Abstract
Mutations in PIGN, resulting in multiple congenital anomalies-hypotonia-seizures syndrome, a glycosylphosphatidylinositol anchor deficiency, have been published in four families to date. We report four patients from three unrelated families with epilepsy and hypotonia in whom whole exome sequencing yielded compound heterozygous variants in PIGN. As with previous reports Patients 1 and 2 (full siblings) have severe global developmental delay, gastroesophageal reflux disease, and minor dysmorphic features, including high palate, bitemporal narrowing, depressed nasal bridge, and micrognathia; Patient 3 had early global developmental delay with later progressive spastic quadriparesis, intellectual disability, and intractable generalized epilepsy; Patient 4 had bilateral narrowing as well but differed by the presence of hypertelorism, markedly narrow palpebral fissures, and long philtrum, had small distal phalanges of fingers 2, 3, and 4, absent distal phalanx of finger 5 and similar toe anomalies, underdeveloped nails, unusual brain anomalies, and a more severe early clinical course. These patients expand the known clinical spectrum of the disease. The severity of the presentations in conjunction with the patients’ mutations suggest a genotype–phenotype correlation in which congenital anomalies are only seen in patients with biallelic loss-of-function. In addition, PIGN mutations appear to be panethnic and may be an underappreciated cause of epilepsy.
Keywords: epilepsy, intractable, spasms, infantile, PIGN, GPI ethanolamine phosphate transferase 1, human, glycosylphosphatidylinositol anchors, hypotonia, genotype-phenotype association, congenital disorders of glycosylation
INTRODUCTION
Multiple congenital anomalies-hypotonia-seizures syndrome associated with mutations in PIGN was initially described in 2011 after isolating the gene via homozygosity mapping of a large consanguineous family with seven affected individuals [Maydan et al., 2011]. A Japanese sibling pair, a North African fetus and a Mexican-American boy with PIGN mutations have since been published [Brady et al., 2014; Ohba et al., 2014; Couser et al., 2015]. Mutations in PIGN have been associated with epilepsy, hypotonia, global developmental delay, gastroesophageal reflux (GERD), and congenital anomalies of the hands, feet, heart, gastrointestinal system, genitourinary system, and brain [Maydan et al., 2011; Brady et al., 2014; Ohba et al., 2014].
PIGN is one of more than 20 genes in the phosphatidylinositol family involved in glycosylphosphatidylinositol (GPI) biosynthesis. GPI acts as a cell surface anchor for over 150 proteins that are involved in cell–cell interaction, signal transduction, cell-adhesion, and host-defense; defects in this family are classified as GPI-anchor defects [Fujita and Jigami, 2008; Maeda and Kinoshita, 2011]. Like glycosphingolipids, GPI-anchor glycans are lipid-bearing glycans [Freeze et al., 2012]. At least eight GPI anchor defects (PIGA, PIGL, PIGM, PIGN, PIGO, PIGV, PIGT, and PGAP2) have been associated with neurologic abnormalities in humans [Freeze et al., 2012; Brady et al., 2014; Ohba et al., 2014]. The backbone of GPI is composed of alternating phosphoethanolamine and sugar moieties bound to phosphatidylinositol. Located on chromosome 18q21.33, PIGN encodes GPI ethanolamine phosphate transferase one, which adds phosphoethanolamine to the first mannose in GPI [Gaynor et al., 1999; Hong et al., 1999]. As such, it is part of the growing class of congenital disorders of glycosylation [Freeze et al., 2012; Hennet, 2012].
PATIENTS AND METHODS
Patient 1 was the second child of a non-consanguineous Caucasian union, born at full term with normal birth parameters after an uncomplicated pregnancy. She presented at 3 weeks of age with back arching and irritability thought to be associated with feeds, as well as intermittent eye and asynchronous limb twitching. A head CT, brain MRI, and EEG obtained at the time were normal. Gastrointestinal evaluation in the first 3 months of life, including upper GI series, abdominal ultrasound, esophagogastroduodenoscopy with biopsy and flex sigmoidoscopy with biopsy was essentially normal/non-diagnostic. At 2 months Patient 1 began having clusters of nystagmus-like eye-movements and clonic upper body movements. She continued to be very irritable and have poor feeding; a gastrostomy tube was placed at 3 months. Her physical exam was notable for a high arched palate, cupped ears, weak suck, and axial hypotonia with appendicular hypertonia of both upper and lower extremities (Fig. 1). Plain films showed a small scapula and pointed inferior iliacs. She developed infantile spasms at 9 months of age. The seizures were multi-focal and intractable to numerous therapies including ketogenic diet, systemic steroids, levetiracetam, valproate, vigabatrin, and topiramate. An EEG at 28 months of age showed persistent bilateral epileptiform activity with right-sided predominance; MRI showed normal cerebellum, corpus callosum, and white matter maturation.
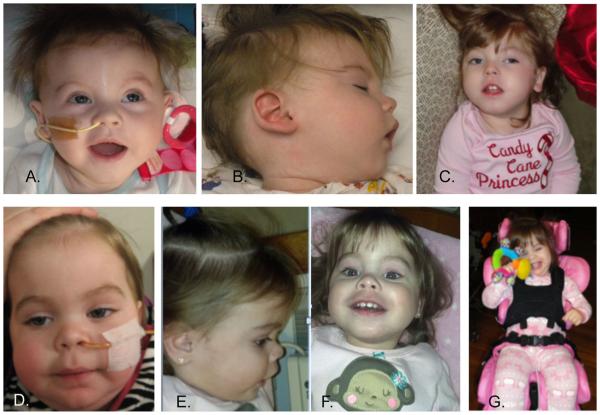
FIG. 1.
Images of siblings, Patients 1 and 2 show distinctive facial features. Patient 1 at (A) 4 months (B) 9 months, and (C) 2 years. Patient 2 at (D) 11 months (E, F) 18 months, and (G) 22 months. Notable features include facial coarsening, hypotonia, bitemporal narrowing, depressed nasal bridge, and micrognathia. [Color figure can be seen in the online version of this article, available at http:// wileyonlinelibrary.com/journal/ajmga]
Patient 1 had global developmental delay and regression. Although she initially made some progress after seizure onset (showing improved head control and eye contact, as well as cooing and batting at toys), she lost these skills with time. She remained irritable and difficult to console. Several months before her death at 2 years 4 months of age, physical examination revealed an unresponsive lethargic child with roving eye movements, bilateral facial weakness, somewhat coarse facial features, hypertonia of all extremities, and brisk reflexes. Laboratory evaluations revealed normal plasma amino acids, urine organic acids, very long chain fatty acids, acylcarnitine profile, karyotype, Angelman/Prader-Willi methylation studies, microarray, and POLG sequencing. Serum alkaline phosphatase levels obtained as part of her clinical care between the ages of 9–27 months, ranged between 126–180 U/L (reference range 100–320 U/L).
Patient 2, the younger sister of Patient 1, was the third child of the same non-consanguineous union, born at term via elective repeat C-section after a pregnancy complicated only by maternal anti-protein antibodies. After a period of normal development, Patient 2 presented at 3 months of age with twitching movements, left-sided rhythmic movements of her upper and lower extremities, bicycling movements, and abnormal eye movements. At the time of her presentation, Patient 2 had a normal EEG and brain MRI. Her physical exam was remarkable for coarse facial features, light hair, slight bitemporal narrowing, depressed nasal bridge, high arched palate with wide alveolar ridge, fifth finger clinodactyly, micrognathia, head lag, good peripheral tone, and symmetric reflexes without clonus (Fig. 1).
Patient 2’s seizures progressed from multifocal to infantile spasms by 9 months of age. Her seizure types include staring episodes, cluster seizures, and spasms. Treatment included ketogenic diet, a course of steroids, levetiracetam, oxcarbazepine, and topiramate. She developed severe gastroesophageal reflux disease (GERD) and generalized hypotonia. At 18 months of age, she continues to have daily seizures, on levitiracetam, oxcarbazepine, and topiramate. She has severe axial and appendicular hypotonia, but is engaged and not irritable. Given her sister’s similar history and prior negative workup, clinical WES was done using samples from Patient 2 and her parents and disease-associated mutations were confirmed in Patient 1 using frozen banked DNA collected prior to her death. Serum alkaline phosphatase levels obtained as part of her clinical care between the ages of 2.5–26 months ranged between 82–296 U/L (reference 100–320 U/L).
Patient 3 was born to a 24 year old G1P0 mother and a 37 year old father, both of African American ancestry. Delivery was induced just prior to term following a largely unremarkable gestation due to concern for lagging head size. Patient 3 was vigorous in the newborn period, although she had left hip dysplasia treated with a Pavlik harness. Concerns for global developmental delay were noted by 6 months of age. She began walking around the age of 2 years. At the age of 5 years she was evaluated by a developmental pediatrician at and diagnosed with spastic diplegia. Brain and spine imaging at that time was notable for mild prominence of the cerebellar folia. Formal neuropsychological testing at the age of 9 years demonstrated moderate intellectual disability. At the age of 11 years she developed worsening gait abnormalities, followed by explosive onset of intractable generalized seizures. Repeat brain imaging revealed development of mild global cerebral volume loss and moderate cerebellar atrophy (Fig. 2).
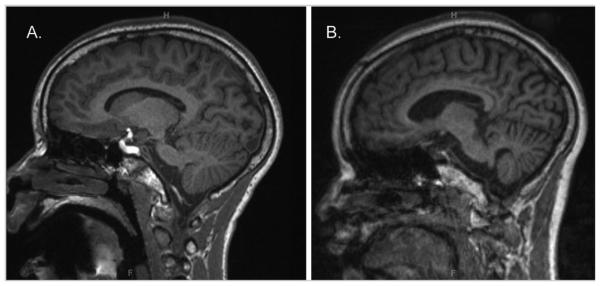
FIG. 2.
MRI of Patient 3. Parasagittal MRI T1 sequences of Patient 3 at (A) age 11 years and (B) age 13 years. Note the increased cortical sulcal prominence and cerebellar atrophy with time.
At the age of 13 Patient 3 demonstrated progressive spasticity and intractable epilepsy. She was alert, could respond in the affirmative or negative to questions, albeit with very poor articulation and intelligibility, and was able to ambulate with assistance. Physical exam was notable for high arched palate, moderate scoliosis, spastic quadriparesis, increased reflexes, and up-going toes. Her weight was 50th centile, height was 75th centile, and head circumference was 10th centile. Plain film showed narrow inferior iliacs. A SNP microarray revealed mosaic absence of heterozygosity, likely due to uniparental isodisomy in the peripheral blood at 25% for 3p26.3p23.21, a region currently without known imprinted genes. Serum alkaline phosphatase levels obtained as part of Patient 3’s clinical care between the ages of 11.5 and 14.1 years ranged between 27–240 and were persistently low (92–27) from age 13.3– 14.1 (reference 100–320 U/L). Patient 3 died at age 14 secondary to left-ventricular non-compaction complicating a pneumonia that had developed during a metapneumoviral infection.
Patient 4 was born at term to a 26 year old G1P1 Caucasian mother and a 25 year old African–American father, delivered via Cesarean section, indicated for fetal distress, after a pregnancy complicated by diet-controlled gestational diabetes with birth parameters at the 95 percentile. Her immediate newborn period was complicated by hypotonia and poor respiratory effort, requiring intubation by day of life (DOL) 1. Patient 4 was transferred to a quaternary care center at DOL 4 with presumed sepsis (organism not found, required inotropic support). Her course was complicated by severe GERD, resulting in Nissen fundoplication and gastrostomy tube, ongoing poor respiratory drive ultimately managed via tracheostomy and assisted ventilation. At 2 months of age, she developed clinical seizures. EEG additionally revealed frequent subclinical seizures and multifocal epileptiform activity. Brain MRI was notable for progressive volume loss, small midbrain, and diffusion restriction in the bilateral globi pallidi and corticospinal tracts (Fig. 3).
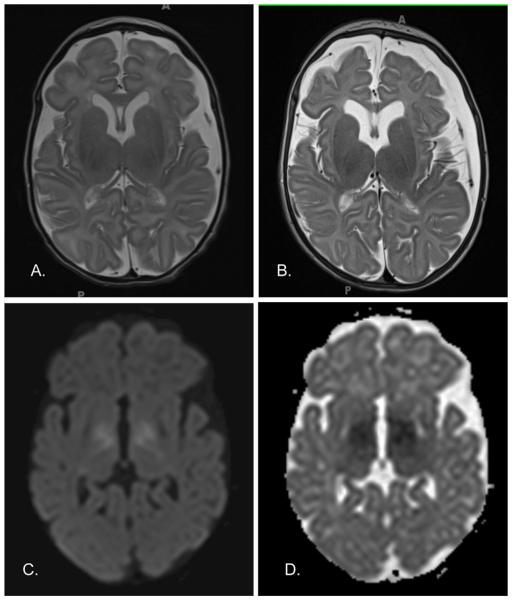
FIG. 3.
MRI of Patient 4. Axial T2-weighted MRI sequence at (A) 2 weeks and (B) 10 weeks of age. Note the increased sulcal and ventricular prominence, and development of left extraaxial collection at 10 weeks of age. Diffusion trace (C) and Apparent diffusion coefficient (D) MRI sequences at 2 weeks of age. Note the diffusion restriction in the bilateral globi pallidi and corticospinal tracts.
At 4 months of life Patient 4 had coarse facial features, bitemporal narrowing, high hair line, low set posteriorly rotated ears with overfolded helices and prominent anterior crus, depressed nasal bridge, high arched intact palate, micrognathia, bilateral single palmar creases, absent fifth distal phalanx, hypoplastic second through fourth distal phalanx, anonychia of the second and third digits and hypoplastic nails of the thumb, fourth and fifth fingers and all toe nails, bilateral accessory nipples, hepatomegaly of unclear etiology, atopic dermatitis and prominent dermal melanosis on her extremities as well as the more usual sacral distribution (Fig. 4). Her plain films confirmed the absence of the fifth distal phalanx and hypoplasia of the second through fourth distal phalanges of the right hand and demonstrated inferior iliac narrowing. Abdominal ultrasound demonstrated partially resolved splenomegaly, resolved bilateral hydronephrosis, and mild left pelviectasis. Ophthalmology evaluation at 2 weeks of life demonstrated cloudy corneas bilaterally, with increased central cornea thickness. Her neurologic exam was notable for absent visual fixation, nystagmus, severe hypotonia, and frequent myoclonus. She passed her newborn hearing exam. Her cardiac exam at 6 months was notable for worsening cardiac function, predicted to progress to cardiac non-compaction. Serum alkaline phosphatase levels obtained as part of her clinical care between the ages of 5 days and 6.5 months ranged from 104–505 U/L, with the highest recorded value at the time of her presentation with severe dehydration secondary to gastroenteritis (reference 100–320 U/L). Evaluation for an inborn error of metabolism was unrevealing. Chromosome microarray and screening metabolic evaluation were unrevealing of an explanation for Patient 4’s presentation. The microarray did reveal an interstitial deletion between BP1 and BP2 of chromosome 15q11.2 (22,750,305-23,226,254); this is a deletion that contains several RefSeq genes, has been seen in normal individuals and well as in association with psychomotor delays and/or neuropsychiatric disorders, and may be a susceptibility locus [Burnside et al., 2011; Stefansson et al., 2014; Cafferkey et al., 2014].
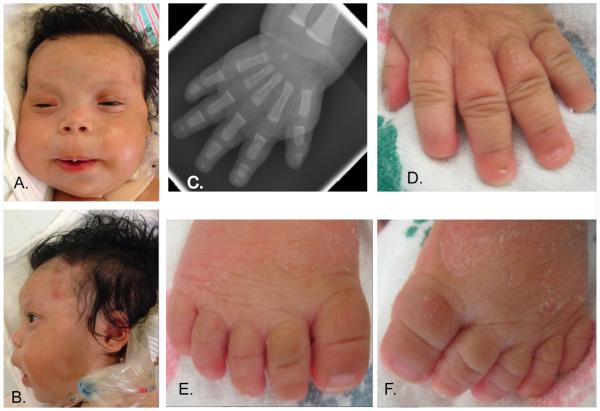
FIG. 4.
Images and plain film of Patient 4 at 4 months of age show distinctive features of the face, hands, and feet. (A, B) Note coarse facial features, bitemporal narrowing, depressed nasal bridge, micrognathia. (C) Absent distal phalanx of the 5th digit and hypoplastic distal phalanges of the 2nd–4th digits on PA view of right hand. (D–F) Hypoplastic 2nd through 4th distal phalanx, anonychia of the 2nd and 3rd digits, and hypoplastic nails of the thumb, 4th and 5th digits (left hand) and all toe–nails. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmga]
RESULTS
Trio Whole Exome Sequencing (WES) was performed commercially at Baylor College of Medicine Molecular Genetics Laboratories/Baylor Miraca Genetics Laboratories on Patient 2 and her parents, as well as Patient 4 and her parents [Yang et al., 2013]. Variants in PIGN, CPA6, and SCN1B found in patient 2 were confirmed in patient 1 (full sibling of pt 2). Compound heterozygous mutations in trans in PIGN in patients 1 and 2 were found; a maternally inherited c.2340T>A (p.Y780X), and paternally inherited c.1434 + 5G>A (NM_176787). The maternally inherited mutation has not been seen in the National Heart, Lung and Blood Institute Exome Sequencing Project (ESP) European Americans (EA) or African Americans (AA) populations, but was seen in ExaAC in 1/13412 of the European non-Finnish population; the paternally inherited mutation has been reported in 1/3039 ESP5400 AA and 0/6595 EA, and in ExAC was seen in 1/5512 African population (A) and 1/28268 EnF. Patients 1 and 2 were also found to have a paternally inherited mutation in SCN1B, associated with type 1 generalized epilepsy with febrile seizures plus (GEFS+), as well as a maternally inherited mutation in CPA6, also associated with familial febrile seizures. The father does not have a personal history of seizures, nor is there a family history of seizures. Trio WES performed commercially at GeneDx in 2013 on Patient 3 and her parents detected compound heterozygous mutations in trans in PIGN, which were called as variants of unknown significance; a maternally inherited c.709 G>A (G273R) and paternally inherited c.2411_2412delTAinsAG (I804K) (NM_176787.4). The variants in PIGN were reclassified as likely pathogenic variants in a disease gene associated with the reported phenotype following exome reanalysis in 2014. The maternally inherited variant has been observed in 1/8157 ESP6500 EA alleles and 0/3682 AA alleles and in ExAC in 1/21566 European non-Finnish alleles; it is predicted to be damaging in SIFT and Mutation Taster, and probably damaging in PolyPhen-2 (score 0.967) [Ng and Henikoff, 2001; Adzhubei et al., 2010; Schwarz et al., 2014]. The paternally inherited variant has not been observed in the ESP5400 AA or EA populations, however, in ExAC a missense variant resulting in the same amino acid substitution has been seen at 6/2202 African alleles; it is predicted to be tolerated in SIFT, disease causing in Mutation Taster and benign in PolyPhen-2 (score 0.221). In CLUSTALW2 both the p.I804, and the p.G273 amino acid residues were conserved across the Norwegian rat (Rattus norvegicus), dog (Canis lupus familiaris), and the house mouse (Mus musculus). The p.G237 residue was not conserved in the cow (Bos taurus). Patient 3 was also found to have a heterozygous de novo missense variant of unknown significance (G361D) in ELN, mutations in which can be associated with cutis laxa, supravalvular aortic stenosis and Williams syndrome, Patient 3 does not have a connective disorder phenotype other than congenital hip dislocation, and does not have supravalvular aortic stenosis. Patient 4 has a maternally inherited c.548_549 + 6del (L183fs) mutation in PIGN detected by WES and a paternally inherited deletion of PIGN encompassing part of exon 5 as well as exons 6 and 7 (chr18:59,821,582-59,824,939 onhg19/CRCh37). The maternally inherited mutation has not been observed in the ESP 5400 AA or EA populations or within ExAC. Based upon the location of the probes used for deletion analysis, whether the paternally inherited deletion is in or out of frame, and its precise length cannot be determined. The two probes within exon 5 are deleted. Of two additional probes that border and extend partially into exon 5, one is absent, and the other appears to be normal. Table I provides a comparison of the main clinical features of patients reported to date. Table II shows the published mutations in PIGN, including this case series. Table SI in supplementary information shows variants other than those in PIGN found in the course of WES.
TABLE 1.
Patient Characteristics
| Maydan Family 1 |
Ohba, Family 2 |
Family 3 |
Family 4 |
Family 5 |
Family 6 |
Family 7 |
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V-1 | V-2 | V-4 | V-5 | V-8 | V-9 | V-10 | 0-1 | 0-2 | Brady* | Couser | Pt 1 | Pt 2 | Pt 3 | Pt 4 | 15 | |
| Age | A. 29m | D. 14m | D. 1m | D. 5m | D. 3m | D. 17m | D. 39m | A. 9y | A. 2y | D. 16 w g | A 2y | D. 30m | A. 18m | D. 14y | A. 4m | |
| Sex | Male | Male | Male | Female | Female | Female | Male | Female | Male | Male | Male | Female | Female | Female | Female | 8F/7M |
| Birth | ||||||||||||||||
| Weight (g) | 3,566 | 4,065 | 3,850 | 3,410 | 4,250 | 4,300 | 4,800 | 3,390 | 3,252 | NA | 4,271 | 3,350 | 3,147 | 2,756 | 4,008 | |
| OFC (cm) | 37 | 37 | 35.5 | 34.5 | NR | NR | NR | 35 | 35 | NA | 36.8 | 35 | 36.5 | NA | 36 | |
| Dysmorphic features |
||||||||||||||||
| Palate | + | + | NR | − | − | − | Other | + | + | + | NR | + | + | + | + | 10/13 (77%) |
| Ears | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | 14/15 (93%) |
| Fingers/ feet | + | + | + | − | − | + | + | + | + | + | + | − | − | − | + | 10/15 (67%) |
| Congenital anomalies |
Other | Other4 | Other5 | |||||||||||||
| Cardiac1 | + | + | + | + | − | + | − | − | − | + | + | − | − | − | − | 7/15 (47%) |
| Urinary2 | + | + | + | − | − | − | − | + | − | + | + | − | − | − | − | 6/15 (40%) |
| Gl (inc GERD]3 | + | + | + | + | − | − | − | + | − | + | + | + | + | − | + | 10/15 (67%) |
| Neurologic | ||||||||||||||||
| Developmental delay |
+ | + | + | + | + | + | + | + | + | NA | + | + | + | + | + | 14/14 (100%) |
| Hypotonia | + | + | + | + | + | + | + | + | + | NA | + | + | + | + | + | 14/14 (100%) |
| Nystagmus | + | + | − | + | + | + | + | + | + | NA | − | + | + | − | + | 11/14 (796%) |
| Tremor | + | + | + | + | + | − | − | + | + | NA | − | − | − | − | 7/14 (50%) |
|
| Seizure | + | − | + | + | + | + | + | + | + | NA | + | + | + | + | + | 13/14 (93%) |
| Feeding | NR | NR | NR | − | − | + | + | + | NR | NA | + | + | + | − | + | 6/10 (60%) |
| Gait | NR | NR | NA | NA | NA | NR | NR | NR | NR | NA | Abnl | Abnl | Abnl | Abnl | NA | 4/4 (100%) |
| Brain MRI | Abnl (1.5 m) |
NR | NR | Abnl (4 m) |
NR | NR | NR | Abnl (6y) |
Nl (2 m) |
Nl | Nl (4d) | Nl (28 m) |
Nl (3 m) |
Abnl (13y) |
Abnl (4 m) |
5/10 (50%) |
| Corpus callosum |
− | + | + | + | 3/9 (33%) |
|||||||||||
| Cerebellar atrophy |
− | + | − | − | − | 1/9 (11%) |
||||||||||
| Cerebral volume loss |
+ | − | + | + | − | 3/9 (33%) |
||||||||||
| BG/CS diffusion restriction |
+ | 1/9 (11%) |
||||||||||||||
Clinical features of the 14 individuals (13 Live born and one fetus) with mutations in PIGN leading to Multiple congenital anomalies hypotonia seizures syndrome/PIGN associated epilepsy. BG/CS = basal ganglia, corticospinal tract. NR, Not reported; NA, Not applicable.
Exam completed on autopsy, features include intestinal malrotation, congenital diaphragmatic hernia, VSD, overriding aorta, and hypoplastic pulmonary trunk, segmental renal dysplasia.
VSD, ASD, PFO, over-riding aorta, and hypoplastic pulmonary trunk.
Renal dysplasia, hydrocele, lobulated kidneys with a duplicated collecting system and hydronephrosis.
GERD, intestinal malrotation, anal stenosis or atresia.
Microphallus, Cryptorchidism, supranumery nipples, wandering eye movements, strabismus.
Hip dysplasia.
TABLE II.
Mutation Table
| Ref Seq ID | Exon | Codon | Protein | Predicted effect | Domain | |
|---|---|---|---|---|---|---|
| Patient 1 and 2 | NM_176787 | exon 25 | c.2340 T>A | Y780X | stop | PigN |
| Patient 1 and 2 | NM_176787 | intron 17 | c.1434+5 G>A | splice site |
||
| Patient 3 | NM_176787.4 | exon 9 | c.709 G>A | G237R | missense | phosphodiesterase |
| Patient 3 | NM_176787.4 | exon 26 | c.2411_2412delT AinsAG |
I804K | missense | PigN |
| Patient 4 | NM_176787 | deletion exons 5–7 |
||||
| Patient 4 | NM_176787 | exon 7 | c.548_549+6del | L183fs | frameshift | phosphodiesterase |
| Maydan V-1, V-2, V-4, V-5, V-8, V-9, V-10 |
NM_176787 | exon 23 | c.2126 G>A | R709Q | missense | PigN |
| Ohba 1 and 2 | NM_176787.4 | exon 9 | c.808 T>C | S270P | missense | phosphodiesterase |
| Ohba 1 and 2 | NM_176787.4 | exon 10 | c.963 G>A | spice site |
stop | phosphodiesterase |
| Brady 1 | NM_012327.5 | exon 16 | c.1574+1G>A | splice site |
stop | PigN |
| Couser 1 | NM_176787 | exon 6 | c.406T>G | p. W136G |
missense | phosphodiesterase |
| Couser1 | NM_176787 | exon 28 | c.2576C>G | p.S859T | missense | PigN |
Published mutations to date, including this report. Mutations are located in both the phosphodiesterase and PigN domains.
DISCUSSION
We report four patients with intractable epilepsy, who were found on whole exome sequencing (WES) to have bilallelic PIGN mutations or deletions. As with previously reported patients, Patients 1, 2, and 4 also demonstrated significant global developmental delay, severe gastroesophageal reflux disease, and minor dysmorphic features, including bitemporal narrowing, depressed nasal bridge, high arched palate, and micrognathia, whereas Patient 3 presented with early global developmental delay, and developed progressive spastic quadriparesis, intellectual disability, and intractable generalized epilepsy over a period of years. Unlike previous reports [Maydan et al., 2011; Brady et al., 2014; Couser et al., 2015], Patients 1, 2, and 3 did not have other major visceral congenital anomalies. Patient 3’s brain MRI showed cerebellar atrophy and hypoplasia of the corpus callosum; Patient 4’s MRI showed diffusion restriction in the bilateral globi pallidi and corticospinal tracts as well as a small midbrain, and both showed cerebral atrophy. Both, Patients 3 and 4 developed cardiac dysfunction, which led to Patient 3’s death secondary to left-ventricular non-compaction during a metapneumoviral pneumonia, and which is predicted to progress to ventricular non-compaction in the case of Patient 4.
With the addition of our patients there have now been six reported ethnicities with PIGN-associated epilepsy/Multiple congenital anomalies-hypotonia-seizures syndrome, including the Arab-Israeli family, Japanese siblings, the North African fetus, a patient of Mexican-American descent, the Northern European descent US siblings (Patients 1 and 2), a patient of African American descent (Patient 3) and a patient of mixed Northern European/African American descent (Patient 4). Each of the seven families has private PIGN mutations.
The clinical severity of the cases reported to date seems to correlate with the predicted functional severity of the mutations seen in PIGN. Patient 3 showed the mildest phenotype, with mild to moderate global developmental delay, no dysmorphic features, and a later onset of seizures and spastic quadriparesis in the presence of two non-conservative missense mutations resulting in replacement of non-polar Glycine with a positively charged Arginine in the phosphodiesterase domain and non-polar Isoleucine with positively charged Lysine in the PigN domain. Patient 3’s second missense mutation, resulting in the replacement of Isoleucine with Lysine, although not seen in the homozygous state, is seen in the heterozygous state with relatively high frequency in the ExAC African population (1/367), and whereas evolutionarily conserved, had a mixed prediction for pathogenicity with SIFT and PolyPhen2 predicting it to be tolerated or benign, and Mutation Taster predicting pathogenicity. These factors suggest that while the variant may contribute to disease it is likely to be better tolerated then other variants. The Mexican–American patient had compound heterozygous non-conservative missense mutations in trans, one in the phosphodiesterase domain and one in the PigN domain as well as several minor dysmorphisms (short, broad thumbs, prominent knuckle pads) which were familial; however, other than the lobulated kidneys and duplicated ureter which were not reported to have resulted in renal failure, he does not have major congenital anomalies, including anomalies of the brain [Couser et al., 2015]. The previously reported Japanese siblings had one non-conservative missense mutation in the phosphodiesterase domain and one mutation resulting in a premature stop codon [Ohba et al., 2014]. Patients 1 and 2 had one non-conservative missense mutation in the PigN domain and one mutation resulting in a premature stop codon. In the initial report of a consanguineous family with multiple congenital anomalies, a homozygous non-conservative missense mutation, resulted in a change from a positively charged Arginine to polar uncharged Glutamine within the transmembrane PigN domain [Maydan et al., 2011]. The most severely affected patients are Patient 4 and the fetal case. Other than her brain MRI findings, Patient 4 is without major structural anomalies; however, she has multiple dysmorphic features and minor skeletal anomalies, including striking digit anomalies as well as a severe clinical course including early onset of intractable seizures and central respiratory failure in the presence of a frameshift mutation in trans with a partial gene deletion of exons 5–7. The fetal case had major congenital anomalies including congenital diaphragmatic hernia, VSD, overriding aorta, intestinal malrotation, segmental renal dysplasia, and oligodactyly of the left foot with digital hypoplasia and anonychia of the remaining toes, in the presence of a homozygous mutation resulting in a premature stop [Brady et al., 2014].
The patients we report and the second published report [Ohba et al., 2014] suggest that major congenital anomalies are not a core feature of PIGN-associated epilepsy/multiple congenital anomalies-hypotonia-seizures syndrome, rather that the phenotypic spectrum of PIGN-associated epilepsy/multiple congenital anomalies-hypotonia-seizures syndrome includes major congenital anomalies only in the presumed absence of the protein. Although elevations in alkaline phosphatase have been associated with mutations in a number of GPI-anchor defect genes, including PIGA, PIGM, PIGO, PIGW, PGAP2, PGAP3, such elevations have not been reported in previous cases of MCAHS1, and were not seen in the patients we describe [Krawitz et al., 2010; Krawitz et al., 2012; Krawitz et al., 2013; Chiyanabu et al., 2014; Howard et al., 2014; Tarailo-Graovac et al., 2015]. In addition to Patient 4’s findings, nine of eleven previously reported patients with MCAHS1 had anomalies of the hands and feet (see Table I) including brachydactyly, broad thumbs, a deep plantar groove, tapered fingers, and oligodactyly with toe hypoplasia and anonychia [Maydan et al., 2011; Brady et al., 2014; Ohba et al., 2014; Couser et al., 2015]. Three of the four patients we describe had anomalies of the iliac bones, suggesting that minor skeletal anomalies beyond those of the digits may be a common feature of the disorder. This requires further study.
Patients 1 and 2 had two additional heterozygous familial mutations in genes associated with familial febrile seizures. CPA6 is autosomal recessive, whereas SCN1B is autosomal dominant with variable penetrance. Although it is possible that these mutations may have contributed to the overall seizure phenotype, we think it unlikely that they have had a major effect as the seizures seen in Patients 1 and 2 are consistent with the previously described PIGN-associated epilepsy and have not been associated with fever [Wallace et al., 1998]. Patient 3 had a single mutation in ELN, associated-disorders include Williams syndrome, cutis laxa, and supravalvular aortic stenosis; whereas this mutation may have contributed to her risk for congenital hip dysplasia, it would not explain her neurologic phenotype. WES for Patient 4 showed a large number of variants, the majority of which were familially inherited or had been seen previously and were not thought to be associated with disease, however, she did have a single novel variant in each of two disease-causing genes, DPYS associated with dihydropyrimidinuria, and FRAS1 associated with Fraser syndrome, these are autosomal recessive and we think are unlikely to have contributed to her phenotype. Patient 4 had a number of overlapping features with DOORS syndrome (deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures), including onychodystrophy, osteodystrophy, and seizures, however, she passed her newborn hearing exam and variants were not seen at greater then 20x coverage of the sequencing region in TBC1D24, the gene associated with DOORS syndrome in 50% of affected families in which all five criteria are present [Campeau et al., 2014]. Although we find this partial overlap in phenotype between Patient 4 and patients with DOORS syndrome intriguing, seizures are a core feature of PIGN-associated epilepsy/MCAHS1 syndrome and as noted in the previous paragraph, onychodystrophy and osteodystrophy have been seen in an additional patient with PIGN variants [Brady et al., 2014]. We think it most likely based on Patient 4’s phenotype as well as the presence of biallelic variants in PIGN that she has one of the more phenotypically severe cases described of PIGN-associated epilepsy/MCAHS1, however, we cannot rule out the possibility that genetic contributors unrevealed by WES, including an as yet unknown gene involved in DOORS syndrome, could contribute to her phenotype. It is also possible that some patients with partial manifestations of DOORS syndrome who have an undetermined molecular etiology will be found to have variants in PIGN.
Evaluation for mutations in PIGN, causing PIGN-associated epilepsy/multiple congenital anomalies-hypotonia-seizures syndrome, should be considered in patients of all ethnicities with epilepsy, with or without additional features. Given the discovery of four cases over a relatively brief period of time at our medical center, we suspect that PIGN is an under-appreciated cause of epilepsy. In addition to intractable epilepsy and global developmental delay, other core features of PIGN-associated epilepsy/ multiple congenital anomalies-hypotonia-seizures syndrome appear to be hypotonia, nystagmus, gastroesophageal reflux disease, and minor facial dysmorphisms. Depending upon mutation severity, major congenital anomalies of the heart, gastrointestinal tract, brain and urinary tract may also be present, but are not obligate features of the syndrome.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the families of patients 1, 2, and 4 for sharing numerous clinical photos, some of which are shown in Figures 1 and 4. We would like to thank Denise Batista, PhD and Elizabeth Wohler, MS of Kennedy Krieger Institute Cytogenetic and Microarray Laboratory for performing SNP microarray and providing an estimate of the mosaicism in Patient 3.
Footnotes
Conflict of interest: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady PD, Moerman P, De Catte L, Deprest J, Devriendt K, Vermeesch JR. Exome sequencing identifies a recessive PIGN splice site mutation as a cause of syndromic Congenital Diaphragmatic Hernia. Eur J of Med Genet. 2014;57:487–493. doi: 10.1016/j.ejmg.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Burnside RD, Pasion R, Mikhail FM, Carroll AJ, Robin NH, Youngs EL, Gadi IK, Keitges E, Jaswaney VL, Papenhausen PR, Potluri VR, Risheg H, Rush B, Smith JL, Schwartz S, Tepperberg JH, Butler MG. Microdeletion/microduplication of proximal 15q11.2 between BP1 and BP2: A susceptibility region for neurological dysfunction including developmental and language delay. Hum Genet. 2011;130:517–528. doi: 10.1007/s00439-011-0970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferkey M, Ahn JW, Flinter F, Ogilvie C. Phenotypic features in patients with 15q11.2(BP1-BP2) deletion: Further delineation of an emerging syndrome. Am J Med Genet Part A. 2014;164A:1916–1922. doi: 10.1002/ajmg.a.36554. [DOI] [PubMed] [Google Scholar]
- Campeau PM, Kasperaviciute D, Lu JT, Burrage LC, Kim C, Hori M, Powell BR, Steward F, F'elix TM, van den Ende J, Wisniewska M, Kayserili H, Rump P, Nampoothiri S, Aftimos S, Antje M, Nair LDV, Begleiter ML, De Bie I, Meenakshi G, Murray ML, Repetto GM, Golabi M, Blair E, Male A, Giuliano F, Karimineejad A, Newman WG, Bhaskar SS, Dickerson JE, Kerr B, Banka S, Giltay JC, Wieczorek D, Tostevin A, Wiszniewska J, Cheune SW, Hennekam RC, Gibbs RA, Lee BH, Sisodiya SM. The genetic basis of DOORS syndrome: An exome-sequencing study. Lancet Neuro. 2014;13:44–58. doi: 10.1016/S1474-4422(13)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyanabu T, Inoue N, Morimoto M, Kinoshita T, Murakami Y. Glycosylphosphatidylinositol (GPI) anchor deficiency caused by mutations in PIGW is associated with West syndrome and hyperphosphatasia with mental retardation syndrome. J Med Genet. 2014;51:203–207. doi: 10.1136/jmedgenet-2013-102156. [DOI] [PubMed] [Google Scholar]
- Couser NL, Masood MM, Strande NT, Foreman AK, Crooks K, Weck KE, Lu M, Wilhelmsen KC, Roche M, Evans JP, Berg JS, Powell CM. The phenotype of multiple congenital anomalies-hypotonia-seizures syndrome 1: Report and review. Am J Med Genet. Part A. 2015;167:2176–2181. doi: 10.1002/ajmg.a.37129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11:453–466. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Jigami Y. Lipid remodeling of GPI-anchored proteins and its function. Biochim Biophys Acta. 2008;1780:410–420. doi: 10.1016/j.bbagen.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Mond'esert G, Grimme SJ, Reed SI, Orlean P, Emr SD. MCD4 encodes a conserved endoplasmic reticulum membrane protein essential for Glycosylphosphatidylinositol anchor synthesis in yeast. Mol Bio of Cell. 1999;10:627–648. doi: 10.1091/mbc.10.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet T. Diseases of glycosylation beyond classical congenital disorders of glycosylation. Biochim Biophys Acta. 2012;1820:1306–1317. doi: 10.1016/j.bbagen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Hong Y, Maeda Y, Watanabe R, Ohishi K, Mishkind M, Riezman H, Kinoshita T. Pig-n, a mammalian homologue of yeast mcd4p, is involved in transferring phosphoethanolamine to the first mannose of the glycosylphosphatidylinositol. J Biol Chem. 1999;274:35099–35106. doi: 10.1074/jbc.274.49.35099. [DOI] [PubMed] [Google Scholar]
- Howard MF, Murakami Y, Pagnamenta AT, Daumer-Haas C, Fischer B, Hecht J, Keays DA, Knight SJ, Kölsch U, Krüger U, Leiz S, Maeda Y, Mitchell D, Mundlos S, Phillips JA, Robinson PN, Kini U, Taylor JC, Horn D, Kinoshita T, Krawitz PM. Mutations in PGAP3 impair GPI-anchor maturation, causing a subtype of hyperphosphatasia with mental retardation. Am J Hum Genet. 2014;94:278–287. doi: 10.1016/j.ajhg.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz PM, Murakami Y, Hecht J, Krüger U, Holder SE, Mortier GR, Delle Chiaie B, De Baere E, Thompson MD, Roscioli T, Keilbasa S, Kinoshita T, Mundlos S, Robinson PN, Hom D. Mutations in PIGO, a member of the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation. Am J Hum Genet. 2012;13:146–151. doi: 10.1016/j.ajhg.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz PM, Murakami Y, Rie A, Hietala M, Krüger U, Zhu Na, Kinoshita T, Mundlos S, Hecht J, Robinson PN, Horn D. Pgap2 mutations, affecting the gpi-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation syndrome. Am J of Hum Genet. 2013;92:584–589. doi: 10.1016/j.ajhg.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz PM, Schweiger MR, Rödelsperger C, Marcelis C, Kölsch U, Meisel C, Stephani F, Kinoshita T, mMurakami Y, Bauer S, Isau M, Fischer A, Dahl A, Kerick M, Hecht J, Köhler S, Jäger M, Grünhagen J, de Condor BJ, Doelken S, Brunner HG, Meinecke P, Passarge E, Thompson MD, Cole DE, Horn D, Roscioli T, Mundlos S, Robinson PN. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet. 2010;42:827–829. doi: 10.1038/ng.653. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kinoshita T. Structural remodeling, trafficking and functions of glycosylphosphatidylinositol-anchored proteins. Prog in Lip Res. 2011;50:411–424. doi: 10.1016/j.plipres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Maydan G, Noyman I, Har-Zahav A, Neriah ZB, Pasmanik-Chor M, Yeheskel A, Albin-Kaplanksi A, Maya I, Magal N, Birk E, Simon AJ, Halevy A, Rechavi G, Shohat M, Straussberg R, Basel-Vanagaite L. Multiple congenital anomalies-hypotonia-seizures syndrome is caused by a mutation in PIGN. J Med Genet. 2011;48:383–389. doi: 10.1136/jmg.2010.087114. [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba C, Okamoto N, Murakam Y, Suzuki Y, Tsurusaki Y, Nakashima M, Miyake N, Tanaka F, Kinoshita T, Matsumoto N, Saitsu H. PIGN mutations cause congenital anomalies, developmental delay, hypotonia, epilepsy, and progressive cerebellar atrophy. Neurogen. 2014;15:85–92. doi: 10.1007/s10048-013-0384-7. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Meyer-Lindenber A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, Bjornsdottir G, Walters GB, Jonsdottir GA, Doyle OM, Tost H, Grimm O, Kristjansdottir S, Snorrason H, Davidsdottir SR, Gudmundsson LJ, Jonsson GF, Stefansdottir B, Helgadottir I, Haraldsson M, Jonsdottir B, Thygesen JH, Schwarz AJ, Didriksen M, Stensbøl TB, Brammer M, Kapur S, Halldorsson JG, Hreidarsson S, Saemundsen E, Sigurdsson E, Stefansson K. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–366. doi: 10.1038/nature12818. [DOI] [PubMed] [Google Scholar]
- Tarailo-Graovac M, Sinclair G, Stockler-Ipsiroglu S, Van Allen M, Rozmus J, Shyr C, Biancheri R, Oh T, Sayson B, Lafek M, Ross CJ, Robinson WP, Wasserman WW, Rossi A, van Karnebeek CDM. The genotypic and phenotypic spectrum of PIGA deficiency. Orphanet J of Rare Dis. 2015;10:23–35. doi: 10.1186/s13023-015-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic EF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, Braxton A, Beuten J, Xia F, Niu Z, Hardison M, Person R, Bekheirnia MR, Leduc MS, Kirby A, Pham P, Scull J, Wang M, Ding Y, Plon SE, Lupski JR, Beaudet AL, Gibbs RA, Eng CM. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.