INTRODUCTION
In the past few decades, various methods of functional brain imaging have been used to study cerebral control of the bladder and urethra.1–9 Recent advances in the understanding of functional disorders such as urgency incontinence have been based largely on functional magnetic resonance imaging (fMRI) of regional brain responses to infusion of liquid into the bladder.10,11 Studies of older female subjects with urgency incontinence12,13 have linked bladder filling sensations such as desire to void and urgency14 to activation of a region near the insula and a region near the dorsal anterior cingulate cortex (dACC) and supplementary motor area (SMA). Similar bladder filling sensations are sometimes reported during daily life15,16 but in healthy individuals there is little awareness of bladder filling until a substantial volume of urine has accumulated, a desire to void is felt, and an opportunity to empty the bladder is sought.16 In normal subjects, brain responses to filling of the bladder when sensation is weak (or absent) have not been systematically investigated by functional imaging. In positron emission tomography studies1–4,17 bladder filling was continued until sensation was present; one fMRI study reported results for normal subjects, but not specifically when sensation was weak18; another was based on infusion and withdrawal of liquid by a rotary peristaltic pump.12 Its pulsatile flow may have provoked unrealistically strong sensations, making it unsuitable for addressing sensation-free brain responses. We therefore performed a study of brain responses to bladder filling in subjects without signs or symptoms of overactive bladder function. We included only older females. We carried out fMRI measurements of brain responses to bladder filling (taking care to avoid flow pulsations) at small bladder volumes, a situation which corresponds to weak or absent filling sensation. For comparison, we made similar measurements with full bladder (defined by the subject’s report of strong desire to void). We hypothesized that, with small bladder volume, responses to bladder filling in the insula and dACC/SMA regions would be weak or absent, while subcortical responses, if evident, would reflect activity related to unconscious monitoring of bladder events. With full bladder we expected to observe activation of insula and dACC/SMA.11,12,18
MATERIALS AND METHODS
Subjects were cognitively and functionally intact, community-dwelling female volunteers aged x60 years, without overactive bladder symptoms, recruited by word of mouth or newspaper advertisement. All gave written informed consent prior to study procedures, which were approved by the University of Pittsburgh Institutional Review Board. There are divergent definitions of normal bladder function, especially in the elderly. For this study all subjects completed a 3-day bladder diary to rule out urgency incontinence. Post-void residual urine was measured by ultrasound after a catheter-free uroflow performed in private, to rule out significant residual urine. Participants underwent comprehensive video urodynamics (conforming to International Continence Society standards19) to exclude involuntary bladder contractions (detrusor overactivity) or other significant bladder dysfunction, and also to familiarize them with a urethral catheter and the sensation of a strong desire to void when the bladder was full. The sensation reported by the subjects during testing was then reproduced during the fMRI study. Detrusor overactivity in the scanner (see below) was a further exclusion criterion.
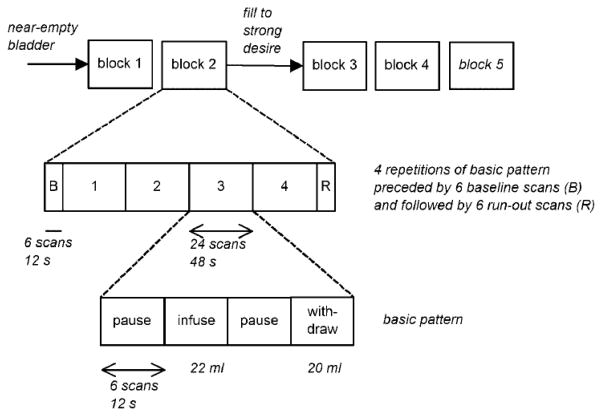
fMRI with simultaneous urodynamic monitoring was performed using a modified version of a protocol published in 2005.11 Subjects lay in the scanner with their head held in place by cushions in a standard head coil. Earplugs were worn but voice communication was possible when not scanning. Subjects held a push-button to signal a strong desire to void. Two 8 F urethral catheters were introduced for infusion and withdrawal of sterile water and for bladder pressure measurement. They were connected by stiff, water-filled tubes to urodynamic equipment (Laborie Medical Avanti) in the adjacent control room, with 2 pressure transducers at the level of the symphysis pubis to measure the pressures in the bladder and at the pump. A small volume of sterile water (10–20 ml) was introduced into the empty bladder and correct pressure measurement was confirmed by recording a cough. A structural MRI was then recorded (duration about 30 min, allowing for accumulation of a small amount of urine in the bladder, perhaps 100 ml; we refer to this situation as a near-empty bladder). It was followed by functional MRI while fluid was repeatedly infused into and withdrawn from the bladder by a computer-controlled syringe pump (Harvard PHD 22/2000), driven by the MRI scanner. The intravesical pressure recording was used to rule out detrusor overactivity in the scanner. The pump pressure was used to indicate the timing and direction of flow, enabling later synchronization with the scanner blood-oxygen-level-dependent (BOLD) signal, which is believed to measure changes in cerebral blood flow that correspond to neuronal activity.20–22 We used a Siemens Trio scanner with a 3 T magnet and an echo-planar imaging protocol with flip angle = 858; echo time TE =27 msec; one scan per 2 sec. Data were obtained from 40 brain slices with thickness 3.2 mm, 3.3 mm × 3.3 mm resolution, and field of view 210 mm × 210 mm. Thus the BOLD signal was determined in near-cubical volume elements (voxels) that covered approximately the whole brain from brainstem to superior cortical boundary. Each infusion/withdrawal cycle comprised: pause (12 sec); infusion (22 ml in 12 sec = 110 ml/min); pause (12 sec); and withdrawal (20 ml in 12 sec). Four such cycles, preceded by a 12 sec measurement to establish a baseline free of mechanical disturbance by the pump, and followed by a further 12 sec of scanning, formed one measurement block (Fig. 1). After 2 measurement blocks, the bladder was filled, with a hand-operated syringe and without scanning, until the subject signaled strong desire to void. After each block the subject was asked about comfort and sensation and, with her permission, further measurement blocks were recorded until she stopped the study because of the intensity of the sensation. After catheter removal the subject voided in private into a container to establish the final volume in the bladder. The BOLD signals acquired in the second measurement block (because the first might not be representative) and the last block (with very strong desire to void, usually the fourth or fifth block) were used for analysis.
Fig. 1.
Bladder infusion/withdrawal protocol. The basic pattern of infusion and withdrawal, separated by pauses, is shown in the bottom row of boxes. Twenty-two milliliters is infused and 20 ml withdrawn, to avoid accommodation. Each phase of the pattern lasts 12 sec and comprises 6 whole-brain scans. In the standard analysis used in previous studies, the brain response to bladder filling at any given voxel is taken to be the contrast infusion– withdrawal, that is, the average of the BOLD signal during infusion minus the average during withdrawal (allowing for hemodynamic smoothing and delay in the response to neural activity changes). Four repetitions of the basic pattern, preceded by 6 baseline scans and followed by 6 more scans to allow for calculations during the hemodynamic delay (middle row), make up each measurement block. Blocks 1 and 2 (top row) are performed with only a small volume in the bladder. Block 3 is performed after filling the bladder sufficiently to evoke a strong desire to void. One or two further blocks are performed with the subject’s permission until the desire to void is too intense to continue. Blocks are separated by about 30 sec for voice communication to ask about the subject’s comfort and the intensity of the sensation.
Data Analysis
Scans were preprocessed with statistical parametric mapping (SPM5)23 as follows. Within each measurement block, successive scans were aligned with the first scan, to correct for head-movement artifacts. They were then coregistered with the same subject’s structural MRI, normalized to the standard SPM brain by an affine transformation, and smoothed with an 8 mm Gaussian filter. First-level (single-subject) analysis was performed with a block design matrix based on 108 scans, shown in Figure 1, using the standard SPM hemodynamic response function and a low-frequency cut-off at 128 sec. For each voxel in the brain the difference between the mean and standard deviation of the BOLD signal during infusion and the mean and standard deviation during withdrawal (i.e., the contrast infusion–withdrawal) was calculated and expressed as a t-value, as we have done previously.11 Voxels where the signal is significantly greater during infusion than during withdrawal (i.e., t is positive and significantly greater than zero) have been called “activated.” If the signal is smaller during infusion than during withdrawal, then the value of t for that voxel is negative, and such voxels have been referred to as “deactivated.” Because some features of the results suggested confounding of the regional BOLD signal by global changes affecting the whole brain (see Discussion Section), the analyses were repeated with the global normalization option provided by SPM, to remove global and non-specific changes and isolate the bladder-filling changes.24 In addition, the coefficient of correlation was calculated between the mean global cerebral activity and a 108-element vector representing the state of the pump (infusion =1, pause = 0, and withdrawal =x 1). Further, the first 36 scans of each measurement block (which included the baseline recording and one infusion/withdrawal cycle; see Fig. 1) were reanalyzed to compare the signal during infusion and the signal during withdrawal with that during the prior baseline (i.e., the contrasts infusion-baseline and withdrawal-baseline). This allowed us to determine whether infusion or withdrawal truly increased or reduced the BOLD signal.
Group analyses of single-subject images were performed with the SPM random-effects model. Because calculations on a voxel-by-voxel basis involve a very large number of statistical comparisons it is important to correct the raw P values for multiple comparisons. We therefore reported activation peaks (in Table II or Fig. 2), defined by thresholding at P < 0.01 (uncorrected), only if they were part of an activation cluster that was significant at P < 0.05 after correction for multiple comparisons. To enable global comparisons, the numbers of significantly activated or deactivated voxels were calculated for each contrast (Table II).
TABLE II.
Main Effects of Bladder Filling (Infusion) at Low and High Bladder Volumes, for the Contrast (Infusion Minus Withdrawal) With and Without Global Normalization, and for Comparisons of Infusion and Withdrawal With Baseline Activity
| Small bladder volume | Large bladder volume | |||
|---|---|---|---|---|
| Contrast | # Activated and deactivated voxels, sign of contrast (# of significant clusters), corrected P-value of most significant cluster | Location of peak in most significant cluster BA MNI coords |
# Activated and deactivated voxels, sign of contrast (# of significant clusters), corrected P-value of most significant cluster | Location of peak in most significant cluster BA MNI coords |
| Infusion–withdrawal, no global normalization | Activated 0 Deactivated 49,327 −(1) P = 0.000 |
R parietal postcentral BA 40 −32, −34, 54 |
Activated 29 294 Deactivated 119 +(1) P = 0.000 |
R frontal precentral BA 4 −34, −18, 52 |
| Infusion–withdrawal, with global normalization | Activated 11 198 Deactivated 4,912 +(1) P = 0.000 |
L temporal subgyral BA 21 −44, −12, −12 |
Activated 1,532 Deactivated 15,900 −(1) P = 0.000 |
L temporal middle BA 39 48, −74, 10 |
| Withdrawal-baseline | Activated 49 116 Deactivated 975 +(1) P = 0.000 |
R frontal medial BA 6 6, −16, 70 |
Activated 4,335 Deactivated 589 (0) |
n/a |
| Infusion-baseline | Activated 3,293 Deactivated 19,132 −(1) P = 0.000 |
R occipital precuneus BA 31 22, −60, 22 |
Activated 7,568 Deactivated 2,500 +(1) P = 0.03 |
L frontal precentral BA 4 −48, −6, 50 |
BA, Brodmann area. MNI coords, Montreal Neurological Institute coordinates.
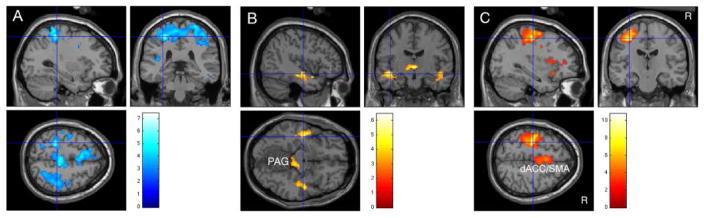
Fig. 2.
Results at small and large bladder volumes displayed on a standard brain at threshold P = 0.01. R, right side. Color bars show values of student’s t. Crosshairs mark the most significant peak in a significant cluster(P < 0.05, corrected at cluster level). A: With small volume and without global normalization, activity during infusion was less than during withdrawal in a right frontoparietal region (apparent deactivation). B: With small volume and with global normalization, activity during infusion was greater than during withdrawal (activation) in a left inferior temporal region and, as a trend, on the right and near the midbrain PAG. C: Results at large volume (strong desire to void) without global normalization. Activity during infusion was greater than during withdrawal in a right frontoparietal region and, less prominently, the dACC/SMA complex.
RESULTS
Eleven healthy older women without significant residual urine, urgency incontinence, or detrusor overactivity, whether on history, on bladder diary, during urodynamics or in the scanner, fulfilled our criteria for normal bladder function and completed the study. Selected demographic, clinical, and urodynamic data are shown in Table I. Note that urodynamic variables were measured on different occasions and cannot be directly compared. During scanning subjects reported no pain or discomfort other than strong desire to void when the bladder was well filled.
TABLE I.
Demographic, Clinical, and Urodynamic Data for the 11 Female Subjects
| Mean | SD | Median | Range | #/11 | |
|---|---|---|---|---|---|
| Age (y) | 65.1 | 4.2 | 63 | 60–71 | |
| MMSE (/30) | 29.0 | 1.9 | 30 | 24–30 | |
| Independently mobile | 11/11 | ||||
| Pregnancies, deliveries | 2/11, 2/11 | 0–4, 0–4 | |||
| History of stress incontinence | 3/11 | ||||
| Current anti-muscarinic or similar medication | 0/11 | ||||
| Hysterectomy | 1/11 | ||||
|
| |||||
| Comorbidities | Depression | 3/11 | |||
| COPD | 1/11 | ||||
| Vaginal atrophy | 1/11 | ||||
| History of seizure | 1/11 | ||||
|
| |||||
| First desire to void (ml) | 309 | 174 | 350 | 80–550 | |
| Strong desire to void (ml) | 510 | 95 | 530 | 350–650 | |
| PVR after free flow in private (ml) | 2.3 | 5.2 | 0 | 0–15 | |
| Volume voided post-fMRI (ml) | 507 | 244 | 450 | 200–1,100 | |
| Maximum cystometric capacity (ml) | 568 | 108 | 610 | 360–680 | |
| Max voided volume on diary (ml) | 647 | 174 | 690 | 300–870 | |
| Daytime voiding frequency on diary (#/24 hr) | 6.2 | 0.9 | 6.0 | 5.0–7.7 | |
| Nocturia on diary (#) | 0.75 | 0.77 | 0.70 | 0–2.0 | |
Small Bladder Volume (Second Measurement Block)
When the bladder volume was small the contrast infusion– withdrawal (without global normalization) identified no significant activations (Table II). There were however numerous voxels where this contrast had a negative value (49,000 out of a total of 170,000), including one highly significant cluster (P = 0.000 corrected for multiple comparisons), centered in the right frontoparietal cortex (Fig. 2A). Further investigation revealed the following.
If a correction was made for global blood flow changes (by performing global normalization), there were fewer voxels with negative values, and none significant after correction for multiple comparisons. Instead there were many activated voxels (Table II and Fig. 2B): some in the midbrain near the periaqueductal gray, and also bilaterally in an inferior temporal region that was significant at cluster level on the right. There was no frontal or parietal activation.
Using the baseline established prior to the infusion/withdrawal cycles (period B in Fig. 1), the contrast (withdrawal-baseline) showed stronger signal during withdrawal than during baseline in numerous voxels, with one significant cluster (P = 0.000) after correction for multiple comparisons (Table II). Thus the apparent deactivations suggested by the contrast (infusion–withdrawal) in Figure 2A and Table II were associated with increased fMRI signal during withdrawal.
The contrast (infusion-baseline) showed that more voxels were deactivated (infusion < baseline) than were activated. Deactivation reached significance in one cluster (Table II).
On average, mean global cerebral blood flow was negatively correlated with the 108-element vector representing the infusion/withdrawal cycle (R = x0.34 to þ0.24, mean =x 0.14, two-tailed P =0.02). This implies that the blood flow tended to decrease during infusion and increase during withdrawal.
Large Bladder Volume (Strong Desire to Void)
In these normal subjects, when the bladder was well-filled and infusion evoked a strong desire to void, the contrast (infusion–withdrawal; without global normalization) showed a predominance of activated voxels, in a pattern different from that reported for urgency-incontinent subjects.12 There was a significant frontoparietal cluster, centered on the left frontal precentral gyrus, and a trend to activation in the dACC/SMA complex (Fig. 2C). There were no significant clusters of deactivated voxels.
Activity during infusion or withdrawal differed from baseline in relatively few voxels. Infusion exceeded baseline in only one significant cluster at a relatively low level of significance (P =0.03, corrected; Table II), and there was no convincing evidence for increased response during withdrawal. Global normalization merely led to weaker significance levels in the left frontoparietal region and the dACC/SMA complex and suggested some deactivation elsewhere (Table II). Correlation of mean global blood flow with the infusion/withdrawal cycle was weaker than at small volumes and on average not significantly different from zero (R =x 0.26 to þ0.14, mean x0.03, two-tailed P =0.5).
DISCUSSION
In normal female individuals with only a small amount of urine in the bladder we observed no significant response to bladder filling in either insular or dACC/SMA complexes, with or without correction for global blood flow changes. This finding is consistent with weakness or absence of bladder filling sensation.13,25 More precise identification of the response to bladder filling requires consideration of the following:
Firstly, the prominence of apparent deactivations seen in Table II and Figure 2A is a characteristic signature of confounding of the BOLD signal by changes in global blood flow.24
Secondly, confounding can occur if these global changes are correlated with the experimental paradigm. In this case, with a small amount of urine in the bladder, there was on average a significant correlation between global blood flow and the infusion/withdrawal cycle.
Thirdly, the apparent deactivations occurred because of an increased fMRI signal during withdrawal of liquid from the bladder. It seems implausible that withdrawal should generate intense neuronal afferents (although it has been proposed 26). A speculative physiological explanation is that pump-induced changes in the bladder and abdominal volumes affect the cardiovascular system and alter global blood flow. Because the increased fMRI signal occurred during withdrawal (state of the pump =x 1), one would expect a negative correlation between the state of the pump and the globally averaged fMRI signal, as observed.
Thus, although an unidentified technical cause of this behavior cannot be completely ruled out, the above observations suggest that global blood flow changes can lead to serious artifacts when the bladder is nearly empty and the real BOLD signal is weak. This real BOLD signal is a measure of cerebral blood flow changes that vary from region to region, according to where neural activity is occurring. A strong BOLD signal corresponds to a change of about 1% and weak signals are smaller still. Thus even small changes in global cerebral blood flow can lead to a spurious BOLD signal that affects all parts of the brain. This becomes a problem (a) if the global changes non-specifically mask the brain responses to bladder filling or (b) if the global changes are correlated with the experimental paradigm used to analyze the data (in this case the infusion/withdrawal cycle). BOLD changes involving the whole brain, especially negative signals or “deactivations,” are physiologically improbable, and likely to be an artifact of global blood flow changes.24 Normalization of scan-to-scan variability to a constant global blood flow can reduce this artifact, especially when the BOLD signal is weak, as at small bladder volumes in this study and in women with Fowler’s syndrome27 (whom it would be interesting to re-study using global normalization). If the BOLD signal is strong (as in the urge-incontinent women we have examined previously11,13,25), global normalization has less effect. If the real and spurious BOLD signals are similar in size, as is the case at large bladder volumes in this study, then global normalization does not make a significant difference.
In this study, recalculation of the results at small bladder volume using global normalization eliminated the apparent deactivations,24 and unmasked activation in the midbrain and inferior temporal regions (Table II and Fig. 2B). The latter region is part of the parahippocampal complex. The midbrain region appears to correspond to the periaqueductal gray (PAG; and possibly the nearby parabrachial nucleus28).
Changes in activation of a similar subcortical circuit have been observed in women following restoration of pathologically absent bladder sensation.27 Thus this subcortical activity presumably represents sensation-free monitoring of bladder events by a neural circuit that automatically ensures continence, as well as safety during voiding.29 It may mimic the situation during normal daily life, when continence is maintained without conscious awareness.
With full bladder and strong sensation the situation is different: activations are strong; there is no significant deactivation; the average correlation of mean global activity with the state of the pump is very close to zero; and the fMRI signal is not markedly increased during withdrawal. These observations suggest that confounding by global blood-flow changes is not an important factor in this case. In confirmation, “correction” using global normalization merely weakened left frontoparietal and dACC/SMA responses, and introduced some questionable temporal deactivation (Table II).
This study highlights the limitations of fMRI when the BOLD signal is weak, especially if, as here, potential confounders are correlated with the paradigm (Fig. 1) that generates the fMRI signal. Because of the design of our paradigm, comparisons involving the baseline depend on relatively few scans, limiting their statistical power. A further limitation of the study is that subjects reported desire to void only if it was strong, so that direct evidence that sensation with near-empty bladder was weak or absent is lacking. However, this limitation had the advantage of precluding further confounding by a conscious attempt to recognize and report weak sensations.
CONCLUSION
During much of normal daily life, continence is maintained without awareness of the bladder even though it is being filled with urine. Under analogous circumstances in the laboratory, filling of a near-empty bladder evokes subcortical activity near the periaqueductal gray (the rostral terminus of the voiding reflex), which may reflect unconscious monitoring and relaying of ascending bladder signals. Meanwhile, cortical regions involved in bladder filling sensation—near the insula and in the dorsal anterior cingulate cortex and supplementary motor area—are not detectably activated. If the bladder is well-filled, however, infusion of additional liquid activates these cortical regions and evokes strong bladder sensation.
The contrast (infusion–withdrawal) used in this and our previous fMRI studies of brain-bladder control seems to adequately represent regional neuronal activation when there is strong bladder sensation. However, if sensation is weak or if the contrast has widespread negative values (“deactivations”), the possibility of confounding by global changes, especially in blood flow, should be considered. In this study a correction for global changes was required to reveal the subcortical activation at small bladder volumes that appears to be an important element of the continence mechanism.
Acknowledgments
We are grateful to Mary Alyce Riley, Mary Jo Sychak, Megan Kramer, Becky Clarkson, Werner Schaefer, and the staff of the Pittsburgh Magnetic Resonance Research Center for their enthusiastic help with this study. We thank our volunteers for taking part and the anonymous reviewers for helpful comments.
Grant sponsor: NIH K23; Grant number: AG31916; Grant sponsor: R01; Grant number: AG20629; Grant sponsor: John A. Hartford Center of Excellence in Geriatric Medicine.
Footnotes
Conflict of interest: Yes, Derek Griffiths is a consultant for J&J and for Laborie Medical.
References
- 1.Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–21. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–42. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- 3.Nour S, Svarer C, Kristensen JK, et al. Cerebral activation during micturition in normal men. Brain. 2000;123:781–9. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- 4.Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–77. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- 5.Blok B, Groen J, Veltman D, et al. Brain plasticity and urge incontinence: PET studies during the first hours of sacral neuromodulation. Neurourol Urodyn [Abstract] 2003;22:490–1. [Google Scholar]
- 6.Zhang H, Reitz A, Kollias S, et al. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. NeuroImage. 2005;24:174–80. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Mehnert U, Boy S, Svensson J, et al. Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve—An fMRI study in healthy women. NeuroImage. 2008;41:682–9. doi: 10.1016/j.neuroimage.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Seseke S, Baudewig J, Kallenberg K, et al. Voluntary pelvic floor muscle control—An fMRI study. NeuroImage. 2006;31:1399–407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Kuhtz-Buschbeck JP, van der Horst C, Pott C, et al. Cortical representation of the urge to void: A functional magnetic resonance imaging study. J Urol. 2005;174:1477–81. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- 10.Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths D, Derbyshire S, Stenger A, et al. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–7. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths D, Tadic SD, Schaefer W, et al. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths D. Imaging bladder sensations. Neurourol Urodyn. 2007;26:899– 903. doi: 10.1002/nau.20488. [DOI] [PubMed] [Google Scholar]
- 14.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 15.Van Meel TD, Wyndaele JJ. Reproducibility of urodynamic bladder filling sensation at weekly interval in healthy volunteers and women with detrusor overactivity. Neurourol Urodyn. 2011;30:1586–90. doi: 10.1002/nau.21100. [DOI] [PubMed] [Google Scholar]
- 16.de Wachter SG, Heeringa R, van Koeveringe GA, et al. On the nature of bladder sensation: The concept of sensory modulation. Neurourol Urodyn. 2011;30:1220–26. doi: 10.1002/nau.21038. [DOI] [PubMed] [Google Scholar]
- 17.Matsuura S, Kakizaki H, Mitsui T, et al. Human brain region response to distention or cold stimulation of the bladder: A positron emission tomography study. J Urol. 2002;168:2035–9. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths DJ, Tadic SD, Schaefer W, et al. Cerebral control of the lower urinary tract: How age-related changes might predispose to urge incontinence. NeuroImage. 2009;47:981–6. doi: 10.1016/j.neuroimage.2009.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodynam. 2002;21:261–274. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 20.Aizenstein HJ, Clark KA, Butters MA, et al. The BOLD hemodynamic response in healthy aging. J Cogn Neurosci. 2004;16:786–93. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- 21.Heeger DJ, Huk AC, Geisler WS, et al. Spikes versus BOLD: What does neuroimaging tell us about neuronal activity? Nat Neurosci. 2000;3:631–3. doi: 10.1038/76572. [DOI] [PubMed] [Google Scholar]
- 22.Norris DG. Principles of magnetic resonance assessment of brain function. J Magn Reson Imaging. 2006;23:794–807. doi: 10.1002/jmri.20587. [DOI] [PubMed] [Google Scholar]
- 23.Wellcome Department of Imaging Neuroscience. Statistical parametric mapping: SPM2. 2004 Jan 7; http://wwwfilionuclacuk/spm/spm2html [internet]. 2003.
- 24.Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage. 1998;8:302–6. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–74. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 26.Tannenbaum C, Madjar C, Doyon J, et al. A better understanding of the perceptual psychophysics of brain responses in fMRI studies of urinary urgency in healthy older subjects. Neurourol Urodyn; Abstract 228, International Continence Society annual meeting; Glasgow, Scotland. 2011. pp. 1116–7. [Google Scholar]
- 27.Kavia RB, DasGupta R, Critchley HD, et al. An fMRI study of the effect of sacral neuromodulation on brain responses in women with Fowler’s Syndrome. BJU Int. 2010;105:366–72. doi: 10.1111/j.1464-410X.2009.08819.x. [DOI] [PubMed] [Google Scholar]
- 28.Cechetto DF, Shoemaker JK. Functional neuronatomy of autonomic regulation. NeuroImage. 2009;47:795–803. doi: 10.1016/j.neuroimage.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths DJ. Use of functional imaging to monitor central control of voiding in humans. In: Michel MC, Andersson K-E, editors. Urinary tract. Berlin, Heidelberg: Springer; 2011. pp. 81–97. [DOI] [PubMed] [Google Scholar]