Abstract
The brain is a plastic organ with a capability to reorganize in response to behavior and/or injury. Following injury to the motor cortex or emergent corticospinal pathways, recovery of function depends on the capacity of surviving anatomical resources to recover and repair in response to task-specific training. One such area implicated in poststroke reorganization to promote recovery of upper extremity recovery is the premotor cortex (PMC). This study reviews the role of distinct subdivisions of PMC: dorsal (PMd) and ventral (PMv) premotor cortices as critical anatomical and physiological nodes within the neural networks for the control and learning of goal-oriented reach and grasp actions in healthy individuals and individuals with stroke. Based on evidence emerging from studies of intrinsic and extrinsic connectivity, transcranial magnetic stimulation, functional neuroimaging, and experimental studies in animals and humans, the authors propose 2 distinct patterns of reorganization that differentially engage ipsilesional and contralesional PMC. Research directions that may offer further insights into the role of PMC in motor control, learning, and poststroke recovery are also proposed. This research may facilitate neuroplasticity for maximal recovery of function following brain injury.
Keywords: premotor cortex, motor control learning, poststroke recovery
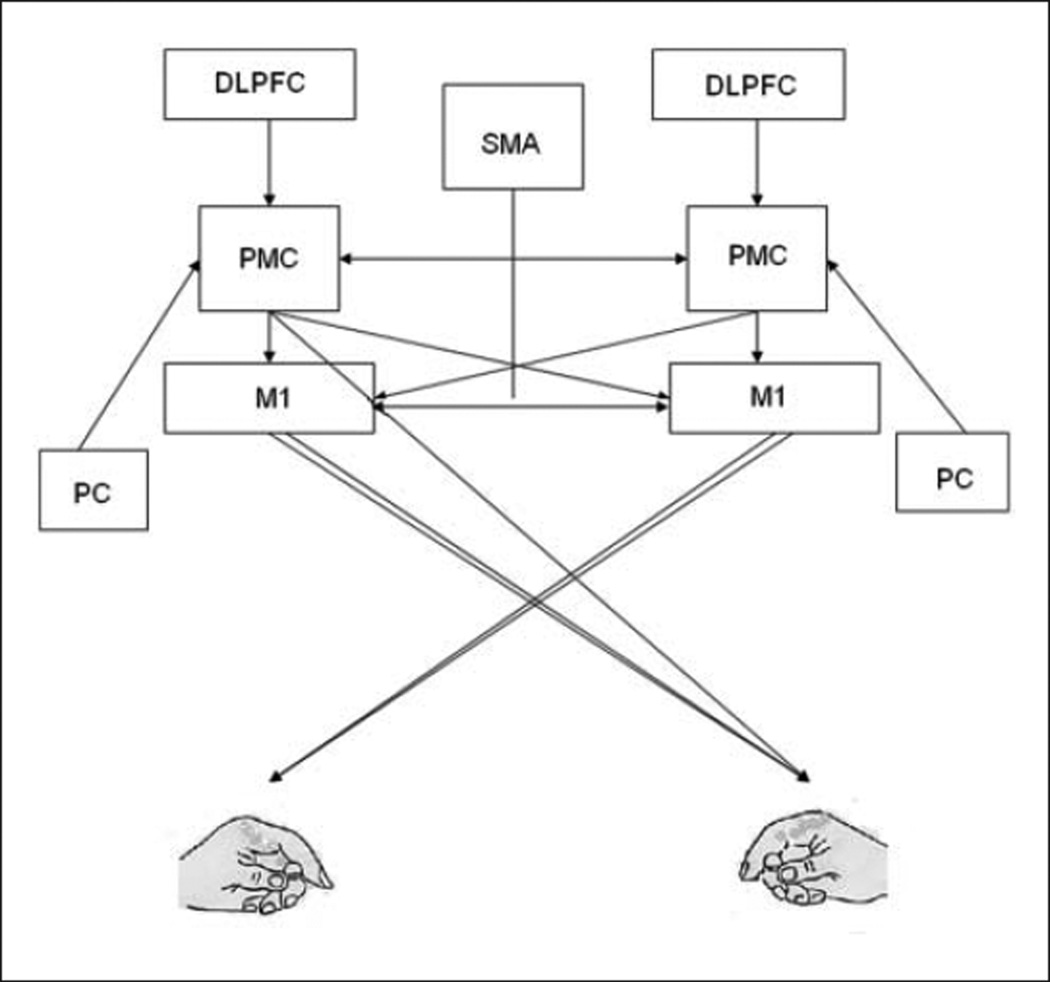
Seemingly effortless goal-directed actions are a final product of complex interactions between multiple sensory, cognitive, and motor areas of the central nervous system. Brain imaging during purposeful actions suggests a functional gradient of cognitive–motor function within the frontal brain regions. The primary motor cortex (M1) is a source of motor commands, whereas the more anterior frontal areas are involved in higher level cognitive aspects of motor control such as decision making, movement selection, and planning. One area critical to motor control and learning of goal-oriented actions is the premotor cortex (PMC). The PMC encompasses the anterior lip of the precentral gyrus, the posterior portion of the middle frontal gyrus, and superior frontal gyrus on the superolateral surface of the brain, corresponding to part of Brodmann’s cytoarchitectonic area (BA) 6. PMC is anatomically positioned between the dorsolateral prefrontal cortex (DLPFC) anteriorly and M1 posteriorly. Functionally, this position within the motor hierarchy allows the PMC to receive direct inputs from the DLPFC and posterior parietal cortex, process this information, and project the output to M1 for movement execution (Figure 1). This functional specialization within the frontal brain areas is plastic such that these brain regions show reorganization with learning, brain injury, and recovery from brain injury. For example, because of its close proximity and similarities in function, PMC is thought to play a significant role in reorganization following injury to M1.1 The new maps created by reorganization may form neural substrates critical for functional recovery following injury to M1.2
Figure 1.
Premotor cortex (PMC) forms a part of the neural network involved in integration of sensory and cognitive information into goal-directed actions. PMC receives sensory information from the parietal cortex (PC), cognitive information from the dorsolateral prefrontal cortex (DLPFC) and supplementary motor cortex (SMA), and projects to the primary motor cortex (M1). In addition, it also has direct projections to the spinal cord via the corticospinal tract. These connections within the neural networks are plastic and are modified in response to injury, learning, and training/therapy.
We examine the role of PMC as a critical node in the neural networks that implement control and learning of goal-oriented actions in healthy individuals and individuals with brain damage to identify future directions for research that can be harnessed to design innovative approaches for enhancing recovery of function following stroke.
Premotor Cortex: Anatomical and Physiological Considerations
The areal boundaries dividing dorsal (PMd) and ventral (PMv) premotor cortices, as well as separating them from surrounding regions, have historically been defined with a rather high degree of variability.3 More recent parcellation attempts, which have used a multimodal approach combining data obtained from complimentary cytoarchitectonic, histochemical, receptor autoradiographic, immunohistochemical, and axonal fiber tracing techniques, have helped resolve these discrepancies.4 This section briefly outlines the differences in intrinsic anatomical organization and extrinsic connectivity patterns between PMd and PMv.
PMd and PMv both can be further subdivided into caudal and rostral parts. The caudal portions of PMd (PMd-C; putatively, area F2 in the macaque brain) and PMv (PMv-C; area F4) are poorly laminated with scattered giant pyramidal cells. These cells are primarily located closer to the border with M1, with overall density being higher in PMd-C relative to PMv-C. The rostral portions of PMd (PMd-R; area F7) and PMv (PMv-R; area F5) are both clearly laminated with respect to their caudal counterparts, and each possesses a prominent layer V. PMd is thought to possess a functional gradient along this rostral–caudal axis. The rostral part (PMd-R) is more engaged in cognitive aspects of motor control; whereas the caudal portion (PMd-C) controls actual movement.5,6
In addition to the distinct cytoarchitecture, PMd and PMv also differ in their extrinsic connectivity with other cortical and subcortical structures.7–10 The PMv is densely interconnected with the hand area of the M1, Supplementary motor area (SMA), the frontal area immediately rostral to the PMv, the ventral portion of BA46, orbitoprefrontal areas 12m and 13l, somatosensory cortex (BAs 3a, 1, 2), area 7b, the anterior intraparietal area (AIP), and parieto-occipital areas. These connections make PMv an important node in the circuit that is critical for sensorimotor processing of grasping. In contrast, PMd-R is densely interconnected with the dorsal aspect of BA46 and the lateral intraparietal area (LIP). PMd-C is interconnected with M1, SMA, the cingulate gyrus, area 5, and the medial intraparietal area (MIP). Differences in the organization of corticospinal projections originating from PMd and PMv have also been observed.11 First, projections from both regions terminate primarily in the intermediate zone of cervical segments of the spinal cord where they synapse directly onto propriospinal neurons.12 This is in stark contrast to the corticomotoneuronal cells located in a caudal subregion of M1 that synapse directly onto alpha motoneurons, providing direct cortical control of muscle activation.13 PMd projections are much more numerous than PMv, and pyramidal cells located in both proximal and distal “arm” representations terminate in upper and lower cervical segments, respectively. In contrast, PMv corticospinal projections only originate from “hand” representations, and counterintuitively terminate within upper, as opposed to lower, cervical segments. Instead of innervating distal arm and hand muscles, the alpha motor neurons within these upper segments innervate neck and shoulder girdle muscles. Thus, corticospinal projections from PMv may play an important role in spinal circuits that orient the head during prehension behavior. Combined, these differences in intrinsic organization and extrinsic connectivity between PMd and PMv reflect their unique functional role in motor control and learning.
Role of PMd and PMv in Motor Control
Accurate performance of a goal-oriented action requires integration of visual and somatosensory information into appropriate motor commands. For example, reaching to grasp a coffee cup on the table requires the information about the location of both the cup and hand in space; their relative location to each other; the size, mass of the cup, and even the temperature of the coffee.14 With this information being relayed by multiple sensory systems, the frontal brain networks plan and generate appropriate motor commands. PMC forms an important node within this neural network. In this section, we discuss the specific role of PMd and PMv in control of goal-oriented actions.
PMd Plays a Role in Goal-Directed Reach Actions
PMd receives integrated visual and somatosensory information from MIP that is used to plan forthcoming arm movement trajectories. Single cell recordings have demonstrated that PMd neurons encode the relative position of the target, hand, and eye in preparation for goal-oriented reach actions.15 Once a forthcoming movement has been instructed, PMd neurons were active during the preparatory phase as well as during online control of reaching movements.16–18 Perturbing human PMd activity using transcranial magnetic stimulation (TMS) increased reaction times, further supporting its critical role in motor planning.19,20 The activity of the PMd neurons during the preparatory phase is thought to reflect 2 main roles: (a) integration of sensory information into motor commands and (b) specification of movement parameters such as amplitude, direction, and speed of movement.
PMd Is Engaged in Action Selection
TMS applied over PMd slows responses to a choice-reaction time task only when applied early during the reaction time (approximately 100–140 ms after the “go” signal). This is in contrast to M1 stimulation that delays the response only if applied approximately 200 ms after the “go” cue. These time-specific effects of PMd and M1 stimulation reflect the distinct roles of the 2 areas: PMd is involved in movement selection, whereas M1 is involved in movement execution.20 Temporary downregulation of PMd with rTMS selectively impaired the learned ability to select a motor response to an arbitrary sensory (visual or auditory) cue.19,21,22 This suggests that PMd is engaged in selection of movement responses based on previously learned arbitrary associations between cues and responses (eg, go when green, stop when red).21 This is likely why patients who have PMD lesions demonstrate deficits in planning and learning of goal-oriented movements as well as anticipatory postural responses.23–25
PMv Forms an Important Part of the “Grasping Network”
PMv is a critical part of the neural network that controls the hand movements for prehension and manipulation of objects. To grasp an object, we preshape our hand to match the structure of the object such that the fingers are positioned around the center of mass of the object. This preshaping of hand occurs early during the reach and relies on feed-forward control mechanisms that transform the visual information of object properties into motor commands of hand muscles. This visuomotor transformation for grasping relies on a neural network that engages the PMv. Transient inactivation of PMv-R in macaque monkeys (functionally homologous area to human PMv) markedly impaired hand preshaping preceding a grasp leading to an inappropriate hand positioning over the object.26 Similarly, a virtual lesion in human PMv using rTMS altered the correct positioning of the fingers on the object as humans reached to grasp it,27 suggesting that visuomotor transformation required for an accurate preshaping of hand to objects of different shapes and sizes relies on PMv.28,29 In addition to feed-forward control, PMv also modulates its influence over M1 to update motor strategies during grasping to accommodate for sudden changes in object properties.30
In addition to effective grasping, manipulation of objects also requires accurate scaling of forces in both a predictive and a reactive manner. As we handle objects in daily life, our nervous system establishes object-specific internal models that are based on the mass, shape, and frictional characteristics of the object. This internal model acquired with experience allows scaling of grip forces in a predictive manner depending on object properties. Single pulse TMS perturbation applied over PMv at the time of peak grasp aperture during a reach-to-grasp task disrupted the predictive scaling of the grip force based on the internal model from previous experience with the object.31 These findings provide support for the notion that PMv is involved in processing object properties relevant for grasping, and relays the information to M1 for generation of motor command for predictive scaling of precision grip during object lift.
PMv and Cognitive Aspects of Motor Control
PMv is also thought to be involved in cognitive aspects of goal-oriented actions. PMv is an integral part of the mirror neuron system: a frontoparietal network that is active when we perform an action and also when we observe a similar action being performed by others.32 This dual activation of PMv mirror neurons during action execution and action observation suggests that these neurons may encode a more abstract representation of motor actions such as understanding the intentions of other’s actions.33 A detailed account of the properties of mirror neurons in PMv is beyond the scope of this review,32,34 but research suggests that the mirror neurons in the PMv encode higher order, abstract representations for goal-oriented actions.
Distinct Contribution of PMv and PMd in Motor Control
Hoshi and Tanji35 directly contrasted the functional role of PMd and PMv in context of a goal-oriented reaching task. They compared preparatory neuronal activity in the PMd and PMv while monkeys were getting prepared to start a reaching action that was selected using instruction cues given before the preparatory period. The majority of PMd neurons exhibited activity tuned to both target location and arm use in the preparatory period for a subsequent arm-reaching action. In contrast, the majority of PMv neurons predominantly showed selectivity for target location, and much less selectivity for arm use. These findings highlight differences between PMd and PMv within the context of a single task: PMv is engaged in encoding the target to be reached, regardless of the arm used to achieve it; whereas PMd is actually engaged in preparation of action once the effector (arm) is specified.35 This also indicates that the PMv is critical for abstract level representation and higher level planning of reaching actions.
In addition to reaching, PMd and PMv, together with AIP are involved in the planning and execution of grasp. Similar to PMv, PMd may also encode an abstract level representation of object–grasp relationship.36 Neuronal recordings suggest that whereas PMv-R neurons encode the object properties for planning and preshaping of the grasp, PMd-C neurons are thought to be important for visuomotor control of wrist and hand orientation for object-specific grasping.37,38 These findings bring into question the apparent dichotomy that PMd is involved in reach and PMv is involved in grasp. This has implications in rehabilitation, given the fact that most of our daily actions involve a combination, that is, reach-to-grasp actions.
PMd and PMv play distinct roles in the control of goal-oriented actions. Whereas PMd is more involved in planning, selection, and preparation of reach-to-grasp actions, PMv is more engaged in the planning and control of prehension and the manipulation of objects. PMv also contains mirror neurons that encode a higher level representation of the motor actions. In the next section, we review evidence related to the role of PMd and PMv in the acquisition of new motor skills.
Role of PMd and PMv in Motor Learning
Learning a motor skill is a cognitive–motor process that leads to acquisition of complex goal-oriented movement skills with practice. Motor learning is characterized by at least 2 distinct time scales: a fast within-practice phase when the performance improves during practice and a slow delayed phase that occurs postpractice leading to performance gains evident during subsequent practice/ testing sessions.39,40 This slow stage involves time- and sleep-dependent consolidation processes. It is evident from animal and human models that a broad neural network, including cortical and subcortical structures, is involved during the 2 phases of motor learning.41–46 The neural structures that are engaged during learning depend on multiple factors such as the type of motor skill (procedural or declarative),47 the task (sequence learning, adaptation, associative learning),48 learner characteristics (novice vs expert),49,50 and structure of practice (random order vs blocked order).51–55
PMd Is Involved in Associative Learning
Neuroimaging studies have reported activation of the PMd during early phases of motor learning.44 This early activation may be associated with increased cognitive processing of information during early phases of motor learning. Specifically, PMd activity is likely related to the development of an internal representation or a spatial map that associates spatial cues with appropriate motor commands.56–58 PMd is critical for associative learning where an internal representation is developed over practice between arbitrary, yet behaviorally relevant cues and appropriate motor commands. Interference to PMd activity in healthy individuals after associative learning impaired the predictive selection of lifting parameters based on the learned associations between color codes and different masses of the object.56 The specialized role of the PMd in associative learning is also supported by clinical observations that patients with PMd lesions are not able to associate sensory stimuli with previously learned movements.59
PMd Activation During Learning Is Related to Cognitive Demands
As discussed before, PMd is critical for premovement cognitive processes such as action selection and planning that also form important elements of motor learning.35,60,61 PMd is actively engaged when the learner has to select one of many action plans based on the sensory cue provided.17,51 Specifically, PMd is thought to be involved when practice invokes a higher cognitive effort. Cross et al51 reported that PMd and M1 demonstrated significantly higher blood oxygen level–dependent (BOLD) activation on functional magnetic resonance imaging (fMRI) during random-order compared with blocked-order practice. Random interleaving of tasks during random-order practice (eg, ACBCABC …) requires the learner to select and plan a new action at every trial compared with blocked-order practice where a single task is repeated for multiple trials in a row before switching to a different task (eg, AAA … BBB … CCC). This implies that PMd is actively engaged in the higher order cognitive processes (eg, action selection and planning) that enhance motor learning. Indeed, in individuals who showed higher performance improvements across practice, there was higher BOLD signal activation within the PMd.62 Interestingly, these “fast learners” also demonstrated higher white matter connectivity of the PMd than those who demonstrated lower improvements in performance (slow learners) across practice.62 All the evidence above strongly suggests engagement of PMd during early cognitive stages of motor learning that is likely related to action selection and planning. Recent evidence has indicated that PMd function can be enhanced during postpractice consolidation phase to improve retention of motor skills.63 These findings indicate noninvasive brain stimulation may influence PMC to facilitate learning when applied immediately after physical practice, a possibility relevant to neurorehabilitation.
PMv Is Engaged in Observational Motor Learning
PMv is engaged in motor learning.64,65 Being well connected with sensory, motor, and high-level cognitive areas, PMv is critical for learning sensorimotor transformations for visually guided actions. PMv neurons are active during performance monitoring and decision making for movement selection as well as processing the outcomes, which are critical elements in practice.66,67 Recent evidence indicates that mirror neurons within the PMv may play a potentially significant role in some forms of motor learning such as observational learning and imitation learning.68
The mirror neurons within the PMv are known to be a part of the mirror neuron network that links observation and action. These mirror mechanisms may form the physiological basis for motor memory and learning by action observation.69 Direct evidence for the role of PMv neurons in observational learning comes from the work of Cross et al.51 Participants underwent fMRI before and after 5 days of physical practice of one dance sequence, and observation of a different dance sequence. BOLD activity in the PMv was evident during observation of both the danced and observed sequence but not during observation of unfamiliar dance sequences. The finding that the activity in PMv did not differ between the danced and observed sequence suggests an overlap of neural substrates between observational and physical practice. These common substrates, that is, PMv may be harnessed to improve motor recovery by combining movement observation and physical practice.34,70
Role of PMd and PMv in Recovery of Function Following Injury to M1
Recovery of patients following stroke is known to involve motor relearning and neuroplasticity that occur within a partially disrupted nervous system.34,71 Fregni and Pascual-Leone72 describe a conceptual framework for brain reorganization for stroke recovery. Recovery following an episode of stroke is implemented partly by reorganization of the intact residual brain areas to enhance relearning of desired goal-directed behaviors. For example, following injury to the M1, parallel networks may emerge at multiple levels of the neuraxis such as corticospinal, corticosubcortical, and corticocortical levels. This section discusses the evidence emerging from animal and human research that indicates reorganization may involve PMC following injury to M1 and its relationship to motor learning and recovery of function.
Using microelectrode stimulation techniques, Frost et al73 examined functional remodeling in cerebral cortex after an experimental ischemic infarct in the hand representation of M1 in adult squirrel monkeys. Improvements in hand motor performance following stroke was associated with reorganization within the PMC. Worthy of note was that the amount of reorganization in PMv was proportional to the extent of injury in M1 hand area. That is, smaller lesions in M1 hand area resulted in small expansions of PMv area devoted to the hand. This suggests that PMv may contribute as a neural substrate for reorganization following injury to M1.73
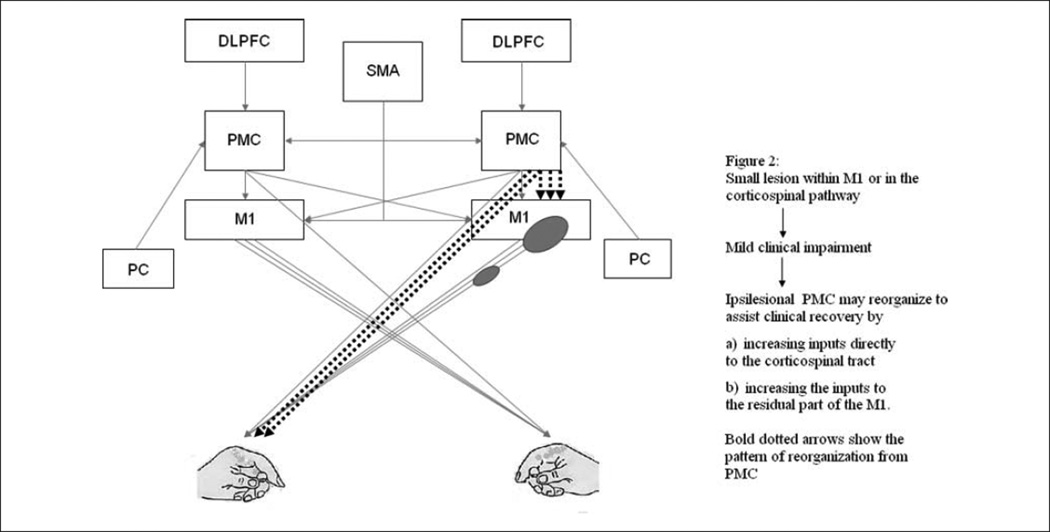
Functional imaging has demonstrated that human brain reorganization following stroke engages a dynamic neural network that includes PMC of the ipsilesional and contralesional hemisphere.61,73–78 However, the precise functional role of PMC reorganization in motor recovery is not well understood. The relative contribution of ipsilesional versus contralesional PMC is likely to depend on the location and size of the stroke and the degree of clinical impairment. When a stroke damages a small part of M1 or corticospinal projections but spares the ipsilesional PMC, the ipsilesional PMC may be more likely to reorganize itself to promote motor recovery (Figure 2). Fridman et al76 reported that in well-recovered chronic patients with stroke disrupting the corticospinal outflow originating in M1, motor potentials evoked by TMS stimulation to the ipsilesional PMd were larger and had a shorter latency than those evoked by stimulation to ipsilesional M1. This indicates that following an injury to M1 or its pathway, ipsilesional PMd may “take over” the motor function of M1 by increasing the strength of PMd projection to the corticospinal tract. This is likely to occur via 2 mechanisms: through increased inputs from the PMC to the spinal cord and through increased inputs to the residual part of M1 (Figure 2). Furthermore, a single TMS pulse disruption applied over the ipsilesional PMd at 120 ms after the “go” signal delayed simple reaction time (RT) of the paretic hand in patients with mild impairments post-stroke. In contrast, there was no effect on the RT when the TMS perturbation was applied over PMv, contralesional M1, PMd, and PMv.76 This indicates that following a small stroke within M1 or corticospinal tract, the ipsilesional PMd reorganizes itself, and this reorganization is functionally relevant for motor function of the paretic hand.
Figure 2.
Figures 2 and 3 show 2 possible ways in which PMC could contribute to functional recovery. The relative engagement of ipsilesional versus contralesional PMC in recovery is likely influenced by the extent and location of the stroke. However, it is important to understand that performance improvements and recovery may emerge from activity in more widespread networks than the ones depicted in Figures 2 and 3. Figure 2 depicts how ipsilesional PMC may reorganize following a small lesion in M1 or corticospinal pathway. Abbreviations: PMC, premotor cortex; DLPFC, dorsolateral prefrontal cortex; SMA, supplementary motor cortex; M1, primary motor cortex.
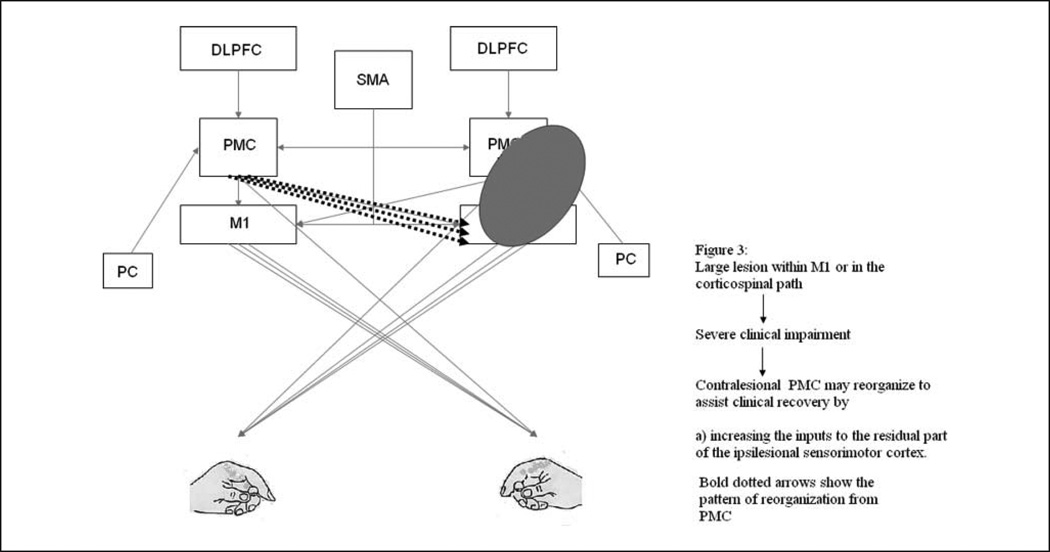
In contrast, when a stroke lesion involves a larger portion of M1 and PMd, it appears that the contralesional PMC may be critical for recovery-related reorganization74,77 (Figure 3). When left PMd activity was perturbed using rTMS prior to an action selection task, a compensatory activity within the right PMd was observed on fMRI and there was no effect on motor performance. Subsequently, when this compensatory activity within the right PMd was perturbed with rTMS, motor performance was attenuated. This suggests that PMd may show functionally specific reorganization following damage to contralateral PMd. Johansen-Berg et al77 reported a slowing of RT when contralesional PMd was perturbed with single-pulse TMS approximately 100 ms after the “go” signal but not when contralesional M1 was perturbed in patients with stroke. This delay in simple RT was not evident in healthy subjects. In patients poststroke with greater clinical impairment, the contralesional PMd is known to exert a less inhibitory/more facilitatory effect over the ipsilesional sensorimotor cortex than that observed in patients with less clinical impairment (Figure 3). More important, this facilitatory influence of contralesional PMd over the ipsilesional sensorimotor cortex is state dependent, that is, the effect is stronger during a grip action with the paretic hand than at rest. It is plausible that the facilitatory input from contralesional PMd to ipsilesional sensorimotor cortex, most likely mediated via the transcallosal pathways, may assist the ipsilesional brain in movement production.74 Furthermore, contralesional PMd may also assist motor recovery via uncrossed pyramidal tract fibers that often innervate proximal arm and leg muscles.
Figure 3.
Figure 3 illustrates how a large lesion in M1 or the corticospinal pathway and subsequent severe impairment may lead to reorganization predominantly involving the contralesional PMC.
These reorganization patterns are crucial for functional recovery after brain damage. An understanding of neural reorganization patterns based on the lesion location, size, and integrity of corticospinal tract is critical to predicting behavioral outcomes and therapy effects in patients poststroke. Tests of motor impairment combined with neuroimaging, genotyping, and neurophysiological assessment of corticospinal integrity and plasticity may allow for improved prognostic accuracy, rehabilitation planning, treatment response, and outcomes.79 For example, injuries to tracts descending from M1 and PMd have been shown to be strongly and negatively correlated with treatment gains in response to robot-aided upper extremity therapy in chronic stroke survivors.80
Finally, not all reorganization may help recovery of motor function. Recently, dynamic causal modeling with functional imaging and connectivity analysis showed that enhancement of paretic hand performance was associated with reduction in overactivity within ipsilesional PMv following single oral dose of reboxetine, a selective noradrenaline reuptake inhibitor.81 This may suggest that some neuroplastic changes within the PMC may actually impede recovery. Further research is required to identify reorganization and connectivity patterns that promote or impede motor recovery post-stroke.82 This information will be critical in planning and testing innovative rehabilitation strategies to promote neuroplastic changes and augment functional outcomes.
Future Directions
Future research needs to investigate the nature of reorganization and identify crucial areas within the reorganized neural networks that constitute the neural substrates of functional recovery.
Hemispheric Specialization of the Premotor Cortex
Hemispheric specialization studies suggest that left PMd activity is associated with unilateral motor performance of either hand, whereas right PMd is involved in bimanual actions.83 For example, left PMv was more active when subjects used their hand or a tool for grasping, reflecting a role of left PMv in abstract-level planning of grasping.84,85 This specialization may be modulated by handedness and the nature of the task being performed.86 It is critical to investigate interactions between handedness, task characteristics, and changes in the nature of hemispheric specialization following unilateral brain damage.
Nature of Reorganization in PMd and PMv Following Brain Damage
Reorganization of interregional influences from and to the PMC follows brain damage, but the cause is unknown. Is the reorganization of PMd a direct consequence of disruption to the corticospinal pathway? Or, is it a compensatory mechanism that is task specific? Alternatively, this reorganization may be driven by training or therapy, constituting an epiphenomenon of performance improvements. Longi tudinal studies using paired-pulse paradigms that investigate the time course of specific interactions between PMC and M1 with practice and/or therapy may allow us in the future to address these questions.87
Can Neuroplasticity Within the PMC Be Enhanced to Improve Learning and Recovery?
Animal and human research suggests that PMd and PMv may demonstrate functionally relevant reorganization during learning and recovery of function after brain damage. It is unclear how training paradigms may be adapted to exploit the beneficial neuroplastic PMC changes for functional motor recovery. For instance, bimanual training has been shown to benefit motor performance after stroke.88 A likely mechanism involves engagement of the contralesional PMC and M1. Furthermore, it is unknown if PMC reorganization can be further exploited by combining noninvasive brain stimulation with intensive task practice to facilitate neuroplasticity and improvement. Imperative to this is the identification of patients who may benefit from such an approach.
The engagement of neural substrates underlying different forms of motor learning and recovery is task specific. With learning, the skill becomes represented within functionally relevant neural networks. Little is known about task characteristics (eg, reach vs grasp) that may preferentially engage PMd or PMv during functional recovery, an issue to be addressed in the future.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- 2.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol. 1991;311:445–462. doi: 10.1002/cne.903110402. [DOI] [PubMed] [Google Scholar]
- 4.Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- 5.Boussaoud D. Attention versus intention in the primate premotor cortex. Neuroimage. 2001;14(1 pt 2):S40–S45. doi: 10.1006/nimg.2001.0816. [DOI] [PubMed] [Google Scholar]
- 6.Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J Neurophysiol. 2002;88:2047–2057. doi: 10.1152/jn.2002.88.4.2047. [DOI] [PubMed] [Google Scholar]
- 7.Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- 8.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 9.Kurata K. Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci Res. 1991;12:263–280. doi: 10.1016/0168-0102(91)90116-g. [DOI] [PubMed] [Google Scholar]
- 10.Stepniewska I, Preuss TM, Kaas JH. Ipsilateral cortical connections of dorsal and ventral premotor areas in New World owl monkeys. J Comp Neurol. 2006;495:691–708. doi: 10.1002/cne.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- 13.Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on corticomotoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey SH, Fogassi L, Grafton S, et al. Neurological principles and rehabilitation of action disorders: computation, anatomy, and physiology (CAP) model. Neurorehabil Neural Repair. 2011;25(5 suppl):6S–20S. doi: 10.1177/1545968311410940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron. 2006;51:125–134. doi: 10.1016/j.neuron.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, van Donkelaar P. The human dorsal premotor cortex generates on-line error corrections during sensorimotor adaptation. J Neurosci. 2006;26:3330–3334. doi: 10.1523/JNEUROSCI.3898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol. 2007;97:188–199. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- 18.Kurata K. Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J Neurophysiol. 1993;69:187–200. doi: 10.1152/jn.1993.69.1.187. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki H, Franca M, Huang YZ, Rothwell JC. The role of dorsal premotor area in reaction task: comparing the “virtual lesion” effect of paired pulse or theta burst transcranial magnetic stimulation. Exp Brain Res. 2005;167:414–421. doi: 10.1007/s00221-005-0047-5. [DOI] [PubMed] [Google Scholar]
- 20.Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121(pt 5):785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- 21.Chouinard PA, Leonard G, Paus T. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci. 2005;25:2277–2284. doi: 10.1523/JNEUROSCI.4649-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- 23.Chang WH, Tang PF, Wang YH, Lin KH, Chiu MJ, Chen SH. Role of the premotor cortex in leg selection and anticipatory postural adjustments associated with a rapid stepping task in patients with stroke. Gait Posture. 2010;32:487–493. doi: 10.1016/j.gaitpost.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116(pt 1):243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- 25.Halsband U, Passingham R. The role of premotor and parietal cortex in the direction of action. Brain Res. 1982;240:368–372. doi: 10.1016/0006-8993(82)90239-6. [DOI] [PubMed] [Google Scholar]
- 26.Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- 27.Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586(pt 11):2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–1057. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dafotakis M, Sparing R, Eickhoff SB, Fink GR, Nowak DA. On the role of the ventral premotor cortex and anterior intraparietal area for predictive and reactive scaling of grip force. Brain Res. 2008;1228:73–80. doi: 10.1016/j.brainres.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Iacoboni M, Mazziotta JC. Mirror neuron system: basic findings and clinical applications. Ann Neurol. 2007;62:213–218. doi: 10.1002/ana.21198. [DOI] [PubMed] [Google Scholar]
- 33.Umiltà MA, Kohler E, Gallese V, et al. I know what you are doing. A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 34.Garrison KA, Winstein CJ, Aziz-Zadeh L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair. 2010;24:404–412. doi: 10.1177/1545968309354536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshi E, Tanji J. Contrasting neuronal activity in the dorsal and ventral premotor areas during preparation to reach. J Neurophysiol. 2002;87:1123–1128. doi: 10.1152/jn.00496.2001. [DOI] [PubMed] [Google Scholar]
- 36.Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- 37.Fattori P, Raos V, Breveglieri R, Bosco A, Marzocchi N, Galletti C. The dorsomedial pathway is not just for reaching: grasping neurons in the medial parieto-occipital cortex of the macaque monkey. J Neurosci. 2011;30:342–349. doi: 10.1523/JNEUROSCI.3800-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raos V, Umiltá MA, Gallese V, Fogassi L. Functional properties of grasping-related neurons in the dorsal premotor area F2 of the macaque monkey. J Neurophysiol. 2004;92:1990–2002. doi: 10.1152/jn.00154.2004. [DOI] [PubMed] [Google Scholar]
- 39.Karni A, Meyer G, Rey-Hipolito C, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS Biol. 2009;7(1):e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian SK, Massie CL, Malcolm MP, Levin MF. Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence. Neurorehabil Neural Repair. 2010;24:113–124. doi: 10.1177/1545968309349941. [DOI] [PubMed] [Google Scholar]
- 43.Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 44.Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci. 2010;30:8332–8341. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Askim T, Indredavik B, Vangberg T, Haberg A. Motor netwrok changes associated with successful motor skill relearning after acute ischemic stroke: a longitudinal fMRI study. Neurorehabil Neural Repair. 2009;23:295–304. doi: 10.1177/1545968308322840. [DOI] [PubMed] [Google Scholar]
- 46.Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Destrebecqz A, Peigneux P, Laureys S, et al. The neural correlates of implicit and explicit sequence learning: Interacting networks revealed by the process dissociation procedure. Learn Mem. 2005;12:480–490. doi: 10.1101/lm.95605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghilardi M, Ghez C, Dhawan V, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 49.Landau SM, D’Esposito M. Sequence learning in pianists and nonpianists: an fMRI study of motor expertise. Cogn Affect Behav Neurosci. 2006;6:246–259. doi: 10.3758/cabn.6.3.246. [DOI] [PubMed] [Google Scholar]
- 50.Tracy J, Flanders A, Madi S, et al. Regional brain activation associated with different performance patterns during learning of a complex motor skill. Cereb Cortex. 2003;13:904–910. doi: 10.1093/cercor/13.9.904. [DOI] [PubMed] [Google Scholar]
- 51.Cross ES, Schmitt PJ, Grafton ST. Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci. 2007;19:1854–1871. doi: 10.1162/jocn.2007.19.11.1854. [DOI] [PubMed] [Google Scholar]
- 52.Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ. Neural substrates of motor memory consolidation depend on practice structure. Nat Neurosci. 2010;13:923–925. doi: 10.1038/nn.2596. [DOI] [PubMed] [Google Scholar]
- 53.Onlaor S, Winstein CJ. Determining the optimal challenge point for motor skill learning in adults with moderately severe Parkinson’s disease. Neurorehabil Neural Repair. 2008;22:385–395. doi: 10.1177/1545968307313508. [DOI] [PubMed] [Google Scholar]
- 54.Lin CH, Fisher BE, Wu AD, Ko YA, Lee LY, Winstein CJ. Neural correlate of the contextual interference effect in motor learning: a kinematic analysis. J Mot Behav. 2009;41:232–242. doi: 10.3200/JMBR.41.3.232-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka S, Honda M, Hanakawa T, Cohen LG. Differential contribution of the supplementary motor area to stabilization of a procedural motor skill acquired through different practice schedules. Cereb Cortex. 2010;20:2114–2121. doi: 10.1093/cercor/bhp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowak DA, Berner J, Herrnberger B, Kammer T, Gron G, Schonfeldt-Lecuona C. Continuous theta-burst stimulation over the dorsal premotor cortex interferes with associative learning during object lifting. Cortex. 2009;45:473–482. doi: 10.1016/j.cortex.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Taubert M, Dafotakis M, Sparing R, et al. Inhibition of the anterior intraparietal area and the dorsal premotor cortex interfere with arbitrary visuomotor mapping. Clin Neurophysiol. 2010;121:408–413. doi: 10.1016/j.clinph.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Buch ER, Brasted PJ, Wise SP. Comparison of population activity in the dorsal premotor cortex and putamen during the learning of arbitrary visuomotor mappings. Exp Brain Res. 2006;169:69–84. doi: 10.1007/s00221-005-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halsband U, Freund HJ. Premotor cortex and conditional motor learning in man. Brain. 1990;113(pt 1):207–222. doi: 10.1093/brain/113.1.207. [DOI] [PubMed] [Google Scholar]
- 60.Hoshi E, Tanji J. Functional specialization in dorsal and ventral premotor areas. Prog Brain Res. 2004;143:507–511. doi: 10.1016/S0079-6123(03)43047-1. [DOI] [PubMed] [Google Scholar]
- 61.O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007;26:2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- 62.Tomassini V, Jbabdi S, Kincses ZT, et al. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyd LA, Linsdell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci. 2009;10:72. doi: 10.1186/1471-2202-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol. 1997;78:977–991. doi: 10.1152/jn.1997.78.2.977. [DOI] [PubMed] [Google Scholar]
- 65.Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. J Cogn Neurosci. 2004;16:127–138. doi: 10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- 66.Pardo-Vazquez JL, Leboran V, Acuna C. Neural correlates of decisions and their outcomes in the ventral premotor cortex. J Neurosci. 2008;28:12396–12408. doi: 10.1523/JNEUROSCI.3396-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pardo-Vazquez JL, Leboran V, Acuna C. A role for the ventral premotor cortex beyond performance monitoring. Proc Natl Acad Sci U S A. 2009;106:18815–18819. doi: 10.1073/pnas.0910524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogt S, Buccino G, Wohlschläger AM, et al. Prefrontal involvement in imitation learning of hand actions: effects of practice and expertise. Neuroimage. 2007;37:1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Stefan K, Cohen LG, Duque J, et al. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cross ES, Kraemer DJ, Hamilton AF, Kelley WM, Grafton ST. Sensitivity of the action observation network to physical and observational learning. Cereb Cortex. 2009;19:315–326. doi: 10.1093/cercor/bhn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 72.Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 74.Bestmann S, Swayne O, Blankenburg F, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30:11926–11937. doi: 10.1523/JNEUROSCI.5642-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125(pt 4):773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 76.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(pt 4):747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 77.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 79.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- 80.Riley JD, Le V, Der-Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol. 2011;69:375–388. doi: 10.1002/ana.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kokotilo KJ, Eng JJ, McKeown M, Boyd LA. Greater activation of secondary motor areas is related to less arm use after stroke. Neurorehabil Neural Repair. 2010;24:78–87. doi: 10.1177/1545968309345269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kermadi I, Liu Y, Rouiller EM. Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosens Mot Res. 2000;17:255–271. doi: 10.1080/08990220050117619. [DOI] [PubMed] [Google Scholar]
- 84.Jacobs S, Danielmeier C, Frey SH. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J Cogn Neurosci. 2010;22:2594–2608. doi: 10.1162/jocn.2009.21372. [DOI] [PubMed] [Google Scholar]
- 85.Martin K, Jacobs S, Frey SH. Handedness-dependent and -independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. Neuroimage. 2011;57:502–512. doi: 10.1016/j.neuroimage.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van den Berg FE, Swinnen SP, Wenderoth N. Hemispheric asymmetries of the premotor cortex are task specific as revealed by disruptive TMS during bimanual versus unimanual movements. Cereb Cortex. 2010;20:2842–2851. doi: 10.1093/cercor/bhq034. [DOI] [PubMed] [Google Scholar]
- 87.Boorman ED, O’Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2010;17:1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 88.Lin KC, Chen YA, Chen CL, Wu CY, Chang YF. The effects of bilateral arm training on motor control and functional performance in chronic stroke. Neurorehabil Neural Repair. 2010;24:42–51. doi: 10.1177/1545968309345268. [DOI] [PubMed] [Google Scholar]