Abstract
Numerous metals are well-known human bladder carcinogens. Despite the significant occupational and public health concern of metals and bladder cancer, the carcinogenic mechanisms remain largely unknown. Chromium, in particular, is a metal of concern as incidences of bladder cancer have been found elevated in chromate workers, and there is an increasing concern for patients with metal hip implants. However, the impact of Cr(VI) on bladder cells has not been studied. We compared chromate toxicity in two bladder cell lines; primary human urothelial cells and hTERT-immortalized human urothelial cells. Hexavalent chromium (Cr(VI)) induced a concentration- and time-dependent increase in chromosome damage in both cell lines, with the hTERT-immortalized cells exhibiting more chromosome damage than the primary cells. Chronic exposure to Cr(VI) also induced a concentration-dependent increase in aneuploid metaphases in both cell lines which was not observed after a 24 h exposure. Aneuploidy induction was higher in the hTERT-immortalized cells. When we correct for uptake, Cr(VI) induces a similar amount of chromosome damage and aneuploidy suggesting that the differences in Cr(VI) sensitivity between the two cells lines were due to differences in uptake. The increase in chromosome instability after chronic chromate treatment suggests this may be a mechanism for chromate-induced bladder cancer specifically and may be a mechanism for metal-induced bladder cancer in general.
Keywords: Chromium, chromate, urothelial, bladder cancer, aneuploidy, genotoxicity
Introduction
Hexavalent chromium [Cr(VI)] is a well-established human carcinogen with most of the focus and attention has been on Cr(VI) acting as a lung carcinogen (IARC, 1990). This primary focus is based, in part, on epidemiology studies, which consistently show Cr(VI) to be a human lung carcinogen; and human pathology studies, which show lung tumors form where Cr(VI) particles impact and persist (Cohen, et al 1993; Ishikawa, et al, 1994). But, this focus is also based, in part, on the toxicology of Cr(VI) which indicates that when reduced extracellularly to Cr(III) there is a loss in toxicity, especially genotoxicity (Xie et al., 2004). In other words, Cr(VI) has been thought to be a site-of-exposure carcinogen because the expectation has been that Cr(III) cannot induce carcinogenesis as it is too poorly absorbed and any Cr(VI) that enters the body would simply be reduced after penetrating the site of exposure.
However, while epidemiology studies have focused attention on the lung, some also consistently show that metal workers with Cr(VI) exposure also have an elevated rate of bladder cancer (IARC 1990). For example, a large study of welders showed an elevation of lung and bladder cancer, that correlated with Cr and nickel (Ni) exposure (Milham, 1983). A study of chrome tannery workers also showed an elevated bladder cancer risk (Montanaro et al., 1997). In addition, two other studies found correlations with occupations involving chromate and bladder cancer (Claude, et al., 1986; Claude et al., 1988). To be clear, these studies do not definitively show Cr(VI) causes bladder cancer. There are power limitations and co-exposures to additional carcinogens that confound their full interpretation, however, at the same time, these confounders do not rule out the possibility that Cr(VI) can cause bladder cancer, and they do suggest such an outcome is possible.
This possibility has become more urgent with the increased use and emerging health concerns of metal-on-metal hip implants. There are several types of metal-on-metal implants with cobalt chrome, titanium-based alloy or stainless steel in the stem. The epidemiology on whether a specific implant confers a greater cancer risk than another is currently poorly developed. However, concern is focused on the implants made of cobalt/chromium (CoCr) alloy, because they are extensively used and the CoCr alloy wears over time and releases a known carcinogen [chromium (Cr)] and a suspected carcinogen [cobalt (Co)] along with CoCr nanoparticles (Keegan, et al., 2007, 2008; Case, et al., 1994). Moreover, the new generation of these CoCr implants are failing in very high numbers, perhaps as high as 50% in some cases (Langton, et al., 2010). Clinical data clearly show the bladder in these patients are directly exposed to high levels of Cr based on blood and urinary excretion levels (Keegan, et al., 2007, 2008; Lhotka, et al., 2003; Pilger, et al., 2002). The valence of Cr released is unclear at this time, but some studies do indicate that Cr(VI) might be released (Keegan, G.M., et al., 2007, 2008; Case, et al., 1994).
Thus, this new public health concern combined with the previous possibility that Cr(VI) causes bladder cancer indicates a need to better understand the impact of Cr(VI) on the bladder. Moreover, our recent data showing a carcinogenic mechanism of Cr(VI)-induced lung cancer that involves chromosome instability (Xie et al., 2007, 2009; Holmes et al., 2006a, 2008, 2010; Wise and Wise, 2010, 2012) is consistent with the fact that chromosome instability is considered a key mechanistic and diagnostic event in bladder carcinogenesis (Florl and Schulz, 2008). Currently, there appears to be no data on the effects of Cr(VI) in bladder cells. Thus, the focus of this study was to investigate the ability of Cr(VI) to induce chromosomal instability in human urothelial cells.
Materials and Methods
Chemicals and Reagents
Sodium chromate, Triton X-100, demecolcine and potassium chloride (KCl) were purchased from Sigma Chemical (St. Louis, MO). Giemsa stain was purchased from Ricca Chemical Company. (Arlington, TX). Crystal violet, sodium dodecyl sulfate (SDS), and acetic acid were purchased from J.T. Baker (Phillipsburg, NJ). Nitric acid and methanol were purchased from BDH Chemicals (Radnor, PA). Gurr’s buffer, Keratinocyte-SFM, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen Corporation (Grand Island, NY). Tissue culture dishes, flasks and plasticware were purchased from BD (Franklin Lakes, NJ). Urothelial cell medium, trypsin/EDTA and trypsin neutralizing solution were purchased from ScienCell Research Laboratories (Carlsbad, CA). Penicillin/streptomycin was purchased from Mediatech (Manassas, VA). Paraformaldehyde 4% was purchased from Alfa Aesar (Ward Hill, MA). Phospho-histone H2A.X (Ser139) antibody was purchased from Cell Signaling (Beverly, MA). AlexaFluor 488-conjugated goat anti-rabbit IgG secondary antibody was purchased from ThermoScientific (Rockford, IL).
Cells and cell culture
Normal human urothelial cells (HUC) were purchased from ScienCell. These cells were maintained in urothelial cell medium supplemented with 1% penicillin/streptomycin and urothelial cell growth supplement and incubated in a 5% CO2 humidity environment at 37°C. hTERT expressing human urothelial cells, hTU1, were obtained as a generous gift from Dr. Louis Liou (described in Kim et al., 2011). hTU1 cells were further subcloned and a stable clone was selected (hTUC1-38). Cells were maintained in keratinocyte-SFM and incubated in a 5% CO2 humidified environment at 37°C. Cells were maintained as adherent subconfluent monolayers, fed twice weekly and subcultured at least once weekly. Cells were routinely tested for mycoplasma contamination.
Chemical treatments
Sodium chromate (CAS #7775-11-3, ACS reagent minimum 98% purity), a soluble hexavalent Cr compound was administered as a solution in water as previously described (Wise J et al., 2002). A stock solution of sodium chromate was suspended in double distilled sterile water the day of treatment. Dilutions were made from the stock solution and administered to the cells for final concentrations ranging from 1–5 uM chromate. Cells were exposed to Cr(VI) for 24 and 120 h.
Intracellular Chromium Ion Measurement
Intracellular chromium ion levels were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) using standard methods (Holmes, et al., 2005). Briefly, a monolayer of cells was treated for 24 or 120 h with varying concentrations of sodium chromate. The cells were harvested by trypsinization and treated with a hypotonic solution followed by 2% SDS. This solution was sheered through a needle seven times and filtered into a vial. Samples of intracellular fluids were diluted 5x in 0.16 M aqueous HNO3 prior to analysis. Chromium concentrations in the intracellular samples were then measured using a Perkin Elmer Optima 2000, equipped with a gem cone low flow nebulizer. Solutions were introduced to the nebulizer using a peristaltic pump operating at 2 mL/min. Chromium was determined using emission wavelength at 267.716 with a minimum detection limit of 2 ppb. Yttrium (Y) was used as an internal standard for chromium determinations. The intracellular concentrations were corrected by converting from ug/L to uM by dividing by the volume of the sample, the atomic weight of the chemical, the number of cells in the sample and the average cell volume for the given cell line.
Immunofluoresence for Gamma-H2A.X Foci Formation
Immunofluorescent staining was performed as previously published (Wise S et al., 2010). Briefly, after a 24 or 120 h sodium chromate treatment, cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Cells were then incubated with an anti-gamma-H2A.X antibody, washed, and incubated with an AlexaFluor 488-conjugated goat anti-rabbit IgG secondary antibody. DNA was counterstained with DAPI. Slides were then coverslipped and analyzed using a laser scanning confocal microscope. One hundred cells were analyzed per concentration using Image J software.
Chromosome Instability Analysis
Structural and numerical chromosome instability was determined using a chromosomal aberration assay based on our published methods (Wise et al., 2002). Briefly, log phase cells were seeded into 100 mm dishes and treated for 24 and 120 h with sodium chromate. Demecolcine (0.1 g/ml) was added 1 h before the end of treatment to arrest the cells in metaphase. Cells were then collected by trypsinization, spun down and resuspended in a 0.075M KCl hypotonic solution for 17 min followed by fixation with 3:1 methanol:acetic acid. The fixative was changed twice then cells were dropped onto clean wet slides and stained with 5% Giemsa stain in Gurr’s buffer. A minimum of one hundred metaphases per treatment were analyzed for aneuploidy and chromosome aberrations scored using standard criteria (Wise J. et al., 2004; Holmes et al., 2010). Each experiment was repeated three times.
Statistical Analysis
We used a one-way ANOVA and SNK method to compare each dose to the control and to compare each dose to each other. P-values of < 0.05 are considered statistically significant.
Results
Intracellular Cr Ion Concentrations Are Higher in hTERT-Immortalized Urothelial Cells
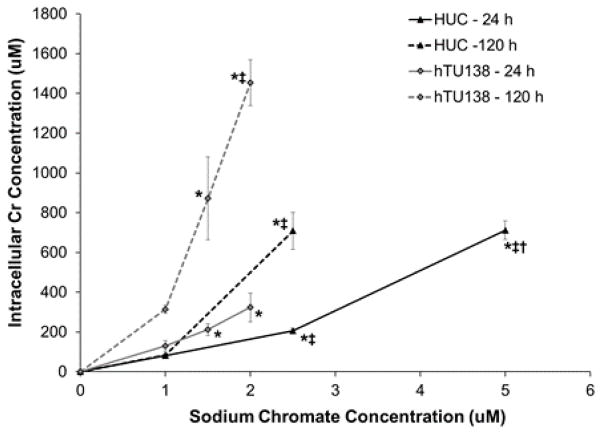
Hexavalent chromium induced a time- and concentration-dependent increase in intracellular Cr ion levels in both primary and hTERT-immortalized urothelial cells (Fig. 1). The hTERT-immortalized cells take up less chromium than primary cells. Following 24 h treatment, concentrations of 0, 1, 2.5 and 5 uM sodium chromate resulted in intracellular Cr ion concentrations of 20, 81, 206, and 712 uM, respectively, in HUCs. At the same time point, hTUC1-38 cells had intracellular Cr ion concentrations of 0, 130, 212, and 324 uM, respectively, with treatment concentrations of 0, 1, 1.5 and 2 uM sodium chromate. After 120 h treatment with concentrations of 0, 1, and 2.5 uM sodium chromate, HUCs had intracellular Cr ion concentrations of 0, 84, and 709 uM, respectively. After 120 treatment with concentrations of 0, 1, 1.5, and 2 uM sodium chromate, hTUC1-38 cells had intracellular Cr ion concentrations of 0, 315, 872, and 1454 uM, respectively.
Figure 1.
hTERT-Immortalized Human Urothelial Cells Take Up More Chromium Than Primary Cells.
This figure shows sodium chromate induced a concentration- and time-dependent increase in intracellular Cr ion concentrations in human urothelial cells with hTERT-immortalized cells taking up more chromium than primary cells. Data represent an average of three experiments ± the standard error of the mean. Statistics: * indicates statistically different from control (p < 0.05); ‡ indicates statistically different from 1 uM sodium chromate (p < 0.05); † indicates statistically different from 2.5 uM sodium chromate (p < 0.001).
Hexavalent Chromium Induces DNA Damage in Urothelial Cells
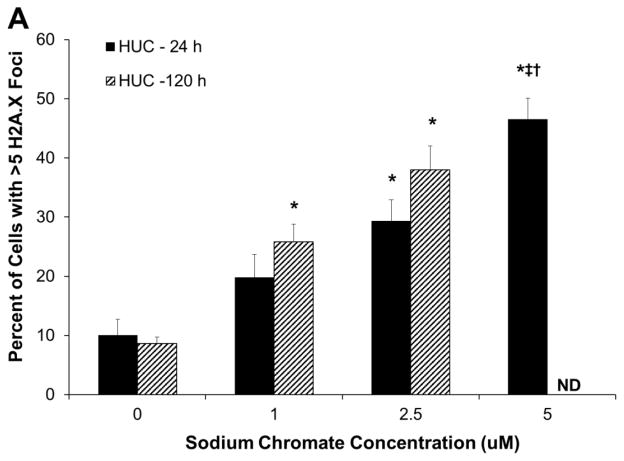
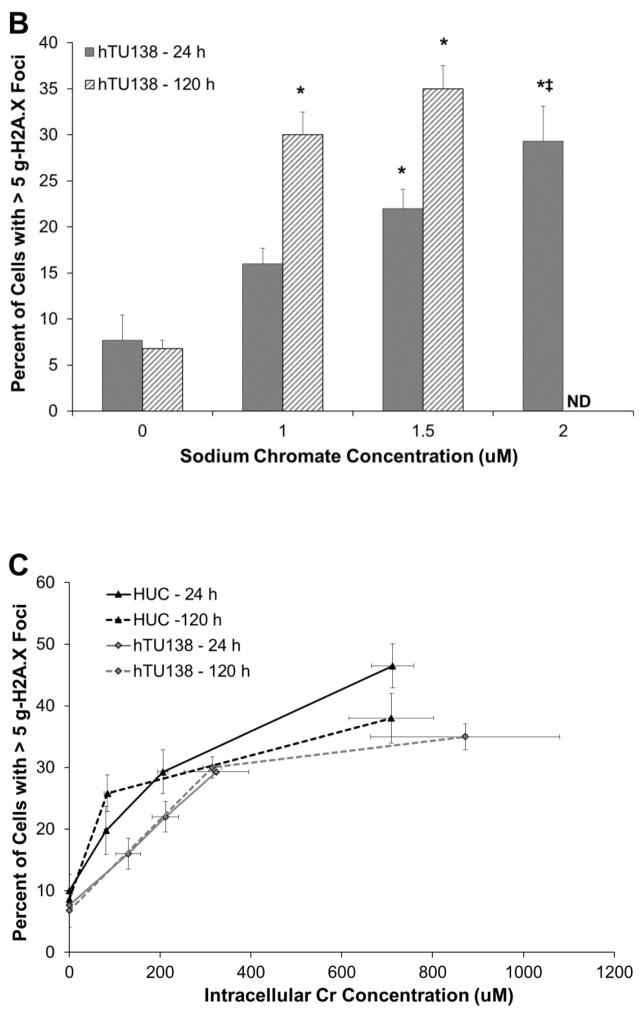
Hexavalent chromium induces a concentration- and time-dependent increase in gamma-H2A.X foci in both primary and hTERT-immortalized urothelial cells. As administered, sodium chromate induced more DNA damage in hTUC1-38 cells than in HUCs (Fig. 2A and 2B). At concentrations of 0, 1, 2.5, and 5 uM sodium chromate there were 7.3, 22.3, 25.7 and 48 percent of cells with greater than 5 foci, respectively, after 24 h exposure; and 8.6, 25.8, and 38 percent of cells with greater than 5 foci with treatments of 0, 1, and 2.5 uM sodium chromate, respectively, after 120 h exposure in HUCs (Fig. 2A). In hTUC1-38 cells, concentrations of 0, 1, 1.5, and 2 uM sodium chromate induced 7.7, 16, 22, and 29.3 percent of cells with greater than 5 foci, respectively, after 24 h of exposure, and concentrations of 0, 1, and 1.5 uM sodium chromate induced 6.8, 30, and 35 percent of cells with greater than 5 foci, respectively, after 120 h of exposure (Fig. 2B). When corrected for intracellular Cr ion levels, both primary and hTERT-immortalized cells had similar amounts of DNA damage (Fig. 2C).
Figure 2.
Hexavalent Chromium Induces DNA Double Strand Breaks in Human Urothelial Cells.
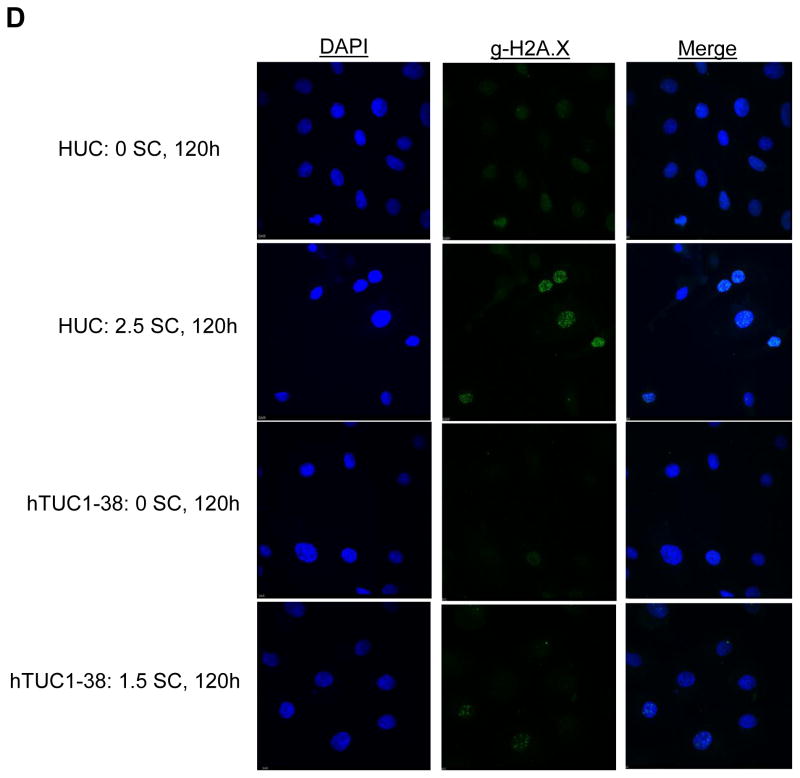
This figure shows acute and chronic exposure to sodium chromate induces DNA double strand breaks in human urothelial cells. A) Primary human urothelial cells. B) hTERT-immortalized human urothelial cells. Based on administered concentration, sodium chromate induces more DNA double strand breaks in hTERT-immortalized cells than in primary cells. ND = Not done. C) When corrected for uptake differences, sodium chromate induces similar levels of gamma-H2A.X foci in primary and hTERT-immortalized human urothelial cells. D) Representative images of gamma-H2A.X foci in primary and hTERT-immortalized human urothelial cells. Data represent an average of at least three experiments ± the standard error of the mean. Statistics: * indicates statistically different from control (p < 0.05); ‡ indicates statistically different from 1 uM sodium chromate (p < 0.05); † indicates statistically different from 2.5 uM sodium chromate (p < 0.01).
Hexavalent Chromium is Clastogenic to Urothelial Cells
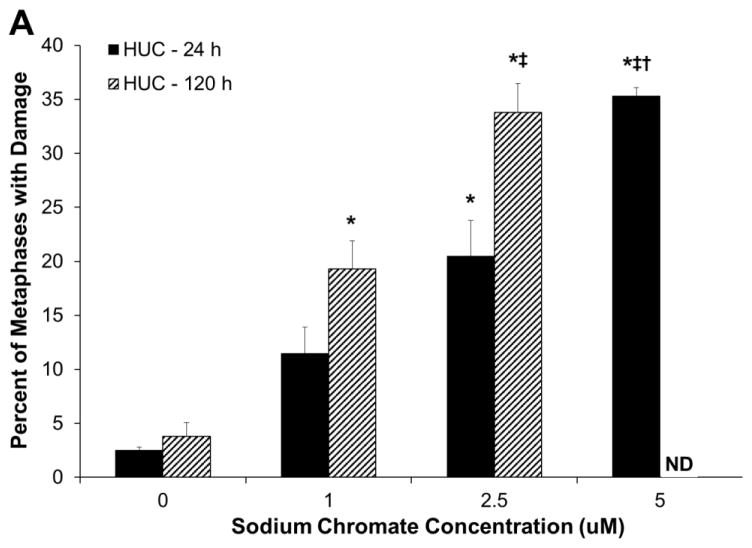
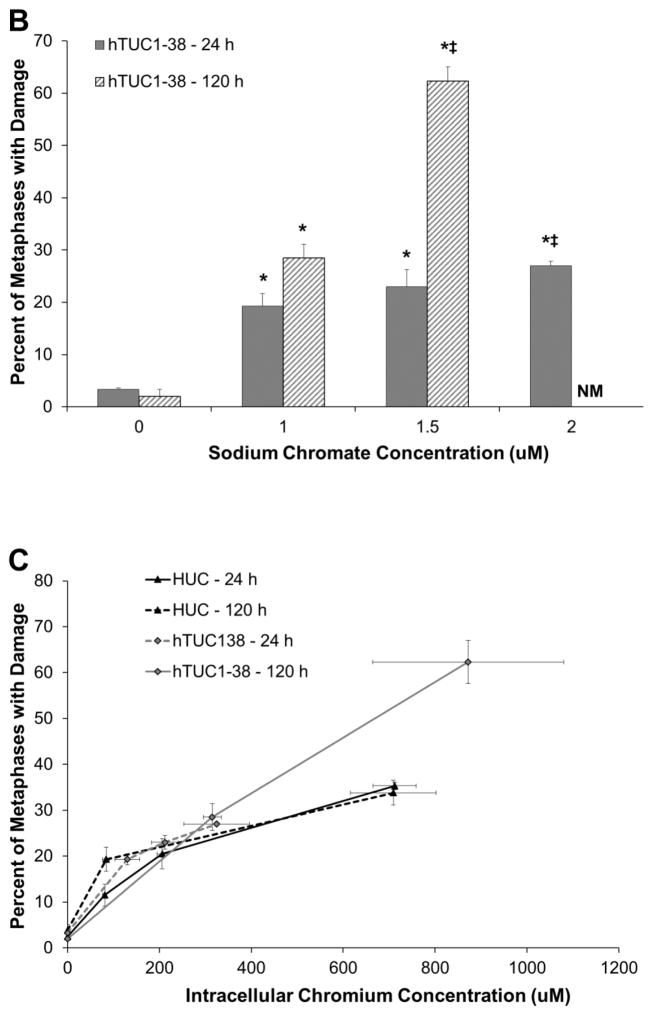
Hexavalent chromium induces a concentration- and time-dependent increase in chromosome damage in both primary and hTERT-immortalized urothelial cells. Based on administered concentration, sodium chromate induces more chromosome damage in hTERT-immortalized cells than in primary cells (Fig. 3A and 3B). HUCs had 2.5, 11.5, 20.5, and 35.3 percent of cells with damage when treated with 0, 1, 2.5 and 5 uM sodium chromate for 24 h and 3.8, 19.3, and 33.8 percent of cells with damage when treated with 0, 1, and 2.5 uM sodium chromate for 120 h. hTUC1-38 cells had 3.3, 19.3, 23, and 27 percent of cells with damage when treated with 0, 1, 1.5 and 2 uM sodium chromate for 24 h and 2, 28.5, and 62.3 percent of cells with damage when treated with 0, 1, and 1.5 uM sodium chromate for 120 h. At concentrations higher than 1.5 uM for 120 h, no metaphases were observed due to cell cycle arrest. Based on intracellular Cr concentrations, sodium chromate induces similar levels of chromosome damage in primary and hTERT-immortalized human urothelial cells (Fig. 3C). Interestingly, there was no effect of time on the amount of damage seen after correcting for chemical uptake in the primary urothelial cells.
Figure 3.
Hexavalent Chromium Induces Chromosome Damage in Human Urothelial Cells.
This figure shows acute and chronic exposure to sodium chromate induces chromosome damage in human urothelial cells. A) Primary human urothelial cells. B) hTERT-immortalized human urothelial cells. Based on administered concentration, sodium chromate induces more chromosome damage in hTERT-immortalized cells than in primary cells. ND = not done. NM = No metaphases. C) Based on intracellular Cr concentrations, sodium chromate induces similar levels of chromosome damage in primary and hTERT-immortalized human urothelial cells. Data represent an average of at least three experiments ± the standard error of the mean. Statistics: * indicates statistically different from control (p < 0.005); ‡ indicates statistically different from 1 uM sodium chromate (p < 0.05); † indicates statistically different from 2.5 uM sodium chromate (p < 0.005).
Hexavalent Chromium is Aneugenic to Urothelial Cells
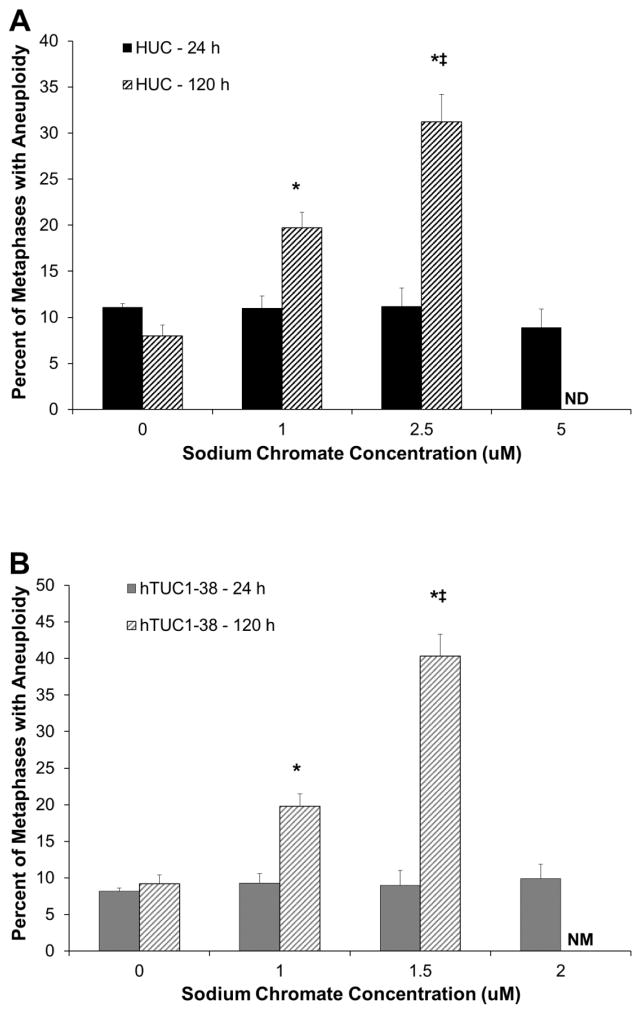
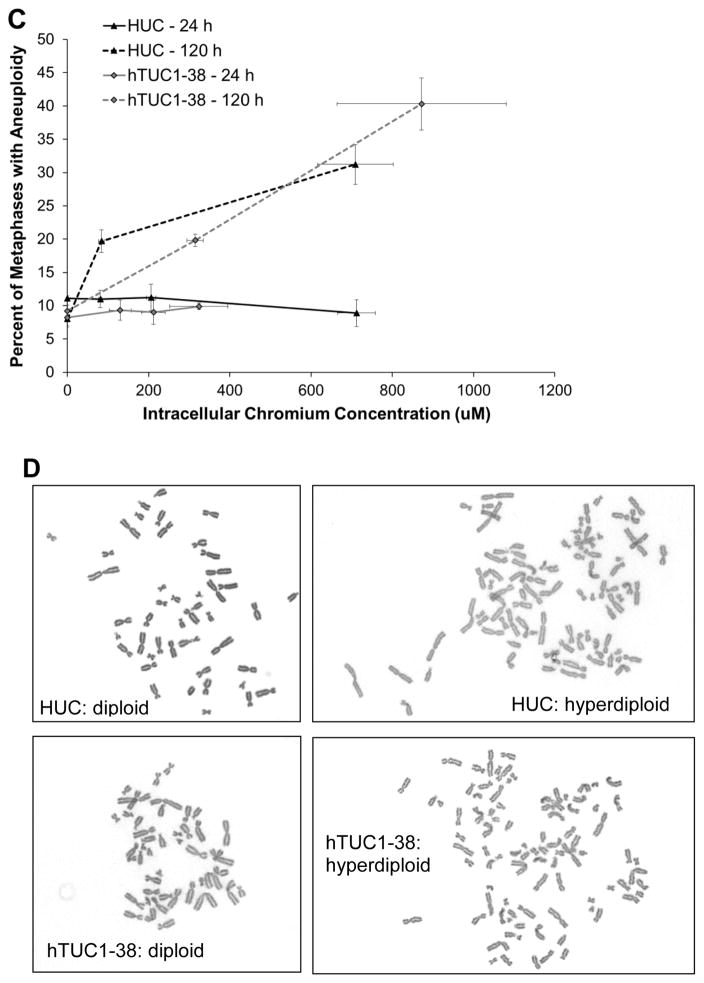
Chronic exposure to Cr(VI) induces a concentration-dependent increase in aneuploidy in both primary and hTERT-immortalized urothelial cells. After 24 h treatment with sodium chromate, no increase in aneuploidy was observed for any concentration in either cell line (Fig. 4 A and B). However, after 120 h, we observed that 8, 19.7, and 31.2 percent of metaphases were aneuploid with treatments of 0, 1, and 2.5 uM sodium chromate in HUCs. In hTUC1-38 cells, we found 9.2, 19.8, and 40.3 percent of metaphases were aneuploid after 120 h treatment with 0, 1, and 1.5 uM sodium chromate for 120 h. Based on intracellular Cr concentrations, there were similar amounts of aneuploidy in both primary and h-TERT-immortalized urothelial cells (Fig. 4C).
Figure 4.
Hexavalent Chromium Induces Aneuploidy in Human Urothelial Cells.
This figure shows chronic exposure to hexavalent chromium induces aneuploidy in human urothelial cells. Exposure to sodium chromate for 24 h does not induce aneuploidy; however, a 120 h exposure induces a concentration-dependent increase in aneuploidy. A) Primary human urothelial cells. B) hTERT-immortalized human urothelial cells. Based on administered concentration, sodium chromate induces more aneuploidy in hTERT-immortalized cells than in primary cells. ND = not done. NM = No metaphases. C) Based on intracellular Cr concentrations, sodium chromate induces similar levels of aneuploidy in primary and hTERT-immortalized human urothelial cells. D) Representative images of aneuploidy in primary and hTERT-immortalized human urothelial cells. Data represent an average of at least three experiments ± the standard error of the mean. Statistics: * indicates statistically different from control (p < 0.05); ‡ indicates statistically different from 1 uM sodium chromate (p < 0.05).
Discussion
Cr(VI) is a potent and known human lung carcinogen and some epidemiologic data suggest that it may also have a role in bladder cancer (IARC, 1990). Recently there has been increased concern for metal-exposed individuals; specifically for patients receiving total hip replacement and other osteo-replacement therapies. While there is, as yet, limited evidence for carcinogenic outcomes for these patients, there is evidence of exposure to known and suspected carcinogens (Cr and Co) as indicated by increased metal levels in urine. Thus, there is increased concern for possible carcinogenic outcomes. This is the first study to investigate the impact of Cr(VI) exposure specifically in human urothelial cells.
We found that Cr(VI) induced genotoxicity in two human urothelial cell lines. We used primary urothelial cells and h-TERT immortalized urothelial cells to establish that these cells have the same response to Cr(VI). We found that the hTERT-immortalized human urothelial cells were more sensitive to Cr(VI). Sodium chromate induced more aneuploidy, chromosome damage and DNA double strand breaks in hTERT-immortalized human urothelial cells compared to primary cells based on the administered sodium chromate doses. However, when we corrected for differences in chromium uptake between the two cell lines, we found that Cr(VI) induced a similar amount of aneuploidy, chromosome damage and DNA double strand breaks indicating that the differences in Cr(VI) sensitivity between the two cells lines were due to differences in uptake. This serves as confirmation that the induction of chromosome instability is a universal mechanism as well as confirming that the hTERT-immortalized cell line had a similar response to metals and will serve as a good tool for studying metal-induced bladder carcinogenesis in future investigations.
Our study is the first study to show that Cr(VI) induces DNA damage in urothelial cells. Zinc chromate and lead chromate have been show to induce DNA double strand breaks in human lung and skin cells using gamma-H2A.X foci formation and single cell gel electrophoresis (Comet assay) (Ha et al., 2004; Xie et al., 2005, 2009). DNA double strand breaks are one of the most dangerous forms of cellular damage. Unrepaired DNA double strand breaks are lethal to the cell and mis-repaired double strand breaks can lead to mutations or chromosome instability.
This study is the first to show that Cr(VI) induces chromosome damage in human urothelial cells. These data are not surprising and are consistent with historical literature showing Cr(VI) is a clastogen (IARC 1990, De Flora et., 1990). Our study is also consistent with current studies using primary and immortalized lung and skin cell lines (Wise, J. et al., 2002; Wise, S. et al. 2004, 2006a; Holmes et al, 2006a, 2006b; Xie et al., 2015). Difficulties arise in trying to compare the urothelial cells to these studies as different cell types require different media which can affect Cr(VI) reduction and dissolution. For example, there was more chromosome damage seen in primary human lung fibroblasts treated with 1 and 2.5 uM sodium chromate, than in the primary urothelial cells; 18 and 33 percent of cells were damaged, respectively, in human lung cells compared to 12 and 20 percent of cell damaged, respectively, in human urothelial cells (Wise, S. et al, 2002). The reasons for these differences are unclear without further investigation but could be due to differences in the cell type (epithelial vs fibroblast), origin (lung vs bladder), or due to differences in culture media (with or without serum). One study used a lung epithelial model using serum free media (Wise, S. et al., 2006a) and showed cells treated with 1, 2.5 and 5 uM sodium chromate induced 25, 34, and 41 percent of metaphases with damage whereas our current study found 12, 20, and 35 percent of metaphases with damage at the same administered concentrations of sodium chromate in primary urothelial cells. Again, the reason for the difference are unclear without further investigation but could be due to differences in cell origin (lung vs bladder) or differences in the model (primary vs immortalized).
Our data are the first to show that Cr(VI) induces aneuploidy in human urothelial cells. These data are consistent with data in human lung cells showing that chronic exposure to Cr(VI) induces chromosome instability and aneuploidy (Wise, S. et al., 2006b; Holmes et al., 2006, 2010; Rodrigues et al., 2009). Our data are also consistent with a study using hTERT-immortalized skin cells (Figgitt et al., 2010) which showed increases in complex aneuploidy following chronic treatment with physiologically relevant doses of Cr(VI) that were equivalent to levels of Cr found in blood of patients with worn metal on metal orthopedic implants. Interestingly, this increased aneuploidy was exacerbated by co-exposure to cobalt, another metal of concern for joint replacement patients.
In sum, we found that Cr(VI) induced DNA double strand breaks and chromosome damage in both urothelial cell lines. We also found that Cr(VI) was aneugenic with prolonged exposures. Chromosome instability is of particular concern as this is considered an initiating event for carcinogenesis. More work will be needed to fully elucidate this potential mechanism for Cr(VI)-induced urothelial cancer, including the role of DNA repair as well as the possible role of centrosome amplification in the induction of aneuploidy and further chromosome instability. However, these data demonstrate that Cr(VI) has a strong likelihood of initiating carcinogenic events leading to bladder cancer.
Highlights.
Hexavalent chromium is genotoxic to human urothelial cells
Hexavalent chromium induces aneuploidy in human urothelial cells
hTERT-immortalized human urothelial cells model the effects seen in primary urothelial cells
Hexavalent chromium has a strong likelihood of being carcinogenic for bladder tissue
Acknowledgments
The authors would like to thank Melanie Hebert and Christy Gianios, Jr for technical assistance. This work was supported by National Institute of Environmental Health Sciences (NIEHS) grant ES016893 (J.P.W.), Army Research Office (ARO) Grant #W911NF-09-1-0296 (J.P.W.), and the Maine Center for Toxicology and Environmental Health.
Footnotes
Conflict of Interest Statement
Dr. John Wise reports grants from National Institute of Environmental Health Sciences (NIEHS), grants from Army Research Office, during the conduct of the study. There are no other conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L. Widespread dissemination of metal debris from implants. Journal of Bone & Joint Surgery, British Volume. 1994;76(5):701–712. [PubMed] [Google Scholar]
- Claude J, Kunze E, Frentzel-Beyme R, Paczkowski K, Schneider J, Schubert H. Life-style and occupational risk factors in cancer of the lower urinary tract. American Journal of Epidemiology. 1986;124(4):578–589. doi: 10.1093/oxfordjournals.aje.a114430. [DOI] [PubMed] [Google Scholar]
- Claude JC, Frentzel-Beyme RR, Kunze E. Occupation and risk of cancer of the lower urinary tract among men. A case-control study. International Journal of Cancer. 1988;41(3):371–379. doi: 10.1002/ijc.2910410309. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. CRC Critical Reviews in Toxicology. 1993;23(3):255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- De Flora S, Bagnasco M, Serra D, Zanacchi P. Genotoxicity of chromium compounds. A review. Mutation Research/Reviews in Genetic Toxicology. 1990;238(2):99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Figgitt M, Newson R, Leslie IJ, Fisher J, Ingham E, Case CP. The genotoxicity of physiological concentrations of chromium (Cr (III) and Cr (VI)) and cobalt (Co (II)): an in vitro study. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2010;688(1):53–61. doi: 10.1016/j.mrfmmm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Florl AR, Schulz WA. Chromosomal instability in bladder cancer. Archives of Toxicology. 2008;82(3):173–182. doi: 10.1007/s00204-008-0280-3. [DOI] [PubMed] [Google Scholar]
- Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr (VI) exposure: involvement of ATM in Cr (VI) induction of γ-H2AX. Carcinogenesis. 2004;25(11):2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicology and Applied Pharmacology. 2005;203(2):167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Sandwick SJ, Wise JP. The clastogenic effects of chronic exposure to particulate and soluble Cr (VI) in human lung cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2006a;610(1):8–13. doi: 10.1016/j.mrgentox.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Sandwick SJ, Lingle WL, Negron VC, Thompson WD, Wise JP. Chronic exposure to lead chromate causes centrosome abnormalities and aneuploidy in human lung cells. Cancer Research. 2006b;66(8):4041–4048. doi: 10.1158/0008-5472.CAN-05-3312. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Wise JP., Sr Carcinogenicity of hexavalent chromium. Indian Journal of Medical Research. 2008;128(4):353. [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Pelsue SC, Aboueissa AM, Lingle W, Salisbury J, … Wise JP., Sr Chronic exposure to zinc chromate induces centrosome amplification and spindle assembly checkpoint bypass in human lung fibroblasts. Chemical Research in Toxicology. 2009;23(2):386–395. doi: 10.1021/tx900360w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans: Chromium, nickel and welding. Vol. 49. International Agency for Cancer Research; Lyons, France: 1990. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Research. 1994;54(9):2342–2346. [PubMed] [Google Scholar]
- Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient A REVIEW OF CURRENT KNOWLEDGE AND FUTURE STRATEGIES. Journal of Bone & Joint Surgery, British Volume. 2007;89(5):567–573. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- Keegan GM, Learmonth ID, Case C. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Critical Reviews in Toxicology. 2008;38(8):645–674. doi: 10.1080/10408440701845534. [DOI] [PubMed] [Google Scholar]
- Kim J, Ji M, DiDonato JA, Rackley RR, Kuang M, Sadhukhan PC, Mauney JR, Keay SK, Freeman MR, Liou SL, Adam RM. An hTERT-immortalized human urothelial cell line that responds to anti-proliferative factor. In Vitro Cellular & Developmental Biology-Animal. 2011;47(1):2–9. doi: 10.1007/s11626-010-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AVF. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement A CONSEQUENCE OF EXCESS WEAR. Journal of Bone & Joint Surgery, British Volume. 2010;92(1):38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- Lhotka C, Szekeres T, Steffan I, Zhuber K, Zweymüller K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements. Journal of Orthopaedic Research. 2003;21(2):189–195. doi: 10.1016/S0736-0266(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Milham S. Occupational mortality in Washington state. 83-1. NIOSH publication; 1983. p. 16. [Google Scholar]
- Montanaro F, Ceppi M, Demers PA, Puntoni R, Bonassi S. Mortality in a cohort of tannery workers. Occupational and Environmental Medicine. 1997;54(8):588–591. doi: 10.1136/oem.54.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilger A, Schaffer A, Rüdiger HW, Osterode W. Urinary 8-hydroxydeoxyguanosine and sister chromatid exchanges in patients with total hip replacements. Journal of Toxicology and Environmental Health Part A. 2002;65(9):655–664. doi: 10.1080/15287390252900359. [DOI] [PubMed] [Google Scholar]
- Rodrigues CFD, Urbano AM, Matoso E, Carreira I, Almeida A, Santos P, Botelho F, Carvalho L, Alves M, Montiero C, Costa AN, Moreno V, Alpoim MC. Human bronchial epithelial cells malignantly transformed by hexavalent chromium exhibit an aneuploid phenotype but no microsatellite instability. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2009;670(1):42–52. doi: 10.1016/j.mrfmmm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutation Research. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Wise SS, Elmore LW, Holt SE, Little JE, Antonucci PG, Bryant BH, Wise JP., Sr Telomerase-mediated lifespan extension of human bronchial cells does not affect hexavalent chromium-induced cytotoxicity or genotoxicity. Molecular and Cellular Biochemistry. 2004;255(1–2):103–112. doi: 10.1023/b:mcbi.0000007266.82705.d9. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Ketterer ME, Hartsock W, Fomchencko E, Katsifas SP, Thompson WD, Wise JP., Sr Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutation Research. 2004;560:79–89. doi: 10.1016/j.mrgentox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2006a;610(1):2–7. doi: 10.1016/j.mrgentox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Xie H, Thompson WD, Wise JP. Chronic exposure to particulate chromate induces spindle assembly checkpoint bypass in human lung cells. Chemical Research in Toxicology. 2006b;19(11):1492–1498. doi: 10.1021/tx0601410. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Qin Q, Xie H, Katsifis SP, Thompson WD, Wise JP., Sr Comparative Genotoxicity and Cytotoxicity of Four Hexavalent Chromium Compounds in Human Bronchial Cells. Chemical Research in Toxicology. 2010;23(2):365. doi: 10.1021/tx900363j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SS, Wise JP., Sr Aneuploidy as an early mechanistic event in metal carcinogenesis. Biochemical Society Transactions. 2010;38(6):1650. doi: 10.1042/BST0381650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SS, Wise JP. Chromium and genomic stability. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2012;733(1):78–82. doi: 10.1016/j.mrfmmm.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Wise SS, Gordon N, Wise JP. Lead chromate-induced chromosome damage requires extracellular dissolution to liberate chromium ions but does not require particle internalization or intracellular dissolution. Chemical Research in Toxicology. 2004;17(10):1362–1367. doi: 10.1021/tx0498509. [DOI] [PubMed] [Google Scholar]
- Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SC, Singh NP, Wise JP. Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2005;586(2):160–172. doi: 10.1016/j.mrgentox.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Wise SS, Huang S, Peng C, Wise JP., Sr Neoplastic transformation of human bronchial cells by lead chromate particles. American Journal of Respiratory Cell and Molecular Biology. 2007;37(5):544–552. doi: 10.1165/rcmb.2007-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Young JL, Qin Q, Joyce K, Pelsue SC, Peng C, Wise SS, Jeevarajan AS, Wallace WT, Hammond D, Wise JP. Zinc chromate induces chromosome instability and DNA double strand breaks in human lung cells. Toxicology and Applied Pharmacology. 2009;234(3):293–299. doi: 10.1016/j.taap.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Wise SS, Young JL, Wise JT, Wise JP., Sr Human Skin Cells Are More Sensitive than Human Lung Cells to the Cytotoxic and Cell Cycle Arresting Impacts of Particulate and Soluble Hexavalent Chromium. Biological Trace Element Research. 2015:1–8. doi: 10.1007/s12011-015-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]