Summary
Background
Improving quality of healthcare is a global priority. Before quality benchmarks are established, we first must understand rates of adverse events. This project assessed risk-adjusted rates of inpatient adverse events for soft tissue reconstructive procedures.
Methods
Patients receiving soft tissue reconstructive procedures from 2005–2010 were extracted from the Nationwide Inpatient Sample. Inpatient adverse events were identified using patient safety indicators (PSI), established measures developed by Agency for Healthcare Research and Quality.
Results
We identified 409,991 patient with soft tissue reconstruction and 16,635 (4.06%) had a PSI during their hospital stay. PSIs were associated with increased risk-adjusted mortality, longer length of stay, and decreased routine disposition (p<.01). Patient characteristics associated with a higher risk-adjusted rate per 1,000 patients at risk (RAR) included older age, men, non-white, and public payer (p<.05). Overall, plastic surgery patients had significantly lower RAR compared to other surgical inpatients for all events evaluated except for failure to rescue and postoperative hemorrhage or hematoma, which were not statistically different. RAR of hematoma hemorrhage were significantly higher in patients receiving size-reduction surgery, and these rates were further accentuated when broken down by gender and payer.
Conclusions
In general, plastic surgery patients had lower rates of in-hospital adverse events than other surgical disciplines, but PSIs were not uncommon. With the establishment of national basal PSI rates in plastic surgery patients, benchmarks can be devised and target areas for quality improvement efforts identified. Further prospective studies should be designed to elucidate the drivers of adverse events identified in this population.
Keywords: Patient Safety, Plastic soft tissue reconstructive surgery, adverse events, outcomes research
Introduction
The Institute of Medicine’s (IOM) reports on healthcare delivery 1,2 estimated approximately 200,000 patient deaths yearly attributable to system related deficiencies. Though plastic reconstructive procedures generally have low mortality, the same system failures noted in the IOM reports that result in complication and mortality also impact plastic surgical patients. Recent articles highlight the importance of risk-limiting techniques for modern plastic surgeons.3,4 To apply these techniques and improve plastic surgery care, we must first understand the current state of the field by measuring and monitoring rates of preventable adverse events.
Adverse events are defined as unintended injuries caused by medical care rather than underlying disease. Rates of adverse events vary substantially between hospitals and surgery types.5–8 Adverse events are not rare; 3.7% of all hospital admissions experience an adverse event and the majority of these events are considered preventable. 9,10 Beyond the impact on the patient and their family, adverse events increase hospital resource utilization and associated costs.11 Given the broad impact of adverse events, there has been global prioritization of patient safety and associated hospital performance.
Plastic surgery is a unique field and likely has a different profile of adverse events compared to other subspecialties.7,12–14 Before establishing quality benchmarks; we first must understand national rates of adverse events. Risk-adjusted rates for individual surgical procedures provide information on which patients undergoing what procedures are at higher risk. Identifying these high-risk patients help guide future clinical studies focused on patient safety. Our specific aim was to assess risk-adjusted rates of inpatient adverse events for general reconstructive soft tissue procedures using established measures. Our secondary goal was to perform a detailed evaluation of these rates in a specific procedure, dermolipectomy, to highlight at-risk patient populations.
Methods
Data Source
We extracted data from the 2005–2009 Nationwide Inpatient Sample database (NIS), developed by the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project. NIS contains hospital discharge records for over 8 million hospital stays. Data are collected from over 1,000 different hospitals across the US and represent approximately 20% of US community hospitals. Data from the NIS are weighted to represent all US hospital stays.15
Patient Safety Indicators
To identify adverse events (AE) during an inpatient hospital stay, we used Patient Safety Indicators (PSI). These measures use standardized methodology to identify possible AEs using hospital ICD-9 codes. AHRQ created these measures by using input from literature review, clinicians, and coding specialists. These indicators are based on Medicare Severity Diagnosis Related Groups (MS-DRGs) and ICD-9-CM codes.16 AHRQ has developed software to identify PSIs and apply risk adjustments to each event, which include age, sex, age-sex interactions, diagnostic related group (DRG), and comorbidities.17 Each AE has a unique set of inclusion and exclusion criteria and risk-adjustors. We used PSI software version 4.3 and all rates reported use the risk-adjustment provided by the AHRQ software. Only PSIs applicable to plastic surgeries were included (pressure ulcer, death among surgical inpatients with serious treatable conditions, post-operative hemorrhage or hematoma, post-operative respiratory failure, post-operative PE/DVT, and post-operative sepsis). As PSIs identify complications of care, by definition they are limited to secondary diagnoses, thus a patient admitted with a pressure ulcer as the principle diagnosis would not be considered at-risk for a pressure ulcer acquired in-hospital.
Study Sample
The study sample included adults who underwent soft tissue plastic surgery procedures that required an inpatient hospital stay. We focused upon soft tissue reconstructive procedures because these represent the core of plastic surgery and could be performed by any surgeon trained in plastic surgery. Patients were identified by ICD-9-CM procedure codes for soft tissue reconstructive operations. To be included in the cohort the plastic surgery procedure code had to be identified as the principal procedure. We excluded patients with secondary reconstructive procedures, as the adverse events identified may not be related to the soft tissue reconstruction, as in the following example: a trauma patient who had a flap to cover a wound and also had a post-operative pulmonary embolism (PE). Our analysis would not be able to discriminate whether the PE was associated with the flap reconstruction or a previous surgery within the same hospital stay the patient underwent for their trauma. Therefore, to minimize capture of adverse events associated with non-plastic reconstructive surgical care, we included only patients whose primary/first procedure was soft tissue reconstruction.
Our plastic surgery cohort included patient records with the following primary procedures identified using ICD-9-CM codes: Hand Skin Graft (86.61, 86.62, 86.63); Free Skin Graft NEC (86.69); Pedicle Graft/Flap (86.70, 86.71, 86.72, 86.74, 86.75); Size Reduction (86.83); Skin Repair & Plasty NEC(86.89); and Breast Reconstruction (85.7X). To allow reconstructive patients to be compared to all other adult surgical patients, we established a comparison group using the 2009 NIS database that included all other surgical inpatients with elective admission.
We sub-analyzed patients who underwent size reduction surgery (ICD-9 86.83). With the increase of patients receiving bariatric surgery, there has been an increase in elective size reduction surgeries, such as dermilipectomy or liposuction. The patients in this cohort had an inpatient stay with a median length of stay (LOS) of 2 days, thus eliminating any who had elective office procedures. This population has high reported rates of adverse events, such as hematoma rates from 5% to 50%.18,19 Therefore we felt this sub-population of elective procedures should undergo more in-depth analyses.
Procedure Indicators
To better understand the case-mix of patients undergoing the selected reconstructive procedures of interest, we identified common diagnoses for our patients. The diagnoses included burn patients (ICD-9-CM 94x), post-operative complications (ICD-9-CM 998), Osteomyelitis, periostitis, and other infections involving bone (ICD-9-CM 730), and spinal cord injury patients (ICD-9-CM 344.0, 344.1, 806, 907.2, 952).
Surgeon Specialty
To understand whether these procedures were performed by a plastic or general surgeon, we used an approach previously defined to obtain surgeon information in the NIS database using de-identified physician identifiers.20 A physician was classified as a “plastic” surgeon if s/he performed a breast reduction or breast reconstruction surgery during the same year s/he performed one of the identified procedures in our study. Likewise, if the surgeon performed an appendectomy s/he was classified as a “general” surgeon.
Statistical Analysis
As the NIS database is survey-based, all analyses accounted for the study design using weights, clusters, and stratum. Univariate comparison between patients with and without a PSI event were made using Rao-Scott χ2 for categorical variables and Student’s t-test for normally distributed continuous variables. The Wilcoxon-Rank Sum test was used for analyses of length of stay and total hospital charges, as these data are not normally distributed. All PSI rates are reported per 1,000 patients at risk. The risk adjusted rates account for age, sex, comorbidities, severity of illness, etc to allow fair comparisons across groups. As our multivariate analysis contained both patient and hospital characteristics, a mixed model was used which accounted for hospital random effects. The use of a mixed model accounts for clustering of patients within hospitals and unmeasured hospital effects. The model included age, gender, race, primary payer, hospital bedsize, hospital teaching status, hospital location (urban vs. rural) and hospital region. These variables were chosen based on the univariate analysis. Comorbidities were not included in the regression model, as the adverse events themselves (and associated complications) are among those codes. All analyses used SAS software 9.2 (SAS Institute Inc, Cary NC).
Results
A total of 409,991 patient records in the NIS database included a principal soft tissue reconstructive procedure between 2005 and 2010. Table 1 presents the breakdown of patients by different procedures. Free skin graft NEC (not elsewhere classified) was the most frequent procedure, representing 37.92% of all patients in the study followed by patients undergoing a pedicle graft or flap (24.74%) and size reduction (20.64%). The least frequent operations were skin repair and plasty, NEC (1.83%). Overall 16,635 (4.06%) patients developed an AE during their hospital stay. Patients receiving a pedicle graft or flap had the highest percentage of AEs (5.08%). Breast reconstruction had the lowest percentage reported AEs (1.97%). To better understand the diagnosis necessitating the soft tissue reconstruction, we assessed the most common patient diagnoses coded during the patient’s hospital stay. (Table 2) This showed a diverse set of diagnoses (spinal cord injury, burns and post-operative complications) for the most common procedures skin graft and flaps.
Table 1.
Inpatient Plastic Surgery Procedures in the US from 2005–2010 and Frequency of PSI Development.
| ICD-9-CM Codes | Procedure Name | N | Performed by Plastic Surgeon | N with ≥1 PSI, (%) |
|---|---|---|---|---|
| 86.61, 86.62, 86.63 | Hand Skin Graft | 25,457 | 58.79% | 958 (3.76) |
| 86.69 | Free Skin Graft NEC | 155,472 | 58.65% | 7834 (5.04) |
| 86.70, 86.71, 86.72, 86.74 | Pedicle Graft/Flap | 101,424 | 54.53% | 5,149 (5.08) |
| 86.83 | Size Reduction | 84,605 | 47.87% | 1869 (2.21) |
| 86.89 | Skin Repair & Plasty NEC | 7,501 | 54.18% | 126 (1.68) |
| 85.7x | Breast Reconstruction | 35,532 | 73.33% | 699 (1.97) |
| Total | 409,991 | 56.59% | 16,635 (4.06) |
Table 2.
Indicators of Procedure Plastic Surgery Procedure
| Surgical Procedure | Median LOS | Post Operative Complication | Spinal Cord Injury | Burn | Bone Infection |
|---|---|---|---|---|---|
| Hand Skin Graft | 6.0 | 9.35% | 0.65% | 44.49% | 2.72% |
| Free Skin Graft NEC | 9.0 | 15.83% | 1.71% | 30.23% | 5.38% |
| Pedicle Graft/Flap | 7.0 | 24.45% | 13.51% | 0.92% | 17.51% |
| Size Reduction | 2.0 | 5.36% | 0.04% | 0.00% | 0.01% |
| Skin Repair & Plasty NEC | 2.0 | 9.75% | 1.40% | 0.43% | 1.65% |
| Breast Reconstruction | 4.0 | 6.84% | 0.00% | 0.10% | 0.01% |
| Total | 5.0 | 14.51 | 4.06% | 14.47% | 6.57% |
Table 3 displays demographics stratified by patients with and without any PSI during their hospital stay. The majority of patients receiving soft tissue reconstruction are between 40–64 years of age and patients with a PSI were more frequently older (p<.0001). Patient with a PSI were more likely to be male, non-white with Medicare or Medicaid (government insurance) (p<.05). Most patients had few noted comorbidities; of patients who had a PSI, 92.6% had no recorded comborbidity, compared to 96.2% of patients with no PSI (p<.0001).
Table 3.
Characteristics of the study population stratified by PSI development.
| Patient Characteristics | No PSI n=393,357 | Any PSI n=16,635 | P-Value |
|---|---|---|---|
| Age, % | <.0001 | ||
| 18–39 | 26.3 | 20.6 | |
| 40–64 | 52.4 | 51.5 | |
| 65+ | 21.3 | 27.8 | |
| Male | 45.7 | 55.2 | <.0001 |
| Race | |||
| White | 70.7 | 70.0 | 0.0498 |
| Black | 13.1 | 14.9 | |
| Hispanic | 10.3 | 8.9 | |
| Asian/Pacific Islander | 1.7 | 1.0 | |
| Native American | 0.8 | 1.3 | |
| Other | 3.5 | 3.9 | |
| Primary Payer | <.0001 | ||
| Medicare | 28.4 | 36.9 | |
| Medicaid | 11.2 | 14.5 | |
| Private | 36.5 | 34.4 | |
| Other | 23.9 | 14.2 | |
| Comorbidity+ | |||
| No Comorbidity | 96.2 | 92.6 | <.0001 |
| # of comorbidities, mean (SD) | 0.2 (0.5) | 0.4 (0.6) | <.0001 |
| # of diagnoses, mean (SD) | 7.3 (4.8) | 12.7(6.4) | <.0001 |
| Outcome | |||
| Risk-Adjusted Mortality | 0.6 | 7.5 | <.0001 |
| LOS, mean (SD) | 9.6 (14.7) | 27.1 (30.1) | <.0001 |
| Total Charges, Mean (SD) | $69.1K ($110K) | $195.7K ($234K) | <.0001 |
| Routine Disposition | 64.0 | 35.5 | <.0001 |
| Hospital Characteristics | |||
| Week-end | 9.4 | 13.8 | <.0001 |
| Hospital Bedsize | 0.0485 | ||
| Small | 11.1 | 9.3 | |
| Medium | 21.3 | 19.2 | |
| Large | 67.7 | 71.5 | |
| Teaching | 67.4 | 70.9 | 0.0195 |
| Urban | 96.0 | 97.8 | <.0001 |
| Region | 0.0008 | ||
| Northeast | 21.6 | 17.0 | |
| Midwest | 20.6 | 24.1 | |
| South | 36.8 | 39.9 | |
| West | 21.1 | 19.0 | |
Outcomes varied significantly between patients with and without a PSI. Risk-adjusted mortality rates for patients with a PSI were 7.5% compared to 0.6% for patients without a PSI (p<.0001). Average length of stay (LOS, in days) was almost triple in patients with a PSI (27.1) compared to those without a PSI (9.6) (p<.0001). Correspondingly, total charges were significantly higher in patients with PSI compared to those without PSI ($195,700 vs. $69,100, respectively) (p<.0001).
Hospital characteristics are shown in Table 2. These examine the association of hospital characteristics. Patients with a PSI were more likely to be discharged from a teaching hospital in an urban area compared to patients without a PSI (p<.05). There were slight differences in the distribution of patients with PSIs compared to those without a PSI by regional area, with the Northeastern US having the fewest patients with a PSI (17.0%, p=0.0008)
Mixed effect models confirmed statistical significance from the univariate results for patient age (p<.0001), race (p<.0001), primary payer (p<.0001) and gender (p<.0001). The odds of developing a PSI during hospitalization were 18% higher in Blacks compared to whites (odds ratio [OR]: 1.179, 95% confidence interval [CI]: 1.121 – 1.241). Both Medicare and Medicaid recipients had higher odds of developing a PSI compared to patients with private insurance (OR: 1.094, CI: 1.047–1.142 and OR: 1.353, CI: 1.287–1.423, respectively). The odds of developing a PSI were 44% greater in males compared to females (OR: 1.444, CI: 1.398–1.491). Receiving an operation in a smaller non-teaching hospitals showed decreased odds for developing a PSI (p<.0001). There were also regional variations. Compared to the western US, patients in the south were 21% more likely to develop a PSI and 22% more likely in the Midwest (OR: 1.207, CI: 1.155–1.126 and OR: 1.224, CI: 1.164–1.288, respectively). Patients in the northeast had decreased odds of developing a PSI compared to patients in the west (OR: 0.852, CI: 0.808–0.898).
Risk-adjusted PSI rates for our reconstructive cohort and all other surgical procedures cohort are compared in Figure 1. Plastic surgery patients had significantly lower rates of most PSI’s compared to all other surgical patients. The only two PSI’s that had similar rates between the plastic surgery group and the other surgical groups were death among surgical inpatients with serious treatable conditions (128.26 vs. 139.45, p=0.848) and postoperative hemorrhage/hematoma (p=0.089). Plastic surgery had significantly lower PSI risk-adjusted rates for pressure ulcer (26.39 vs. 36.11, p<0.0001), PO pulmonary embolism or deep vein thrombosis (PE/DVT) (14.77 vs. 18.01, p<.0001), and PO sepsis (17.84 vs. 21.87, p<.0001). Data for PSI risk-adjusted rates per 1,000 patients at risk for each individual procedure are available as a supplement.
Figure 1.
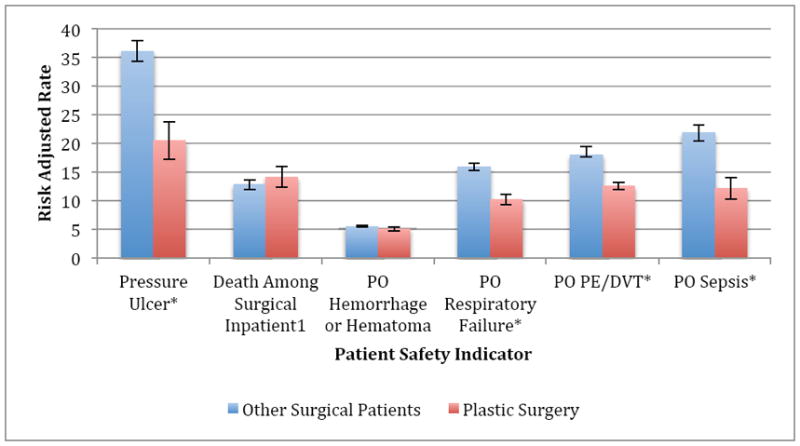
Data for Death Among Surgical Inpatients are shown as risk-adjusted rates per 100 patients at risk for better visualization.
* Statistically significant at p<.0001
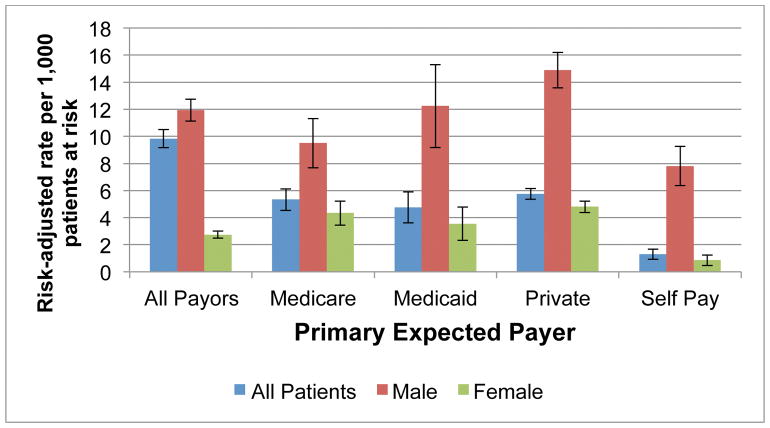
We next separately analyzed the size reduction group. These 84,605 patients represented the third largest group. Size reduction procedures had significantly lower risk-adjusted PSI rates compared to all other surgical inpatients: pressure ulcer (13.68 vs 36.11, p<.0001), PO respiratory failure (7.09 vs 15.89, p<.0001), PO PE/DVT (5.99 vs. 18.01, p<.0001), and PO sepsis (11.09 vs. 21.87, p<.0001). (Table Supplement) However, these patients had significantly higher rates of PO hemorrhage or hematoma (9.83 vs. 5.59, p<.0001) compared to all surgical patients. Further investigation of the higher hematoma rates revealed that men had significantly higher rates compared to females (11.93 vs 2.74, p<.0001). Men with private payer had the highest risk-adjusted rate (14.89), followed by Medicaid (12.24), Medicare (9.50), and self-pay (7.81) (p<.0001) (Figure 2).
Figure 2.
Discussion
Plastic surgery is a unique surgical discipline. There are essentially two groups of patients requiring reconstructive surgery: elective patients who are generally young, healthy adults and complex patients requiring reconstructive surgery due to other conditions, such as wound closure for exposed hardware, reconstruction after tumor extirpation or injury repair such as burns. In our inpatient study population, we found overall reconstructive patients had lower risk-adjusted rates of PSIs than other surgical disciplines, but PSIs were not uncommon. Over this five-year period, a total of 16,635 patients experienced at least one potentially preventable adverse event during their hospital stay. These events led to over seven percent excess mortality, more than double a patient’s length of stay and substantial associated hospital charges.
The first goal of our study was to describe the current rates of adverse events for the field. Not surprisingly, we found that PSI risk-adjusted rate for plastic surgery patients were significantly lower than all other surgical patients with the exception of death among surgical in-patients with serious treatable complications (PSI04) and post-operative hemorrhage/hematoma (PSI09). The finding that the rate of PS104, also known as “failure to rescue”, was similar between the soft tissue group and all other surgical patients was surprising. Failure to rescue is an interesting variable that has received a large amount of attention in health quality research.21 It represents death after surgery from a hospital acquired serious condition, such as DVT or pneumonia (i.e. failure to prevent mortality after a complication has occurred). Some patients who require soft tissue reconstruction, such as burn patients, are at high risk for complications and mortality. Lower rates of failure to rescue seem to be related to hospital factors such as volume of surgery and high nurse to patient ratios.22,23 Thus to improve PSIO4 rates in soft tissue reconstruction, a first step may be to focus on hospital factors in high risk populations.
Pressure ulcers represent an important adverse event in the plastic surgery population, with a risk-adjusted rate of 20.46 per 1,000 patients at risk. Plastic surgeons often care for pressure ulcers but the pressure ulcer diagnoses identified in this study were not the primary diagnosis but developed secondarily after a soft tissue reconstructive procedure: a hospital-acquired pressure sore developed in plastic surgical patients. Pressure ulcers are an important safety indicator as demonstrated by their “never-event” status established by Medicare24 and understanding this adverse event is part of maintaining U.S. plastic surgery certification.25 It is of value to highlight this adverse event occurs in plastic surgery patients because once recognized as an area for improvement, plastic surgeons have the ability and knowledge to prevent this outcome.
Our final analyses looked deeply into a growing plastic surgery subgroup, size reduction patients. This subgroup consists of patients receiving body-contouring procedures commonly performed after significant weight loss. These elective surgeries have been increasing due to increased in bariatric procedures; it represents the third largest subgroup in this analysis.26 We found that this group had high rates of hematoma, which is consistent with published rates in the literature.27,28 This finding of high rates of clinically significant hematomas is related to a topic that has been extensively discussed within the plastic surgery community: post-operative hematoma and the use of chemical DVT prophylaxis.29 Plastic surgeons are concerned that chemical prophylaxis might increase the rate of hematomas and there is evidence that substantial disparities exist in the use of such treatment.30 Indeed many plastic surgeons do not follow the guidelines due to their concern about a high risk of hematoma beneath large undermined areas. 31,32 This data suggests that plastic surgeons’ concerns about hematoma are valid especially for men having size reduction surgery. Thus this analysis helps direct next steps to improve quality of care by highlighting areas where further detailed studies can be focused. Clinically rich data are warranted to validate these increased rates of hematoma and analyze the causality of such events.
Similar to other studies, PSIs among plastic surgery patients were more frequently seen in Blacks with public insurance, both Medicare and Medicaid treated in urban, teaching hospitals.7,33,34 These data might reflect delayed access to care, although further studies are needed in these different patient populations. The disparities shown in our data for these elective procedures highlight the need to establish tangible benchmarks. Our study is the first step at obtaining measurements of these rates in this unique surgical specialty.
Limitations
Our study has important limitations. Using administrative data, we are aware of miscoded events and incomplete risk adjustment due to ICD-9 coding limitations and completeness for secondary diagnoses.14,35 Also, these data have significant noise, certainly for hospitals in the lowest tertile of plastic surgery procedures. Therefore we are only reporting data at the aggregate national level, with low volume hospitals contributing to both the numerator and denominator of each event.
Another concern is the validity of identified adverse events due to the lack of present on admission (POA) coding.36,37 Certain PSIs are greatly influenced by the inclusion of POA information and the validity of these rates is questionable in the absence of POA codes. 38–40 Despite these limitations, PSIs are used for safety monitoring across the nation, and often considered an important first step in identifying clinical targets for more detailed clinical data exploration.
Conclusions
This study establishes current rates for adverse events within specific plastic surgery procedures. As plastic surgery patients are relatively young and healthy, it is important to achieve quality improvements to prevent the long-term costs to society and the patient of these adverse events. Because it is based on robust risk adjustment and stratification, this work can serve as a guideline for targeted quality improvement and used in the establishment of future quality benchmarks. Further prospective studies should be designed to elucidate the drivers of adverse events in this population, as the results may be extrapolated and result in strategies to improve outcomes across many surgical disciplines.
Table 4.
Patient and Hospital Risk Factors and Their Association with Any PSI
| Risk Factor | Odds Ratio | 99% Confidence Intervals | p-value | |
|---|---|---|---|---|
| Age | 1.008 | 1.009–1.011 | <.0001 | |
| Race | White | Ref | <.0001 | |
| Black | 1.179 | 1.121–1.241 | ||
| Hispanic | 0.955 | 0.896–1.018 | ||
| Other* | 1.012 | 0.972–1.053 | ||
| Payer | Private | Ref | <.0001 | |
| Medicare | 1.094 | 1.047–1.142 | ||
| Medicaid | 1.353 | 1.287–1.423 | ||
| Other& | 0.645 | 0.613–0.678 | ||
| Gender | Male vs. Female | 1.444 | 1.398–1.491 | <.0001 |
| Comorbidity Index | 1.791 | 1.669–1.922 | <.0001 | |
| Hospital Bed-size | Large | Ref | ||
| Medium | 0.908 | 0.859–0.959 | ||
| Small | 0.887 | 0.852–0.924 | ||
| Teaching Hospital | Non-Teaching vs Teaching | 0.821 | 0.792–0.851 | <.0001 |
| Hospital Region | West | Ref | <.0001 | |
| Northeast | 0.852 | 0.808–0.898 | ||
| Midwest | 1.224 | 1.164–1.288 | ||
| South | 1.207 | 1.155–1.126 |
Acknowledgments
Funding: This project was supported by grant number K01 HS018558 from the Agency for Healthcare Research and Quality. Dr. Curtin was supported by a VA RR&D career development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the Department of Veterans Affairs.
Footnotes
Meetings: A portion of this work will be presented at: Plastic Surgery The Meeting 2012, October 26 – 30, 2012 in New Orleans, Louisiana
Conflict of Interest. None.
References
- 1.Kohn KT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington DC: Institutes of Medicine, National Academy Press; 1999. [PubMed] [Google Scholar]
- 2.IOM, editor. Crossing the quality chasm: a new system for the 21st century. Washington, DC: National Academic Press; 2001. [PubMed] [Google Scholar]
- 3.Poore SO, Sillah NM, Mahajan AY, Gutowski KA. Patient Safety in the Operating Room: II. Intraoperative and Postoperative Plastic and reconstructive surgery. 2012;130:1048–58. doi: 10.1097/PRS.0b013e318267d531. [DOI] [PubMed] [Google Scholar]
- 4.Poore SO, Sillah NM, Mahajan AY, Gutowski KA. Patient safety in the operating room: I. Preoperative Plastic and reconstructive surgery. 2012;130:1038–47. doi: 10.1097/PRS.0b013e31826945d6. [DOI] [PubMed] [Google Scholar]
- 5.Gawande AA, Thomas EJ, Zinner MJ, Brennan TA. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery. 1999;126:66–75. doi: 10.1067/msy.1999.98664. [DOI] [PubMed] [Google Scholar]
- 6.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg. 2008;207:698–704. doi: 10.1016/j.jamcollsurg.2008.06.138. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Boussard T, McDonald K, Morton J, Dalman RL, Bech F. Determinants of Adverse Events in Vascular Surgery. Journal of the American College of Surgeons. 2012 doi: 10.1016/j.jamcollsurg.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan TA, Hebert LE, Laird NM, et al. Hospital characteristics associated with adverse events and substandard care. Jama. 1991;265:3265–9. [PubMed] [Google Scholar]
- 9.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study. I N Engl J Med. 1991;324:370–6. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 10.Gawande A. Striving for Health Care Excellence through Research. AcademyHealth 2005 Annual Research Meeting; 2005; Boston, MA. 2005. [Google Scholar]
- 11.Rivard PE, Luther SL, Christiansen CL, et al. Using patient safety indicators to estimate the impact of potential adverse events on outcomes. Med Care Res Rev. 2008;65:67–87. doi: 10.1177/1077558707309611. [DOI] [PubMed] [Google Scholar]
- 12.NQF Endorsed Standards. Washington, DC: National Quality Forum; 2011. [Google Scholar]
- 13.National Healthcare Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [Google Scholar]
- 14.Romano PS, Geppert JJ, Davies S, Miller MR, Elixhauser A, McDonald KM. A national profile of patient safety in U.S. hospitals. Health Aff (Millwood) 2003;22:154–66. doi: 10.1377/hlthaff.22.2.154. [DOI] [PubMed] [Google Scholar]
- 15.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Effective clinical practice : ECP. 2002;5:143–51. [PubMed] [Google Scholar]
- 16.McDonald KM, Romano PS, Geppert J, et al. Measures of Patient Safety Based on Hospital Administrative Data - The Patient. Rockville (MD): 2002. [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Murshid M, Khalid KN, Shakir A, Bener A. Abdominoplasty in obese and in morbidly obese patients. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2010;63:820–5. doi: 10.1016/j.bjps.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 19.Zuelzer HB, Ratliff CR, Drake DB. Complications of abdominal contouring surgery in obese patients: current status. Annals of plastic surgery. 2010;64:598–604. doi: 10.1097/SAP.0b013e3181cf9f9e. [DOI] [PubMed] [Google Scholar]
- 20.Cowan JA, Jr, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR., Jr Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2003;37:1169–74. doi: 10.1016/s0741-5214(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 21.Gawande AA. Failure to Rescue. Newyorker; 2012. [Google Scholar]
- 22.Glance LG, Dick AW, Osler TM, Mukamel DB, Li Y, Stone PW. The association between nurse staffing and hospital outcomes in injured patients. BMC health services research. 2012;12:247. doi: 10.1186/1472-6963-12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Medical care. 2011;49:1076–81. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal MB. Nonpayment for performance? Medicare’s new reimbursement rule. N Engl J Med. 2007;357:1573–5. doi: 10.1056/NEJMp078184. [DOI] [PubMed] [Google Scholar]
- 25.Tchanque-Fossuo CN, Kuzon WM., Jr An evidence-based approach to pressure sores. Plastic and reconstructive surgery. 2011;127:932–9. doi: 10.1097/PRS.0b013e3182046a02. [DOI] [PubMed] [Google Scholar]
- 26.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surgical endoscopy. 2005;19:616–20. doi: 10.1007/s00464-004-8827-8. [DOI] [PubMed] [Google Scholar]
- 27.Arthurs ZM, Cuadrado D, Sohn V, et al. Post-bariatric panniculectomy: pre-panniculectomy body mass index impacts the complication profile. American journal of surgery. 2007;193:567–70. doi: 10.1016/j.amjsurg.2007.01.006. discussion 70. [DOI] [PubMed] [Google Scholar]
- 28.Araco A, Sorge R, Overton J, Araco F, Gravante G. Postbariatric patients undergoing body-contouring abdominoplasty: two techniques to raise the flap and their influence on postoperative complications. Annals of plastic surgery. 2009;62:613–7. doi: 10.1097/SAP.0b013e3181856d85. [DOI] [PubMed] [Google Scholar]
- 29.Pannucci CJ, Dreszer G, Wachtman CF, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plastic and reconstructive surgery. 2011;128:1093–103. doi: 10.1097/PRS.0b013e31822b6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broughton G, 2nd, Rios JL, Rohrich RJ, Brown SA. Deep venous thrombosis prophylaxis practice and treatment strategies among plastic surgeons: survey results. Plastic and reconstructive surgery. 2007;119:157–74. doi: 10.1097/01.prs.0000240810.52392.51. [DOI] [PubMed] [Google Scholar]
- 31.Schunemann HJ, Cook D, Guyatt G. Methodology for Antithrombotic and Thrombolytic Therapy Guideline Development*. Chest. 2008;133:113S–22S. doi: 10.1378/chest.08-0666. [DOI] [PubMed] [Google Scholar]
- 32.Clavijo-Alvarez JA, Pannucci CJ, Oppenheimer AJ, Wilkins EG, Rubin JP. Prevention of venous thromboembolism in body contouring surgery: a national survey of 596 ASPS surgeons. Annals of plastic surgery. 2011;66:228–32. doi: 10.1097/SAP.0b013e3181e35c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada SL, Montez-Rath ME, Loveland SA, Zhao S, Kressin NR, Rosen AK. Racial Disparities in Patient Safety Indicator (PSI) Rates in the Veterans Health Administration. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol 1: Assessment) Rockville (MD): 2008. [PubMed] [Google Scholar]
- 34.Clement JP, Lindrooth RC, Chukmaitov AS, Chen HF. Does the patient’s payer matter in hospital patient safety?: a study of urban hospitals. Medical care. 2007;45:131–8. doi: 10.1097/01.mlr.0000244636.54588.2b. [DOI] [PubMed] [Google Scholar]
- 35.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Medical care. 1994;32:700–15. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Bahl V, Thompson MA, Kau TY, Hu HM, Campbell DA., Jr Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission? Medical care. 2008;46:516–22. doi: 10.1097/MLR.0b013e31815f537f. [DOI] [PubMed] [Google Scholar]
- 37.Kaafarani HM, Borzecki AM, Itani KM, et al. Validity of selected patient safety indicators: opportunities and concerns. J Am Coll Surg. 2011;212:924–34. doi: 10.1016/j.jamcollsurg.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Houchens RL, Elixhauser A, Romano PS. How often are potential patient safety events present on admission? Jt Comm J Qual Patient Saf. 2008;34:154–63. doi: 10.1016/s1553-7250(08)34018-5. [DOI] [PubMed] [Google Scholar]
- 39.Henderson KE, Recktenwald A, Reichley RM, et al. Clinical validation of the AHRQ postoperative venous thromboembolism patient safety indicator. Jt Comm J Qual Patient Saf. 2009;35:370–6. doi: 10.1016/s1553-7250(09)35052-7. [DOI] [PubMed] [Google Scholar]
- 40.Romano PS, Mull HJ, Rivard PE, et al. Validity of selected AHRQ patient safety indicators based on VA National Surgical Quality Improvement Program data. Health services research. 2009;44:182–204. doi: 10.1111/j.1475-6773.2008.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]