Abstract
During the last decades, transcranial magnetic stimulation (TMS) has been used as a noninvasive method to investigate motor cortical reorganization and neuroplasticity in humans after stroke. An increasing number of studies in the field of motor control have used TMS to gain an understanding of the different aspects of stroke cortical physiology and motor recovery. This review addresses the effects of corticospinal tract (CST) lesions in humans and nonhuman primates on the functional organization of the motor system. We review information on the physiological mechanisms by which the CST contributes to normal motor control and to central nervous system reorganization following stroke when the CST is injured as measured using TMS. Insight into these physiological mechanisms has led to the development of scientifically sound interventional proposals in the field of neurorehabilitation.
Keywords: motor control, neuroplasticity, primate models, primary motor cortex, rehabilitation
Stroke affects more than 795,000 people each year in the United States, and it is the leading cause of long-term disability.1 Despite advances in acute treatment methods,2 a substantial proportion of patients remain affected by different degrees of motor disability following stroke.3,4 Subsequent cost of care is proportional to the degree of disability, resulting in tremendous physical, psychological, and financial consequences for patients, families, and society.5 It is imperative to develop novel interventional strategies to improve disability and integrate the stroke patient more effectively into society. Such a goal could only be accomplished by understanding the mechanisms underlying recovery of function after brain lesions.6
Transcranial magnetic stimulation (TMS), a noninvasive painless method to stimulate the brain of conscious humans, has emerged as an important tool for investigating motor cortical function. A detailed description of the contribution of TMS to the understanding of physiological mechanisms of motor and cognitive cortical plasticity has been previously described.6–24 The purpose of this review is to describe how TMS contributes to the understanding of cortical plasticity specifically associated with corticospinal tract (CST) function. TMS has been used most extensively to evaluate the corticospinal system, since the output of the primary motor cortex (M1) can be easily assessed in the form of a motor-evoked potential (MEP) by using surface electromyographic (EMG) recording electrodes.25 Initially, we discuss the consequences of CST or M1 lesions in humans and nonhuman primates on motor function and cortical organization. This information provides the basis for discussing which neuronal elements are excited by TMS. The combination of TMS with simultaneous recording of descending volleys in the spinal cord using epidural electrodes and with pharmacological interventions has made it possible to study inhibitory/excitatory circuits in the human cerebral cortex. We then focus on the discussion of some of the most widely used electrophysiological markers available to assess motor cortical function and cortical reorganization after stroke (i.e., motor threshold, short intracortical inhibition, and interhemispheric inhibition) at rest and during different types of motor behavior in humans.
Corticospinal Tract
Lesion studies in nonhuman primates
Previous lesion studies in monkeys have demonstrated that voluntary movements can persist after the M1 or the corticospinal tract is lesioned.26–28 Lawrence and Kuypers26 completed unilateral and bilateral lesions of the hand representation of M1 in adult monkeys and followed up their motor recovery for several months using film recordings. One of the important findings in this investigation was the demonstration that several months after injury, despite animals recovering control over a wide range of arm movements, the capacity to perform individuated finger movements never returned and movement slowness persisted, suggesting an important role of corticospinal pathways in controlling movement speed and in the ability to fractionate individual finger movements. It was proposed that the reason for these deficits was that the CST constituted the main source of direct corticomotoneuronal connections innervating distal hand muscles.29 Hepp-Reymond and Wiesedanger27 reported that after a unilateral lesion of the CST, monkeys trained to perform a precision grip task (i.e., compress a small wafer between the thumb and index finger or another finger) showed impairments in controlling contralateral hand force and movement speed. In this study, after 1 to 2 weeks of retraining, animals showed a gradual improvement in motor performance and a partial recovery was even observed in monkeys with a total or nearly total CST lesion. Of note is that some residual deficits were observed for up to 6 months after the lesion. Monkeys that trained to perform the precision grip task in a reaction time (RT) paradigm showed slower movements after damage of about 75% of the pyramidal tract fibers. Although movements were slower, an equivalent delay was not observed in the onset of EMG latencies, suggesting that the execution but not the initiation of the contraction was altered by the lesion. In addition, monkeys trained to exert increasing levels of force performed successfully at highest levels but showed deficits in modulating EMG activity over time. The authors concluded that the execution rather than the initiation of the contraction was mainly affected by a CST lesion. In a later study, Hoffman and Strick28 performed a unilateral lesion of the hand area of the M1 in a monkey that was trained to make rapid step-tracking movements of the wrist in different directions and examined movement kinematics and EMG activity in forearm muscles. This study demonstrated that a unilateral M1 lesion resulted in an inability to activate arm muscles in a coordinated, time-specific manner. Also, animals were unable to suppress the antagonistic muscle activity at movement onset (see Figure 1). The authors suggested that the fundamental difficulty produced by a lesion over the hand representation of the M1, which was the inability to activate and inactivate muscles in a precise spatiotemporal pattern, was likely to be the basis for the deficits in fractionating finger movements reported by previous studies.
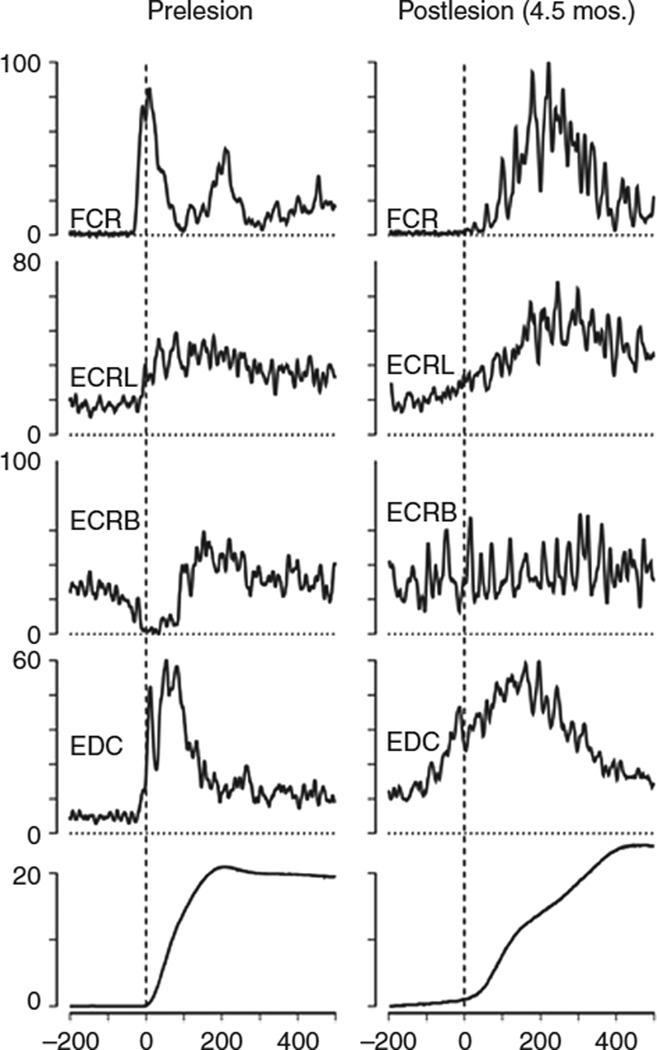
Figure 1.
Electromyographic (EMG) activity after an M1 lesion in an adult monkey. The monkey was trained to perform rapid step-tracking movements of the wrist to eight different target locations. EMG activity was full-wave rectified and averaged before (prelesion, left column) and after (postlesion, right column) the lesion. Averages were normalized, and the maximum EMG activity observed in one of eight wrist movement directions represents 100%. Note that, prelesion, the agonist (flexor carpis radialis [FCR]) burst began 40 ms before movement onset and lasted 100 ms. Activity in synergistics (extensor carpis radialis longus [ECRL] and extensor digitorum communis [EDC]) began 20 ms before movement onset. Activity in antagonistic (extensor carpis radialis brevis [ECRB]) muscle was strongly suppressed. Note that, postlesion, the agonist burst in the FCR was delayed. Synergistics (ECRL and EDC) showed early activity, and the suppression of antagonistic activity in ECRB was absent. Bottom traces showed displacement of the wrist (in degrees). Reproduced, with permission, from Hoffman DS, Strick PL. Effects of a primary motor cortex lesion on step-tracking movements of the wrist. J Neurophysiol. 1995;73: 891–895. Copyright © 1995 by The American Physiological Society.
Motor deficits have also been examined after a partial inactivation of the hand representation of the M1 in monkeys.30,31 Kubota30 injected muscimol, a GABAA agonist, into the hand representation of the M1 in freely moving infant macaque monkeys. After the injection, monkeys tended to use the intact hand, whereas the hand contralateral to the lesion showed a dropped posture in the wrist and fingers with prolonged movement time. It was concluded that GABAA inhibition was important for the initiation of flexion and/or extension movements of the wrist and fingers. In a later study, Schieber and Poliakov31 made single intracortical injections of muscimol when trained monkeys performed individual flexion-extension movements with the fingers and the wrist following a visual cue. The authors found that the location of each injection was unrelated to which finger movement was impaired. This study showed that a flexion movement of a given finger was unaffected even though an extension movement was impaired or vice versa, suggesting that the control of individuated finger movement was widely distributed in the hand representation of the M1.
In addition to deficits in motor performance, M1 or CST lesions result in changes in cortical organization. Glees and Cole32 using superficial stimulation techniques provided some of the first evidence of representational plasticity from brain damage recovery in adult monkeys. Later, intracortical microstimulation (ICMS) techniques were used to develop more detailed maps of limb movement representations in M1 of adult monkeys.33 Nudo and Milliken34 demonstrated clear but transient deficits in motor function in the hand contralateral to a focal M1 ischemic infarct. The authors concluded that substantial functional reorganization occurred in the hand representation of the M1 of adult monkeys following a focal ischemic infarct; and in the absence of training after the infarct, movements previously represented in the infarct zone did not reappear in adjacent cortical regions as reported earlier by Glees and Cole.32 When training was introduced during the first week after the infarct, the spared hand representations adjacent to the injury were retained.35 This is in contrast to the significant reductions in hand area observed in the digit representations following spontaneous recovery.34,36 Spontaneous recovery resulted in an expansion of proximal representations into the former distal representations.34 It was proposed that motor training, which facilitated the functional recovery of motor activity in the impaired hand, might have affected local network interactions within the peri-lesional cortex and therefore contributed to maintain the efficacy of those neurons projecting to hand motoneurons. Current studies support the view that the recovery of function lost due to cortical injury may be related to adaptive plasticity in the remaining cortical motor network. Aizawa et al37 described that over-learning of a motor task resulted in substantial reduction in supplementary motor area (SMA) premovement activity in adult monkeys. After a lesion in M1, premovement activity in SMA returned, indicating that the functional organization of this remote cortical area was altered after a focal cortical lesion. It has also been shown in monkeys that a few days after ischemic injury to the entire hand representation of the M1, there are changes in angiogenesis in this region although there is no real neuronal loss in the ventral premotor cortex (PMv).38 Motor maps of the hand area in PMv undergo nonlinear changes in motor representation after an M1 injury. For example, around 3 weeks after M1 injury, PMv maps reduce in size and the increased excitability in this region does not result in a simple expansion of motor maps at this time point.40 However, 3 to 5 months after M1 injury, even in the absence of postinfarct rehabilitation, the PMv maps often are expanded.39 A more recent study demonstrated that an inactivation of the hand representation of the M1 by a GABAA agonist reduced or abolished responses from PMv (rostral division, area F5), suggesting that the motor effects evoked from F5 depend, at least in part, on interactions with M1.40 All these data support the view that recovery of function lost due to cortical injury may be attributable to adaptive plasticity in the remaining cortical motor network. On the other hand, evidence of a cause-effect link between these various forms of cortical reorganization and performance improvements does not abound. One additional issue to be considered is that, although these are excellent models of cortical plasticity following focal cortical lesions, these lesions are very rare in human stroke. Therefore, the applicability of conclusions drawn from these models to human stroke requires specific testing in humans.
Lesion studies in humans
A common (although somewhat inaccurate) perception has been that severe motor deficits observed following stroke are exclusively a consequence of CST involvement. However, studies in monkeys with CST lesions showed that they recover many aspects of voluntary motor control, keeping only some residual deficits as discussed previously.
Individuals with hemiparesis following stroke can show a decreased capacity to recruit muscles agonistic to the intended action,41,42 and muscle contraction of agonist muscles is frequently accompanied by co-contraction of antagonist muscles.43–46 An excellent description of the time course of motor deficits after stroke has been done by Twitchell.47 Hammond et al42 developed a technique to obtain simultaneous counts of motor unit activity in a wrist flexor and extensor muscle using monopolar needle EMG electrodes. Their results showed that a co-contraction ratio of antagonist activity to total activity (agonist and antagonist) was much greater in patients with stroke compared to controls, suggesting that both agonist recruitment and antagonist inhibition were impaired in the hemiparetic arm. Another important deficit reported after stroke is a failure to increase motor unit discharge rate during voluntary force increments in paretic muscles.41 The decrease in discharge rate might to some extent alter the precise match between motoneuron properties and mechanical properties of the innervated muscle fibers reducing the efficiency of muscle contraction leading to increased effort and muscle fatigue. Although, in general, these reports demonstrate impairment in both agonist recruitment and antagonist inhibition in the hemiparetic arm, these studies involved heterogeneous stroke patients with different lesions and with a variety of motor and cognitive deficits.
To better understand the effect of a lesion of the corticospinal system, Lang and Schieber48 investigated the control of finger movements in a relatively infrequent group of patients with damage relatively restricted to the M1 or corticospinal tract. They studied individuals with pure motor hemiparesis. Pure motor hemiparesis is a relatively homogeneous clinical syndrome characterized by paresis on one side of the body without sensory and cognitive deficits.49,50 This syndrome most frequently results from relatively small ischemic lesions affecting the corticospinal tract unilaterally in the basis pontis or in the posterior limb of the internal capsule. The authors found that individuals with pure motor hemiparesis showed a differential impairment in independent finger movements during individuated flexion/extension contractions.48 More recently, the authors demonstrated that corticospinal tract damage in the same group of individuals reduced the selectivity of finger muscle activation during individual finger abduction/adduction movements, resulting in reduced independence of these movements.51 The present findings extended their previous results demonstrating that loss of finger movement selectivity goes beyond the movements for which the muscle would be either an agonist or an antagonist. In cases where the lesion occurred in the CST, the reduced muscle selectivity may reflect the loss of corticomotoneuronal connections to hand muscles combined with the compensatory capacity of alternative descending pathways.
In other cases, hemiparesis attributed to corticospinal tract lesions is accompanied by preserved activity in the M1.52 It has been proposed that this activity, as measured by magnetoencephalography for example, could control movements of a hand prosthesis attached to a completely paralyzed hand through a brain-computer interface device.53 With damage to the crossed CST, other intact and active motor areas may exert some control over spinal motor neurons by alternative descending pathways.54 These findings imply that, in humans, spared cerebral motor areas and descending pathways that remain may activate finger muscles but do not necessarily compensate fully for the highly selective control provided by the M1 and the crossed corticospinal system.
TMS and the Corticospinal Tract
Motor pathways activated by M1 stimulation with TMS
The M1 is one of the major sources of descending pyramidal tract neurons originated in layer V. There are two types of CST neurons. One type has axons terminating in the intermediate zone of the spinal cord, where they synapse with spinal interneurons. In turn, some of these interneurons make connections with alpha moto-neurons and conduct the descending commands necessary for movement. A second type of CST neurons has axons that terminate in the ventral horn of the spinal cord, where they make monosynaptic connections with motoneurons (called corticomotoneuronal cells). The available data suggest that corticomotoneuronal cells are involved in the generation and control of skilled motor behavior. Electrophysiological studies support the view that with TMS we can assess corticomotoneuronal connections by examining the effect of TMS on (a) the probability of discharge of single motor units voluntarily preactivated (i.e., poststimulus time histograms [PSTHs]55–57), and (b) the amplitude of H-reflexes.58,59 MEPs, on the other hand, most likely result from stimulation of both mono- and polysynaptic corticospinal connections (for review, see ref. 60).
A suprathreshold TMS stimulus results in multiple descending waves as recorded from epidural electrodes positioned over the spinal cord and in a complex configuration of MEP as measured by single motor unit recordings with needle electrodes.61 A short latency direct wave (D-wave) is followed by several longer latency indirect waves (I-waves). The D-wave is thought to result from direct depolarization of the initial axon segment of the CST neuron and is most effectively activated in human subjects by high-intensity TMS or by transcranial electrical stimulation. D- and I-waves are sensitive to the current direction of TMS. The D-wave is preferentially elicited with the induced current flowing in the lateral-medial direction. I-waves, which follow the D-wave, occur sequentially with a periodicity of approximately 1.5 ms and reflect the delay required for synaptic transmission from a corticocortical connection neuron to the CST neuron. The reason for the periodicity is not completely understood at this time. It has been proposed that it may depend on reverberating activity of circuits within the cortex or, alternatively, on changes in the membrane properties of corticospinal neurons which cause it to fire repeatedly after the application of a synchronous depolarizing stimulus. The first I-wave (I1) is thought to be generated through the depolarization of an axon synapsing directly onto a corticospinal neuron, and the following I-waves (I2 and later) may require local polysynaptic circuits.
Several TMS applications have been developed to examine the physiology of the human motor system. These range from simple measurements that are used in clinical practice, such as the assessment of central motor conduction time, to more complex examples that include the use of pairs of TMS stimuli or pairs of TMS and peripheral nerve stimuli to make inferences about excitability of corticospinal neurons, interneurons, and connected structures.60 In the following section, we discuss different TMS methodologies used to examine cortical reorganization in humans following stroke.
Electrophysiological changes in TMS measurements after stroke
Motor cortical reorganization following stroke has been examined with TMS by assessing measures of CST integrity. One of the most common measures of cortical excitability is the motor threshold that can be examined at rest and during a voluntary muscle contraction. The resting motor threshold (RMT) is generally defined as the lowest TMS intensity required for eliciting MEPs of at least 50 µV amplitudes in three of five consecutive trials,62 whereas the active motor threshold (AMT) is usually defined as the lowest TMS intensity required for eliciting MEPs of at least 200 µV amplitudes in three of five consecutive trials during a voluntary contraction.62 It is important to consider that the relative value for the size of the MEP depends on the level of background EMG activity that needs to be standardized within and between subjects. These measurements are simple to assess but they represent complex indexes of cortical function, because MEPs are evoked only after a sequence of synaptic relays occurring both at the cortex and spinal cord. Although threshold of corticospinal axons is likely to be dependent on the level of synaptic activity in the cortex, the MEP threshold will also depend on the excitability of synaptic relays. For example, if a TMS pulse activates a portion of the corticospinal projection generating an excitatory postsynaptic potential (EPSP) in spinal motoneurons and if the spinal motoneurons are far from their threshold, they will not discharge and therefore no MEP will be recorded; but if spinal excitability is high, the same EPSP will discharge the motoneuron and produce an MEP. Pharmacological studies have suggested that motor thresholds reflect to a large extent changes in axonal excitability.63–65 Then, RMT and AMT are susceptible to both synaptic influences at the cortical and spinal cord levels; however, because AMT is measured during muscle contraction, it might be less susceptible to tonic changes in spinal motoneurons activity and allow better assessment of cortical influences.14
Neurophysiological data acquired during the first weeks after stroke have demonstrated an increase in motor thresholds by stimulating the ipsilesional M1 in comparison to the contralesional hemisphere and to healthy controls.66–70 However, Delvaux et al71 reported no changes in motor threshold compared to controls. Swayne et al72 recently described that neurophysiological data acquired within the first 3 weeks after stroke showed great within-subject variability. The authors showed no relationship between the observed physiological variations and motor performance in this period, suggesting that multiple physiological measurements are necessary in the first weeks after stroke to provide a better indicator of motor cortical function. At later stages, although several studies suggest an increase in motor thresholds on the ipsilesional compared to the contralesional side and to healthy controls,73–76 some variability has also been reported. Cicinelli et al76 showed in 10 well-recovered patients (patients with mild to moderate hemiparesis and partially recovered hand movements as measured by the Canadian Scale hand item around 30 days after stroke) that the RMT and AMT to a single magnetic stimulus over the M1 were significantly higher in the affected hemisphere than in the unaffected hemisphere. In contrast, Bütefisch et al77 reported in 13 well-recovered patients (patients with good functional recovery of hand function as defined by the ability to perform selective movements of the fingers within the first month following the event) that the RMT was similar in the affected hemisphere to healthy controls. In agreement, Swayne et al72 also demonstrated that RMT and AMT were similar in the ipsilesional M1 compared to healthy controls (around 30 days after stroke). Studies involving chronic stroke patients encompassed different degrees of motor recovery and, perhaps for that reason, abnormalities in motor thresholds are reported in some studies and not in others.72,76–79 The relationship between changes in motor threshold in the affected hand and motor function has been addressed.67,75,80,81 In general, it is suggested that lower thresholds are associated with better functional outcome. Thickbroom et al79 demonstrated a negative correlation between MEP threshold and grip strength in a group of stroke patients. In this study, MEP parameters did not correlate with dexterity of the affected hand, despite the fact that grip strength and dexterity were correlated, suggesting that motor thresholds may not provide the best index of all aspects of functional improvements in the corticomotor projection.79 Thus, in general, thresholds are generally higher in patients with severe CST impairment, but more work is needed to characterize fully the relationship between motor thresholds and motor function after stroke.
Intracortical function after stroke
Intracortical inhibitory activity changes in the affected and unaffected M1 after stroke (for review, see ref. 14). Paired-pulse TMS protocols have provided insight into the nature of the cortical circuitry that is activated by TMS. A variety of different methods exist to examine the connections within and between primary motor cortices. In this section, we will discuss two of the most widely used paired-pulse TMS protocols in humans: short interval intracortical inhibition (SICI) and interhemispheric inhibition (IHI). For both techniques, direct recordings of the effects on descending volleys have not only confirmed the mechanisms of these effects but also revealed some degree of selectivity for different waves (D, I1, I2, etc.) of the response. It is also known in healthy controls that these two measurements of motor cortical function interact at rest82 and during voluntary movement83; therefore, they might provide relevant information after stroke, although we have to keep in mind that this is only one of the many interactions that could potentially be relevant in stroke recovery.
SICI Technique
SICI was first reported by Kujirai and collaborators in 1993.84 This is a method for probing intracortical synapses in the conscious human brain.84 The authors demonstrated that a conditioning TMS pulse of a subthreshold intensity decreased the size of an MEP elicited by a later suprathreshold test stimulus when applied over the M1. This effect was observed at conditioning time intervals between 1 and 5 ms (see Figure 2A). Two main phases of inhibition have been described at conditioning time intervals of 1 and 2.5 ms.85,86 Since the intensity of the conditioning stimulus was below the active motor threshold, Kujirai et al84 suggested that this effect was occurring at the cortical level and that the conditioning stimulus was suppressing the recruitment of descending volleys by the test stimulus. Later evidence provided by Di Lazzaro and collaborators87 confirmed the cortical location of SICI measurements. They demonstrated that a subthreshold conditioning stimulus, which itself did not evoke descending activity, produced a clear suppression of late I-waves if the interval between the stimuli was between 1 and 5 ms. The I1-wave was nearly unaffected but the I3-wave and later volleys were the most sensitive to the stimulation. These results were supported by the study of Hanajima et al88 who showed that a subthreshold conditioning stimulus given over M1 moderately suppresses I3 waves but did not affect I1 waves. The duration of suppression of the I3 waves supported the idea that this was an effect of GABAergic inhibition within the motor cortex. Kujirai et al84 in the original paper suggested that SICI was GABAergic in origin. Later studies have shown that administration of a single oral dose of lorazepam, a positive allosteric modulator of the GABAA receptor, increases the amount of SICI and also increases the inhibition of later descending I-waves.89 Other studies have confirmed that GABAA agonists enhance SICI.64,90 It has been proposed that inhibition at a conditioning time interval of 1 ms may be related to refractoriness85 and to a synaptic mechanism,91 however, the inhibition observed at 2.5 ms is likely to be mainly mediated by GABAergic inhibition at the intracortical level.85,86 At present, SICI is considered as one of the standard paired-pulse TMS protocols used to examine intracortical function at rest and during voluntary movement in humans.
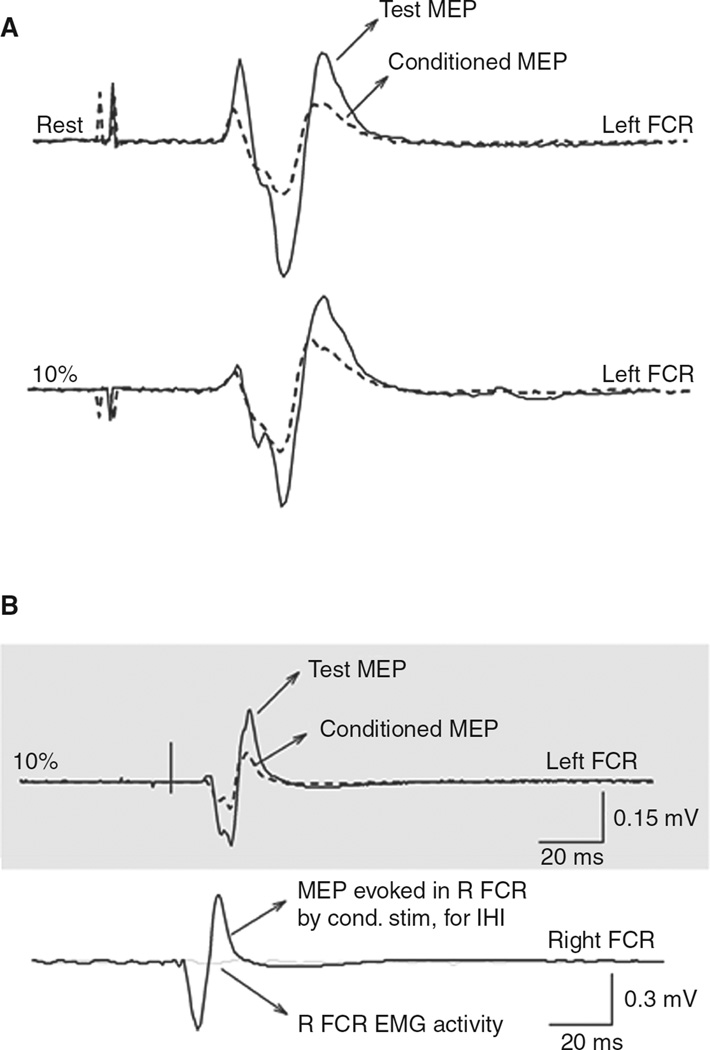
Figure 2.
Short-interval intracortical inhibition (SICI) and interhemispheric inhibition (IHI) measurements. (A) SICI measured in the left flexor carpis radialis (FCR) in a representative subject when the right FCR was at rest or performing 10% of maximal right wrist flexion force. Solid lines indicate test motor-evoked potentials (MEPs); dashed lines indicate conditioned MEPs. The conditioning subthreshold pulse was given 2.5 ms earlier than the test pulse. Note the well-defined SICI at rest and 10% of maximal right wrist flexion force. (B) IHI from the left M1 to the right recorded from the left FCR of a representative subject during performance of 10% of maximal right wrist flexion force. Solid lines indicate test MEPs; dashed lines indicate conditioned MEPs. The conditioning suprathreshold pulse was given 10 ms earlier than the test pulse. Recordings from the right FCR are shown to demonstrate with solid lines the MEP evoked by the conditioning stimulus (for eliciting IHI) and in light gray solid lines the raw EMG activity in the right FCR at the time of application of the test stimulus alone. Note the well-defined IHI at 10% of maximal right wrist flexion force. Modified, with permission, from Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex cortex ipislateral to a moving hand. J Neurosci. 2008;28: 5631–5640. Copyright © 2008 by Elsevier.
SICI After Stroke
Previous studies have reported a decrease in the magnitude of SICI measured in a finger muscle by stimulating the M1 in the affected or the unaffected hemisphere after stroke. We discuss here the extent of this “deficit,” and we ask whether it could be interpreted as a biomarker of stroke recovery.
In the first weeks after stroke, previous studies have reported a decrease in the magnitude of SICI measured by stimulating the M1 in the affected hemisphere,68–70,72,76 although one study reported an increase or no changes in SICI depending on the location of the stroke.92 Liepert et al69 examined SICI in patients 3–14 days after stroke and reported a decrease in SICI by stimulating the ipsilesional M1 compared to the contralesional hemisphere and to healthy controls. One of the interesting findings in this study was that although SICI was decreased the authors still were able to elicit about 30% of inhibition in a finger muscle by stimulating the affected hemisphere and 60% of inhibition was present in the unaffected hemisphere and in healthy controls. Manganotti et al70 also examined SICI 5–7 days after stroke and reported about 15% of inhibition by stimulating the affected hemisphere, 23% in the unaffected hemisphere, and greater inhibition in healthy controls (around 60%). In agreement, Cicinelli et al76 demonstrated a partial loss of SICI (around 45% of inhibition) in the affected hemisphere compared to 30% in the unaffected hemisphere and higher values in healthy controls. Swayne et al72 also reported a decreased in SICI in both hemispheres (approximately 25%–40% of inhibition) compared to healthy controls (approximately 55% of inhibition). Although it is not possible to directly compare the magnitude of inhibition between these studies (i.e., differences might be related to the stimulation parameters), it is important to consider that in the acute stage after stroke a conditioning pulse is still able to inhibit the size of test MEP, suggesting that SICI is to some extent preserved at this early stage.
Bütefisch et al77 published a very elegant paper that provided important insights to better understand the deficits associated with SICI after stroke. In their study (around 30 days after stroke), the authors reported that the magnitude of SICI was completely abolished at certain stimulus intensities but not at others when the contralesional M1 was stimulated compared to healthy controls. The authors demonstrated that part of the input-output intensity curve of SICI was similar in stroke patients and in healthy controls. Swayne et al72 and Cicinelli et al76 also demonstrated a decrease in SICI compared to healthy controls in the contralesional M1 at a similar time period following stroke. However, Manganotti et al70 reported that approximately 30 days after stroke SICI remained decreased in the ipsilesional M1 but values were close to controls in the contralesional hemisphere in patients with good motor recovery. In agreement, Bütefisch et al93 found similar changes in SICI when tested in the ipsilesional M1. At chronic stages, there is less information. Swayne et al72 reported a decrease in SICI as described in the first month after injury, whereas other groups report that the magnitude of SICI tends to reach values similar to those in healthy controls in more chronic stages.94,95 The association between measurements of SICI and motor functional outcomes still remains controversial. Bütefisch et al77 and Shimizu et al94 found no relationship between clinical status and single measurements of SICI. One of the interesting findings in the study by Swayne et al72 was that the relationship between SICI and motor function was present 3 months after stroke but disappeared at 6 months. Therefore, more comprehensive electrophysiological studies are needed to better understand the role of SICI in stroke rehabilitation and motor recovery. Such studies will hopefully include multiple determinations over time after stroke72 using different stimulation parameters77 that are measured in task-relevant settings (associated with performance of the affected voluntary movements).96 A more complicated issue is to interpret the mechanisms by which SICI might be altered after stroke, because most studies included patients with cortical and subcortical lesions. The assessment of patients with damage relatively restricted to the M1 or corticospinal tract might provide more relevant insights to the measurements of SICI after stroke, but on the other hand it may limit the generalizability of the conclusions to wide stroke populations.
IHI Technique
Ferbert and collaborators97 published the first extensive study that reveals powerful interhemispheric interactions between primary motor cortices in intact human subjects by using TMS. It has been demonstrated that a TMS suprathreshold pulse applied over the M1 of one hemisphere can inhibit motor responses evoked in distal and proximal muscles by a magnetic stimulus given 6–50 ms later over the opposite hemisphere (i.e., interhemispheric inhibition [IHI]91,97; see Figure 2B). A single suprathreshold TMS pulse was also capable of inhibiting ongoing voluntary EMG activity when applied to the motor cortex ipsilateral to the contracting arm (i.e., ipsilateral silent period [iSP]). This inhibition lasted for about 30 ms and began 12 to 15 ms after the minimum corticospinal conduction time to the muscle. Ferbert et al97 suggested that the inhibition was produced at cortical level via a transcallosal route. The proposed view that both inhibitory interhemispheric effects were mediated through transcallosal pathways was strongly supported by later studies in patients with agenesis of the corpus callosum,98 but see also Gerloff et al.99 Direct proof of the cortical origin of the inhibition was provided by Di Lazzaro et al100 who recorded descending volleys produced by the test MEP alone and with and without a prior conditioning stimulus to the contralateral motor cortex. In spinal recordings, it was demonstrated that the inhibitory effect was present in the later I3-waves. Pharmacological evidence suggested that IHI measured at short and longer conditioning test intervals is mediated through GABAB receptors,101,102 however, only electrophysiological evidence support the view that the iSP is also mediated by GABAB receptors.103
IHI After Stroke
Previous studies have examined IHI targeting the M1 in the affected or unaffected hemisphere after stroke.93,94,104–107 One important consideration is that in these studies IHI has been measured using a paired-pulse TMS protocol (IHI) and by assessing the iSP. It has been suggested that the inhibition measured by a paired-pulse protocol and the iSP provide information that is complementary but their mechanisms might differ.97,103,108
IHI from the affected to unaffected hemisphere
Around 42 days after stroke, Bütefisch et al93 found in patients with cortical and subcortical lesions that IHI from the affected to the unaffected hemisphere was reduced compared to healthy controls. In contrast, Boroojerdi et al104 found strong IHI from the affected to the unaffected hemisphere (about 50% of inhibition) in patients with subcortical stroke, which did not differ from previous results in healthy controls. In agreement, Lewis and Perrault107 also demonstrated in patients with chronic stroke that IHI from the affected to the unaffected hemisphere was similar to healthy controls at rest. In the study by Boroojerdi et al,104 patients with cortical-subcortical lesions showed inhibition, but this was less pronounced compared to the group with purely subcortical lesions. One important consideration is that Boroojerdi et al104 also measured the iSP. The authors found that in six out of eight patients with subcortical stroke no inhibition was present, and the other two showed about 30% of inhibition in the EMG compared to baseline EMG activity. In the same study, in 14 patients with cortical-subcortical lesions, 5 of them showed no inhibition and all other patients showed an iSP of about 43% of the mean baseline EMG. Shimizu et al94 demonstrated in patients with early (0.6–2.9 months) and late (5.4–12.9 months) cortical stroke no iSP in the unaffected hand after a single TMS pulse to the ipsilesional M1, whereas all the subcortical patients showed a clear iSP in agreement with the results by Boroojerdi et al.104
Task-dependent determinations
One consideration has been that in patients with stroke, motor deficits are present when patients move or intend to move the paretic limbs. On the other hand, most physiological determinations have been made while patients were at rest. Intuitively, it would be desirable to explore these different physiological variables in settings comparable to those in which motor deficits are detected, that is, related to performance of voluntary movements. In this regard, Duque et al106 demonstrated in patients with chronic subcortical stroke that movement of the nonparetic hand was associated with less prominent premovement IHI targeting the unaffected hemisphere as shown in controls. Lewis and Perrault107 demonstrated in patients with chronic stroke that IHI from the affected to the unaffected hemisphere was not increased by voluntary contraction of the paretic hand as demonstrated in healthy subjects.83,97 Clearly, it would represent a step forward to investigate these various physiological parameters in association with performance of motor tasks that are compromised. Such investigations in the future could augment our mechanistic understanding of the mechanisms underpinnings of motor deficits after stroke.
IHI from the unaffected to the affected hemisphere
Bütefisch et al93 showed that regardless of the infarct location IHI from the unaffected to the affected hemisphere was present in patients and their values were close to healthy controls when measured at rest. Similar results at rest were also found by Murase et al.105
Task-dependent determinations
However, deficits have been reported in association with voluntary movements of the paretic hand. Murase et al105 and Duque et al106 showed in patients with chronic subcortical stroke that movements of the paretic hand were associated with persistent premovement IHI targeting the ipsilesional M1 (see Figure 3). More important, the magnitude of this abnormality correlated with the magnitude of motor deficits in the paretic hand in a group of patients with chronic subcortical stroke and relatively good motor recovery. These electrophysiological findings, in line with those in other cognitive domains,109 led to the formulation of possible interventional approaches to facilitate or decrease cortical activity in each hemisphere.12,110–112
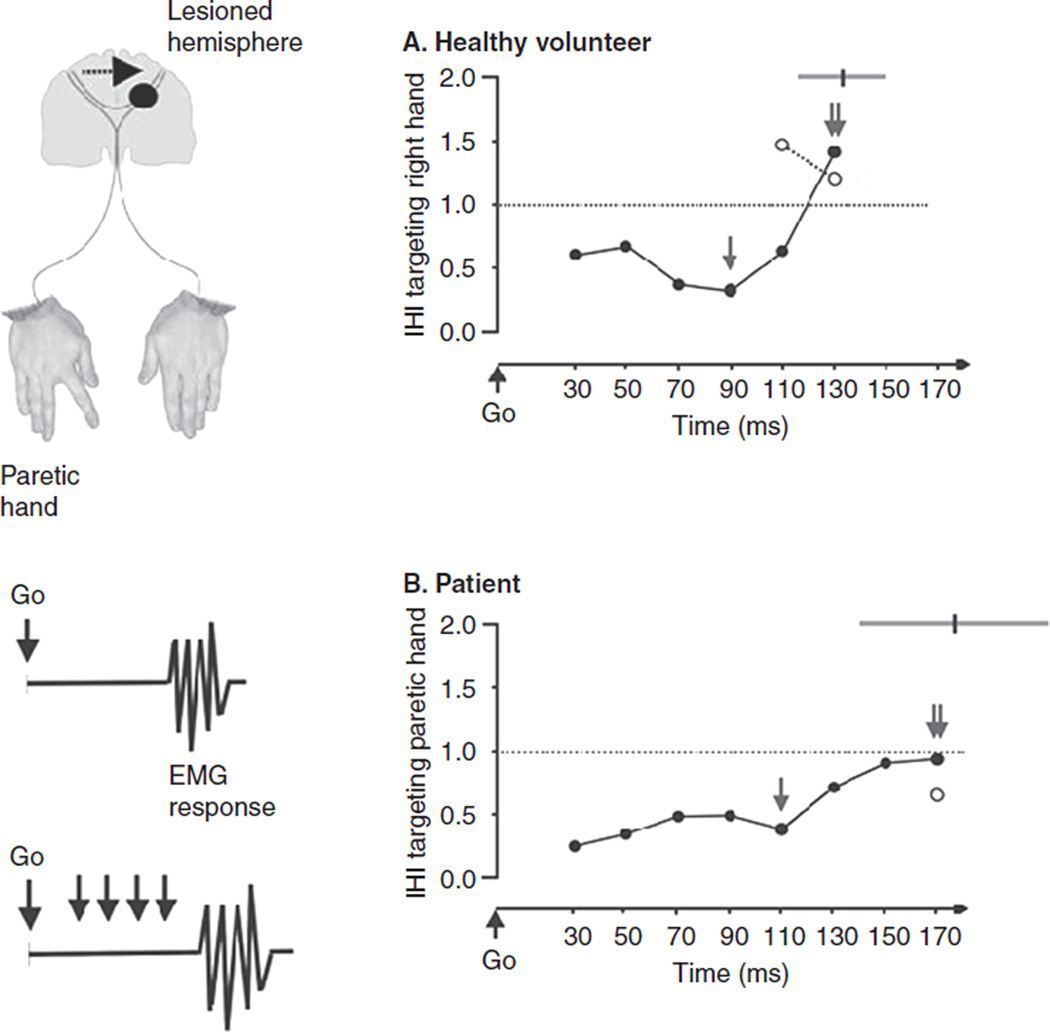
Figure 3.
Left column: Diagram illustrating the proposed hypothesis by Murase et al: enhanced interhemispheric inhibition (IHI) from the intact hemisphere to the M1 in the lesioned hemisphere in patients with subcortical stroke relative to healthy controls. IHI was evaluated in the process of generation of a voluntary movement in a simple reaction time (RT) paradigm. Subjects responded with a brisk voluntary index finger movement to a Go signal. The onset of the electromyogram (EMG) response from first dorsal interosseus (FDI) characterized the RT. IHI in the paretic hand was studied applying a conditioning stimulus to the M1 of the intact hemisphere of patients (right hemisphere of controls) 10 ms preceding a test stimulus applied to M1 of the lesioned hemisphere of patients (or left hemisphere of controls). Right column: IHI targeting the moving hand in a RT paradigm in a healthy volunteer (A) and a stroke patient (B). The abscissa shows the timing (milliseconds) of application of the test stimulus relative to the Go signal. The ordinate shows the magnitude of IHI targeting the moving hand (1 indicates absence of facilitation or inhibition; >1 indicates facilitation; and <1 indicates inhibition). Note the deep maximum IHI (single arrow, <1) that progressively became less prominent in intervals close to movement onset in both subjects. Around movement onset, IHI at movement onset (double arrow) and IHI before movement (open circles) turned to facilitation in the control subject but remained inhibited in the patient. The gray lines in the top right corner indicate mean ±1 SD of RTs in unstimulated trials. Modified, with permission, from Murase N, Duque J, Mazzocchio R, et al. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. Copyright © 2004 by Wiley InterScience.
Relationship between SICI and IHI
Bütefisch et al93 showed in patients with cortical infarct an inverse relationship between SICI and IHI. In this study, patients with less IHI targeting the unaffected hemisphere were those with greater SICI in the same hemisphere, consistent with previous findings in healthy controls.82,83 Butefisch et al93 proposed that, since in their study SICI was decreased in both hemispheres while measurements of IHI were only impaired from the affected to the unaffected hemisphere, it was unlikely that IHI was the only mechanism responsible for changes in SICI. This is in agreement with extensive literature showing that SICI measured at rest is decreased in the affected hemisphere compared to the unaffected hemisphere or controls,68–70,72,76,77 whereas IHI targeting the ipsilesional M1 is similar to control values when tested at rest.93,105 On the other hand, a recent study documented abnormal premovement modulation of SICI in the ipsilesional M1 of patients with chronic stroke. This abnormality might contribute to deficits in motor control of the paretic hand, presenting a possible target for correction.96 Shimizu et al94 also suggested a relationship between SICI and the iSP after stroke, but the authors did not specify whether these two measurements might be directly or indirectly related. However, the lack of iSP in the unaffected hand and the normal resting SICI measure in the unaffected hemisphere, inasmuch as they can be compared, agree with the results of Bütefisch et al,93 suggesting that it is unlikely that IHI is the only mechanism responsible for changes found in SICI. One of our challenges in stroke rehabilitation is to expand our assessment of cortical physiology to multiple sites (other than M1) within the central nervous system that might be affected directly or indirectly through distant transynaptic mechanisms by the lesion. In this regard, it is important to keep in mind that circuits mediating SICI and IHI are affected by sensory inputs113–115 that may be disrupted after stroke. In fact, manipulation of sensory inputs has resulted in improvements in hand motor function after stroke.116,117
Conclusions
This review focused on the description of motor functional deficits after lesions of the CST or M1 in primate models and after stroke in humans. Although it is often thought that severe motor deficits after stroke reflect the involvement of the corticospinal system, studies in monkeys and humans with lesions limited to the CST revealed recovery of voluntary control of movements to a great extent over time. Residual deficits in fractionation of finger movements and agonist contraction/antagonist inhibition persist. We discussed the changes in two crucial neurophysiological measurements—intracortical inhibition and interhemispheric inhibition— in stroke patients at rest and in task-related settings as well as their possible interactions. Clearly, there are many other neurophysiological mechanisms that involve other regions interacting with M1 including nonprimary motor (PMd, PMv, and SMA) and parietal areas that require investigation after stroke. TMS has proved an excellent tool to address these upcoming questions of mechanistic and clinical value. Overall, the available evidence keeps pointing toward the need for more studies that include multiple measurements in the time domain72 and the assessment of multiple stimulation parameters77 in behaviorally relevant movement-dependent domains105 to further our understanding of motor cortical function in stroke recovery.
Contributor Information
Monica A. Perez, University of Pittsburgh, Department of Physical Medicine and Rehabilitation, Center for the Neural Basis of Cognition, Pittsburgh, Pennsylvania.
Leonardo G. Cohen, Human Cortical Physiology and Stroke Neurorehabilitation Section, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.American Heart Association. statistics update. 2009 [Google Scholar]
- 2.van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357:572–579. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- 3.Flynn RW, MacWalter RS, Doney AS. The cost of cerebral ischaemia. Neuropharmacology. 2008;55:250–256. doi: 10.1016/j.neuropharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics–2009 Update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Dec 15; doi: 10.1161/CIRCULATIONAHA.108.191259. E-pub. CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 5.Cheeran B, Cohen L, Dobkin B, et al. The future of restorative neurosciences in stroke: driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabil Neural Repair. 2009;23:97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen LG, Ziemann U, Chen R, Classen J, Hallett M, Gerloff C, Butefisch C. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:305–324. doi: 10.1097/00004691-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Seitz RJ, Bütefisch CM, Kleiser R, Hömberg V. Reorganisation of cerebral circuits in human ischemic brain disease. Restor Neurol Neurosci. 2004;22:207–229. [PubMed] [Google Scholar]
- 9.Ward NS. Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol. 2004;17:725–730. doi: 10.1097/00019052-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Liepert J. Transcranial magnetic stimulation in neurorehabilitation. Acta Neurochir Suppl. 2005;93:71–74. doi: 10.1007/3-211-27577-0_10. [DOI] [PubMed] [Google Scholar]
- 11.Bütefisch CM, Kleiser R, Seitz RJ. Post-lesional cerebral reorganisation: evidence from functional neuroimaging and transcranial magnetic stimulation. J Physiol Paris. 2006;99:437–454. doi: 10.1016/j.jphysparis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Floel A, Cohen LG. Translational studies in neurorehabilitation: From bench to bedside. Cogn Behav Neurol. 2006;19:1–10. doi: 10.1097/00146965-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neuro-rehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 14.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx. 2006;3:474–481. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovasc Dis. 2007;24:157–166. doi: 10.1159/000107392. [DOI] [PubMed] [Google Scholar]
- 17.Butler AJ, Wolf SL. Putting the brain on the map: use of transcranial magnetic stimulation to assess and induce cortical plasticity of upper-extremity movement. Phys Ther. 2007;87:719–736. doi: 10.2522/ptj.20060274. [DOI] [PubMed] [Google Scholar]
- 18.Profice P, Pilato F, Dileone M, et al. Use of transcranial magnetic stimulation of the brain in stroke rehabilitation. Expert Rev Neurother. 2007;7:249–258. doi: 10.1586/14737175.7.3.249. [DOI] [PubMed] [Google Scholar]
- 19.Rossini PM, Altamura C, Ferreri F, et al. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eura Medicophys. 2007;43:241–254. [PubMed] [Google Scholar]
- 20.Chen R, Cros D, Curra A, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Bernad DM, Doyon J. The role of noninvasive techniques in stroke therapy. Int J Biomed Imaging. 2008;2008:672–682. doi: 10.1155/2008/672582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliassen JC, Boespflug EL, Lamy M, Allendorfer J, Chu WJ, Szaflarski JP. Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: a review. Top Stroke Rehabil. 2008;15:427–450. doi: 10.1310/tsr1505-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards LG, Stewart KC, Woodbury ML, Senesac C, Cauraugh JH. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia. 2008;46:3–11. doi: 10.1016/j.neuropsychologia.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;19:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Hepp-Reymond M-C, Wiesendanger M. Unilateral pyramidotomy in monkeys: effect on force and speed of a conditioned precision grip. Brain Res. 1972;36:117–131. doi: 10.1016/0006-8993(72)90770-6. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman DS, Strick PL. Effects of a primary motor cortex lesion on step-tracking movements of the wrist. J Neurophysiol. 1995;73:891–895. doi: 10.1152/jn.1995.73.2.891. [DOI] [PubMed] [Google Scholar]
- 29.Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- 30.Kubota K. Motor cortical muscimol injection disrupts forelimb movement in freely moving monkeys. NeuroReport. 1996;7:2379–2384. doi: 10.1097/00001756-199610020-00020. [DOI] [PubMed] [Google Scholar]
- 31.Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glees P, Cole J. Recovery of skilled motor functions after small repeated lesions in motor cortex in macaque. J Neurophysiol. 1950;13:137–148. [Google Scholar]
- 33.Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- 34.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 35.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 36.Barbay S, Plautz EJ, Friel KM, Frost SB, Dancause N, Stowe AM, Nudo RJ. Behavioral and neuro-physiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2006;169:106–116. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Exp Brain Res. 1991;84:668–671. doi: 10.1007/BF00230980. [DOI] [PubMed] [Google Scholar]
- 38.Stowe AM, Plautz EJ, Eisner-Janowicz I, et al. VEGF protein associates to neurons in remote regions following cortical infarct. J Cereb Blood Flow Metab. 2007:2776–2785. doi: 10.1038/sj.jcbfm.9600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 40.Schmidlin E, Brochier T, Maier MA, Kirkwood PA, Lemon RN. Pronounced reduction of digit motor responses evoked from macaque ventral premotor cortex after reversible inactivation of the primary motor cortex hand area. J Neurosci. 2008;28:5772–5783. doi: 10.1523/JNEUROSCI.0944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 42.Hammond MC, Fitts SS, Kraft GH, Nutter PB, Trotter MJ, Robinson LM. Cocontraction in the hemiparetic forearm: quantitative EMG evaluation. Arch Phys Med Rehabil. 1988;69:348–351. [PubMed] [Google Scholar]
- 43.Bourbonnais D, Vanden Noven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- 44.Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve. 2001;24:673–681. doi: 10.1002/mus.1054. [DOI] [PubMed] [Google Scholar]
- 45.Fellows SJ, Kaus C, Thilmann AF. Voluntary movement at the elbow in spastic hemiparesis. Ann Neurol. 1994;36:397–407. doi: 10.1002/ana.410360311. [DOI] [PubMed] [Google Scholar]
- 46.Gowland C, deBruin H, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Phys Ther. 1992;72:624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- 47.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 48.Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol. 2003;90:1160–1170. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- 49.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 50.Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979;36:65–73. doi: 10.1001/archneur.1979.00500380035003. [DOI] [PubMed] [Google Scholar]
- 51.Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- 52.Cramer SC, Mark A, Barquist K, et al. Motor cortex activation is preserved in patients with chronic hemiplegic stroke. Ann Neurol. 2002;52:607–616. doi: 10.1002/ana.10351. [DOI] [PubMed] [Google Scholar]
- 53.Buch E, Weber C, Cohen LG, et al. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fridman EA, Hanakawa T, Chung M, et al. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 55.Mills KR. Magnetic brain stimulation: a tool to explore the action of the motor cortex on single human spinal motoneurons. Trends Neurosci. 1991;14:401–405. doi: 10.1016/0166-2236(91)90029-t. [DOI] [PubMed] [Google Scholar]
- 56.Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–312. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brouwer B, Ashby P. Corticospinal projections to lower limb motoneurons in man. Exp Brain Res. 1992;89:649–654. doi: 10.1007/BF00229889. [DOI] [PubMed] [Google Scholar]
- 58.Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurons in man. J Physiol. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nielsen J, Petersen N, Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurons in man. J Physiol. 1995;484:791–802. doi: 10.1113/jphysiol.1995.sp020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amassian VE, Cracco RQ, Maccabee PJ. Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:401–416. doi: 10.1016/0168-5597(89)90029-4. [DOI] [PubMed] [Google Scholar]
- 62.Rossini PM, Barker AT, Berardelli A, et al. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 63.Ziemann U, Bruns D, Paulus W. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neurosci Lett. 1996;208:187–190. doi: 10.1016/0304-3940(96)12575-1. [DOI] [PubMed] [Google Scholar]
- 64.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 65.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 66.Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in acute stroke: early excitation threshold and functional prognosis. Electroencephalogr Clin Neurophysiol. 1996;101:233–239. doi: 10.1016/0924-980x(96)95656-8. [DOI] [PubMed] [Google Scholar]
- 67.Traversa R, Cicinelli P, Oliveri M, et al. Neurophysiological follow-up of motor cortical output in stroke patients. Clin Neurophysiol. 2000;111:1695–1703. doi: 10.1016/s1388-2457(00)00373-4. [DOI] [PubMed] [Google Scholar]
- 68.Nardone R, Tezzon F. Inhibitory and excitatory circuits of cerebral cortex after ischaemic stroke: prognostic value of the transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 2002;42:131–136. [PubMed] [Google Scholar]
- 69.Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- 70.Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- 71.Delvaux V, Alagona G, Gerard P, De Pasqua V, Pennisi G, de Noordhout AM. Post-stroke reorganization of hand motor area: a 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin Neurophysiol. 2003;114:1217–1225. doi: 10.1016/s1388-2457(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 72.Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–2192. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 74.Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 75.Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 76.Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34:2653–2658. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- 77.Bütefisch CM, Netz J, Wessling M, Seitz RJ, Hömberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- 78.Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889:278–287. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- 79.Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL. Motor outcome after subcortical stroke: MEPs correlate with hand strength but not dexterity. Clin Neurophysiol. 2002;113:2025–2029. doi: 10.1016/s1388-2457(02)00318-8. [DOI] [PubMed] [Google Scholar]
- 80.Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res. 1999;129:559–572. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- 81.Byrnes ML, Thickbroom GW, Phillips BA, Wilson SA, Mastaglia FL. Physiological studies of the corticomotor projection to the hand after sub-cortical stroke. Clin Neurophysiol. 1999;110:487–498. doi: 10.1016/s1388-2457(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 82.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex cortex ipislateral to a moving hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- 86.Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- 87.DiLazzaro V, Restuccia D, Oliviero A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- 88.Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di Lazzaro V, Oliviero A, Profice P, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- 90.Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. J Neurol Rehabil. 2007;21:568–573. doi: 10.1177/1545968307302438. [DOI] [PubMed] [Google Scholar]
- 93.Bütefisch CM, Wessling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22:4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- 94.Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 95.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 96.Hummel F, Steven B, Hoppe J, Heise K, Thomalla G, Cohen LG, Gerloff C. Deficient short intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009;23(4):366–372. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- 99.Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Di Lazzaro V, Oliviero A, Profice P, et al. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- 101.Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA (A) and GABA (B) agonists on interhemispheric inhibition in man. Clin Neurophysiol. 2007;118:308–316. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 102.Florian J, Müller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 104.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 105.Murase N, Duque J, Mazzocchio R, et al. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 106.Duque J, Hummel F, Celnik P, et al. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 107.Lewis GN, Perreault EJ. Side of lesion influences interhemispheric inhibition in subjects with post-stroke hemiparesis. Clin Neurophysiol. 2007;118:2656–2663. doi: 10.1016/j.clinph.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiology. 2009;587:725–726. doi: 10.1113/jphysiol.2008.166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- 110.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy toimprove neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 111.Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- 113.Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. Erratum in: J Physiol. 2003;552:993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swayne O, Rothwell J, Rosenkranz K. Transcallosal sensorimotor integration: effects of sensory input on cortical projections to the contralateral hand. Clin Neurophysiol. 2006;117:855–863. doi: 10.1016/j.clinph.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 115.Floel A, Hummel F, Duque J, Knecht S, Cohen LG. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 117.Floel A, Nagorsen U, Werhahn KJ, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]