Abstract
Trophoblast stem (TS) cells possess the capacity to differentiate along a multi-lineage pathway yielding several specialized cell types. The regulatory network controlling trophoblast cell differentiation is poorly understood. cAMP-response element binding protein/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain-2 (CITED2) has been implicated in the regulation of placentation; however, we know little about how CITED2 acts to influence trophoblast cells. Rat Rcho-1 trophoblast stem (TS) cells can be manipulated to proliferate or differentiate into specialized trophoblast lineages and are an excellent model for investigating trophoblast differentiation. CITED2 transcript and protein showed a robust induction during Rcho-1 TS cell differentiation. We used a short hairpin RNA knockdown approach to disrupt CITED2 expression in order to investigate its involvement in trophoblast cell differentiation. RNA-sequencing was used to examine the impact of CITED2 on trophoblast cell differentiation. CITED2 disruption affected the differentiating trophoblast cell transcriptome. CITED2 possessed a prominent role in the regulation of cell differentiation with links to several signal transduction pathways and to hypoxia-regulated and coagulation processes. In summary, our findings indicate that CITED2 contributes to the regulation of trophoblast cell differentiation.
INTRODUCTION
The placenta has a diverse set of responsibilities ensuring the survival, growth, and development of the embryo/fetus within the mother’s reproductive tract (Amoroso 1968; Cross et al. 1994; Geogiades et al. 2002). In most primates and rodents these tasks are accomplished through the formation of a hemochorial placenta where maternal blood directly bathes specialized extraembryonic cells termed trophoblast. The hemochorial placenta, through the activities of trophoblast cells, modifies maternal physiology via intrauterine migration and vascular remodeling and the production of hormones/cytokines (Cross et al. 1994; Pijnenborg et al. 2006; Soares et al. 2014). The hemochorial placenta also regulates the bidirectional transport of nutrients and wastes between the mother and fetus (Sibley et al. 1997; Knipp et al. 1999; Watson and Cross 2005; Dilworth and Sibley 2013). Execution of these functions requires coordinated temporal and spatial differentiation of stem and progenitor cells into specialized trophoblast cell types and their organization into a hemochorial placenta (Roberts and Fisher 2011). The rat has proven to be a superb model for investigating hemochorial placentation (Soares et al. 2012).
The mature rat hemochorial placenta consists of two major compartments, the junctional zone and the labyrinth zone (Soares et al. 1996, 2012). The junctional zone lies at the maternal-placental interface and is composed of progenitor cells, which differentiate into four distinct specialized trophoblast cell types. Trophoblast giant cells are large polyploid cells arising by endorepduplication. They possess a remarkable capacity for steroid and peptide hormone biogenesis (Soares et al. 1996; Soares 2004). Spongiotrophoblast cells are a major constituent of the mature junctional zone and produce peptide hormones. Glycogen trophoblast cells accumulate glycogen and are viewed as an energy reservoir for the developing placenta and fetus. Junctional zone progenitors also give rise to invasive trophoblast cells, which exit the placenta and infiltrate the uterine compartment where they target and facilitate uterine spiral artery remodeling and the delivery of nutrients to the placenta (Ain et al. 2003; Soares et al. 2014). In contrast, the labyrinth zone is situated at the placental-fetal interface and consists of progenitor cells, which can differentiate into trophoblast giant cells or fuse to form syncytial trophoblast (Soares et al. 2012). The latter cell layers form the barrier between maternal and fetal compartments and directly connect with fetal vasculature and promote bidirectional transport (Knipp et al. 1999). Trophoblast cell differentiation can be effectively modeled in Rcho-1 trophoblast stem (TS) cells (Faria et al. 1991; Sahgal et al. 2006). Rcho-1 TS cells were derived from a rat choriocarcinoma (Teshima et al. 1983) and can be maintained in the stem/progenitor state or the culture conditions can be modified resulting in their differentiation into specialized trophoblast lineages, including but not limited to trophoblast giant cells (Faria et al. 1991; Sahgal et al. 2006). Transcriptome profiles of Rcho-1 TS cells in the stem and differentiated states, reflect the known behavior of trophoblast cell lineages developing in the rat hemochorial placenta (Kent et al. 2010).
Several signaling pathways have been implicated in the regulation of trophoblast development (Soares et al. 2014). Among these pathways, hypoxia inducible factor (HIF) and fos-like antigen 1(FOSL1)/jun B proto-oncogene (JUNB) transcriptional regulators have been shown to influence the regulation of trophoblast cell differentiation in the mouse, rat, and human (Schorpp-Kistner et al. 1999; Adelman et al. 2000; Caniggia et al. 2000; Schreiber et al. 2000; Chakraborty et al. 2011; Kent et al. 2011; Renaud et al. 2014; Kubota et al. 2015). Both transcription factor complexes recruit histone acetyl transferases, CREB binding protein (CREBBP) and E1A binding protein p300 (EP300), in activating their gene targets (Vo and Goodman 2001; Bedford et al. 2010; Semenza 2010).
CREBBP/EP300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain, 2 (CITED2) is a transcriptional co-regulator that regulates interactions between DNA-binding proteins and histone modifying enzymes, including transcription factor-CREBBP/EP300 interactions (Braganca et al. 2003; Freedman et al. 2003). CITED2 is widely expressed in embryonic and extraembryonic tissues, is required for normal development of the embryo and placenta (Bamforth et al. 2001; Barbera et al., 2002; Yin et al. 2002; Weninger et al., 2005; Withington et al., 2006; Moreau et al., 2014), and is also expressed in the uterus where it is essential for embryo implantation (Yoo et al. 2015). CITED2 has been implicated in the regulation of two transcription factor families: activator protein 2 (TFAP2) and hypoxia inducible factor (HIF) (Bhattacharya et al. 1999; Bamforth et al. 2001; Yin et al. 2002; Braganca et al. 2003; Freedman et al. 2003). Members of these transcription factor families are significant contributors to the regulation of trophoblast cell differentiation (Simon and Keith 2008; Dunwoodie 2009; Chakraborty et al. 2011, 2012; Kuckenberg et al. 2012); however, specific roles for CITED2 in regulating trophoblast cell differentiation have not been determined.
CITED2 is dynamically regulated during rat trophoblast cell development (Kent et al. 2010) and based on mouse mutagenesis experimentation contributes to development of both junctional and labyrinthine trophoblast lineages (Withington et al. 2006; Moreau et al. 2014). In this study, we explore roles for CITED2 in the regulation of trophoblast cell differentiation. We utilize an in vitro loss-of-function approach and RNA-sequencing (RNA-seq) to identify targets downstream of CITED2 action.
MATERIALS AND METHODS
Rcho-1 trophoblast stem (TS) cell culture
Rcho-1 TS cells are an effective model system for interrogating regulatory pathways controlling rat trophoblast cell differentiation (Faria and Soares 1991; Kent et al. 2010, 2011; Kubota et al. 2015) and were used to investigate a role for CITED2 in the regulation of trophoblast cell differentiation. Rcho-1 TS cells were maintained in Stem State Medium [RPMI-1640 culture medium (Gibco-Life Technologies, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS: Atlanta Biologicals, Norcross, GA), 50 µM 2-mercaptoethanol (Sigma-Aldrich, St Lois, MO), 1 mM sodium pyruvate, 100 µM penicillin, and 100 U/ml streptomycin], as previously reported (Faria and Soares 1991; Sahgal et al. 2006). Rcho-1 TS cells were grown to near confluence and differentiation was induced by replacing the Stem State Medium with Differentiation State Medium [NCTC-135 medium (Sigma-Aldrich) supplemented with 1% horse serum (HS; Atlanta Biologicals), 50 µM 2-mercaptoethanol, 1 mM sodium pyruvate, 10 mM HEPES, 4-(2-hydroxyethy)-1-piperazineethanesulfonic acid, 38 mM sodium bicarbonate, 100 µM penicillin and 100 U/ml streptomycin.
CITED2 knockdown
A short hairpin (shRNA) knockdown approach was used to disrupt CITED2 expression in Rcho-1 TS cells and to investigate the role of CITED2 in trophoblast cell differentiation. Three different Cited shRNAs (shCited2-1: 5’gaagctcaacaaccagtatttc3’; shCited2-2: 5’catcgacgaggaagtgcttatctc3’; shCited2-3: 5’agaagctcaacaaccagtatt3’; Sigma-Aldrich) and a control shRNA (shCtrl, plasmid No. 1864; Addgene, Cambridge, MA) were packaged into lentiviral vectors and used to produce lentiviral particles in 293FT cells (Kent et al. 2011; Asanoma et al. 2012). Culture supernatants containing lentiviral particles were harvested, centrifuged to remove cell debris, filter sterilized, concentrated by ultracentrifugation, and stored at −80°C until used. Rcho-1 TS cells maintained in Stem State Medium were exposed to lentiviral particles, selected with puromycin dihydrochloride (Sigma-Aldrich, 2 µg/µl) for two days, and then maintained in a lower concentration of the antibiotic (1 µg/µl). Puromycin selection was removed during in vitro differentiation. Knockdown efficiencies were monitored using quantitative RT-PCR (qRT-PCR) and western blotting.
qRT-PCR
Transcript levels were measured by qRT-PCR. RNA was extracted using TRI reagent (Sigma-Aldrich) according to the manufacturer's instructions. cDNAs were reversed transcribed by using a High-Capacity cDNA Reverse Transcription kit from Applied Biosystems (Foster City, CA) as instructed by the manufacturer. Power SYBR Green PCR Master Mix (Applied Biosystems) was used in the PCR. Primers utilized in the analyses are provided in Table 1. Amplification and fluorescence detection were carried out using an ABI Prism 7500 real-time PCR system (Applied Biosystems). The thermal profile for real-time PCR consisted of an initial hold step (95 °C for 10 min) and 40 cycles at 95 °C for 15 sec and annealing at 60 °C for 1 min. Amplification efficiencies of each target and the reference gene, 18S rRNA, were examined through their calibration curves and found to be comparable. Average threshold (Ct) values for each target were determined by Sequence Detection System software v1.2 (Applied Biosystems). Each run was completed with a melting curve analysis to confirm the specificity of amplification and the absence of primer dimer.
Table 1.
Primers for qRT-PCR analysis
| Target gene |
Forward primer | Reverse primer | Accession number |
|---|---|---|---|
| Apoa4 | ACAGCTCAATACCCTCTTCCAG | GGCTCACTTTGTTGGCATGG | NM_012737 |
| Ass1 | AAAAAGGGGTCCCTGTGAAG | ATGAGCGTGGTAAAGGATGG | NM_013157 |
| Cited2 | GAAGGACTGGAAATGGCAGA | GCGCCGTAGTGTATGTGCT | NM_053698 |
| Egln3 | CTCCTATGCCACCAGGTACG | ACAAGGTAGGGAGCCAAACG | NM_019371 |
| F3 | GTGTCCTGGGAGAAACACTCAT | CCAGCAGAGGTCTCGGTAAC | NM_013057 |
| Il1r2 | CATGGGAGATGCAGGCTATT | TACCAGTTCCCAGGAACACC | NM_053953 |
| Krt15 | GATCCAGGGGCTCATTACCG | AGCAGCCATCTTAGCATCCTG | NM_001004022 |
| Mest | CAAGCCGAGACCACATCAGT | GTGAGACGGCCAGAACGATT | NM_001009617 |
| Mmp12 | ACATGAAGCGTGCGGATGTA | AGGAACAGGTTTGTGCCTTGA | NM_053963 |
| Prl8a5 | CCTCCGGAAGCATTTAACAG | GATTTGGGTTTTGCACGATT | NM_173110 |
| Prl8a9 | GGCACATTCCTGATGCTTGC | CCTAATCAGTTTTGAGTTAAGAGCC | XM_006253905 |
| Ptprk | ACTACATCGATGGCTACCAGAG | AGCATTTCACCCGACCAACT | XM_008758616 |
| Pxdc1 | CAACCCAGCTTTCAAAGTCC | CTGGGTCATCACCATCTTCC | NM_001025719 |
| Thbd | CACTGGACTCGGGAAGTGAC | TGGTAGCCTGTTTCGCACAT | NM_031771 |
| Timp3 | ACAGACGCCAGAGTCTCCTA | ACCTCAAGTCTGTCCGGGTA | NM_012886 |
| Top2a | CAGCGTGTTGAGCCTGAATG | TAACTTGGGAGCATGGGCAG | NM_022183 |
| Tpbpa | TGAATTGCAAGAGCAGAAGGGTA | CATCGCCAAGTGACTGTGCT | NM_172073 |
| 18S rRNA | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA | NR_046237 |
Western blotting
Cell lysates were prepared in buffer containing 62.5 mM Tris-HCl (pH 6.8), 10% glycerol, 2% sodium dodecyl sulfate (SDS), and 50 mM dithiothreitol. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Total proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. CITED2 was detected using anti-CITED2 antibody (ab108345; Abcam, Cambridge, MA) at a dilution of 1:500.
RNA-seq analysis
Transcriptomic profiles in control shRNA treated Rcho-1 TS cells and Cited2 shRNA knockdown Rcho-1 TS cells (n=3 each) were performed using RNA-seq analysis. Complementary DNA libraries from total RNA samples were prepared with Illumina TruSeq RNA sample preparation kits (Illumina, San Diego, CA). Five hundred ng of total RNA were used as input. Poly-A containing RNAs were purified with oligo-dT-coated magnetic beads. RNA fragmentation, first and second strand cDNA synthesis, end repair, adaptor ligation, and PCR amplification were performed according to the manufacturer’s recommendations. The cDNA libraries were validated for RNA integrity using an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA) before sequencing.
cDNA libraries were clustered onto a TruSeq paired-end flow cell, and sequenced (100 bp paired-end reads) using a TruSeq 200 cycle SBS kit (Illumina). Samples were run on an Illumina HiSeq2000 sequencer located at the University of Kansas Medical Center Genome Sequencing Facility, and sequenced in parallel with other samples to ensure the data generated for each run were accurately calibrated during data analysis. Following generation of sequencing images, the pixel-level raw data collection, image analysis, and base calling were performed by Real Time Analysis software (Illumina). The base call files (*.bcl) were converted to *.qseq files by Illumina's BCL Converter, and the *.qseq files were subsequently converted to *.fastq files for downstream analysis. Reads from *.fastq files were mapped to the rat reference genome (Ensembl Rnor_5.0.78) using CLC Bio Genomics Workbench 7.0 (CLC Bio, Aarhus, Denmark). The mRNA abundance was expressed in reads per kilobase of exon per million reads mapped (RPKM). Statistical significance was calculated by empirical analysis of digital gene expression in the CLC Bio Genomics Workbench. A corrected false discovery rate (FDR) of 0.05 was used as a cutoff for significant differential expression (control vs Cited2 knockdown). Functional patterns of transcript expression were further analyzed using Ingenuity Pathway Analysis (IPA, Qiagen, Redwood City, CA) and Database for Annotation, Visualization and Integrated Discovery (DAVID v6.7; https://david.ncifcrf.gov/). Results from the RNA-seq analysis were validated using qRT-PCR. Primer sets for the qRT-PCR are shown in Table 1.
Statistical analysis
Values are expressed as the mean ± the standard error of the mean (SEM). Comparisons between more than two groups were made using one-way analysis of variance following log10 transformation of the raw data and multiple comparisons were done using Dunnett’s post hoc test. The procedure for analyzing the RNA-seq data is described in the preceding paragraph.
RESULTS
CITED2 is a transcriptional co-regulator implicated in the regulation of placentation (Withington et al. 2006; Moreau et al. 2014). We sought to gain insight into the role of CITED2 in trophoblast development using a loss-of-function approach in rat Rcho-1 TS cells.
Cited2 expression in differentiating trophoblast cells
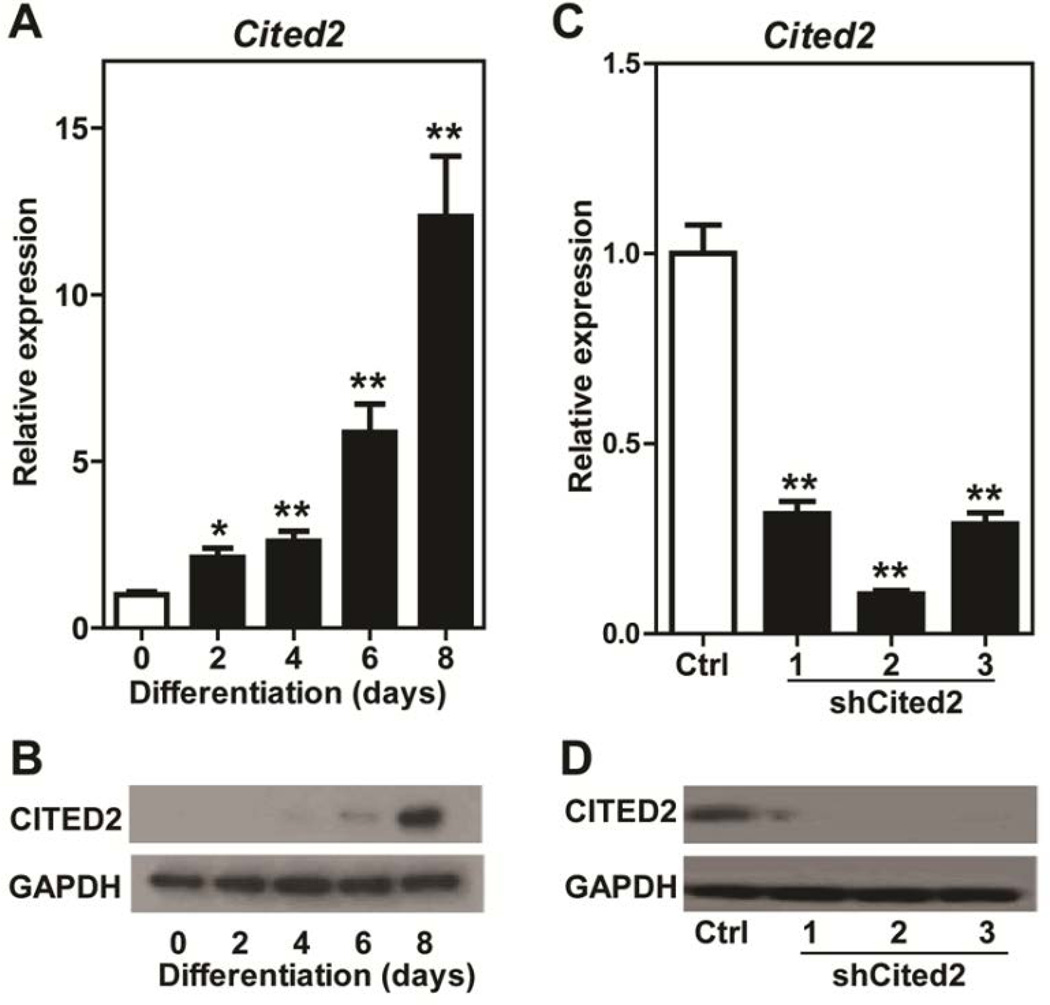
Initially, we profiled the expression of CITED2 transcript and protein in stem and differentiating Rcho-1 TS cells using qRT-PCR and western blotting, respectively. Both CITED2 mRNA and protein exhibited a robust increase in expression as Rcho-1 TS cells underwent differentiation (Fig. 1A,B). These observations are consistent with earlier findings correlating Cited2 expression with trophoblast differentiation (Kent et al. 2010) and directed us to examine the developmental consequences of CITED2 knockdown in differentiating Rcho-1 TS cells.
Figure 1.
Identification of CITED2 downstream targets in differentiating trophoblast cells
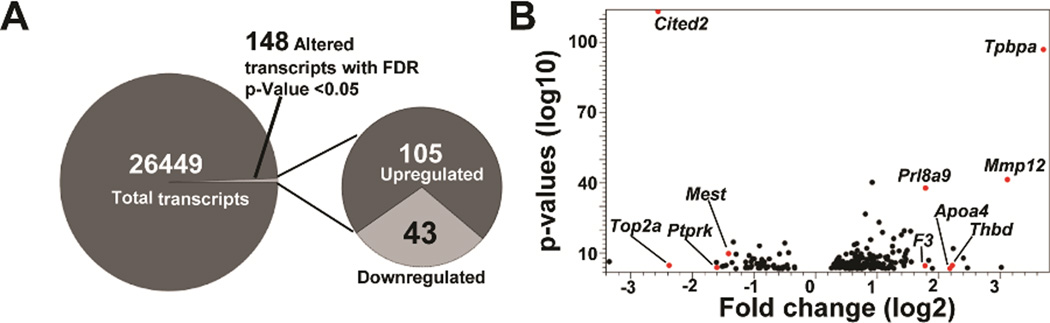
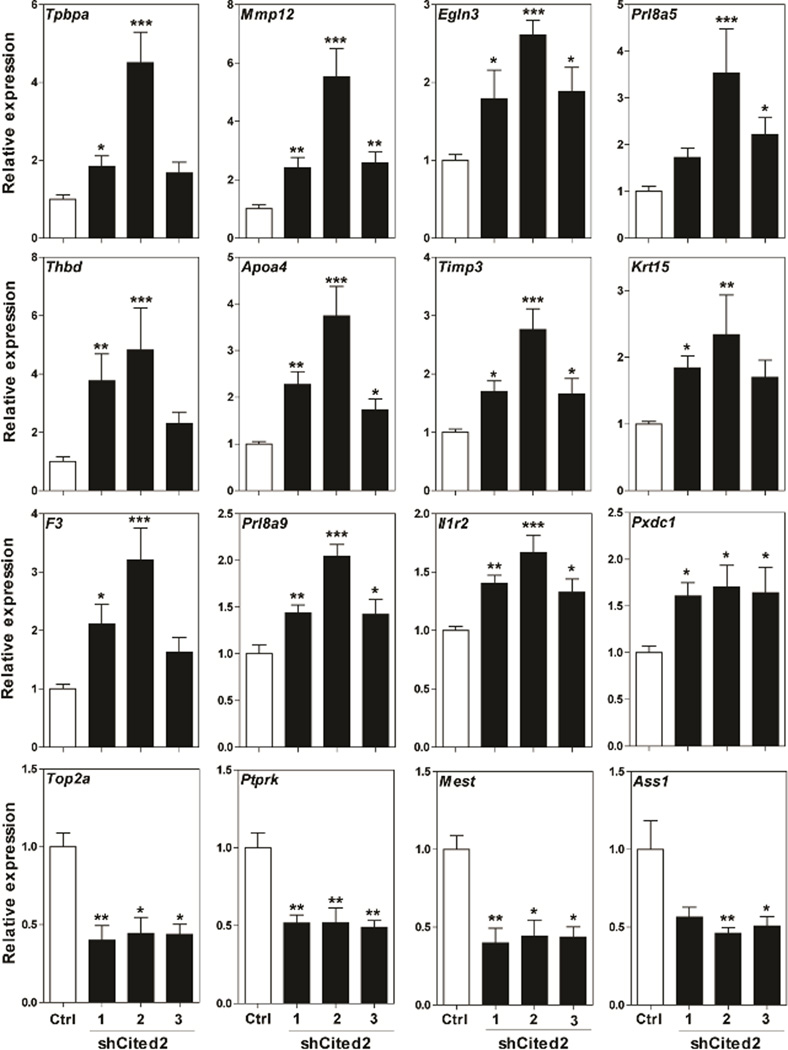
We utilized an shRNA approach to inhibit the expression of CITED2 in Rcho-1 TS cells and RNA-seq to characterize the transcriptome of the manipulated cells. Rcho-1 TS cells were stably infected with lentiviral constructs containing shRNAs specifically targeting three different and independent sequences within Cited2 (termed: shCited2-1, shCited2-2, or shCited2-3) or a control shRNA and exposed to differentiation culture conditions. CITED2 shRNAs effectively inhibited the expression of the differentiation-dependent upregulation of CITED2 mRNA and protein (Fig. 1C,D). Disruption of CITED2 expression did not interfere with trophoblast cell proliferation or morphological indices of trophoblast giant cell formation. It is important to appreciate that trophoblast cell differentiation is more than the formation of trophoblast giant cells. Trophoblast giant cells can possess distinct phenotypes (Simmons et al. 2007) and other distinct differentiated trophoblast cell lineages can arise from TS cells (Gardner and Beddington 1988; Soares et al. 1996, 2012; Simmons and Cross 2005). Thus, RNA-seq was performed on Rcho-1 TS cells stably expressing control shRNA versus CITED2 shRNA (shCited2-2) on day 8 of differentiation to gain additional insights into the role of CITED2 in the regulation of trophoblast cell differentiation. Results from the RNA-seq analysis are deposited in the Gene Expression Omnibus (Accession No. GSE74748). A total of 148 known transcripts (105 upregulated versus 43 downregulated) exhibited a significant change in expression (false discovery rate, FDR, P<0.05) in control versus CITED2 shRNA cells (Fig. 2A,B). Table 2 includes a list of selected upregulated and downregulated transcripts. Canonical pathways sensitive to CITED2 disruption included signal transduction cascades (e.g. extracellular signal-regulated kinase/mitogen-activated protein kinase, peroxisome proliferator-activated receptor, eukaryotic initiation factor 2, paxillin, integrin-linked kinase, phospholipase C, and integrin) and processes associated with leukocyte extravasation and phagocytosis (Table 3). Additional insights from the pathway analyses were not evident. qRT-PCR was used to validate expression of a set of selected upregulated and downregulated transcripts (Fig. 3). Several features characterized subsets of upregulated transcripts: i) transcripts expressed by non trophoblast giant cell lineages of differentiated trophoblast cells (Tpbpa, Mmp12, Prl8a5, Prl8a9); ii) known hypoxia/HIF-responsive transcripts (Tpbpa, Mmp12, and Egln3); iii) transcripts encoding proteins associated with coagulation/thrombosis (Mmp12, F3, and Thbd). A prominent feature associated with several downregulated transcripts (Top2a, Ptprk, and Mest) is their known linkage with the Rcho-1 TS cell stem state (Kent et al. 2010). Mitogen removal promotes differentiation of Rcho-1 TS cells and is also compatible with the survival of quiescent TS cells, which can be revived by reintroduction of a mitogenic stimulus (Sahgal et al. 2006). Collectively, our findings indicate that CITED2 may restrain expansion of TS cells in the stem state, promote TS cell quiescence, and/or direct trophoblast cell differentiation.
Figure 2.
Table 2.
Selected transcripts identified by RNA-seq in differentiating control and Cited2 shRNA-treated Rcho-1 TS cells
| Gene Description | Gene Symbol | Biological properties | Chromosome | Cited2 shRNA/ Control shRNA |
|---|---|---|---|---|
| Trophoblast specific protein alpha | Tpbpa | Related to cysteine-type endoprotease | 17 | 12.1 |
| Matrix metallopeptidase 12 | Mmp12 | Metalloelastase, ECMa, coagulation | 8 | 8.1 |
| Egl-9 family hypoxia-inducible factor 3 | Egln3 | Prolyl hydroxylase, hypoxia signaling | 6 | 5.5 |
| Prolactin family 8, subfamily a, member 5 | Prl8a5 | Cytokine, hormone | 17 | 5.0 |
| Thrombomodulin | Thbd | Thrombin receptor, coagulation | 3 | 4.5 |
| Apolipoprotein A-IV | Apoa4 | Lipid metabolism | 8 | 4.4 |
| TIMP metalloproteinase inhibitor 3 | Timp3 | Inhibitor of matrix metallopeptidases, ECM |
7 | 3.6 |
| Keratin 15 | Krt15 | Intermediate filaments, epithelial cells | 10 | 3.3 |
| Coagulation factor 3 | F3 | Coagulation | 2 | 3.2 |
| Prolactin family 8, subfamily a, member 9 | Prl8a9 | Cytokine, hormone | 17 | 3.2 |
| Interleukin 1 receptor, type II | Il1r2 | Decoy receptor for IL-1 ligands | 9 | 2.6 |
| PX domain containing 1 | Pxdc1 | Phosphatidylinositol binding | 17 | 2.5 |
| Argininoscuccinate synthase 1 | Ass1 | Arginine biosynthetic pathway | 3 | 0.37 |
| Mesoderm specific transcript | Mest | Hydrolase superfamily, imprinted, developmentally regulated |
4 | 0.34 |
| Protein tyrosine phosphatase, receptor type K | Ptprk | Signaling pathway controlling cell-cell adhesion, growth control, invasion |
1 | 0.30 |
| Topoisomerase (DNA) II alpha | Top2a | Nuclear protein regulating chromosome dynamics |
10 | 0.17 |
| CREBBP/EP300-interacting transactivator, with Glu/Asp-rish carboxy-terminal domain, 2 |
Cited2 | Transcription co-regulator | 1 | 0.16 |
ECM, extracellular matrix
Table 3.
Analysis of pathways affected for CITED2 disruption in differentiating Rcho-1 TS cells
| Canonical pathwaysa | -Log (P- value) |
Ratio | zScore | Molecules |
|---|---|---|---|---|
| ERK/MAPK signaling | 1.44E00 | 3.09E-02 | −0.45 | ITGB1, FOS, H3F3A/H3F3B, PRKCI, SOS1 |
| PPAR signaling | 2.53E00 | 5.75E-02 | −1.34 | IL1R2, FOS, HSP90B1, SOS1, CITED2 |
| EIF2 signaling | 3.68E00 | 5.59E-02 | 1.34 | PABPC1, RPL14, RPL22, SOS1, RPL17, RPL10, EIF4A2, RPS25 |
| Leukocyte extravasation | 2.56E00 | 4.19E-02 | 1.63 | ITGB1, TIMP3, PRKCI, CDH5, MMP12, ACTG1, ACTN1 |
| Paxillin signaling | 4.17E00 | 7.78E-02 | 1.63 | ITGB1, SOS1, ARHGEF6, ITGAV, PTPN12, ACTG1, ACTN1 |
| Fcγ receptor-mediated phagocytosis in macrophages and monocytes |
1.78E00 | 4.60E-02 | 2.00 | HMOX1, PRKCI, ARPC2, ACTG1 |
| ILK signaling | 3.39E00 | 5.06E-02 | 2.12 | ITGB1, FOS, FN1, ARHGEF6, FERMT2, TMSB10/TMSB4X, ACTG1, ACTN1 |
| Phospholipase C signaling | 1.64E00 | 3.08E-02 | 2.24 | ITGB1, HMOX1, PRKCI, AHNAK, SOS1, ARHGEF6 |
| Integrin signaling | 2.39E00 | 3.91E-02 | 2.65 | ITGB1, ASAP1, ARPC2, SOS1, ITGAV, ACTG1, ACTN1 |
Abbreviations: ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferator-activated receptor; EIF2, eukaryotic initiation factor 2; ILK, integrin-linked kinase.
Figure 3.
DISCUSSION
CITED2 is an established regulator of cell growth and differentiation and has been implicated in a broad range of developmental events, including neural tube, heart, lung, liver, hematopoietic, gonad, adrenal, and placental morphogenesis (Bamforth et al. 2001; Barbera et al. 2002; Yin et al. 2002; Withington et al. 2006; Qu et al. 2007; Val et al. 2007; Xu et al. 2008; Combes et al. 2010; Du and Yang 2013). In this report, we provide insight into the modulatory actions of CITED2 on trophoblast cell differentiation.
A null mutation at the Cited2 locus of the mouse leads to defects in placentation (Withington et al. 2006; Moreau et al. 2014). Cited2 null placentas are smaller than placentas from wild type littermates with a profound disruption in the development of the junctional zone (Withington et al. 2006). Each differentiated trophoblast cell lineage is present in the Cited2 null placenta; however, the absence of CITED2 affects the organization and function of the differentiated trophoblast cell lineages. Differentiating Rcho-1 TS cells exhibit many features of junctional zone trophoblast cell development (Soares et al. 1996). Some of the most prominent effects of disrupting CITED2 in differentiating Rcho-1 TS cells was upregulation of Tpbpa, Mmp12, Timp3, and Egln3 expression. Tpbpa expression identifies a progenitor population of trophoblast cells within the developing junctional zone possessing the capacity to differentiate into spongiotrophoblast, glycogen cells, invasive trophoblast cells, and subsets of trophoblast giant cells (Hu and Cross 2011). MMP12 localizes to invasive endovascular trophoblast lining uterine spiral arterioles (Harris et al. 2010; D. Chakraborty and M.J. Soares, unpublished) and is viewed as a contributor to pregnancy-dependent structural changes associated with these vessels that are critical for hemochorial placentation (Harris et al. 2010). MMP12 is a member of the matrix metalloproteinase family and among its targets is elastin, a prominent constituent of the arterial vasculature (Van Doren 2015). Paradoxically, the transcript for TIMP3, a broad spectrum inhibitor of MMPs (Brew and Nagase 2010), is also upregulated by CITED2 knockdown, reflecting a level of complexity to CITED2 action in differentiating trophoblast cells. Expression of Tpbpa and Mmp12 are known hypoxia and HIF-regulated genes in rat TS cells (Chakraborty et al. 2011). Egln3 is a hypoxia/HIF responsive transcript and encodes for a negative modulator of HIF activities (Lee and Percy 2011). Even though Rcho-1 TS cells were not cultured in hypoxic conditions in the present work, there is some evidence for the involvement of HIF signaling in TS cell differentiation exposed to atmospheric conditions (Maltepe et al. 2005). Thus upregulation of Tpbpa, Mmp12, and Egln3 in the present work may reflect removal of CITED2 antagonism of HIF actions.
CITED2 knockdown also led to an increase in the expression of trophoblast cell transcripts possessing ties to blood coagulation (Mmp12, F3, and Thbd). MMP12 has both pro- and anti-coagulant actions via inactivation of tissue factor pathway inhibitor (Belaaouaj et al. 2000) and the inhibition of circulating fibrinogen levels (Motterle et al. 2012), respectively. F3 (also known as tissue factor) is a pro-coagulant (Pawlinski et al. 2004) and THBD is an inhibitor of blood coagulation (Isermann et al. 2001, 2003; Weiler 2004). Thus, CITED2 negatively regulates multiple transcripts encoding regulatory components of the blood coagulation pathway with apparently opposing outcomes.
Trophoblast differentiation is a multi-lineage process (Gardner and Beddington 1988; Simmons and Cross 2005; Soares et al. 2012). In vitro differentiation of Rcho-1 TS cells favors trophoblast giant cell differentiation (Faria and Soares 1991; Sahgal et al. 2006; Kent et al. 2010). Transcripts exhibiting some of the most dramatic changes following CITED2 manipulation are characteristic of trophoblast lineages other than the trophoblast giant cell lineage. Thus, at least in the context of differentiating Rcho-1 TS cells, CITED2 appears to guide/restrict the specialization of specific differentiated trophoblast cell populations, potentially favoring trophoblast giant cell development and inhibiting the development of Tpbpa-positive progenitors and their descendants.
A key mechanism of action of CITED2 is its modulation of transcription factor interactions with the histone acetyl transferases, CREBBP and EP300 (Bhattacharya and Ratcliffe 2003; Du and Yang 2013). These histone acetyl transferases govern gene activation of numerous transcription factors (Vo and Goodman 2001; Bedford et al. 2010), including many that have been implicated in regulating trophoblast cell differentiation (GATA2, GATA3, AP-1, TFAP2C, ETS2, HIF1, PPARG, SATB1; Soares et al. 2014; Soncin et al. 2015). Thus CITED2 has the potential to modulate a wide spectrum of biological processes associated with trophoblast development. However, at this juncture, only two known modulators of trophoblast development, HIF1 and TFAP2C, have been directly connected to CITED2 (Bhattacharya et al. 1999; Bamforth et al. 2001; Yin et al. 2002; Braganca et al. 2003; Freedman et al. 2003). The precise mechanism(s) underlying the effects of CITED2 on the differentiated trophoblast cell transcriptome remains to be determined.
Acknowledgments
We thank Stacy McClure for administrative assistance.
FUNDING
This work was supported by the National Institutes of Health (HD020676, HD079363).
Footnotes
DECLARATION OF INTEREST
There are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Amoroso EC. The evolution of viviparity. Proc R Soc Med. 1968;61:1188–1200. [PMC free article] [PubMed] [Google Scholar]
- Asanoma K, Kubota K, Chakraborty D, Renaud SJ, Wake N, Fukushima K, Soares MJ, Rumi MA. SATB homeobox proteins regulate trophoblast stem cell renewal and differentiation. J Biol Chem. 2012;287:2257–2268. doi: 10.1074/jbc.M111.287128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Bragança J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- Barbera JP, Rodriguez TA, Greene ND, Weninger WJ, Simeone A, Copp AJ, Beddington RS, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11:283–293. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- Bedford DC, Kasper LH, Fukuyama T, Brindle PK. Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics. 2010;5:9–15. doi: 10.4161/epi.5.1.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaaouaj AA, Li A, Wun T-C, Welgus HG, Shapiro SD. Matrix metalloproteinases cleave tissue factor pathway inhibitor. Effects on coagulation. J Biol Chem. 2000;275:27123–27128. doi: 10.1074/jbc.M004218200. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Ratcliffe PJ. ExCITED about HIF. Nat Struct Biol. 2003;10:501–503. doi: 10.1038/nsb0703-501. [DOI] [PubMed] [Google Scholar]
- Bragança J, Eloranta JJ, Bamforth SD, Ibbitt JC, Hurst HC, Bhattacharya S. Physical and functional interactions among AP-2 transcription factors, p300/CREB-binding protein, and CITED2. J Biol Chem. 2003;278:16021–16029. doi: 10.1074/jbc.M208144200. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. Journal of Clinical Investigation. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Rumi MAK, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating HIF-dependent trophoblast lineage decisions. Proc Natl Acad Sci USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes AN, Spiller CM, Harley VR, Sinclair AH, Dunwoodie SL, Wilhelm D, Koopman P. Gonadal defects in Cited2-mutant mice indicate a role for SF1 in both testis and ovary differentiation. Int J Dev Biol. 2010;54:683–689. doi: 10.1387/ijdb.092920ac. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Dilworth MR, Sibley CP. Transport across the placenta of mice and women. Placenta. 2013;34(Suppl):S34–S39. doi: 10.1016/j.placenta.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Du J, Yang YC. Cited2 in hematopoietic stem cell function. Curr Opin Hematol. 2013;20:301–307. doi: 10.1097/MOH.0b013e3283606022. [DOI] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Faria TN, Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- Freedman SJ, Sun ZY, Kung AL, France DS, Wagner G, Eck MJ. Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nat Struct Biol. 2003;10:504–512. doi: 10.1038/nsb936. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Beddington RS. Multi-lineage ‘stem’ cells in the mammalian embryo. J Cell Sci Suppl. 1988;10:11–27. doi: 10.1242/jcs.1988.supplement_10.2. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knofler M, Cartwright JE, Whitley GSJ, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol. 2010;177:2103–2115. doi: 10.2353/ajpath.2010.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Cross JC. Ablation of Tpbpa-positive trophoblast precursors leads to defects in maternal artery remodeling in the mouse placenta. Dev Biol. 2011;358:231–239. doi: 10.1016/j.ydbio.2011.07.036. [DOI] [PubMed] [Google Scholar]
- Isermann B, Hendrickson SB, Hutley K, Wing M, Weiler H. Tissue-restricted expression of thrombomodulin in the placenta rescues thrombomodulin-deficient mice from early lethality and reveals a secondary developmental block. Development. 2001;128:827–838. doi: 10.1242/dev.128.6.827. [DOI] [PubMed] [Google Scholar]
- Isermann B, Sood R, Pawlinski R, Zogg M, Kalloway S, Degen JL, Mackman N, Weiler H. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat Med. 2003;9:331–337. doi: 10.1038/nm825. [DOI] [PubMed] [Google Scholar]
- Kent LN, Konno T, Soares MJ. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10:97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent LN, Rumi MA, Kubota K, Lee DS, Soares MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipp GT, Audus KL, Soares MJ. Nutrient transport across the placenta. Adv Drug Deliv Rev. 1999;38:41–58. doi: 10.1016/s0169-409x(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Kubota K, Kent LN, Rumi MAK, Roby KF, Soares MJ. Dynamic regulation of AP-1 transcriptional complexes directs trophoblast differentiation. Mol Cell Biol. 2015;35:3163–3177. doi: 10.1128/MCB.00118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckenberg P, Kubaczka C, Schorle H. The role of transcription factor Tcfap2c/TFAP2C in trophectoderm development. Reprod Biomed Online. 2012;25:12–20. doi: 10.1016/j.rbmo.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Lee FS, Percy MJ. The HIF pathway and erythrocytosis. Annu Rev Pathol Mech Dis. 2011;6:165–192. doi: 10.1146/annurev-pathol-011110-130321. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Artap ST, Shi H, Chapman G, Leone G, Sparrow DB, Dunwoodie SL. Cited2 is required in trophoblasts for correct placental capillary patterning. Dev Biol. 2014;392:62–79. doi: 10.1016/j.ydbio.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Motterle A, Xiao Q, Kiechl S, Pender SLF, Morris GE, Willeit J, Caulfield MJ, Ye S. Influence of matrix metalloproteinase-12 on fibrinogen level. Atherosclerosis. 2012;220:351–354. doi: 10.1016/j.atherosclerosis.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Pawlinski R, Pedersen B, Erlich J, Mackman N. Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thromb Haemost. 2004;92:444–450. doi: 10.1160/TH04-05-0309. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Qu X, Lam E, Doughman Y-Q, Chen Y, Chou Y-T, Lam M, Turakhia M, Dunwoodie SL, Watanabe M, Xu B, Duncan SA, Yang Y-C. Cited2, a coactivator of HNF4α, is essential for liver development. EMBO J. 2007;26:4445–4456. doi: 10.1038/sj.emboj.7601883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud SJ, Kubota K, Rumi MA, Soares MJ. The FOS transcription factor family differentially controls trophoblast migration and invasion. J Biol Chem. 2014;289:5025–5039. doi: 10.1074/jbc.M113.523746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RM, Fisher SJ. Trophoblast stem cells. Biol Reprod. 2011;84:412–421. doi: 10.1095/biolreprod.110.088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal N, Canham LN, Canham B, Soares MJ. Rcho-1 trophoblast stem cells: a model system for studying trophoblast cell differentiation. Methods Mol Med. 2006;121:159–178. [PubMed] [Google Scholar]
- Schorpp-Kistner M, Wang ZQ, Angel P, Wagner EF. JunB is essential for mammalian placentation. EMBO J. 1999;18:934–948. doi: 10.1093/emboj/18.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularization requires the AP-1 component fra1. Development. 2000;127:4937–4948. doi: 10.1242/dev.127.22.4937. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- Sibley C, Glazier J, D'Souza S. Placental transporter activity and expression in relation to fetal growth. Exp Physiol. 1997;82:389–402. doi: 10.1113/expphysiol.1997.sp004034. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;5:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Chakraborty D, Kubota K, Renaud SJ, Rumi MA. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int J Dev Biol. 2014;58:247–259. doi: 10.1387/ijdb.140083ms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Chakraborty D, Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Chapman BM, Rasmussen CA, Dai G, Kamei T, Orwig KE. Differentiation of trophoblast endocrine cells. Placenta. 1996;17:277–289. doi: 10.1016/s0143-4004(96)90051-x. [DOI] [PubMed] [Google Scholar]
- Soncin F, Natale D, Parast MM. Signaling pathways in mouse and human trophoblast differentiation: a comparative review. Cell Mol Life Sci. 2015;72:1291–1302. doi: 10.1007/s00018-014-1794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima S, Shimosato Y, Koiede T, Kuroki M, Kikuchi Y, Aizawa M. Transplantable choriocarcinoma of rats induced by fetectomy and its biological activities. Gann. 1983;74:205–212. [PubMed] [Google Scholar]
- Val P, Martinez-Barbera J-P, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- Van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015;44–46:122–129. doi: 10.1016/j.matbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- Weiler H. Mouse models of thrombosis: thrombomodulin. Thromb Haemost. 2004;92:467–477. doi: 10.1160/TH04-05-0307. [DOI] [PubMed] [Google Scholar]
- Weninger WJ, Lopes Floro K, Bennett MB, Withington SL, Preis JI, Barbera JP, Mohun TJ, Dunwoodie SL. Cited2 is required both for heart morphogenesis and establishment of the left-right axis in mouse development. Development. 2005;132:1337–1348. doi: 10.1242/dev.01696. [DOI] [PubMed] [Google Scholar]
- Withington SL, Scott AN, Saunders DN, Lopes Floro K, Preis JI, Michalicek J, Maclean K, Sparrow DB, Barbera JP, Dunwoodie SL. Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev Biol. 2006;294:67–82. doi: 10.1016/j.ydbio.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Xu B, Qu Z, Gu S, Doughman Y-Q, Watanabe M, Dunwoodie SL, Yang Y-C. Cited2 is required for fetal lung maturation. Dev Biol. 2008;317:95–105. doi: 10.1016/j.ydbio.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Haynie J, Yang X, Han B, Kiatchoosakun S, Restivo J, Yuan S, Prabhakar NR, Herrup K, Conlon RA, Hoit BD, Watanabe M, Yang YC. The essential role of Cited2, a negative regulator for HIF-1alpha, in heart development and neurulation. Proc Natl Acad Sci USA. 2002;99:10488–10493. doi: 10.1073/pnas.162371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J-Y, Kim TH, Lee JH, Dunwoodie SL, Ku BJ, Jeong J-W. Mig-6 regulates endometrial genes involved in cell cycle and progesterone signaling. Biochem Biophys Res Commun. 2015;462:409–414. doi: 10.1016/j.bbrc.2015.04.146. [DOI] [PMC free article] [PubMed] [Google Scholar]