
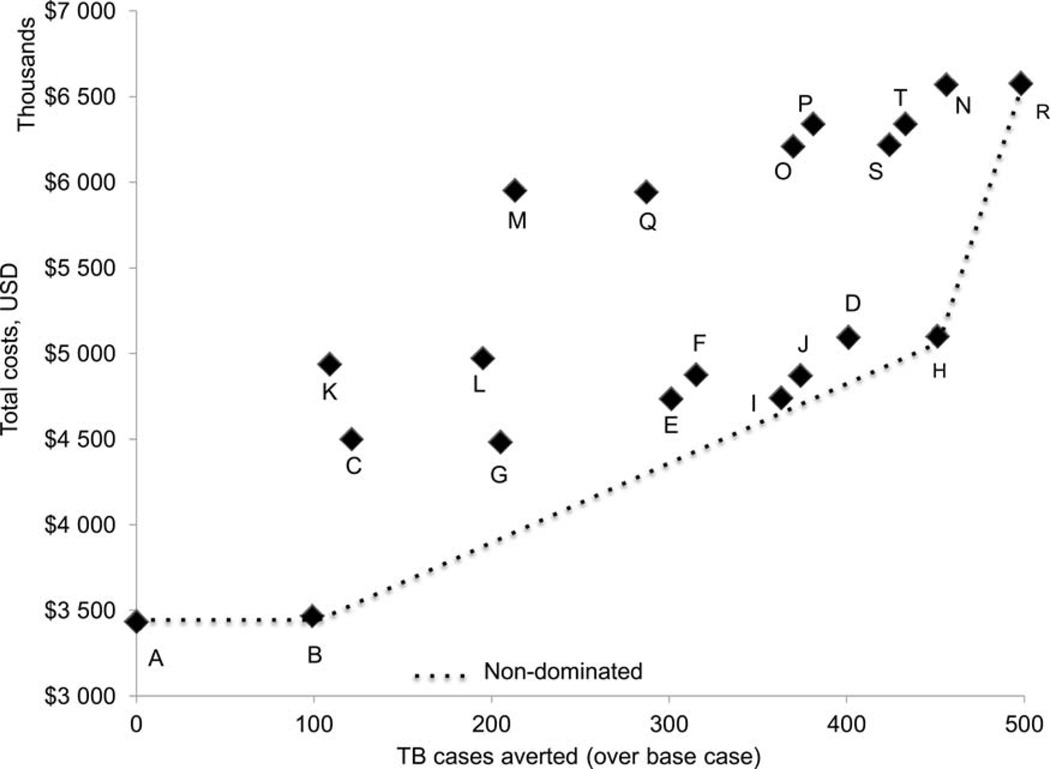
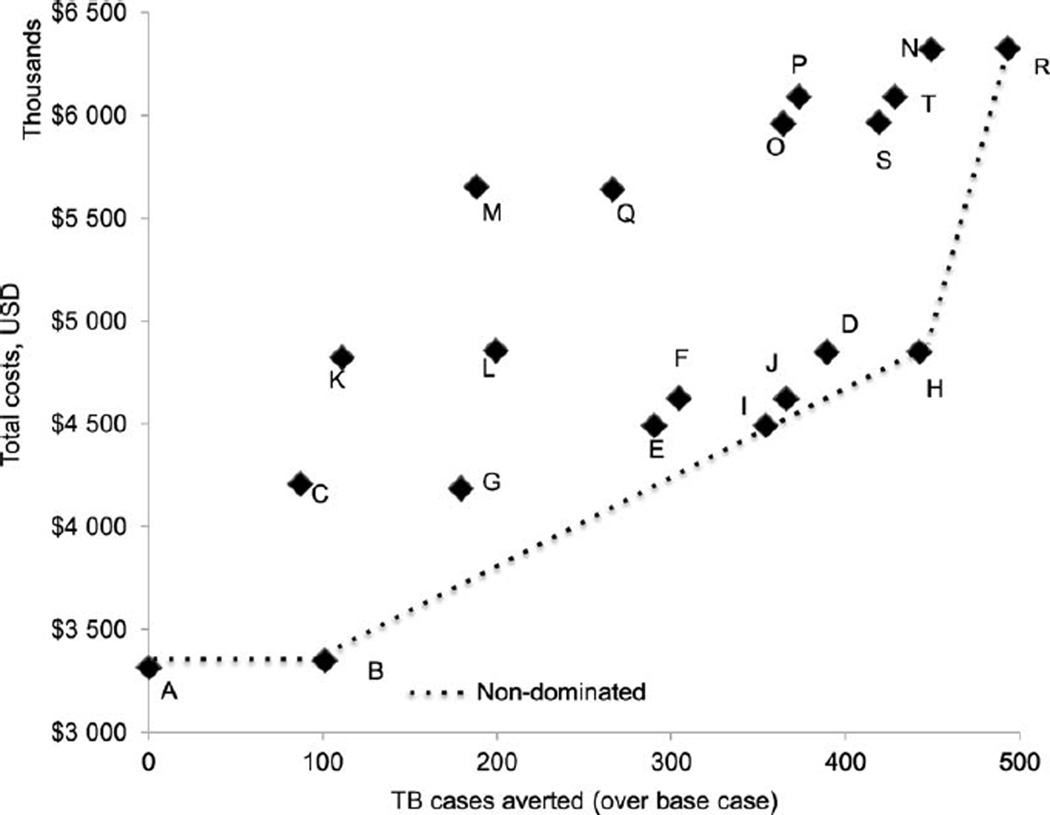
Considering sputum smear and CXR as the diagnostic tool for TB, the ‘base scenario’ cost US$3.4M and resulted in 1289 TB cases per 10 000 HIV-positive people. The most cost-effective strategies included 1) 55% ART coverage and IC (‘ART IC’), 2) 55% ART coverage, IC and 36-month IPT (‘ART IC ICF IPT 36’) and 3) expanded ART coverage with IC and 36-month IPT (‘ARTexp IC ICF IPT 36’) (Appendix Figure A.3). Adding IC averted 99 TB cases at an additional cost of US$34 000 (ICER = US$342). Compared to the ‘ART IC ICF IPT 36′ scenario, the ‘ARTexp IC ICF IPT 36′ scenario averted 47 TB cases and cost an additional US$1.5M. This resulted in an ICER of US$31 463 (Appendix Table A.1). The ICER would likely be far less if it took into account the other benefits of ART beyond TB prevention.
Strategies with ICF using four-symptom screening and IPT for 6 or 36 months were weakly dominated when compared with corresponding strategies with IC, which prevented additional TB cases at a small net cost. Increasing the duration of IPT from 6 to 36 months prevented a substantial number of TB cases, such that the strategies with 6-month IPT were weakly dominated. Although TB incidence was not significantly lower in TST-negatives receiving IPT, strategies with IPT for all people living with HIV dominated the strategies that provided IPT to TST-positive people living with HIV.
TB diagnostic algorithm: Xpert MTB/RIF
The results remained consistent with Xpert as the TB diagnostic tool. The base scenario cost US$3.3M, and resulted in 1308 TB cases per 10 000 HIV-positive people (Appendix Figure A.4). The ‘ART IC’ scenario averted 101 TB cases at an additional cost of US$33 900 (ICER = US$336). The ‘ARTexp IC ICF IPT 36’ scenario prevented 51 additional TB cases over the ‘ART IC ICF IPT 36’ scenario at an incremental cost of US$1.5M, resulting in an ICER of US$28 936 (Appendix Table A.1). This ICER would likely be markedly lower if we consider the benefits of ART beyond TB prevention.
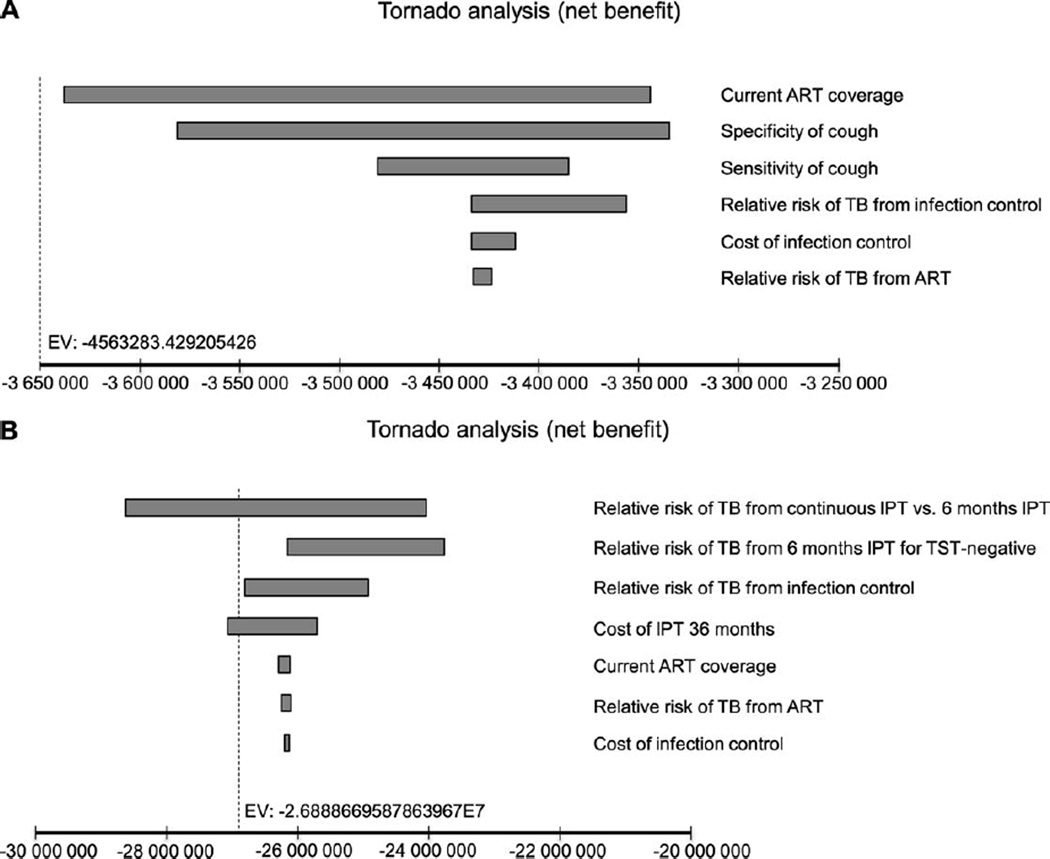
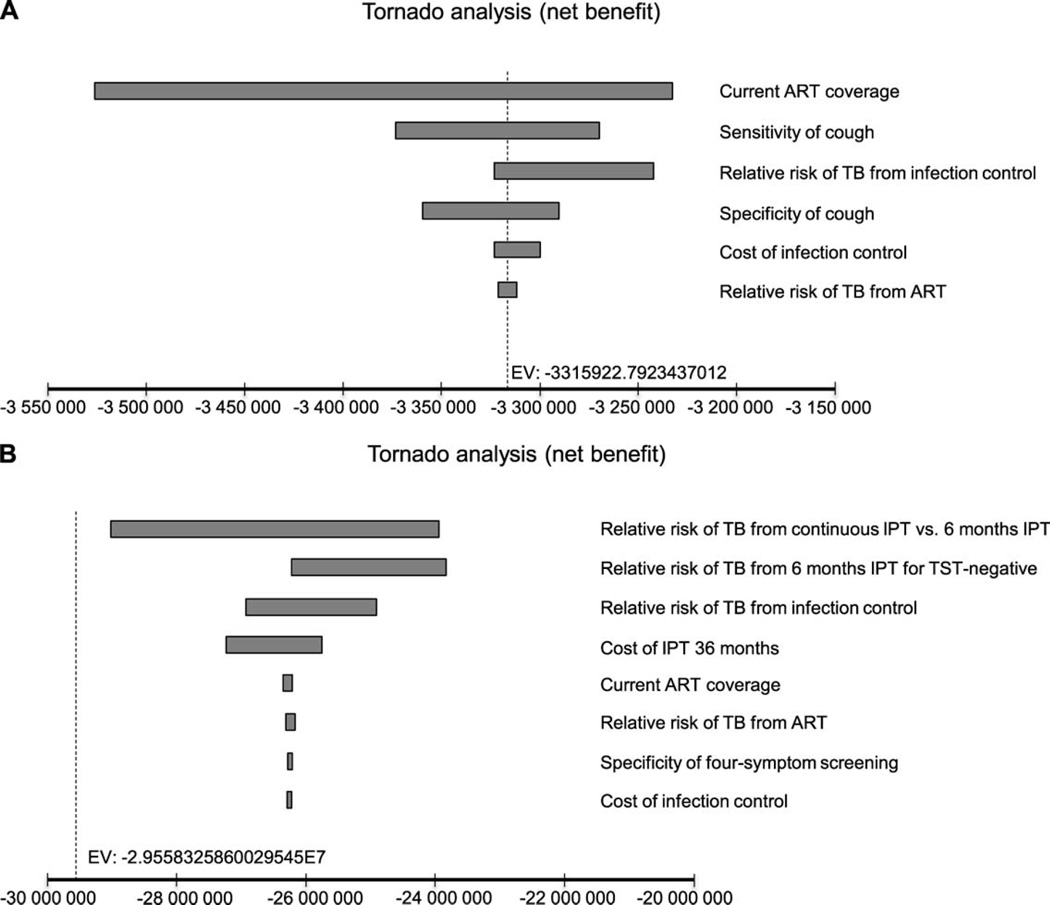
Sensitivity analysis
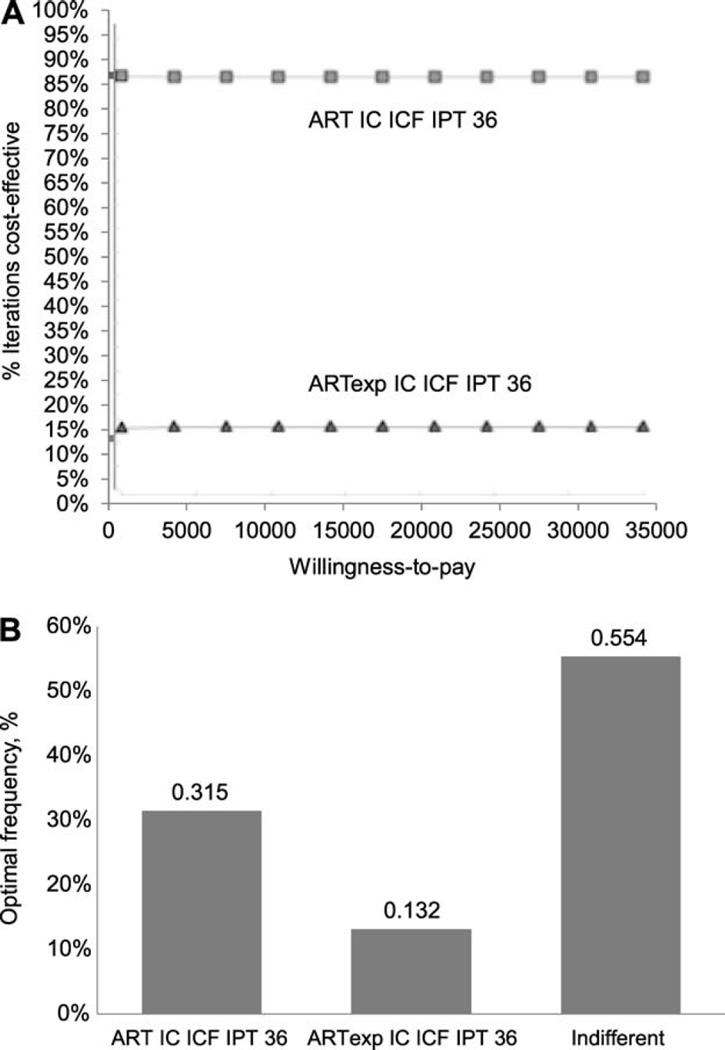
For both the diagnostic algorithms, six parameters accounted for variations in cost-effectiveness results at a WTP threshold of US$1000: ART coverage, sensitivity and specificity of TB screening with cough, the RR of TB from ART and from IC, and cost of IC. At the higher WTP threshold of US$35 000; the RR of TB from 1) continuous IPT vs. 6-month IPT, 2) 6-month IPT for TST-negative and 3) IC, and cost of 36-month IPT were responsible for maximum variation in the ICERs (Appendix Figures A.5 and A.6). Threshold analyses showed that for an RR of TB from IC of >0.985 (at WTP threshold of US$1000) and 0.994 (at WTP threshold of US$35 000), the alternatives without IC would be cost-effective compared to the corresponding scenarios with IC. At a high WTP for a TB case averted (US$35 000), PSA revealed that the ‘ARTexp IC ICF IPT 36′ strategy would be preferred to the ‘ART IC ICF IPT 36’ scenario 13% of the time (Appendix Figure A.2). However, the probability of choosing the expanded ART strategy would likely be much higher if we include benefits beyond preventing TB.
DISCUSSION
Effective anti-tuberculosis treatment remains a key strategy to reduce TB incidence, but this approach is unlikely to contain the rise in incidence resulting from HIV. In high HIV burden settings, the health systems need to be strengthened to provide the Three I’s for HIV/TB, including early ART for people living with HIV. We calculated the cost-effectiveness of these WHO-recommended TB prevention strategies using a decision-analytic model.
This is the first study to estimate the costs and benefits of the Three I’s for HIV/TB including ART together under different WHO-recommended TB diagnostic algorithms. Our findings suggest that in resource-constrained settings with a high burden of HIV and TB, a complete TB prevention package with 90% ART coverage at CD4 ≤350 cells/mm3, IC interventions and IPT for 36 months averted more TB cases than other scenarios with increased ART coverage, IC, 6-month IPT and/or IPT for TST-positives. This scenario, along with the ‘ART IC and ‘ART IC ICF IPT 36’ scenarios, was among the most cost-effective strategies.
The health care utilisation costs from South Africa may be higher than in other countries. Our results were therefore probably an overestimation of the true cost of TB diagnosis and treatment in many TB-HIV-endemic countries. While the portability of health care costs may restrict the interpretation of the costs and cost-effectiveness ratios to countries with higher costs, it does not change our conclusions about the effectiveness of the interventions evaluated in this study.
The figures for scenarios involving ART at 90% coverage need to be interpreted with caution, as we did not consider the impact of expanded access to ART on other HIV-related illnesses. Studies from similar settings have shown that increasing the provision of ART is highly cost-effective or cost saving for reducing the HIV- and TB-related burden (e.g., ICERs of US$530–590 per life-year saved in India and South Africa).31,32 Thus, the scenario with 90% ART coverage, IC and 36-month IPT will likely be more cost-effective when prevention benefits of ART beyond TB are considered. We also did not explore the potential impact of using the WHO 2013 guidelines or the initiation of ART regardless of CD4 count on TB incidence. We assumed the ART eligibility criteria to be CD4 count ≤350 cells/mm3, as recommended by the 2013 ART guidelines from South Africa. As ART prevents TB across all CD4 cell counts,4 it is likely that the scenarios with ART at CD4 count ≤500 cell/mm3 or regardless of CD4 count along with the Three I’s for HIV/TB will be cost-effective. In addition, we did not consider the effects of expanded ART coverage on the prevention of HIV transmission to sexual partners and children, which could have a considerable impact on the TB burden.33
The effect of IC measures on nosocomial transmission of TB and the costs of these interventions are not well documented, and the assumption that a TB prevention package with respirators, surgical masks and natural ventilation will cost US$3.4 per person is an estimate. We kept our model simple by assuming that TB prevention interventions are independent of one another. We did not model long-term TB or HIV transmission dynamics, and estimated the costs and benefits of one-time TB screening over a period of 3 years. We did not consider the potential impact of Xpert on TB incidence by reducing the time between detection and initiation of appropriate treatment for TB.
Our study highlights the importance of the Three I’s for HIV/TB, including expanded ART coverage to address TB, in communities with high HIV and TB prevalence. The availability of these interventions has significantly improved in sub-Saharan Africa. In 2012, according to the WHO 2010 guidelines, ART coverage was >80% in six countries.2 An estimated 2.3 million people were screened for TB, a two-fold increase from 860 000 in 2009. IPT was initiated among 470 000 people newly registered in HIV care in 2012, compared to <50 000 people in 2009.1
However, despite this remarkable progress, the vast majority of people who could benefit from these interventions do not have access. Likewise, IC for TB is often not a priority for many HIV and TB programmes. Expanding access to HIV treatment remains a priority, as coverage of the 28.6 million people eligible for ART (per the WHO 2013 guidelines) is less than 40%. Our study highlighted the potential cost-effectiveness of the Three Is for HIV/TB, including early ART, in preventing TB from a health sector perspective. However, it is likely to be even more cost-effective to prevent both TB and HIV with these interventions if we take the significant societal and individual costs of these diseases into consideration. In addition to the health benefits for the individual and the community, national TB and HIV/AIDS (acquired immune-deficiency syndrome) programmes may benefit from considering the economic implications of TB prevention as they develop an integrated approach to ensuring people living with HIV have access to high-quality HIV and TB prevention, care and treatment services.
Acknowledgments
This study was presented at the International AIDS Society (IAS) Conference, 3 July 2013, Kuala Lumpur, Malaysia, and the 44 Union World Conference on Lung Health, 2 November 2013, Paris, France.
The findings, opinions and statements in this article are those of the authors and do not represent the official policy, endorsement or views of the Joint United Nations Programme on HIV/AIDS (Geneva, Switzerland) or the US Centers for Disease Control and Prevention (Atlanta, GA, USA).
APPENDIX
Appendix Table A.1.
Costs, outcomes and ICERs (in USD spent in health care costs/TB case averted)
| A) TB diagnostic algorithm: sputum smear and chest radiography | |||||
|---|---|---|---|---|---|
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1289 | 3 434 704 | |||
| ART IC* | 1190 | 3 468 604 | 99 | 33 900 | 342 |
| ARTexp | 1180 | 4 941 979 | 10 | 1 473 375 | 147 338 |
| ART ICF IPT 6* | 1168 | 4 502 542 | 12 | −439437 | (36 620) |
| ARTexp IC | 1094 | 4 975 879 | 74 | 473 337 | 6 396 |
| ART IC ICF IPT 6* | 1084 | 4 485 802 | 10 | −490 077 | (49 008) |
| ARTexp ICF IPT 6 | 1076 | 5 954 018 | 8 | 1 468 216 | 183 527 |
| ARTexp IC ICF IPT 6 | 1002 | 5 943 379 | 74 | −10 639 | (144) |
| ART ICF TST IPT 36* | 988 | 4 739 030 | 14 | −1 204 349 | (86 025) |
| ART ICF IPT 6 TST IPT 36 | 974 | 4 874 713 | 14 | 135 683 | 9 692 |
| ART IC ICF TST IPT 36* | 926 | 4 743 516 | 48 | −131 197 | (2 733) |
| ARTexp ICF TST IPT 36 | 919 | 6 212 020 | 7 | 1 468 504 | 209 786 |
| ART IC ICF IPT 6 TST IPT 36* | 915 | 4 872 908 | 4 | −1339 112 | (334 778) |
| ARTexp ICF IPT 6 TST IPT 36 | 908 | 6 341 615 | 7 | 1 468 707 | 209815 |
| ART ICF IPT 36* | 888 | 5 097 662 | 20 | −1 243 953 | (62 198) |
| ARTexp IC ICF TST IPT 36 | 865 | 6 220 368 | 23 | 1 122 706 | 48813 |
| ARTexp IC ICF IPT 6 TST IPT 36 | 856 | 6 344 626 | 9 | 124 258 | 13 806 |
| ART IC ICF IPT 36* | 838 | 5 101 438 | 18 | −1243 188 | (69 066) |
| ARTexp ICF IPT 36 | 833 | 6 571 933 | m | 1 470 495 | 294099 |
| ARTexp IC ICF IPT 36* | 791 | 6 580182 | 42 | 8 249 | 196 |
| Ai) After removing the dominated strategies from incremental analysis: | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1289 | 3 434 704 | |||
| ART IC* | 1190 | 3 468 604 | 99 | 33 900 | 342 |
| ART ICF IPT 6 | 1168 | 4 502 542 | 22 | 1 033 938 | 46 997 |
| ART IC ICF IPT 6* | 1084 | 4485 802 | 84 | −16 740 | (199) |
| ART ICF TST IPT 36 | 988 | 4 739 030 | 96 | 253 228 | 2 638 |
| ART IC ICF TST IPT 36* | 926 | 4 743 516 | 62 | 4 486 | 72 |
| ART IC ICF IPT 6 TST IPT 36 | 915 | 4 872 908 | 11 | 129 392 | 11 763 |
| ART ICF IPT 36 | 888 | 5 097 662 | 27 | 224754 | 8 324 |
| ART IC ICF IPT 36* | 838 | 5 101 438 | 50 | 3 776 | 76 |
| ARTexp IC ICF IPT 36* | 791 | 6 580182 | 47 | 1 478 744 | 31 463 |
| Aii) | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1289 | 3 434 704 | |||
| ART IC* | 1190 | 3 468 604 | 99 | 33 900 | 342 |
| ART IC ICF IPT 6 | 1084 | 4485 802 | 106 | 1 017198 | 9 596 |
| ART IC ICF TST IPT 36* | 926 | 4 743 516 | 158 | 257714 | 1 631 |
| ART IC ICF IPT 36* | 838 | 5 101 438 | 88 | 357 922 | 4 067 |
| ARTexp IC ICF IPT 36* | 791 | 6580 182 | 47 | 1 478 744 | 31 463 |
| Aiii) | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1289 | 3 434 704 | |||
| ART IC* | 1190 | 3 468 604 | 99 | 33 900 | 342 |
| ART IC ICF TST IPT 36 | 926 | 4 743 516 | 264 | 1 274912 | 4 829 |
| ART IC ICF IPT 36* | 838 | 5 101 438 | 88 | 357 922 | 4 067 |
| ARTexp IC ICF IPT 36* | 791 | 6 580182 | 47 | 1 478 744 | 31463 |
| B) Incremental cost-effectiveness ratios for the cost-effective strategies | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1289 | 3 434 704 | |||
| ART IC* | 1190 | 3 468 604 | 99 | 33 900 | 342 |
| ART IC ICF IPT 36* | 838 | 5 101 438 | 352 | 1 632 834 | 4639 |
| ARTexp IC ICF IPT 36* | 791 | 6 580182 | 47 | 1 478 744 | 31463 |
| C) TB diagnostic algorithm: Xpert® MTB/RIF | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1308 | 3 315 687 | |||
| ART ICF IPT 6 | 1221 | 4 207 407 | 87 | 891 720 | 10250 |
| ART IC* | 1207 | 3 349 587 | 14 | −857 820 | (61 273) |
| ARTexp | 1197 | 4 822 962 | 10 | 1 473 375 | 147 338 |
| ART IC ICF IPT 6* | 1129 | 4185 320 | 68 | −637 642 | (9 377) |
| ARTexp ICF IPT 6 | 1120 | 5 652 851 | 9 | 1 467 531 | 163 059 |
| ARTexp IC* | 1109 | 4 856 862 | 11 | −795 989 | (72 363) |
| ARTexp IC ICF IPT 6 | 1042 | 5 639 057 | 67 | 782 195 | 11 675 |
| ART ICF TST IPT 36* | 1018 | 4490 753 | 24 | −1 148 304 | (47 846) |
| ART ICF IPT 6 TST IPT 36 | 1004 | 4 626 362 | 14 | 135 609 | 9 686 |
| ART IC ICF TST IPT 36* | 954 | 4493 283 | 50 | −133 079 | (2 662) |
| ARTexp ICF TST IPT 36 | 944 | 5 960 442 | 10 | 1 467 1 59 | 146716 |
| ART IC ICF IPT 6 TST IPT 36* | 942 | 4 622 591 | 2 | —1 337 851 | (668 926) |
| ARTexp ICF IPT 6 TST IPT 36 | 935 | 6 091 225 | 7 | 1 468 634 | 209 805 |
| ART ICF IPT 36* | 919 | 4 849 293 | 16 | —1 241 932 | (77621) |
| ARTexp IC ICF TST IPT 36 | 889 | 5 967 624 | 30 | 1 118331 | 37278 |
| ARTexp IC ICF IPT 6 TST IPT 36 | 880 | 6 091 822 | 9 | 124198 | 13 800 |
| ART IC ICF IPT 36* | 866 | 4851 163 | 14 | —1 240 659 | (88 619) |
| ARTexp ICF IPT 36 | 859 | 6319731 | 7 | 1 468 568 | 209795 |
| ARTexp IC ICF IPT 36* | 815 | 6 326 899 | 44 | 7 168 | 163 |
| Ci) After removing the dominated strategies from incremental analysis: | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1308 | 3 315 687 | |||
| ART IC* | 1207 | 3 349 587 | 101 | 33 900 | 336 |
| ART IC ICF IPT 6* | 1129 | 4185 320 | 78 | 835 733 | 10715 |
| ARTexp IC | 1109 | 4 856 862 | 20 | 671 542 | 33 577 |
| ART ICF TST IPT 36* | 1018 | 4490 753 | 91 | −366109 | (4 023) |
| ART IC ICF TST IPT 36* | 954 | 4493 283 | 64 | 2 530 | 40 |
| ART IC ICF IPT 6 TST IPT 36 | 942 | 4 622 591 | 12 | 129308 | 10 776 |
| ART ICF IPT 36 | 919 | 4 849 293 | 23 | 226702 | 9 857 |
| ART IC ICF IPT 36* | 866 | 4851 163 | 53 | 1 870 | 35 |
| ARTexp IC ICF IPT 36* | 815 | 6 326 899 | 51 | 1 475 736 | 28 936 |
| Cii) TB cases Total cost Incremental cases averted Incremental costs | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1308 | 3 315 687 | |||
| ART IC* | 1207 | 3 349 587 | 101 | 33 900 | 336 |
| ART IC ICF IPT 6 | 1129 | 4185 320 | 78 | 835 733 | 10715 |
| ART ICF TST IPT 36 | 1018 | 4490 753 | 111 | 305 433 | 2 752 |
| ART IC ICF TST IPT 36* | 954 | 4493 283 | 64 | 2 530 | 40 |
| ART IC ICF IPT 36* | 866 | 4851 163 | 88 | 357 880 | 4 067 |
| ARTexp IC ICF IPT 36* | 815 | 6 326 899 | 51 | 1 475 736 | 28936 |
| Ciii) | |||||
| Strategy | TB cases n |
Total cost USD |
Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1308 | 3 315 687 | |||
| ART IC* | 1207 | 3 349 587 | 101 | 33 900 | 336 |
| ART IC ICF TST IPT 36 | 954 | 4493 283 | 253 | 1 143 696 | 4 521 |
| ART IC ICF IPT 36* | 866 | 4851 163 | 88 | 357 880 | 4 067 |
| ARTexp IC ICF IPT 36* | 815 | 6 326 899 | 51 | 1 475 736 | 28936 |
| D) Incremental cost-effectiveness ratios for the cost-effective strategies | |||||
| Strategy | TB cases n | Total cost USD | Incremental cases averted n |
Incremental costs USD |
ICER/case averted |
| Base* | 1308 | 3 315 687 | |||
| ART IC* | 1207 | 3 349 587 | 101 | 33 900 | 336 |
| ART IC ICF IPT 36* | 866 | 4851 163 | 341 | 1 501 576 | 4 403 |
| ARTexp IC ICF IPT 36* | 815 | 6 326 899 | 51 | 1 475 736 | 28936 |
Non-dominated strategies (defined in the main paper)
ICER = incremental cost-effectiveness ratio; USD = US dollars; TB = tuberculosis; ART =antiretroviral therapy; IC = infection control; ARTexp = expanded ART (90% coverage); IPT = isoniazid preventive therapy; ICF = intensified TB case finding; TST = tuberculin skin test.
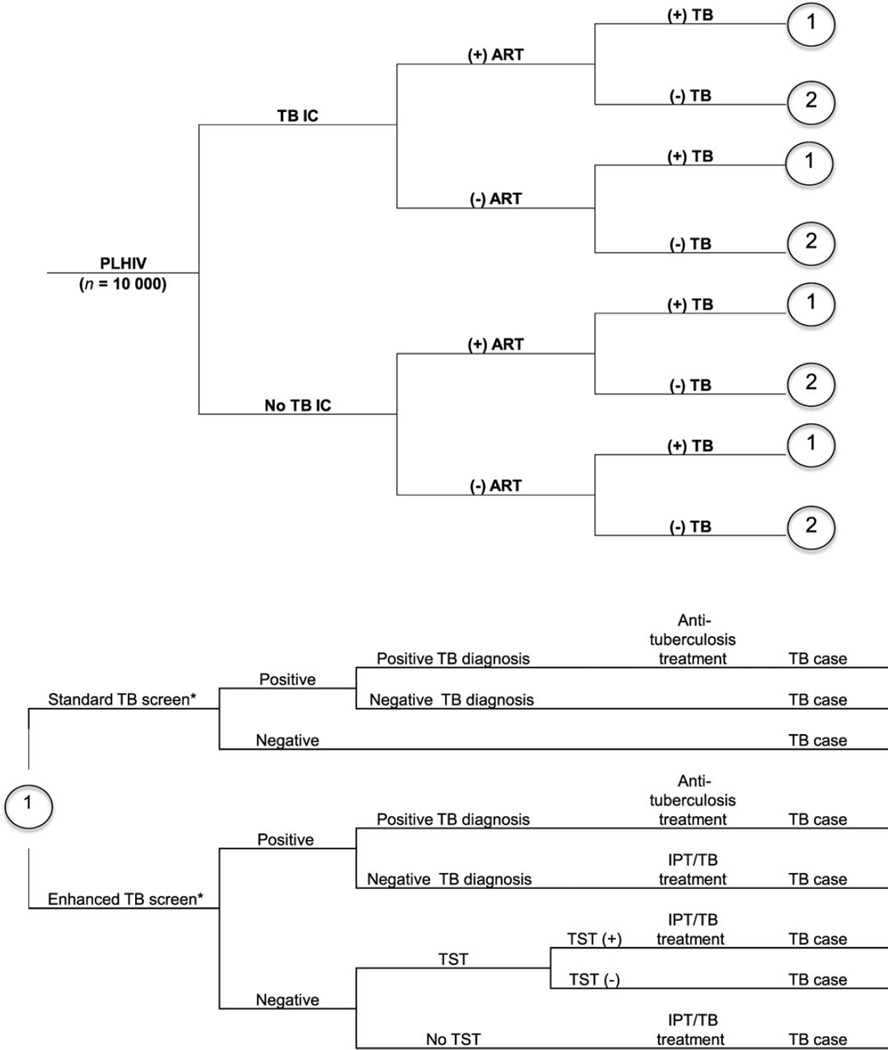
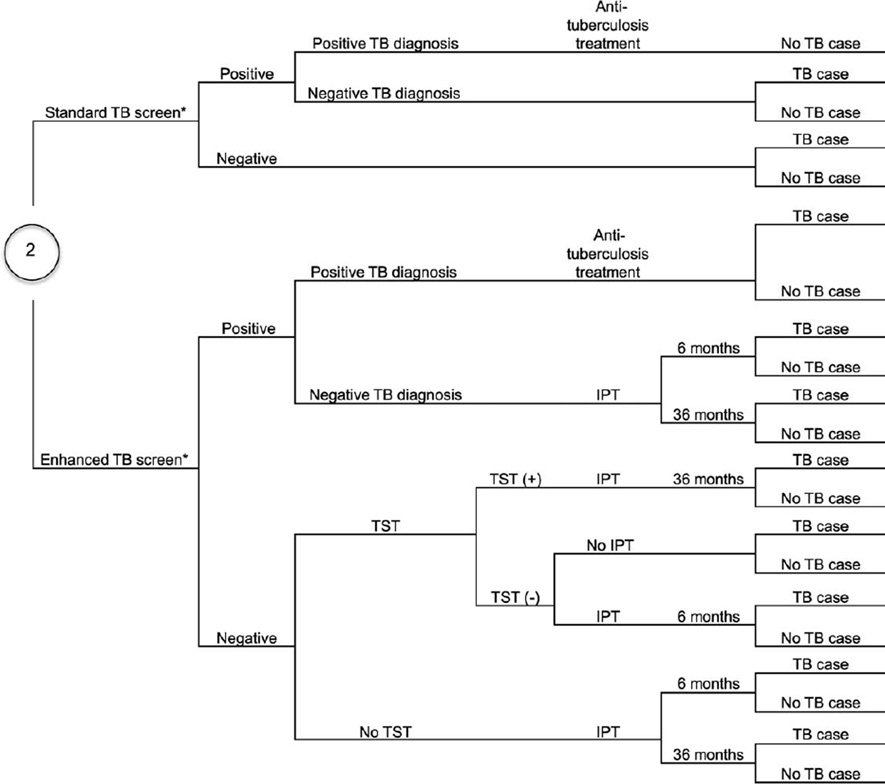
Figure A.1.
Decision tree. TB was diagnosed using sputum smear and chest radiography or Xpert® MTB/RIF. * Standard TB screening is using cough; enhanced TB screening refers to the WHO-recommended four-symptom screening algorithm. PLHIV = people living with HIV; TB = tuberculosis; IC = infection control;+= positive;-= negative; ART=antiretroviral therapy; TST = tuberculin skin test; IPT = isoniazid preventive therapy; WHO = World Health Organization.
Figure A.2.
Probabilistic sensitivity analysis. A) Cost-effectiveness acceptability curve; B) strategy selection (willingness-to-pay/TB case averted = USD35 000). We ran 10 000 iterations of the model for Year 1 using Monte Carlo simulation probabilistic sensitivity analysis and compared the two most comprehensive strategies that were also among the cost-effective strategies (ART IC ICF IPT 36 and ARTexp IC ICF IPT 36, Xpert TB diagnostic algorithm). We set distribution for only those variables found to be influential in the oneway sensitivity analysis under the two willingness-to-pay thresholds of USD1000 and 35 000 (ART coverage, relative risk of TB from ART and from IC, cost of IC interventions and cost of IPT). The result of the probabilistic sensitivity analysis using the willingness-to-pay threshold of USD35 000 indicated that we will choose the ART IC ICF IPT 36 strategy 32% of the time and we will be indifferent between both the strategies 55% of the time. We will choose the ARTexp IC ICF IPT 36 strategy 13% of the time. Note that strategy selection is based on costs and outcomes of the scenarios in Year 1. The probability of choosing ARTexp IC ICF IPT 36 strategy is likely to be higher over a 3-year period. Furthermore, the probability of choosing the expanded ART strategy would likely be much higher if we include benefits of ART beyond the prevention of TB. ART=antiretroviral therapy; IC = infection control; ICF = intensified case finding; PT = isoniazid preventive therapy; ARTexp = expanded ART (90% coverage); USD = US dollars.
Figure A.3.
Expected costs and TB cases (TB diagnostic algorithm: sputum smear and chest radiography). Note: The description of the strategies (A to T) is given in Table 1. Base (A), ART IC (B), ART IC ICF IPT 36 (H) and ARTexp IC ICF IPT 36 (R) scenarios were most cost-effective. The total TB cases and total costs under each strategy are available in the Online Appendix. USD = US dollars; TB = tuberculosis; ART =antiretroviral therapy; IC = infection control; ICF = intensified case finding; IPT = isoniazid preventive therapy; ARTexp = expanded ART (90% coverage).
Figure A.4.
Expected costs and TB cases (TB diagnostic algorithm: Xpert® MTB/RIF assay). Note: The description of the strategies (A to T) is given in Table 1. Base (A), ART IC (B), ART IC ICF IPT 36 (H) and ARTexp IC ICF IPT 36 (R) scenarios were most cost-effective. The total TB cases and total costs under each strategy are available in the Online Appendix. USD = US dollars; TB = tuberculosis; ART=antiretroviral therapy; IC = infection control; ICF = intensified case finding; IPT= isoniazid preventive therapy; ARTexp = expanded ART (90% coverage).
Figure A.5.
Tornado diagram of univariate analyses for strategies with sputum smear and chest radiography for TB diagnosis. A) Willingness-to-pay threshold of USD1000. Note: the diagram shows the degree to which uncertainty in current ART coverage, specificity and sensitivity of TB screening with cough, the RR of TB from IC and ART and cost of IC interventions accounted for 100% variation in cost-effectiveness results. Variations in other parameters (cost of TST, IPT and anti-tuberculosis treatment, sensitivity and specificity of four-symptom screening, the RR of TB from 6-month or 36-month IPT, proportion of people testing TST-positive of those with active TB and without active TB, and expanded ART coverage) had no effect on the results. B) Willingness-to-pay threshold of USD35 000. Note: this diagram shows the degree to which uncertainty in RR of TB from continuous IPT vs. 6-month IPT, 6-month IPT for TST-negatives and IC and cost of 36-month IPT accounted for nearly 100% variation in cost-effectiveness results. Variations in other parameters (cost of TST and anti-tuberculosis treatment, sensitivity and specificity of TB screening using cough or four-symptom screening, proportion of people testing TST-positive of those with active TB and without active TB, and expanded ART coverage) had no effect on the results. ART = antiretroviral therapy; RR = relative risk; TB = tuberculosis; IC = infection control; IPT = isoniazid preventive therapy; TST = tuberculin skin test; USD = US dollars.
Figure A.6.
Tornado diagram of univariate analyses for strategies with Xpert® MTB/RIF for TB diagnosis. A) Willingness-to-pay threshold of USD1000. Note: the diagram shows the degree to which uncertainty in current ART coverage, specificity and sensitivity of TB screening with cough, RR of TB from IC and ART, and cost of IC interventions accounted for 100% variation in cost-effectiveness results. Variations in other parameters (cost of TST, IPT and anti-tuberculosis treatment; sensitivity and specificity of four-symptom screening; RR of TB from 6-month or 36-month IPT; proportion of people testing TST-positive of those with active TB and without active TB; and expanded ART coverage) had no effect on the results. B) Willingness-to-pay threshold of USD35 000. Note: this diagram shows the degree to which uncertainty in RR of TB from continuous IPT vs. 6-month IPT, 6-month IPT for TST-negative, and IC and cost of 36-months IPT accounted for nearly 100% variation in cost-effectiveness results. Variations in other parameters (cost of TST and anti-tuberculosis treatment; sensitivity and specificity of TB screening using cough; sensitivity of four-symptom screening; proportion of people testing TST-positive of those with active TB and without active TB; and expanded ART coverage) had no effect on the results. ART =antiretroviral therapy; RR = relative risk; TB = tuberculosis; IC = infection control; IPT = isoniazid preventive therapy; TST = tuberculin skin test; USD = US dollars.
Footnotes
The Appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2014/00000018/00000010/art00006.
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2013. WHG7HTM/TB/2013.11. Geneva, Switzerland: WHO; 2013. [Accessed June 2014]. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic 2013. UNAIDS/JC2502/1/E. Geneva, Switzerland: UNAIDS; 2013. 2013. [Accessed June 2014]. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 3.World Health Organization. Policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. WHO/HTM/TB/2012.1. WHO/HIV/2012.1. Geneva, Switzerland: WHO; 2012. [Accessed June 2014]. http://whqlibdoc.who.int/publications/2012/9789241503006_eng.pdf. [PubMed] [Google Scholar]
- 4.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLOS MED. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middelkoop K, Bekker LG, Myer L, et al. Antiretroviral therapy and TB notification rates in a high HIV prevalence South African community. J Acquir Immune Defic Syndr. 2011;56:263–269. doi: 10.1097/QAI.0b013e31820413b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLOS ONE. 2007;2:e826. doi: 10.1371/journal.pone.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Geneva, Switzerland: WHO; 2010. [Accessed June 2014]. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [Google Scholar]
- 8.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. [Accessed June 2014]. http://apps.who.int/iris/bitstream/10665/85321/l/9789241505727_eng.pdf. [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines on intensified tuberculosis case finding and isoniazid preventive therapy for people living with HIV in resource constrained setting. Geneva, Switzerland: WHO; 2011. [Accessed June 2014]. http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. [Google Scholar]
- 10.World Health Organization. WHO/HTM/TB/2009.419. Geneva, Switzerland: WHO; 2009. [Accessed June 2014]. WHO policy on TB infection control in health-care facilities, congregate settings and households. http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf. [PubMed] [Google Scholar]
- 11.UNAIDS Report on the Global AIDS Epidemic 2012. Geneva, Switzerland: UNAIDS; 2012. [Accessed June 2014]. Joint United Nations Programme on HIV/AIDS. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf. [Google Scholar]
- 12.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. Preventing tuberculosis among health workers in Malawi. Bull World Health Organ. 2002;80:526–531. [PMC free article] [PubMed] [Google Scholar]
- 13.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha RK, Mugisha B, Bunnell R, et al. Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis. 2007;11:747–754. [PubMed] [Google Scholar]
- 15.Lawn SD, Harries AD, Williams BG, et al. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis. 2011;15:571–581. doi: 10.5588/ijtld.10.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV-infected persons. Cochrane Database Syst Rev. 2010;(1):CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monkongdee P, McCarthy KD, Cain KP, et al. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am J Respir Crit Care Med. 2009;180:903–908. doi: 10.1164/rccm.200905-0692OC. [DOI] [PubMed] [Google Scholar]
- 20.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLOS MED. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangaka MX, Diwakar L, Seldon R, et al. Clinical, immunological, and epidemiological importance of anti-tuberculosis T-cell responses in HIV-infected Africans. Clin Infect Dis. 2007;44:1639–1646. doi: 10.1086/518234. [DOI] [PubMed] [Google Scholar]
- 24.Kerkhoff AD, Kranzer K, Samandari T, et al. Systematic review of TST responses in people living with HIV in under-resourced settings: implications for isoniazid preventive therapy. PLOS ONE. 2012;7:e49928. doi: 10.1371/journal.pone.0049928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high-burden countries: a cost-effectiveness analysis. PLOS MED. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairall L, Bachmann MO, Zwarenstein M, et al. Cost-effectiveness of educational outreach to primary care nurses to increase tuberculosis case detection and improve respiratory care: economic evaluation alongside a randomised trial. Trop Med Int Health. 2010;15:277–286. doi: 10.1111/j.1365-3156.2009.02455.x. [DOI] [PubMed] [Google Scholar]
- 27.Dowdy DW, O’Brien MA, Bishai D. Cost-effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12:1021–1029. [PubMed] [Google Scholar]
- 28.World Health Organization. Country-specific unit costs 2008. Geneva, Switzerland: WHO; 2008. [Accessed June 2014]. CHOosing Interventions that are Cost Effective (WHO-CHOICE). Costs and prices. http://www.who.int/choice/country/country_specific/en/index.html. [Google Scholar]
- 29.Arnold DT, Bentham LM, Jacob RP, Lilford RJ, Girling AJ. Should patients with abnormal liver function tests in primary care be tested for chronic viral hepatitis: cost minimisation analysis based on a comprehensively tested cohort. BMC Fam Pract. 2011;12:9. doi: 10.1186/1471-2296-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granich R, Kahn JG, Bennett R, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011–2050. PLOS ONE. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings—the case of Cote d’lvoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 32.Walensky RP, Ross EL, Kumarasamy N, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369:1715–1725. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci USA. 2010;107:19485–19489. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]