Abstract
Background and Purpose
In AIM-HIGH addition of extended-release niacin (ERN) to simvastatin in participants with established CV disease, low HDL-C and high triglycerides, there was no incremental benefit despite increases in HDL-C, preliminary analysis based on incomplete endpoint adjudication suggested increased ischemic stroke risk among participants randomized to ERN.
Methods
This final analysis was conducted after complete AIM HIGH event ascertainment to further explore potential relationship between niacin therapy and ischemic stroke risk.
Results
There was no group difference in trial primary composite endpoint at a mean 36-month follow-up among 3,414 patients (85% male; mean age: 64±9 years) randomized to simvastatin plus ERN (1,500–2,000mg/day) versus simvastatin plus matching placebo. In the intention to treat analysis, there were 50 fatal or non-fatal ischemic strokes, 18 (1.06%) in placebo arm versus 32 (1.86%) in ERN arm: age-adjusted hazard ratio, HR 1.78 (95% CI 1.00–3.17, p=0.050). Multivariate analysis showed independent associations between ischemic stroke risk and age > 65 years (HR 3.58, 95% CI 1.82–7.05, p=0.0002), history of stroke/TIA/carotid disease (HR 2.18, 95% CI 1.23–3.88, p=0.0079), elevated baseline Lp(a) (HR 2.80, 95%CI 1.25 – 6.27, middle vs. lowest tertiles, HR 2.31, 95% CI 1.002 – 5.30 highest vs lowest tertiles, overall p=0.042), but a non-significant association with ERN (HR 1.74 95% CI .97–3.11, p=0.063).
Conclusions
Although there were numerically more ischemic strokes with addition of ERN to simvastatin that reached nominal significance, number was small and multivariable analysis accounting for known risk factors did not support a significant association between niacin and ischemic stroke risk.
Clinical Trial Registration
AIM-HIGH ClinicalTrials.gov number, NCT00120289
Keywords: niacin therapy, lipid lowering, clinical trial, stroke
Observational epidemiological studies show that low high-density lipoprotein cholesterol (HDL-C) levels are independently associated with an increased risk of cardiovascular disease1.2. In addition, HDL-C remains a strong predictor for cardiovascular disease risk in statin-treated individuals who have reached their target low-density lipoprotein cholesterol (LDL-C) levels3,4. It is less clear whether raising HDL-C levels can reduce this residual risk, although preliminary data and meta-analyses of data from the early niacin trials used to raise HDL-C before the widespread use of statins suggested this potential5–9. As niacin also reduces LDL-C, it is not clear whether these benefits were due to the effects of raising HDL-C or lowering LDL-C. Recently, dalcetrapid, a cholesteryl ester transfer protein (CETP) inhibitor, that raises HDL-C by 31 to 40%, without further changes in low LDL-C levels, had no effect in reducing clinical outcomes, including coronary heart disease (CHD) or stroke in over 13,000 subjects with acute coronary syndrome (ACS)10.
The Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial tested whether extended-release niacin (ERN) added to intensive statin (combination) therapy, as compared with statin therapy alone (monotherapy), would reduce the risk of cardiovascular events in individuals with established atherosclerotic cardiovascular disease and atherosclerotic dyslipidemia (low HDL-C and elevated triglycerides)11,12. On April 25, 2011, at a planned interim analysis, the trial Data and Safety Monitoring Board recommended that the blinded intervention be stopped, 18 months earlier than planned, because of futility for the composite primary endpoint. Of concern also was an unexpected higher rate of ischemic stroke among participants allocated to ERN-statin combination therapy13. The latter assessment was preliminary and based on incomplete outcome event adjudication in the interim report. The objective of this final analysis was to further examine AIM-HIGH data related to the risk of ischemic stroke after complete event ascertainment had been performed.
Methods
The design, organization, and baseline data from the AIM-HIGH Study, an investigator-initiated trial approved and sponsored by the National Heart, Lung, and Blood Institute (NHLBI), have been described previously11,13. The study was reviewed and approved by the Institutional Review Boards of each of the 92 U.S. and Canadian enrolling sites and signed informed consent was obtained from each participant. Enrolled participants were ≥ age 45 years and required to have established cardiovascular disease defined by either documented stable CHD (previous history of myocardial infarction [MI] or documented multivessel coronary artery disease by angiography), cerebrovascular or carotid disease, or peripheral arterial disease (Table 1). All participants had low baseline levels of HDL-C (< 1.0 mmol/L [40 mg/dL] for men; < 1.4 mmol/L [50 mg/dL] for women), elevated triglycerides (1.1–4.5 mmol/L [150–400 mg/dL]), and for the 6% of participants who were not on a statin at entry, LDL-C < 4.7 mmol/L (180 mg/dL). Participants were required to discontinue lipid modifying drugs, except for statins or ezetimibe, at least 4 weeks prior to randomization and had a fasting lipid profile meeting inclusion criteria, verified by the core laboratory. Potential participants were excluded if, within 4 weeks of enrollment, they had been hospitalized for an ACS, had a planned coronary revascularization, or had a stroke within the preceding 8 weeks. Exclusion criteria have been reported previously11,13.
Table 1.
Pertinent baseline characteristics
| Variable | Statin Monotherapy | Combination Therapy |
|---|---|---|
| Number | 1696 | 1718 |
| Age (SD) yrs | 63.7 (8.7) | 63.7 (8.8) |
| Female | 251 (14.8) | 253 (14.7) |
| Risk factors (%): | ||
| Diabetes | 570 (33.6) | 588 (34.2) |
| Hypertension | 1189 (70.1) | 1250 (72.8) |
| Metabolic syndrome | 1353 (79.8) | 1414 (82.3) |
| History (%): | ||
| Myocardial infarction | 955 (56.3) | 968 (56.3) |
| Cerebrovascular disease (stroke/TIA) | 362 (21.3) | 358 (20.8) |
| Atrial fibrillation | 129 (7.6) | 122 (7.1) |
| PVD | 231 (13.6%) | 234 (13.6%) |
| Medications at baseline (%): | ||
| Niacin* | 338 (19.9) | 324 (18.9) |
| Statin | 1601 (94.4) | 1595 (92.8) |
| ASA/Antiplatelet agents | 1595 (94.0) | 1637 (95.3) |
| ACE inhibitors/ARB | 1271 (74.9) | 1258 (73.2) |
| BP lowering meds (beta-blockers/ACE inhibitors/ARB/calcium channel blockers/diuretics) |
1615 (95.2) | 1642 (95.6) |
| LDL Cholesterol (mmol/L) Mean (SD) |
1.91 (0.59) | 1.92 (0.61) |
| Triglycerides (mmol/L) Median (interquartile range) |
1.84 (1.48, 2.44) | 1.89 (1.48, 2.47) |
| HDL Cholesterol (mmol/L) Mean (SD) |
0.90 (0.14) | 0.89 (0.14) |
| ApoB (mmol/L) Mean (SD) |
0.83 (0.21) | 0.83 (0.20) |
| ApoA (mmol/L) Mean (SD) |
1.24 (0.16) | 1.22 (0.16) |
| ApoB/ApoA ratio | 0.68 (0.18) | 0.69 (0.18) |
| Total/HDL Cholesterol ratio | 4.2 (1.0) | 4.3 (1.0) |
| LP (a) (µmol/L) Median (interquartile range) |
1.16 (0.47, 4.29) | 1.29 (0.48, 4.51) |
Note: : Number (percent) for categorical variables or Mean (SD) for continuous normally distributed variables and median (interquartile range) for continuous and skewed variables.
Intervention
Individuals randomized to combination therapy were to receive simvastatin plus ERN 1,500–2,000 mg/day whereas those randomized to monotherapy received matching placebo containing a small (50 mg) dose of immediate-release niacin in each tablet of 500 mg or 1,000 mg placebo to mask the identity of blinded treatment to participants and study personnel. Both treatment arms received simvastatin with the dose adjusted to an on-treatment LDL-C level of 1.0–2.2 mmol/L (40–80 mg/dL). In order to achieve and maintain a similar LDL-C target level in both treatment groups, subjects in either arm could also receive ezetimibe 10 mg/day, as needed. Only LDL-C levels were reported to clinical sites to maintain therapy masking13.
Study Outcomes
The primary trial outcome was the composite of first occurrence of CHD death, non-fatal MI, ischemic stroke, hospitalization for ACS, or symptom-driven coronary or cerebral revascularization. Ischemic stroke was a component of several secondary composite outcomes which included: CHD death, non-fatal MI, ischemic stroke or hospitalization for ACS; CHD death, non-fatal MI or ischemic stroke; and cardiovascular mortality. Tertiary outcomes included total mortality, individual components of the primary endpoint, and the effects of treatment on pre-specified subgroups defined by sex, history of diabetes, and metabolic syndrome11,13.
An ischemic stroke was defined as an acute vascular event with focal neurologic signs lasting more than 24 hours, without evidence of primary intracranial hemorrhage. A transient ischemic attack (TIA) was defined as having focal symptoms of a presumed ischemic basis lasting less than 24 hours. Imaging was not required (if imaging was performed and showed an area of ischemic injury, the event was adjudicated as a TIA if symptoms and signs lasted less than 24 hours). Hemorrhagic stroke was defined as an acute neurological vascular event with focal neurologic signs lasting more than 24 hours or sudden severe headache and meningeal signs, and evidence of intracranial hemorrhage by neuroimaging or autopsy. An acute neurological vascular event that fulfilled the definition of stroke, with focal neurologic signs lasting more than 24 hours, or sudden severe headache followed rapidly by coma, but without neuroimaging or autopsy data to classify as either an ischemic or hemorrhagic stroke was considered as a primary stroke endpoint event.
A Clinical Events Committee, masked to the identity of treatment and included three board certified university-based vascular neurologists, reviewed suspected primary outcomes (including silent MI) with supporting documentation. ECG’s were centrally interpreted; all lipid and serum chemistry analyses were performed by a central laboratory. All neurological events were reviewed by two of three neurologists. Consensus was reached if there were no disagreements after the primary adjudication. At study termination and after database lock, all neurological events which had not been originally classified as strokes were re-adjudicated by the still blinded Clinical Events Committee. The process was performed to distinguish TIA events, from those reported neurologic events initially classified by the Clinical Events Committee as “no neurologic event”.
Statistical Analyses
All analyses were performed using the intention to treat approach. Events adjudicated as ischemic stroke, hemorrhagic stroke, or TIA were included. Analyses of stroke endpoints, individually and in combination, were performed using time to event methods. Kaplan-Meier methods were used to estimate stroke rates, and Cox proportional hazards models to estimate hazard ratios (HR), confidence intervals (CI), and adjusted Wald statistics for stroke endpoints. Cox models were used to test for interactions between randomization assignment and known risk factors for stroke, including the about 20% of participants who had a prior stroke, and characterize the relationship between lipids and stroke without considering randomization assignment. Time-dependent Cox Models were used to adjust for time on and off niacin/placebo. Models were adjusted for age (≥65 vs <65 years) at randomization. A stepwise Cox model was used to identify factors independently associated with a given endpoint. Two sided p-values < 0.05 were considered statistically significant with no adjustments for multiple statistical testing.
Results
Subject characteristics, study and concomitant drug therapy and main outcomes of the AIM-HIGH trial have been reported in detail previously13. Table 1 summarizes pertinent baseline demographic and clinical characteristics, medications, and both lipid and lipoprotein levels for the 3,414 subjects enrolled in the trial, of whom 1,696 were randomized to statin plus placebo (monotherapy) and 1,718 to statin plus ERN (combination therapy). All baseline characteristics were balanced between the two treatment groups.
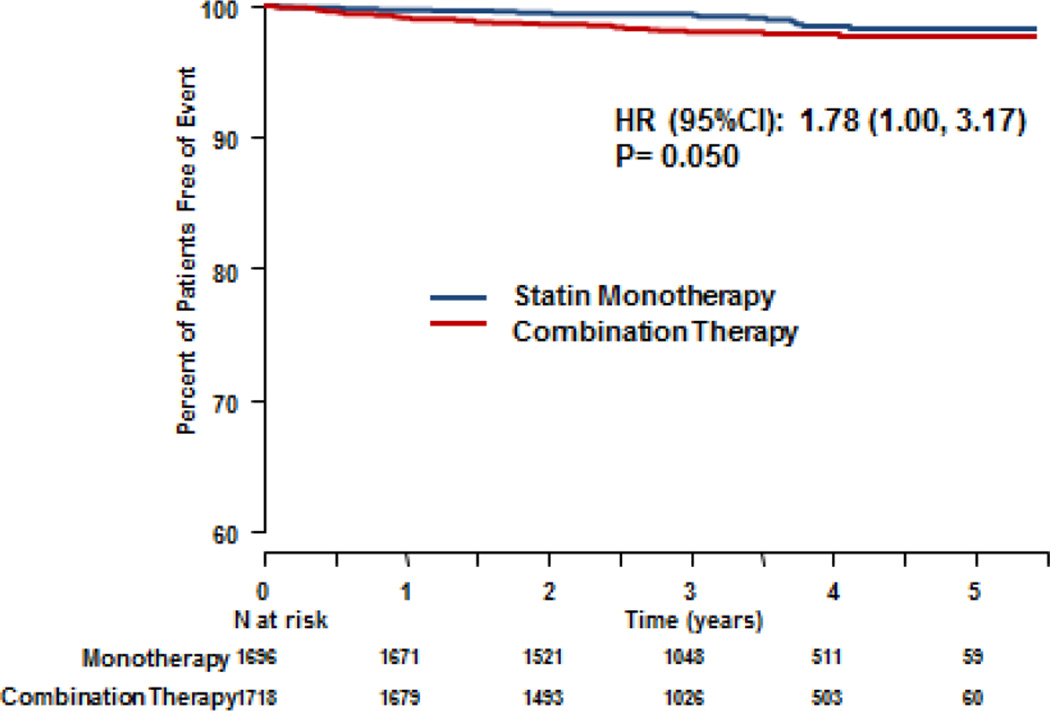
At a mean 36-month follow-up, the composite primary outcome (death from CHD, non-fatal MI, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven coronary or cerebral revascularization) occurred in 274 (16.2%) of those randomized to monotherapy and 282 (16.4%) randomized to combination therapy13. There was no difference between the treatment groups in the primary outcome, which included 15 ischemic strokes in the monotherapy group and 27 in the combination group in the intention to treat analysis (HR 1.02, 95% CI 0.87–1.21, p=0.80)13. Re-review of the previously TIA “non-stroke” neurological events by the Clinical Events Committee, identified an additional 3 ischemic stroke events. A total of 50 ischemic strokes that occurred during the trial, including 5 ischemic strokes that did not qualify as primary study events as they occurred after one of other events in the time to first event in the primary composite endpoint, were identified [total of 18 (1.06%) fatal or non-fatal ischemic strokes in the monotherapy group and 32 (1.86%) in the combination group]. This number also included 8 strokes that occurred 2 months to 4 years after subjects had discontinued ERN therapy. Of the 50 fatal or non-fatal ischemic strokes, there was an excess number of events in the statin/ERN combination group (HR 1.78; 95% CI 1.00–3.17, p=0.050). (Figure 1) There were 7 hemorrhagic strokes and 30 TIAs among participants. The HR for the composite ischemic strokes and TIA was 1.20 (95% CI 0.77–1.88, p=0.428). Table 2 summarizes the stroke events, composite stroke events and the hazard ratios comparing monotherapy and combination therapy. (Table 2)
Figure 1.
Kaplan-Meier Curves for Ischemic Stroke comparing statin monotherapy with ERN-statin combination therapy. Included non-fatal and fatal strokes, including 5 which were not counted in the primary composite endpoints of the trial as these occurred after another event which was counted.
Table 2.
Incidence of various stroke types
| Event | Statin Monotherapy |
Combination Therapy |
Hazard Ratio (95% CI) p-value |
|---|---|---|---|
| Ischemic stroke (fatal or non-fatal) | 18 (1.06%) | 32 (1.86%) | 1.78 (1.00–3.17) P=0.050 |
| Ischemic stroke + hemorrhagic stroke + confirmed TIA |
38 (2.24%) | 46 (2.68%) | 1.21 (0.79–1.87) P=0.377 |
| Ischemic stroke + hemorrhagic stroke | 21 (1.24%) | 36 (2.10%) | 1.73 (1.01–2.96) P=0.047 |
| Ischemic stroke + confirmed TIA | 35 (2.06%) | 42 (2.44%) | 1.20 (0.77–1.88) P=0.428 |
| Hemorrhagic stroke | 3 (0.18%) | 4 (0.23%) | 1.36 (0.30–6.08) P=0.688 |
| TIA | 19 (1.12%) | 11 (0.64%) | 0.57 (0.27–1.21) P=0.143 |
Table 3 shows the univariate associations between baseline parameters and the risk of ischemic stroke, which were significant only for age and a history of prior stroke. The 20% of participants with a history of cerebrovascular disease (stroke/TIA/carotid disease), were at higher risk for ischemic stroke after adjusting for age and randomization assignment (HR 2.08, 95% CI 1.17–3.68, p=0.013) but there was no interaction between history of cerebrovascular disease and treatment group (data not shown).
Table 3.
Association between baseline parameters and risk of ischemic stroke by univariable analyses*
| Parameter | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Age | 4.16 (2.13, 8.137) | <0.0001 |
| Gender (M/F) | 2.07 (0.74–5.74) | 0.164 |
| Previous niacin | 0.56 (0.24–1.32) | 0.186 |
| On statin at baseline | 0.75 (0.27–2.08) | 0.578 |
| Aspirin at baseline | 0.51 (0.24–1.09) | 0.085 |
| Antiplatelet agents at baseline | 0.75 (0.27–2.09) | 0.582 |
| ACE inhibitor/ARB | 0.94 (0.50–1.77) | 0.852 |
| Any BP lowering drugs | 0.69 (0.21–2.20) | 0.526 |
| History of Diabetes | 0.92 (0.51–1.65) | 0.768 |
| History of PVD | 1.02 (0.46, 2.27) | 0.964 |
| Previous MI | 1.17 (0.67–2.062) | 0.576 |
| Previous Stroke/TIA | 2.07 (1.17–3.67) | 0.013 |
| Hypertension | 0.86 (0.47–1.58) | 0.632 |
| Atrial fibrillation | 1.66 (0.74–3.70) | 0.217 |
| Metabolic syndrome | 0.88 (0.44–1.76) | 0.938 |
| LDL cholesterol | 0.740 | |
| Highest vs lowest tertile | 0.76 (0.38–1.52) | |
| Middle vs lowest tertile | 0.87 (0.45–1.68) | |
| Triglycerides | 0.992 | |
| Highest vs lowest tertile | 0.99 (0.50–1.97) | |
| Middle vs lowest tertile | 0.96 (0.49–1.88) | |
| HDL cholesterol | 0.349 | |
| Lowest vs highest tertile | 1.52 (0.78–2.98) | |
| Middle vs highest tertile | 1.01 (0.48–2.13) | |
| Total/HDL cholesterol ratio | 0.909 | |
| Highest vs lowest tertile | 0.86 (0.43–1.71) | |
| Middle vs lowest tertile | 0.94 (0.48–1.82) | |
| ApoB | 0.307 | |
| Highest vs lowest tertile | 1.30 (0.67– 2.50) | |
| Middle vs lowest tertile | 0.74 (0.35 – 1.55) | |
| ApoA | 0.294 | |
| Highest vs lowest tertile | 0.56 (0.28 – 1.16) | |
| Middle vs lowest tertile | 0.81 (0.42 – 1.56) | |
| ApoB/ApoA ratio | 0.234 | |
| Highest vs lowest tertile | 1.31 (0.61 – 2.84) | |
| Middle vs lowest tertile | 1.82 (0.90 – 3.68) | |
| LP(a) | 0.044 | |
| Highest vs lowest tertile | 2.27 (0.99 – 5.22) | |
| Middle vs lowest tertile | 2.78 (1.24 – 6.21) | |
Adjusted for age > 65 years (yes/no)
The previous use of niacin or baseline use of statin, aspirin or other antiplatelet agents, angiotensin converting inhibitor inhibitors or angiotensin receptor blockers or any BP lowering drugs, a history of diabetes or MI, the presence of hypertension, atrial fibrillation or the metabolic syndrome were not associated with an increased risk of ischemic stroke (Table 3).
There was no association between baseline lipids and stroke with the exception of elevated Lp(a), with higher Lp(a) levels associated with higher stroke risk (overall p=0.044, comparing highest vs lowest terttiles and middle vs lowest tertiles). (Table 3) There were no significant associations between the mean lipid levels over time during the study and ischemic stroke risk (Table 4).
Table 4.
Association between mean on-treatment lipids levels during trial and ischemic stroke
| Parameter | Hazard ratio (95% CI) | P-value |
|---|---|---|
| LDL cholesterol | 0.99(0.97, 1.01) | 0.427 |
| Triglycerides | 0.997(0.99, 1.003) | 0.296 |
| HDL cholesterol | 1.03(0.99, 1.07) | 0.105 |
| Total/HDL cholesterol ratio | 0.75(0.50, 1.13) | 0.170 |
| ApoB | 1.01(0.98, 1.03) | 0.674 |
| ApoA | 0.99(0.96, 1.02) | 0.480 |
| ApoB/ApoA ratio | 1.829(0.13, 25.96) | 0.655 |
Adjusted for age and baseline lipid value.
Multivariate stepwise regressions analyses showed independent associations between ischemic stroke risk and older age ≥ 65 (HR 3.58, 95% CI 1.82–7.05, p=0.0002), a history of stroke/TIA/carotid disease (HR 2.18 95% CI 1.23–3.88, p=0.0079), and elevated baseline Lp(a) (HR 2.80, 95% CI 1.25 – 6.27 comparing the middle to the lowest tertile, and HR 2.31 95% CI 1.00 – 5.30 comparing the highest to the lowest tertile, overall p=0.042), but a non-significant association between ischemic stroke and combination therapy (HR 1.74 95% CI .97–3.11, p=0.063). (Table 5). Similar associations are observed when the composite outcome of ischemic stroke and TIA was used in the analysis. (Table 5)
Table 5.
Association of risk factors with ischemic stroke and composite of ischemic stroke and TIA by multivariate analysis*
| Parameter | Hazard Ratio (95% CI) | P value |
|---|---|---|
| ISCHEMIC STROKE | ||
| Age >=65 vs <65 yrs | 3.58 (1.82–7.05) | 0.0002 |
| History of stroke/TIA/presence of carotid disease | 2.18 (1.23–3.88) | 0.008 |
| LPa by Tertiles | ||
| Highest vs lowest tertile | 2.31 (1.00 – 5.30) | |
| Middle vs lowest tertile | 2.80 (1.25–6.26) | 0.042 |
| Randomization assignment: | ||
| Combination vs statin alone | 1.74 (0.97–3.11) | 0.063 |
| ISCHEMIC STROKE OR TIA | ||
| Age >=65 vs <65 yrs | 2.56 (1.54–4.27) | 0.0003 |
| History of stroke/TIA/presence of carotid disease | 2.76 (1.74–4.38) | <0.0001 |
| LPa by Tertiles | ||
| Highest vs lowest tertile | 2.30 (1.19–4.42) | |
| Middle vs lowest tertile | 2.49 (1.31–4.73) | 0.0156 |
Discussion
There was no prior published evidence of an increased risk of ischemic stroke associated with niacin, in combination with statin therapy. The initial observation at the meeting of the DSMB on April 25, 2011 that combination treatment with ERN-statin might be associated with an excess of ischemic stroke was a concern. This analysis, however, was based on preliminary data. In the final analysis based on all finally adjudicated events, there remained an excess number of ischemic strokes associated with addition of ERN to simvastatin compared to simvastatin alone, but there was only a trend towards higher risk in the multivariable analysis. Although not significant, an increased risk with the addition of ERN to statin cannot be completely excluded given the wide confidence interval owing to the relatively small number of outcome events. There was also a non-significant numerical imbalance in the total adjudicated TIA events in the study, with 19 of the 30 occurring in the statin monotherapy group. Because TIA has pathophysiological similarities to ischemic stroke, if the combination therapy was truly harmful, the composite endpoint including both ischemic stroke and TIA would be expected to accentuate the risk, which was not observed. There were very few hemorrhagic strokes and no significant differences between treatments groups for these events.
No previous studies or meta-analyses have found an increased incidence of stroke with niacin in any therapeutic formulation, dose or dosing regimen. Previous trials5–9 carried out since the 1970’s before the introduction of statin therapy, were all small except for the CDP trial. This latter trial reported a significant 25% stroke reduction in subjects randomized to niacin compared to placebo (8.5% vs 11.2% respectively, odds ratio 0.75, 95% CI 0.59–0.92)5,6. A prior meta-analysis of these10 trials evaluating the effects of niacin on clinical outcomes involving 6,545 participants found treatment was associated with 25% reduction in major coronary events, a 26% reduction in stroke, and a 27% reduction of any cardiovascular event8. A more recent meta-analysis of 11 trials on nearly 10,000 subjects, and which included AIM-HIGH plus many of the trials included in the earlier meta-analysis, reported that niacin was associated with significant reductions in composite endpoints of any CVD event (odds ratio [OR] 0.66, 95% CI 0.49–0.89, p=0.007) and in major CHD events (OR 0.75, 95% CI 0.59–0.96, p=0.02) but no significant association between niacin therapy and stroke incidence (OR 0.88, 95% CI 0.50–1.54, p=0.65)9.
There are major differences in background therapy and baseline and on-treatment lipid levels between prior trials and two recent studies. For example, baseline total cholesterol levels in the CDP trial were approximately 250 mg/dL (6.5 mmol/L) with an on-treatment level of approximately 225 mg/dL (5.8 mmol/L). These levels were much higher than the baseline and on treatment levels in AIM HIGH12,13. In AIM-HIGH all participants received intensive statin therapy to reduce their LDL-Cholesterol levels to 1.0–2.2 mmol/L (40–80 mg/dL). Similarly, in the recently reported HPS2-THRIVE randomized placebo-controlled trial of extended release niacin combined with laropiprant in 25,673 participants with pre-existing cardiovascular disease, who all received LDL-lowering therapy with simvastatin 40 mg daily, with or without ezetimibe, baseline total cholesterol was 128 mg/dL (3.32 mmol/L).14 Both AIM-HIGH and the much larger HPS2-THRIVE showed that the addition of niacin was not associated with reductions in vascular events, including no reduction in stroke. HPS2-THRIVE is a large trial, in which participants were followed for four years and during which 804 ischemic strokes occurred. This would have been adequately powered to detect specific clinical endpoints such as stroke. That this trial did not show an excess of ischemic stroke, suggests that there is little risk of excess ischemic stroke with niacin therapy. The addition of niacin may have resulted in little if any reduction of CV events in the more recent trials because of the lower LDL-C levels due to statin therapy.
We also explored the effects of known factors that could influence the risk for ischemic stroke in the AIM-HIGH dataset. Older age and a history of stroke/TIA/carotid disease are established risk factors for first or recurrent stroke and were found to be independently associated with an increased risk of ischemic stroke in AIM-HIGH, regardless of treatment assignment. There was a moderate association between Lp(a) and ischemic stroke comparing the lowest with the middle tertile and between the lowest with the highest tertile of Lp(a) levels. These results were consistent with the Emerging Risk Factors Collaboration investigators report, with pooled data from 36 prospective studies on 126,634 participants and concluded that there were continuous, independent, and modest associations of increasing Lp(a) concentration with increased risk of CHD and stroke15. In our further exploratory analyses in AIM-HIGH, there were more events in the middle tertile than in the highest tertile of Lp(a) levels. This observation is likely due to chance given the small numbers of events. There was also a significant interaction between Lp(a) distribution and treatment assignment on ischemic stroke risk in multivariate analysis. The risk of ischemic stroke with ERN-statin combination therapy compared to simvastatin alone tended to be lower in participants in the lowest (HR 0.62, 95% CI 0.15–2.60) and highest tertiles of Lp(a) (HR 0.76, 95% CI 0.30–1.94) but increased in those in the middle tertile (HR 6.95, 95% CI 2.06–23.38), p value for interaction 0.008. There is no plausible reason for this finding, which may also have been due to chance.
Other factors which may influence the risk of ischemic stroke including previous niacin or statin therapies, background trial therapies with aspirin or other antiplatelet agents, inhibitors of th e renin-angiotensin system, or any blood pressure lowering agents, history of diabetes, previous CHD, hypertension, atrial fibrillation, or metabolic syndrome, other baseline lipid subfractions or lipoproteins were not associated with an increased stroke risk in this study.
AIM-HIGH has several limitations. Although we enrolled 3,414 participants with adequate sample size to detect a 25% reduction in the primary composite endpoint with 85% power between the treatments, the trial was not designed nor powered to detect a treatment effect for each of the components of the composite primary endpoint. For ischemic stroke, the event rate of 0.5 events/year was comparable to the 0.5 events/year reported by the Cholesterol Treatment Trials Collaboration in the group of participants who received intensive statin therapy16. However, with such a low incidence and only 3 years of follow-up, the trial was not adequately powered to detect a difference in long-term treatment effect even if one existed. The absence of excess ischemic stroke risk in the larger HPS2-THRIVE trial further lowers the possibility of this risk being real. The number of hemorrhagic strokes and TIAs was too few to provide adequate power for an analysis of a potential treatment effect. In previous trials of niacin, particularly the CDP trial, baseline LDL-C levels were much higher and participants were not treated with a statin. The benefit of niacin may be limited to individuals with poorly controlled LDL-C levels. Although it is not possible to exclude the possibility that niacin might in some way interfere with the effects of the statin, there is no pharmacologic basis for such an interaction.
Conclusion
Although there was a numerical excess in ischemic strokes associated with the addition of niacin to simvastatin, the number of events was small and multivariate analysis accounting for known risk factors did not support a significant association between niacin and ischemic stroke risk.
Acknowledgments
The patients participating in the AIM-HIGH trial, the AIM-HIGH investigators, their study coordinators and staff of the trial coordinating center.
Sources of funding:
Supported by the National Heart, Lung, and Blood Institute and by an unrestricted grant from AbbVie (formerly Abbott Laboratories). AbbVie donated the extended-release niacin, the matching placebo, and the ezetimibe; Merck donated the simvastatin. Neither of these companies had any role in the oversight or design of the study or in the analysis or interpretation of the data.
LBG received modest honoraria from Pfizer and the SPARCL trial steering committee; BRC served on the consultant/advisory boards and received modest honoraria from Merck, Pfizer and Roche; RJP has full-time employment with AbbVie.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests (disclosures):
KKT, SG, WSW, DCA, KSS, SCF, WJK and WEB have none to declare
References
- 1.Castelli WP. Cholesterol and lipids in the risk of coronary artery disease—the Framingham Heart Study. Can J Cardiol. 1988;4(suppl A):5A–10A. [PubMed] [Google Scholar]
- 2.Assmann G, Schulte H, von EA, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–S20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 3.Waters DD, LaRosa JC, Barter P, Fruchart JC, Gotto AM, Carter R, et al. Effects of high-dose atorvastatin on cerebrovascular events in patients with stable coronary disease in the TNT (Treating to New Targets) Study. J Am Coll Cardiol. 2006;48:1793–1799. doi: 10.1016/j.jacc.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 5.The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 6.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 8.Bruckett E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention. A systematic review and meta-regression. J Am Coll Cardiol. 2013;61:440–446. doi: 10.1016/j.jacc.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 11.The AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-desnsity lipoprotein cholesterol: Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high Triglycerides: Impact on Global Health outcomes (AIM-HIGH) Am Heart J. 2011;161:471–477. doi: 10.1016/j.ahj.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-desnsity lipoprotein cholesterol: Baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high Triglycerides: Impact on Global Health outcomes (AIM-HIGH) Am Heart J. 2011;161:538–543. doi: 10.1016/j.ahj.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 14.Armitage J on behalf of the HPS2-THRIVE Collaborative Group. HPS2-THRIVE: randomized placebo-controlled trial of ER niacin and laropiprant in 25,673 patients with pre-existing cardiovascular disease. Presented at the American College of Cardiology 2013 Annual Scientific Conference; March 2013; San Francisco. [Google Scholar]
- 15.The Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cholesterol Treatment Trialists Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]