Abstract
The ability to generate and maintain stable in vitro cultures of mouse endothelial cells (EC) has great potential for genetic dissection of the numerous pathologies involving vascular dysfunction as well as therapeutic applications. However, previous efforts at achieving sustained cultures of primary stable murine vascular cells have fallen short, and the cellular requirements for EC maintenance in vitro remain undefined. In this study, we have generated vascular ECs from mouse embryonic stem (ES) cells, and show that active Akt is essential to their survival and propagation as homogeneous monolayers in vitro. These cells harbor the phenotypical, biochemical, and functional characteristics of ECs, and expand throughout long-term cultures, while maintaining their angiogenic capacity. Moreover, Akt-transduced embryonic ECs form functional perfused vessels in vivo that anastomose with host blood vessels. We provide evidence for a novel function of Akt in stabilizing EC identity, whereby the activated form of the protein protects mouse ES cell-derived ECs from TGFβ-mediated transdifferentiation by downregulating SMAD3. These findings identify a role for Akt in regulating the developmental potential of ES cell-derived ECs, and demonstrate that active Akt maintains endothelial identity in embryonic ECs by interfering with active TGFβ-mediated processes that would ordinarily usher these cells to alternate fates.
Keywords: endothelial, embryonic, stem, Akt, SMAD3
INTRODUCTION
To date, the ability to generate long-term in vitro cultures of stable mouse embryonic stem (ES) cell-derived endothelial cells (ECs) has been hindered by the inability to faithfully recreate a physiological micro-environment in vitro, including the requisite signaling milieu that is essential to maintain a vascular identity. The development and upkeep of a properly formed vascular system is a tightly regulated process that is dependent on intrinsic genetic determinants and extracellular signals. Although ECs are exposed to a wide repertoire of factors that mediate their maintenance in vivo, only a small fraction of these cues have been identified [1, 2]. When these cells are removed from their physiological environment for in vitro culture, they are no longer subjected to these angiogenic factors, and thus fail to proliferate long-term.
The culture of stable ECs in fully defined conditions has the potential to accelerate drug discovery and enable cell-based therapies, while also improving our understanding of the genesis and homeostasis of the vascular system. Indeed, experiments using human ECs have revealed many factors that govern physiological and pathological vasculogenesis and angiogenesis [3]. However, further study has been restricted by the impracticality of obtaining human ECs from specific genetic backgrounds, as well as by the limited tissue sources from which human vascular cells can be isolated. The culture of stable, bona-fide ECs from mice is an attractive alternative, with a growing library of genetically modified animals from which ECs can be obtained. An in vitro model to study mouse ECs would provide a platform to unveil the genetic contributions to numerous vascular maladies, as well as the effects of therapeutic agents on ECs of specific disease contexts. Current approaches have focused on isolating vascular progenitors from differentiating embryonic stem cells, followed by screening for factors that can enhance vascular specification [4–6]. However, these approaches have yielded modest amounts of ECs in heterogeneous cultures due to the plasticity of embryonic cells, and because the mechanisms governing EC stability have yet to be elucidated. Other methods have focused on the isolation of adult ECs, but these techniques have yielded variable populations of unstable cells [7, 8].
Numerous studies have addressed the molecular circuitry that governs vascular fate during embryonic development. While some attention has been given to determining the proper signaling environments and growth factor requirements for vascular EC specification [9–11], recent studies have interrogated the intrinsic transcriptional programs responsible for vascular identity. The ETS-family of transcription factors has been implicated in various aspects of EC development and angiogenesis [12–15]. In particular, ER71 (ETV2 or etsrp) was identified as an early regulator of endothelial cell fate, through direct control of vascular genes such as VEGFR2 and VE-cadherin [16–18], and through its genetic interactions with other vascular transcription factors [19]. In fact, ER71 was shown to be indispensable for the initial specification of vascular mesoderm during development [20]. As such, ER71 can be placed at the apex of endothelial development, setting in motion downstream events which perpetuate the vascular lineage in those cells. Indeed, the potency of ER71’s inductive capacity was recently demonstrated when the overexpression of ER71 was shown to be crucial to initiate the reprogramming of non-vascular cells into EC’s [21], and therefore might play a key role in the maintenance of endothelial identity in developing embryonic cells.
The Serine/Threonine kinase Akt, a component of the Phosphatidylinositol-3-Kinase (PI3K) signaling axis, is involved in numerous cellular processes such as apoptosis, cell growth, and differentiation [22]. Akt activation is involved in the survival of many cell types, including ECs [23, 24]. While deregulation of the PI3K signaling pathway is implicated in various tumorigenic scenarios, persistent activation of Akt itself was shown to be non-transformative [25, 26]. In addition to its canonical roles, new functions have recently been uncovered whereby signaling through Akt is activated specifically during angiogenesis [27], in part by promoting the elaboration of endothelial-derived angiogenic factors [28].
The TGFβ signaling pathway is involved in numerous aspects of EC biology, including migration, proliferation, and tube formation [29]. Signaling through the TGFβ receptor leads to changes in gene expression by activating SMAD proteins, which convey the signal to the nucleus [30]. In particular, the response to TGFβ is mediated by SMAD2 and SMAD3, which form heteromeric complexes with SMAD4, before translocating to the nucleus and affecting transcription of downstream target genes [31]. TGFβ signaling plays an important role during EC development in regulating VEGF-A expression and bioavailability [32], in addition to downregulating expression of its receptor, VEGFR2/Flk-1 [33]. On the other hand, inhibition of TGFβ signaling in ECs was recently shown to promote their expansion and maintenance [34, 35].
In this study, we report the generation and long-term culture of stable, functional ECs derived from mouse embryonic stem (ES) cells. Using a modified cytokine-mediated differentiation platform, embryonic ECs were generated from mouse ES cells, isolated, and transduced with Akt via lentiviral infection. Akt-transduced ECs are viable in serum-free culture conditions, and are highly proliferative over many months through numerous passages. These ECs exhibit the genetic and phenotypic hallmarks of prototypical ECs, presenting canonical endothelial proteins localized at the proper subcellular domains. When stimulated with angiogenic factors such as VEGF-A, Akt-transduced ECs demonstrate endothelial responses in vitro, such as VEGFR2 phosphorylation and acetylated low-density lipoprotein uptake. Additionally, they form tube-like networks on Matrigel in vitro, and functional, patent vessels in vivo when transplanted into mice in subcutaneous Matrigel or liver regeneration models. Furthermore, we identified the TGFβ pathway (specifically Smad3) as a target for Akt in maintaining ECs in vitro. These data establish Akt as a specific angiogenic effector in ECs, whose activity is sufficient to maintain mouse ES cell-derived ECs in vitro over long term cultures without oncogenic transformation. These findings offer new insights into mouse EC biology, and provide a novel in vitro platform to study stable ECs for biomedical discovery.
MATERIALS AND METHODS
Cells and Cell Culture
Mouse ES cells were maintained on mouse embryonic fibroblasts in ES media. Differentiation of ES cells was carried out in StemPro-34 media for 14 days, supplemented with Bone Morphogenic Protein 4 (BMP4, 5ng/mL), activin A (5ng/mL), Fibroblast Growth Factor (FGF)-2 (10ng/mL), and Vascular Endothelial Growth Factor (VEGF)-A (20ng/mL). ES cells were transferred to StemPro-34 media on Day 0 to begin the differentiation protocol. On day 1, BMP4 was added. On day 2, the media was changed with fresh BMP4, activin A, and FGF-2. On day 3, the media was once again changed, removing activin A, while adding fresh BMP4, FGF-2, and VEGF-A. On day 5, the media was changed, removing BMP4, while adding fresh FGF-2 and VEGF-A. The media was then refreshed every two days with FGF-2 and VEGF-A, to Day 14, when Tie2+ ECs were isolated. Detailed conditions can be found in the supplemental methods.
Lentiviral Transduction
The mouse ER71, myristoylated Akt1 (Akt), K-Ras, polyoma middle-T antigen, and C-RAF cDNAs were cloned into the pCCL.PGK lentiviral vector. Viruses were produced in 293T cells, concentrated, and used singly at a multiplicity of infection (MOI) of 10. Detailed virus production methods can be found in the supplemental methods.
Quantitative PCR and RNA-Sequencing
Total RNA was extracted from cells using the RNeasy Mini kit (Qiagen), followed by cDNA preparation with the Quantitect Reverse Transcription kit (Qiagen), and then by qPCR analysis on a 7500 Fast Real Time PCR System (Applied Biosystems). RNA-Sequencing analysis was performed as previously described [21]. Primers used and detailed conditions can be found in the supplemental methods.
Immunofluorescence
Cultured cells were fixed in 4% paraformaldehyde, and immunocytochemically stained in situ, as previously described [34]. Primary antibodies used were anti-mouse VE-Cadherin and PECAM. Imaging was performed on a Zeiss 710 META confocal microscope (Carl Zeiss, Germany).
Matrigel™ Plug In Vivo Assay
Control, ER71- or Akt-transduced Tie2-ECs were labeled with a PGK-GFP lentivirus. These cells were mixed with Matrigel (BD), then injected subcutaneously to the flanks of NOD-SCID-IL2Rγ−/− (NSG) mice (Jackson Laboratories, ME) (n=5). After two weeks, intravital labeling of the vasculature was achieved with Alexa568-conjugated Isolectin (Invitrogen) (2mg/kg). After 10 minutes, the Matrigel plugs were harvested, fixed in 4% paraformaldehyde overnight, and saturated with 30% sucrose for 48 hours. 30µM sections were stained with PECAM (BD), then counterstained with DAPI. Confocal images were obtained on a Zeiss 710 META confocal microscope (Carl Zeiss, Germany).
Liver Regeneration Engraftment Assay
GFP-labeled Tie2-Akt-ECs were intrasplenically injected into C57BL/6 mice (n=4) immediately following 70% partial hepatectomy, as previously described [36]. Three weeks after injection of the GFP-labeled Tie2-Akt-ECs, intravital labeling of the vasculature was achieved with Alexa568-conjugated Isolectin (Invitrogen) or anti-VE-Cadherin (Biolegend). The mice were then sacrificed, and their livers were harvested, processed, and analyzed as the Matrigel plugs above.
Western Blot
Western blot analysis was performed as previously described [28]. See the supplemental methods for detailed procedures and antibodies used.
RESULTS
Tie2-positive ECs emerge from mouse embryonic stem (ES) cells over a 14-day time interval
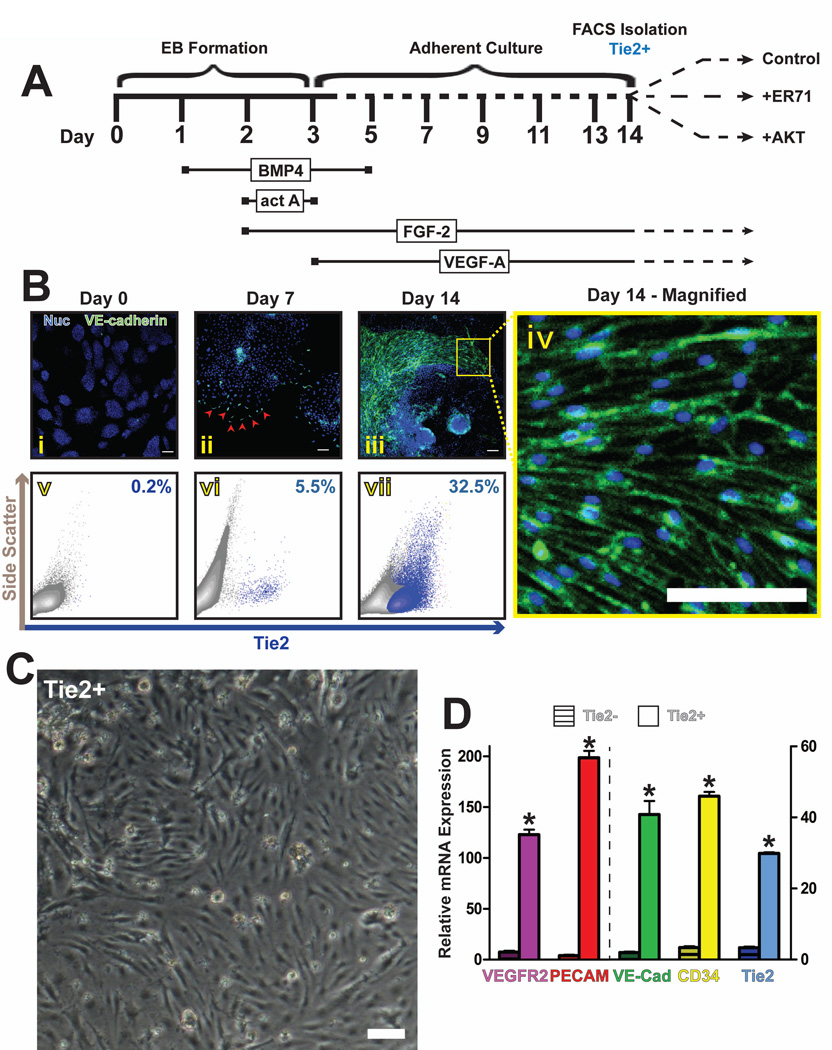
To drive mouse ES cells towards the vascular lineage, we modified previously published protocols [11, 34] to develop a chemically defined, serum-free differentiation timeline. Embryoid body cultures of mouse ES cells were sequentially stimulated with bone morphogenic protein 4 (BMP4), activin A, fibroblast growth factor 2 (FGF-2), and vascular endothelial growth factor A (VEGF-A) (Fig 1A). At the beginning of the differentiation (Day 0), no VE-cadherin protein could be visualized by immunofluorescence (Fig 1Bi). Correspondingly, no surface expression of Tie2 could be detected by flow cytometry on undifferentiated mouse ES cells (Fig 1Bv). Kinetic analysis of differentiation cultures revealed a small, distinct population of Tie2-expressing cells at Day 7 (Fig 1Bvi), but a robust population by Day 14 (Fig 1Bvii), with no significant change afterwards (data not shown). These results correlated with the visualization of sparse VE-cadherin-expressing cells at Day 7 (Fig 1Bii), compared to numerous VE-cadherin-positive EC sheets at Day 14 (Fig 1Biii, iv). To verify the endothelial identity of this cell population, Tie2-positive cells were FACS sorted, and their gene expression profiles were examined for known endothelial genes. Immediately after isolation, Tie2-positive cells exhibit the morphological characteristics of ECs (Fig 1C). They also express typical vascular genes such as VE-cadherin, VEGFR2, and PECAM (Fig 1D). Since Tie2 expression has been reported on a subset of hematopoietic cells [37, 38], flow cytometry analysis was performed to exclude the presence of Tie2-positive hematopoietic cells. Differentiation cultures at day 14 did not exhibit significant populations of CD45, CD41, or CD11b-positive cells (Fig S1A–C), indicating that the isolated Tie2 cells weren’t of hematopoietic identity. As might be expected, the Tie2-negative component of these cultures displayed some expression of Smooth Muscle Actin, though no CD90 expression could be detected (Fig S1D).
Figure 1. Differentiation of mouse ES cells to Tie2+ ECs.
a) Schematic of mouse ES differentiation protocol to Tie2+ ECs. Mouse ES cells were incubated sequentially with BMP4, Activin A, FGF-2, and VEGF-A over a 14-day time course, at which point Tie2+ cells were isolated by FACS. b) Immunofluorescence images show the absence of VE-Cadherin+ ECs at the start of the differentiation protocol (i), the emergence of individual punctuate VE-Cadherin+ ECs at day seven (ii, red arrowheads), and the abundance of VE-Cadherin+ EC sheets at day 14 (iii, iv) of ES cell differentiation. These correlate with a concomitant increase in surface expression of Tie2, as determined by flow cytometry (v–vii). Panel iv is an enlargement of a field from panel iii, showing the membrane localization of the VE-Cadherin complexes. Scale bars – 100µm. c) Phase-contrast microscopy reveals an endothelial morphology of isolated ES cell-derived Tie2+ cells, six hours after sorting. Scale bar – 100µm. d) qPCR analysis was performed to determine the relative mRNA expression levels of canonical EC genes in the ES cell-derived Tie2+ versus Tie2− populations. Error bars represent standard error of experimental values performed in triplicate. (p<0.01 compared to Tie2− cells for all genes tested).
Purified Tie2-positive ECs lose their identity in vitro
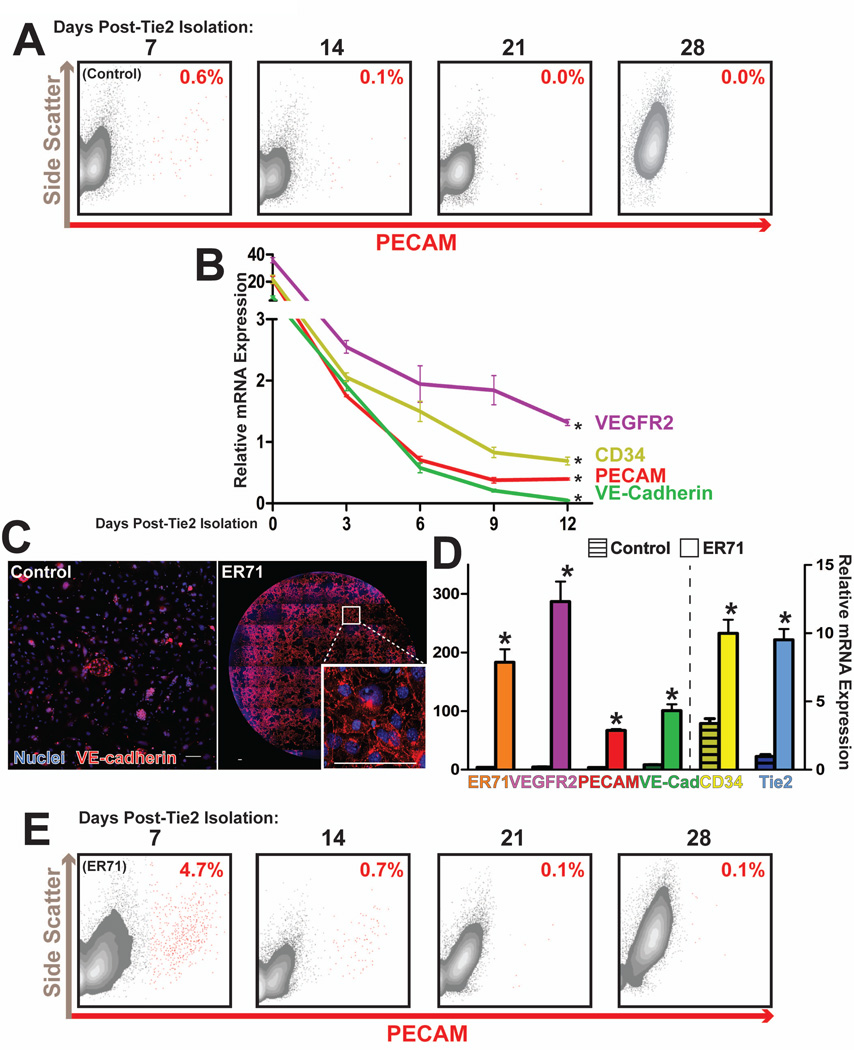
To further study the stability and durability of vascular identity of these embryonic ECs, we attempted to maintain them as a homogeneous monolayer in vitro post-isolation. After returning these ECs to culture, we observed a loss of endothelial identity, as indicated by a loss of surface expression of PECAM (CD31) (Fig 2A, S2A). Concomitantly, we observed a loss of expression of other vascular genes including VE-Cadherin, VEGFR2, and CD34 (Fig 2B). Due to their embryonic origin, we hypothesized that the loss of EC identity was a function of the incomplete specification, and inherent plasticity, of these vascular cells.
Figure 2. ER71 overexpression induces the vascular program, but does not maintain ES cell-derived Tie2-ECs.
a) Flow cytometry of sorted Tie2-ECs shows a rapid loss of PECAM surface expression after further in vitro culture. b) qPCR analysis was performed to determine the prevalence of EC markers over several days after isolation of Tie2-ECs. Error bars represent standard error of experimental values performed in triplicate. (p<0.03 compared to day-0). c) Immunocytostaining of Tie2-ECs two days after isolation demonstrates that ER71 overexpression is capable of sustaining junctional VE-Cadherin complexes, whereas this protein is scarcely detected in control (untransduced) cultures. Scale bars – 100µm. d) ER71 upregulates the expression of EC genes in sorted Tie2-ECs, as measured by qPCR. Error bars represent standard error of experimental values performed in triplicate. (p<0.02 compared to control cells for all genes tested). e) Flow cytometry analysis of ER71-transduced Tie2-ECs reveals a loss of PECAM surface expression after continued in vitro culture.
ER71 overexpression temporarily stabilizes endothelial identity in Tie2-positive ECs
Recent studies have shown that the ETS transcription factor ER71 acts as an early regulator of endothelial fate by coordinating expression of multiple vascular genes [39, 40]. To examine the potential of ER71 to specify an endothelial fate in embryonic-derived mouse cells, the murine ER71 gene was cloned into a lentiviral overexpression vector. Transduction of ES-derived Tie2-positive cells with the ER71 lentivirus resulted in an initial stabilization of EC identity. Compared to control (untransduced) cells, Tie2-positive ECs overexpressing ER71 retained a surface phenotype consistent with endothelial identity, including functional VE-cadherin complexes at intercellular junctions (Fig 2C). Similarly, ER71 overexpression resulted in an increase in the expression of various vascular genes, compared to control cells (Fig 2D).
While ER71 overexpression reinforced vascular identity, it was not sufficient to maintain these ECs for long durations. Upon further culture, ER71-transduced Tie2-ECs lose surface expression of PECAM (Fig 2E, S2B), regressing to a phenotype similar to untransduced Tie2-ECs. These data suggest that the in vitro instability of these Tie2-positive ECs is not a function of their incomplete specification, but rather due to other maintenance-specific mechanisms.
Active Akt promotes the maintenance of Tie2-positive ECs
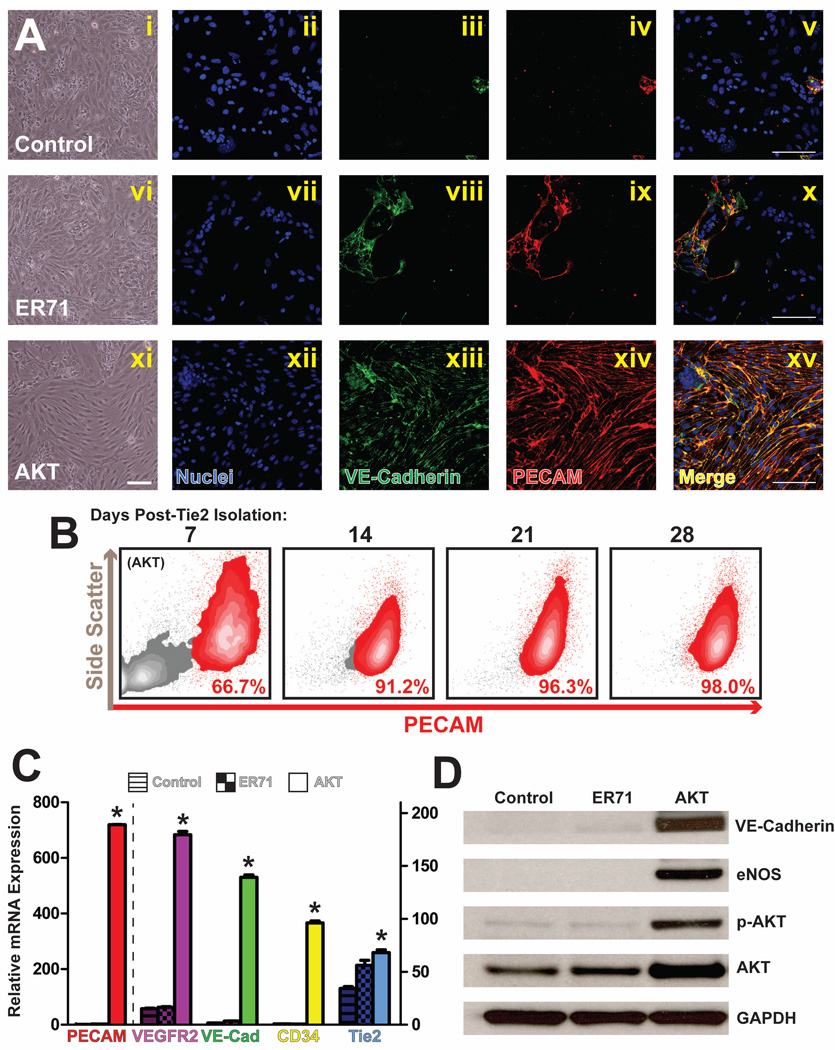
Akt was recently implicated in EC-specific signaling and survival [27], and was thus a physiologically relevant candidate to maintain the vascular identity of ES cell-derived Tie2-ECs in vitro. To test this hypothesis, we transduced purified Tie2-ECs with lentiviral constructs expressing constitutively active Akt1 (Tie2-Akt-ECs). In contrast to control (Fig 3Ai) or ER71-transduced (Fig 3Avi) Tie2+ cells, Tie2-Akt-ECs maintained an endothelial morphology in vitro in isolated culture (Fig 3Axi).
Figure 3. Akt overexpression maintains Tie2-ECs long-term in in vitro culture.
a) Phase-contrast microscopy (i, vi, xi) and immunofluorescence (ii–v, vii–x, xii–xv) imaging of control (untransduced), ER71-transduced, or Akt-transduced Tie2-ECs 28 days after the initial Tie2 isolation shows that the EC membrane proteins PECAM and VE-Cadherin are maintained with Akt overexpression. Scale bars – 100µm. b) Flow cytometry analysis of Tie2-Akt-ECs demonstrates the maintenance of surface PECAM expression over a 28 day time course across the entire culture. c) qPCR analysis was performed 28 days after the initial Tie2-EC isolation, and revealed expression of endothelial genes in Tie2-Akt-ECs, but not in control (untransduced) or ER71-transduced cells. Error bars represent standard error of experimental values performed in triplicate. (p<0.01 compared to control cells for all genes tested). d) Western blot analysis of sorted Tie2-ECs reveals the presence of EC-specific proteins in Tie2-Akt-ECs, but not in control (untransduced), or ER71-transduced cells, 28 days after the initial Tie2 isolation.
To confirm the endothelial identity of the Tie2-Akt-ECs, we performed in situ immunocytostaining for VE-Cadherin and PECAM. Whereas these proteins were not detected in control or ER71-transduced cells, VE-Cadherin and PECAM were abundant in the Akt-transduced cells, and were properly localized at the intercellular junctions (Fig 3Axii–xv). Additionally, these cells exhibited expression of other vascular genes, such as VEGFR2, CD133, Endoglin, and VCAM, among others (Fig S3A).
Furthermore, Tie2-Akt-ECs preserve membrane expression of PECAM over long time periods and through numerous passages. Flow cytometry analysis demonstrates that Tie2-Akt-EC cultures maintain homogeneous monolayers 28 days after the initial Tie2 isolation (Fig 3B).
To confirm that other EC genes were similarly maintained, quantitative-PCR was performed on control cells, ER71-transduced cells, and on Tie2-Akt-ECs. 28 days post-isolation, expression of VEGFR2, PECAM, VE-Cadherin, and CD34 was maintained in Tie2-Akt-ECs, but not in either control group (Fig 3C). Additionally, the presence of EC-specific proteins, including VE-Cadherin and eNOS, was detected by Western blot in Tie2-Akt-ECs, but not in control or ER71-transduced cells, 28 days after Tie2 isolation (Fig 3D). There was no significant change in protein or gene expression levels for all tested parameters past 28 days, as Tie2-Akt-ECs persist for months after the initial Tie2 sort (data not shown). Therefore, Tie2-Akt-ECs are viable in vitro over a long-term, and maintain a surface phenotype, gene expression, and protein expression profile that is consistent with mature ECs.
Tie2-Akt-ECs exhibit a global transcriptional endothelial signature
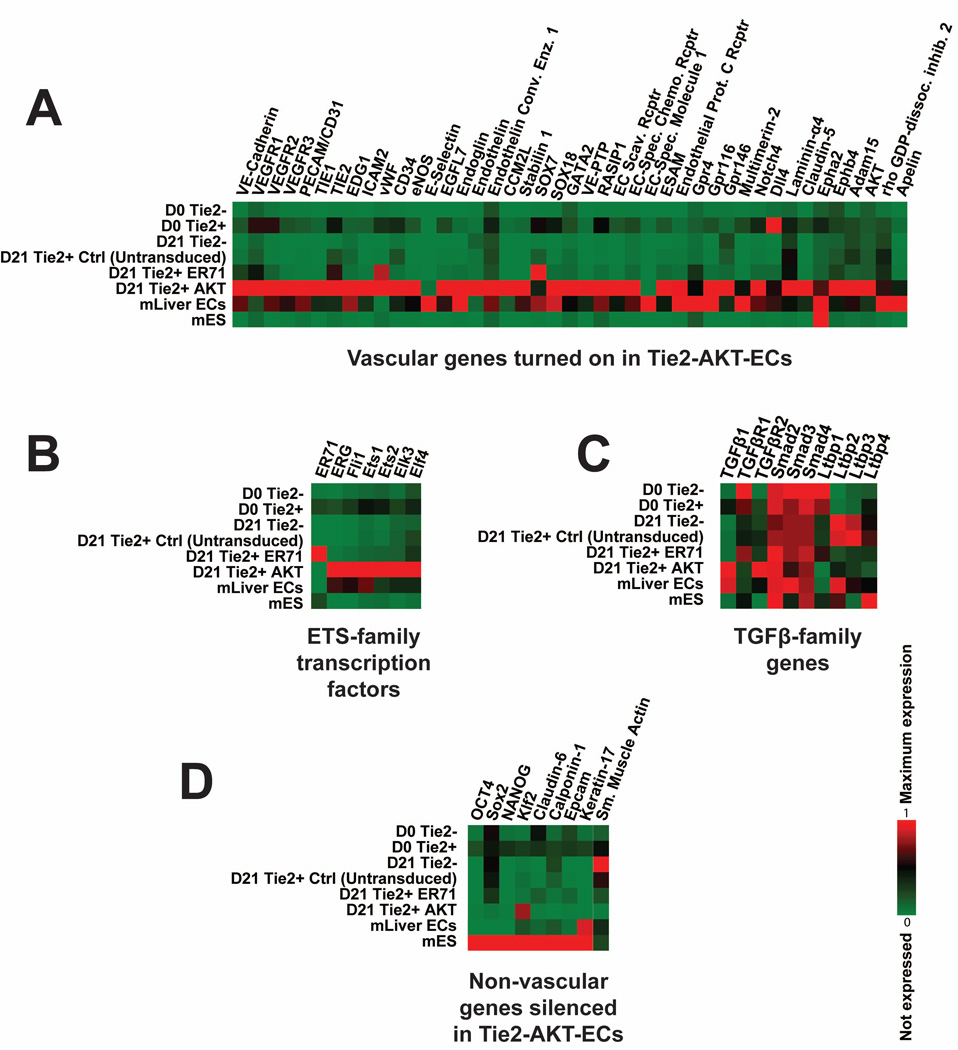
To assess the global gene expression profile of Tie2-Akt-ECs, RNA sequencing was performed. Unlike control or ER71-transduced cells, Tie2-Akt-ECs express a broad array of vascular genes (Fig 4A), and exhibit a total transcriptome signature closely resembling that of mature adult mouse liver ECs. These results were also reflected in the expression of various ETS-family transcription factors (Fig 4B), and members of the TGFβ signaling family (Fig 4C). Importantly, non-vascular genes such as Oct4 and Sox2, which are expressed in the starting population of ES cells, are silenced in the Tie2-Akt-ECs (Fig 4D). Together, these results indicate that Tie2-Akt-ECs express a wide array of vascular genes, and have adopted an endothelial identity similar to adult mouse ECs while suppressing other fates.
Figure 4. Tie2-Akt-ECs exhibit a global transcriptional endothelial signature.
RNA sequencing analysis was performed on Tie2-negative cells immediately after isolation at day-14 of differentiation (D0 Tie2−), Tie2-positive cells isolated at this time point (D0 Tie2+), cultured Tie2-negative and Tie2-positive cells 21 days after isolation (D21 Tie2−, and D21 Tie2+ Ctrl (untransduced)), cultured Tie2-positive cells transduced with ER71 (D21 Tie2+ ER71), cultured Tie2-positive cells transduced with active Akt (D21 Tie2+ Akt), adult mouse liver ECs, and mouse embryonic stem cells (mES). a) Gene expression data shows that Tie2-Akt-ECs and adult mouse liver ECs express a broad array of vascular specific genes, unlike control (untransduced) or ER71-transduced ES-derived cells. b+c) Tie2-Akt-ECs present similar gene expression patterns among the ETS- and TGFβ-family members to adult mouse liver ECs. d) Tie2-Akt-ECs exhibit silencing of non-vascular genes, particularly those of the starting ES cell population.
Tie2-Akt-ECs display endothelial functionality and responsiveness to VEGF-A stimulation in vitro
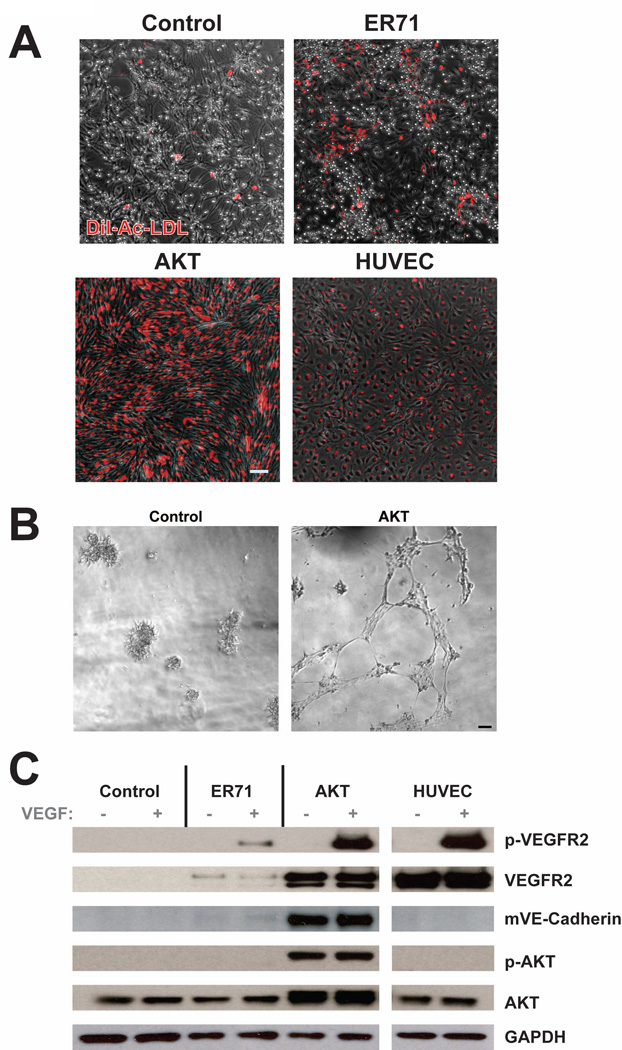
To determine whether Tie2-Akt-ECs exhibit the functional characteristics of ECs, we gauged their capacity to uptake fluorescently labeled (DiI) acetylated LDL (DiI-ac-LDL), as well as their ability to form tube-like structures in vitro. While control or ER71-transduced cells showed only sparse DiI-acetylated-LDL labeling, Tie2-Akt-ECs showed significant intracellular accumulation across the entire culture, in a manner similar to other established EC models (Fig 5A). Additionally, Tie2-Akt-ECs formed networks of tube-like structures when cultured on Matrigel, unlike control cells (Fig 5B).
Figure 5. Tie2-Akt-ECs exhibit endothelial functionality in vitro.
a) Fluorescence imaging shows the uptake of DiI-acetylated-LDL in Tie2-Akt- ECs, but not in control (untransduced), or ER71-transduced cells, 28 days after the initial Tie2 isolation. Scale bar – 100µm. b) Phase-contrast microscopy demonstrates that, unlike control cells, Tie2-Akt-ECs form networks of tube-like structures when cultured on Matrigel in vitro. c) Western blot analysis of control (untransduced), ER71-transduced, or Akt-transduced Tie2-ECs and HUVECs reveals protein levels 28 days after the initial Tie2 isolation. Cells were starved in minimal media for six hours, then stimulated with or without VEGF-A for five minutes. Cell lysates were collected and probed for the indicated proteins.
Another measure of endothelial function and angiogenic capacity is the ability to respond to the major angiogenic cytokine, VEGF-A, by autophosphorylation of its receptor, VEGFR2. Following six hours of starvation in minimal media, control cells, ER71-transduced cells, and Tie2-Akt-ECs were stimulated for five minutes with VEGF-A. Protein analysis revealed that Tie2-Akt-ECs exhibit phosphorylation of VEGFR2 when stimulated with VEGF-A, but not when this cytokine was withheld (Fig 5C), in the same way mature human ECs (HUVECs) respond to VEGF-A stimulation. Conversely, control cells did not demonstrate phosphorylated VEGFR2, or even total VEGFR2 protein. Likewise, ER71-transduced cells displayed insignificant levels of phosphorylated and total VEGFR2.
Another facet of EC functionality involves the endothelial inflammatory response. When challenged with the pro-inflammatory cytokine TNFα, Tie2-Akt-ECs induce surface expression of E-Selectin, unlike control cells (Fig S3B).
Thus, Tie2-Akt-ECs express various functional capacities, including responding to VEGF-A stimulation, uptaking acetylated LDL, forming tube-like structures in an in vitro setting, and responding to pro-inflammatory stimuli.
Tie2-Akt-ECs form functional vessels in vivo
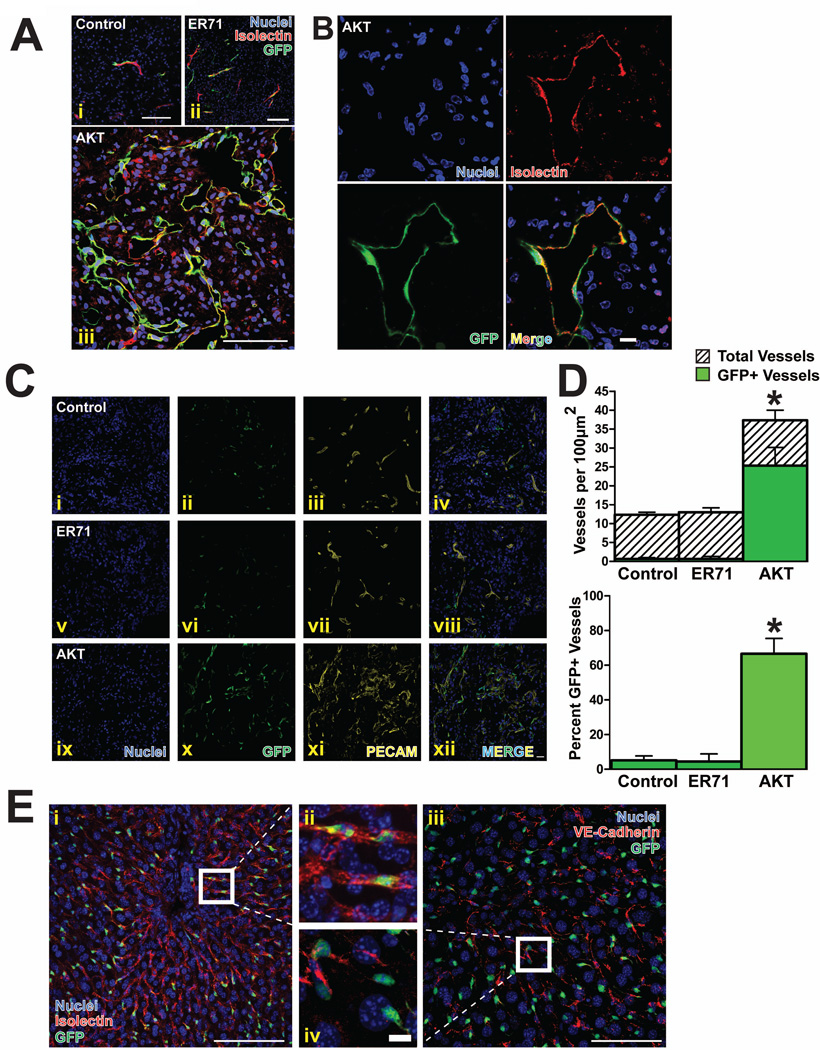
To assess the angiogenic and functional potential of Tie2-Akt-ECs in vivo, a Matrigel plug assay was performed. Tie2-Akt-ECs, as well as control and ER71-transduced cells, were delivered a PGK-GFP expression vector for tracking purposes, then injected with Matrigel™ into immunocompromised mice. After two weeks, intravital labeling of the perfused host vasculature was accomplished by retro-orbital injection of fluorescent Isolectin, followed by retrieval of the plug, and analysis by confocal microscopy. The control cells and the ER71-transduced cells formed mosaic vessels with invading host ECs in a few rare instances (Fig 6Ai–ii). Contrarily, Tie2-Akt-ECs coalesced into numerous vessels (Fig 6Aiii). These tubes anastomosed with the host vasculature, as observed by colocalization of the GFP-labeled Tie2-Akt-ECs with the Isolectin reporter, indicating that blood flowed through these foreign ECs (Fig 6B). Furthermore, these vessels express PECAM (Fig 6C), and quantification of vessel density in these plugs revealed a high percentage of GFP-positive vessels in the Tie2-Akt-EC plugs compared to the control or ER71 plugs (Fig 6D).
Figure 6. Tie2-Akt-ECs exhibit endothelial functionality in vivo.
a) In vivo tube formation assay of control (untransduced) (i), ER71-transduced (ii), or Akt-transduced Tie2-ECs (iii). 28 days after the initial isolation, cells were labeled with a PGK-GFP reporter, then loaded into Matrigel™ plugs (n=5) and implanted subcutaneously into NOD-SCIDIL2Rγ−/− (NSG) mice. After two weeks, the plugs were harvested and sectioned. Immunofluorescence images show control (untransduced), ER71-transduced, and Akt-transduced Tie2-ECs in green (GFP), intravital labeling of the perfused vasculature in red (Isolectin), and a nuclear counterstain (DAPI) in blue. Scale bar – 100µm. b) Magnified view of Tie2-Akt-ECs from the in vivo Matrigel™ plug assay showing a lumenized GFP+ vessel colocalizing with the intravital Isolectin stain. Scale bar – 10µm. c) Immunofluorescence images of PECAM-stained Matrigel™ plugs loaded with control (i-iv), ER71-transduced (v–viii), or Akt transduced Tie2 -ECs (all labeled with GFP) (ix–xii). Scale bar – 20µm. d) Quantification of vessel density from the in vivo Matrigel™ plug assay showing the total number of GFP+ and GFP− vessels per 100µm2 (upper graph), as well as the percentage of GFP+ vessels (lower graph) (*p<0.02 for all groups). e) In vivo engraftment of Tie2-Akt-ECs into the liver vasculature of partially hepatectomized mice (n=4). After performing 70% partial hepatectomy, GFP-labeled Tie2-Akt-ECs were injected intrasplenically. After three weeks, intravital labeling of the vasculature was achieved with either Alexa568-conjugated Isolectin (i, ii) or Alexa568-conjugated anti-VE-cadherin (iii, iv). The mice were then sacrificed, and the livers removed. Immunofluorescence images show GFP-labeled Tie2-Akt-ECs (green), Isolectin labeling of the perfused vasculature (red, i and ii), VE-cadherin labeling of the perfused vasculature (red, iii and iv), and a nuclear counterstain (blue). Scale bars – 100µm (i,iii) and 10µm (ii,iv).
To further test their in vivo potential, Tie2-Akt-EC engraftment was evaluated in a more physiologically relevant model of organ regeneration. Tie2-Akt-ECs were introduced by intrasplenic injection to mice which had undergone a 70% partial hepatectomy [36]. After three weeks, all blood-carrying vessels of the microvasculature were labeled intravitally, achieved by systemic injection of either fluorescent Isolectin or VE-cadherin antibody, followed by retrieval and analysis of the regenerated liver. GFP+ Tie2-Akt-ECs were observed to have contributed to the vasculature of the regenerating liver, and exhibited functionality as determined by colocalization with the systemically-labeled markers Isolectin and VE-cadherin (Fig 6E). Thus, Tie2-Akt-ECs exhibit EC functionality in vivo.
Akt does not merely function as a pro-survival factor
Akt is involved in many cellular processes which endorse cell survival, such as promoting translation and cell growth, and by inhibiting apoptosis [24, 41]. Since there is recent appreciation for novel endothelial-specific functions of Akt [27, 28], we asked whether Akt was acting simply as a pro-survival factor in maintaining Tie2-Akt-ECs, or whether an EC-specific mechanism is operating. To address this question, purified Tie2-ECs were transduced with oncogenes that are commonly used to immortalize ECs, such as polyoma middle T antigen (PymT), constitutively active K-Ras, or constitutively active Raf. Whereas Tie2-Akt-ECs maintained as ECs, Tie2-PymT, Tie2-K-Ras, and Tie2-Raf cells adopted non-EC morphologies (Fig S4A). Moreover, surface expression of VE-Cadherin and PECAM was rarely observed in Tie2-PymT, Tie2-K-Ras, or Tie2-Raf cells, in contrast to the profuse expression observed in Tie2-Akt-ECs (Fig S4B). These observations were supported quantitatively by flow cytometry, which shows insignificant amounts of PECAM-positive cells in cultures lacking Akt (Fig S4C), compared to the essentially homogeneous composition of the Tie2-Akt-EC culture. Notably, Tie2-PymT cultures did exhibit minor amounts of ECs in the immunofluorescence images and by flow cytometry. This finding may be due to a very low activation of Akt by the polyoma middle T antigen (Fig S4B,C). These data indicate that providing ES cell-derived Tie2-ECs with a pro-survival factor is not sufficient to maintain them in vitro, and therefore Akt may work through alternate means to achieve the observed stabilization.
Tie2-Akt-ECs are not a transformed cell line
Other endothelial models involving transduction of mature ECs with the oncogenes polyoma middle T antigen, K-Ras, or Raf are not ideal due to the transformed nature of these cells. Thus, the activation status of the pro-growth mitogen-activated protein kinase (MAPK) pathway was interrogated, in order to assess the proliferative capacity of Tie2-Akt-ECs. While cells infected with PymT, K-Ras, and Raf displayed phosphorylation of the MAPK-effectors ERK1 and ERK2, Tie2-Akt-ECs show a reduced amount of this activation (Fig S4D).
Another characteristic of transformed cells is their ability to proliferate and evade replicative senescence [42]. A commonly used molecular indicator of senescence is expression of pH-dependent β-galactosidase activity [43]. While Tie2-Akt-ECs did not exhibit β-galactosidase activity 14 days after the initial Tie2 isolation, these cells stained positively for this senescence marker 60 days post-isolation (Fig S4E), in addition to adopting an enlarged cell size and a reduced proliferative capacity. Taken together, these observations suggest that Tie2-Akt-ECs undergo replicative senescence, and are therefore not a transformed cell line.
Akt interferes with Tie2-EC responsiveness to TGFβ-stimulation
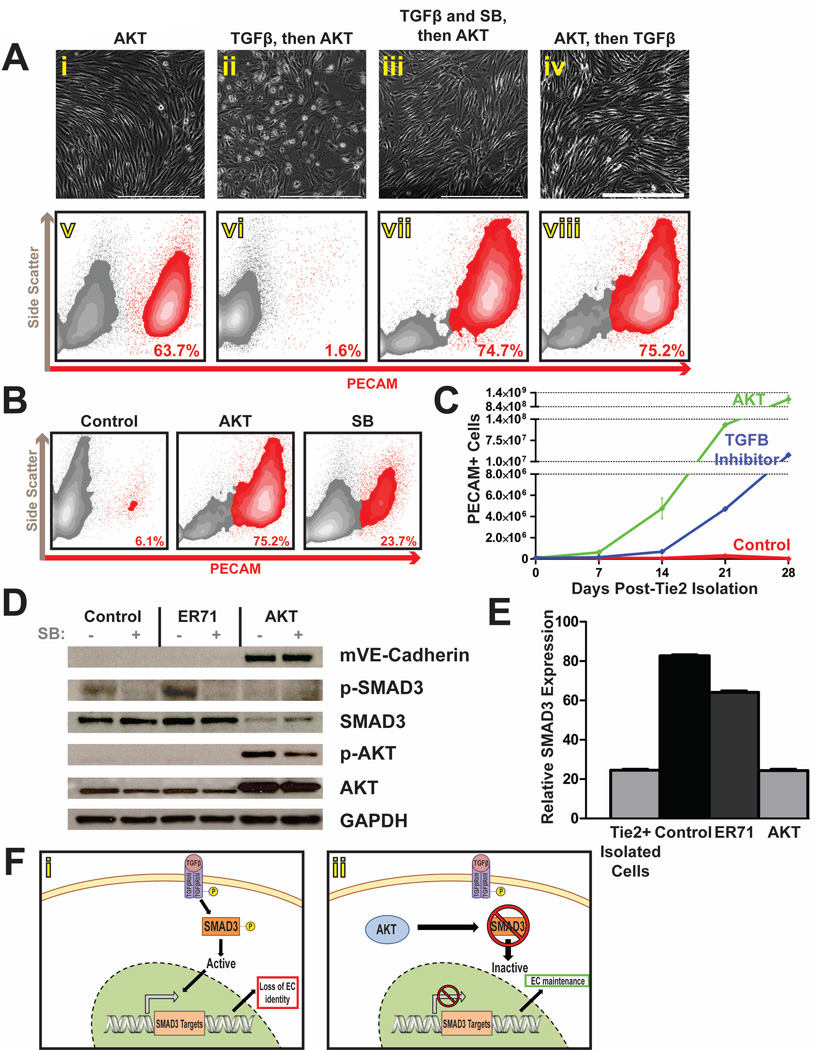
Recent efforts at generating stable cultures of human ES cell-derived ECs have uncovered the importance of TGFβ inhibition to the maintenance of those cells [34]. To address whether Akt might be serving a similar purpose, we first examined what effect stimulation with TGFβ might have on isolated Tie2-ECs. Day-14 sorted Tie2-ECs were transduced with active Akt, and simultaneously stimulated with TGFβ1/3 alone, or with TGFβ1/3 and SB-431542 (SB), an inhibitor of the TGFβ-Receptor-2 (TGFβR2). In contrast to Tie2-Akt-ECs, treatment with TGFβ resulted in the transdifferentiation of these cells to non-EC fates, as a complete loss of endothelial morphology was observed (Fig 7Aii), along with a correlated loss in surface expression of PECAM (Fig 7Avi). This effect was mitigated by SB-432542, as cells treated with both the ligand and the inhibitor retained their EC morphology and PECAM expression (Fig 7Aiii,vi). Notably, when TGFβ ligand was administered to Tie2-Akt-ECs after Akt transduction, their morphology and PECAM expression were unaffected (Fig 7Aiv,viii).
Figure 7. Akt maintains Tie2-ECs in part by interfering with SMAD3-mediated TGFβ signaling.
a) Phase-contrast microscopy (i–iv) and flow cytometry (v–viii) analyses indicate that, while Tie2-Akt-ECs maintain an EC morphology (i) and PECAM surface expression (v), treatment of Tie2-ECs with TGFβ prior to the establishment of Akt signaling yields a loss of EC morphology (ii) and surface PECAM (vi). Tie2-ECs which were simultaneously administered TGFβ and SB431542 (SB), a small molecule inhibitor of TGFβ Receptor 2, exhibited a maintenance of EC morphology (iii) and surface PECAM (vii), as did Tie2-ECs which were treated with TGFβ subsequent to the establishment of Akt signaling (iv, viii). Scale bars – 400um. b) Flow cytometry analysis seven days after Tie2-EC isolation shows a retention of PECAM surface expression upon treatment of cells with SB compared to untreated control cells, though to a lesser extent than with Akt overexpression. c) The total number of PECAM+ ECs is shown for untreated control cells, SB-treated cells, and Tie2-Akt-ECs. Error bars represent standard error of experimental values performed in triplicate. d) Western blot analysis of cell lysates reveals an absence of phosphorylated SMAD3 and a reduction in total SMAD3 protein levels in Tie2-Akt-ECs, compared to control (untransduced) or ER71-transduced cells. e) qPCR analysis revealed reduced SMAD3 mRNA expression in Tie2-Akt-ECs (at levels similar to Tie2-ECs at the time of isolation), compared with control (untransduced) and ER71-transduced cells. f) Proposed model for Tie2-EC maintenance by Akt demonstrating that the presence of SMAD3 regulates the stability of embryonic EC identity. In the absence of Akt (i), available SMAD3 can be phosphorylated and activated, resulting in the regulation of downstream factors that effect a loss of EC identity. However, in the presence of Akt (ii), SMAD3 signaling is abrogated, protecting the identity of these Tie2-ECs.
Furthermore, treatment of Tie2-sorted cells with SB-431542, in the absence of Akt activation, was able to maintain a significant population of PECAM-expressing ECs compared to the untreated and control (untransduced) cells (Fig 7B). However, treatment with SB-431542 was unable to fully recapitulate the phenotype observed with Akt activation, as Tie2-Akt-ECs displayed enhanced stability and proliferation compared with this small molecule inhibitor (Fig 7C). Together, these data suggest that Akt maintains Tie2-ECs in part by modulating their responsiveness to TGFβ-mediated signaling.
Akt interferes with TGFβ signaling by downregulating SMAD3
Recent work has shown that Akt inhibits the phosphorylation and activation of the TGFβ effector SMAD3, though the precise mechanism by which this occurs is still under debate [44, 45]. To determine whether Akt was inhibiting SMAD3 phosphorylation in Tie2-Akt-ECs, western blot analysis was performed. Whereas phosphorylated SMAD3 was detectable in control and ER71-transduced cells, it was absent in Tie2-Akt-ECs (Fig 7D). Unexpectedly, total levels of SMAD3 protein were reduced in Tie2-Akt-ECs, in contrast with control or ER71-transduced cells. To corroborate this reduction in SMAD3, quantitative-PCR analysis was performed. Tie2-Akt-ECs showed reduced gene expression of SMAD3 compared to control or ER71-transduced cells, and showed comparable levels of expression to day-14 Tie2-positive cells at the time of isolation (Fig 7E). Therefore, Akt activation downregulates SMAD3 gene expression in ECs.
Downregulation of SMAD3 by Akt is an EC-specific effect
Since previous reports did not show a change in SMAD3 protein levels with Akt activation [44, 45], we asked whether the downregulation we observed was an endothelial-specific phenomenon. To address this, we transduced EC-deficient bone marrow stromal cells and HUVECs with active Akt, and probed the SMAD3 protein content. As shown in Supplemental Figure 5, active Akt reduced the levels of SMAD3 in HUVECs, but the SMAD3 of the bone marrow stromal cells remained unperturbed. Thus, Akt activation results in a downregulation of SMAD3 specifically in ECs.
Discussion
Constitutive expression of active Akt maintains vascular identity and proliferative potential of mouse ES cell-derived ECs
The ability to study human ECs in defined, in vitro cultures has had a significant impact in advancing our understanding of vascular biology and the various diseases that involve its dysfunction. However, generating stable and proliferative cultures of authentic mouse ECs has remained elusive. Previously, other groups have used Akt to promote the survival of various cell types, including pancreatic islet β cells [46] and cardiomyocytes [47] in vitro and in vivo, and have shown this effect to be mediated in part by the inhibition of the forkhead transcription factor FKHRL1 [48]. Here, we report that overexpression of constitutively active Akt maintains mouse ES cell-derived ECs in vitro, and that these ECs exhibit the morphological, phenotypic, gene expression, and functional profiles of mature ECs. Notably, Tie2-Akt-ECs demonstrate a proliferative capacity unmatched in previous studies, resulting in the generation of an unprecedented large number of stable ECs that have not adopted a transformed phenotype. These data suggest that Akt-activated embryonic ECs may serve as a valid in vitro model to study endothelial cell biology and angiogenesis under wild-type or genetically altered backgrounds. Additionally, these ECs can function as a cellular platform to study the effects of pharmacological agents in various disease models, and can serve as a vascular product for potential cell-based therapies.
ER71 specifies a vascular fate, but is not sufficient to maintain ES cell-derived ECs
Isolation of ES cell-derived Tie2-ECs yields cells that are viable yet unstable, as they rapidly lose their endothelial identity in culture. In accordance with earlier work, overexpression of the transcription factor ER71, an early regulator of the vascular program [16, 49], was able to induce expression of many vascular genes in ES cell-derived ECs. However, the stabilization and further culture of ER71-transduced cells was not demonstrated. Indeed, we also showed that ES cell-derived ECs transduced with ER71 could not maintain an EC fate, despite observing a modest increase in EC marker expression in these cells. Since ER71 upregulates vascular targets via a synergistic interaction with FoxC2 on putative FOX:ETS domains [19], it is plausible that this, or other as yet unidentified cofactors, may be additionally necessary to achieve expression above a threshold required to reinforce EC identity in these embryonic cells. Alternatively, the inability of vascular gene upregulation to stabilize the EC fate in Tie2-ECs may be due to the existence of distinct mechanisms to first specify an endothelial identity, and then to protect against active processes that usher these ECs to other fates.
Active Akt downregulates SMAD3-mediated TGFβ signaling
TGFβ signaling has been implicated in many aspects of development. Notably, signaling through the PI3K-Akt axis has been shown to suppress TGFβ’s effects in particular contexts [50]. Indeed, Tie2-Akt-ECs exhibit protection from TGFβ stimulation, as control Tie2-ECs rapidly lose their endothelial character upon TGFβ administration, whereas those expressing Akt do not. Recent work has uncovered an interaction between Akt and the transcription factor SMAD3, where Akt blocks SMAD3 phosphorylation and activation [44, 45]. These studies show that a physical interaction between Akt and SMAD3 blocks SMAD3 activation and nuclear translocation, and that this interaction is independent of the kinase activity of Akt. This finding is consistent with the absence of phosphorylated SMAD3 in Tie2-Akt-ECs. However, our work revealed a significant reduction of total SMAD3 protein in ECs transduced with Akt (Fig 7E), a finding that is not congruous with those previous reports [44, 45], which saw no change in total SMAD3 protein upon Akt overexpression. Since those experiments were performed in non-ECs, the data support the notion that the downregulation of SMAD3 in Akt-transduced ECs may be an endothelial-specific phenomenon, although the direct or indirect mechanism by which Akt imparts this effect on SMAD3 expression is still under investigation. Gene expression data from qPCR (Fig 7E) and RNA-sequencing (Fig 4C) experiments indicate that Akt may regulate SMAD3 at the transcriptional level, possibly through an as yet unidentified repressor function. Notably, while Tie2-Akt-ECs show reduced SMAD3 expression, adult mouse liver ECs do not, suggesting that embryonic ECs may harbor a specific sensitivity to TGFβ signaling. Similar trends have been observed with human embryonic ECs, where TGFβ inhibition is necessary for their survival in vitro [34], unlike mature HUVECs which do not require TGFβ inhibition for in vitro culture. Additionally, it is possible that Akt could mediate SMAD3 post-translationally via proteasomal targeting [51] in ECs. These possibilities will be the subjects of future studies involving the Akt/SMAD3 association. Taken together, these results suggest that embryonic EC’s may be sensitive to SMAD3-mediated TGFβ signaling, and thus it is plausible that these cells have evolved a unique mechanism to ensure this response is suppressed to maintain their integrity.
Indeed, the diversity and complexity of TGFβ signaling gives credence to the notion that different contexts utilize various components of these pathways for distinct ends. As there are multiple points of variety and integration throughout the TGFβ signaling tree, it is conceivable that different cell types may express varying cofactors such that similar stimulation would yield two entirely different responses. In fact, it is often necessary to take advantage of this complexity, as evidenced by our need to first stimulate differentiation cultures with the TGFβ ligand Activin A, followed by a requirement to remove this ligand from culture downstream in the differentiation timeline.
The EC-specific nature of Akt’s effects can be further clarified by the effect of TGFβ stimulation on Tie2-ECs. While addition of TGFβ to Tie2-ECs prior to Akt-transduction had a deleterious effect on this population, treatment with TGFβ after Akt overexpression did not have the same effect on these cells (Fig 7A). It is likely that TGFβ stimulation of Tie2-ECs results in their rapid deviation to non-EC cell types, at which point overexpression of Akt does not function as in ECs. Moreover, these data indicate that active Akt cannot revert non-ECs back into ECs, supporting the view of Akt as a maintenance mediator, as opposed to an induction factor.
In addition to phosphorylating and activating SMAD3, stimulation of TGFβR2 results in phosphorylation and activation of the related effector SMAD2. Though both genes initially arose from the evolutionary duplication of a single SMAD2/3 gene, recent appreciation has been given to distinct functions of each SMAD protein. Akt overexpression showed no effect on SMAD2 phosphorylation or protein levels (data not shown), which may benefit ECs by its pro-vascular regulation of endothelial Nitric Oxide Synthase [52]. Thus, it is the abrogation of SMAD3-mediated signaling by Akt that may influence the stability and proliferative capacity of Tie2-ECs.
Therefore, we propose a model incorporating a novel function of Akt in ECs, involving the attenuation of TGFβ signaling. In the presence of TGFβ stimulation, available SMAD3 protein is phosphorylated and activated, resulting ultimately in a loss of EC identity (Fig 7Fi). However, when Akt is activated, downregulation of SMAD3 reduces protein available for signal transduction to the nucleus, and thus protects against the deleterious effects this factor exerts on ECs (Fig 7Fii). Furthermore, Akt might also physically interact with residual SMAD3, adding an additional measure of protection to ensure this pathway is efficiently inhibited.
SMAD3 target genes may provide insight into Akt-mediated EC maintenance
Since SMAD3 is a transcription factor, of great interest in dissecting its effects on ECs are the downstream genes and processes that it regulates. One potential response might be an endothelial-specific variation of the epithelial-to-mesenchymal (EMT) transition, termed the endothelial-to-mesenchymal transition (endoMT). This naturally-occurring event was described in vivo for the formation of endocardial cushion mesenchyme from underlying endothelium [53, 54], and in vitro for the transition of bovine aortic ECs into smooth muscle cells [55]. Moreover, SMAD3 has been linked to the regulation of Snail [56] and Twist, two factors central to EMT, which are responsible for some of the changes in gene expression that endow transitioning cells a mesenchymal migratory phenotype. Thus, it is feasible that this pathway is responsible for the developmental transdifferentiation observed in control or ER71-transduced, but not in the Akt-transduced Tie2-EC’s. Investigation into the dynamics of such effectors of endoMT may clarify the relationship between Akt and the maintenance of Tie2-Akt-ECs. Future studies will focus on interrogating expression levels of these factors quantitatively, as well as by assessing the status of other hallmarks of EMT, such as increased cell migration.
Akt performs a multitude of intracellular functions
In this study, we identified a novel function of Akt in ECs, involving the protection from SMAD3-mediated TGFβ signaling. However, Akt signaling in ECs provides numerous additional advantages. For instance, Akt mediates VEGF-A-induced cytoprotection [57, 58], and contributes to EC function by directly phosphorylating eNOS, increasing Nitric Oxide bioavailability [52]. These benefits might help rationalize the disparity between the maintenance observed in Tie2-Akt-ECs compared with that of the Tie2-EC’s treated with the TGFβR2-inhibitor SB-431542. Additionally, the positive influences Akt exerts on protein translation and cell growth (via NF-κB, for example), might account for the considerable proliferative capacity Tie2-Akt-ECs exhibit.
Tie2-Akt-ECs serve as a practical in vitro model of mouse ECs
While there are numerous available mouse EC lines, their study is limited by their immortalized nature. Unlike these cells, Tie2-Akt-ECs do not show the signaling, phenotypic, or functional characteristics of transformed cells, while maintaining the full angiogenic repertoire (Fig 6). Indeed, Tie2-Akt-ECs not only exhibit a limited replicative capacity, but also display molecular characteristics of non-immortalized cells, such as the ability to senesce (Fig 6E). However, since in vivo models of Akt-ECs exhibit increased vascular permeability [59], it may be beneficial to pursue a more physiologically-relevant context, which might be accomplished by modulating Akt activation in an inducible fashion. Nevertheless, the in vitro culture of Tie2-Akt-ECs has provided new insights into EC biology, and has defined a new avenue of research which can be followed to gain deeper understanding of vascular pathologies.
Supplementary Material
Acknowledgments
This study was funded by the Howard Hughes Medical Institute, and the National Institutes of Health – National Heart, Lung, and Blood Institute grant HL007423 (to E. Israely).
Footnotes
- Edo Israely: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
- Michael Ginsberg: Conception and design, data analysis and interpretation
- Daniel Nolan: Collection and/or assembly of data, data analysis and interpretation
- Bi-Sen Ding: Collection and/or assembly of data
- Daylon James: Conception and design
- Olivier Elemento: Data analysis and interpretation
- Shahin Rafii: Conception and design, financial support, administrative support
- Sina Y Rabbany: Conception and design, final approval of manuscript
Disclosures
The authors declare no conflicts of interest.
References
- 1.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 2.Shing Y, Folkman J, Sullivan R, et al. Heparin affinity: Purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984;223:1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 4.Joo HJ, Kim H, Park SW, et al. Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells. Blood. 2011;118:2094–2104. doi: 10.1182/blood-2010-12-323907. [DOI] [PubMed] [Google Scholar]
- 5.Yamamizu K, Kawasaki K, Katayama S, et al. Enhancement of vascular progenitor potential by protein kinase a through dual induction of flk-1 and neuropilin-1. Blood. 2009;114:3707–3716. doi: 10.1182/blood-2008-12-195750. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 7.Fehrenbach ML, Cao G, Williams JT, et al. Isolation of murine lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1096–L1103. doi: 10.1152/ajplung.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzaniga S, Gonzalez L, Mantovani A, et al. Isolation and molecular characterization of a mouse renal microvascular endothelial cell line. In Vitro Cell Dev Biol Anim. 2004;40:82–88. doi: 10.1290/1543-706x(2004)040<0082:iamcoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Nostro MC, Cheng X, Keller GM, et al. Wnt, activin, and bmp signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park C, Afrikanova I, Chung YS, et al. A hierarchical order of factors in the generation of flk1- and scl-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 11.Pearson S, Sroczynska P, Lacaud G, et al. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to bmp4, activin a, bfgf and vegf. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 12.Lelievre E, Lionneton F, Soncin F, et al. The ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- 13.Oikawa T, Yamada T. Molecular biology of the ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y. Role of ets family transcription factors in vascular development and angiogenesis. Cell Struct Funct. 2001;26:19–24. doi: 10.1247/csf.26.19. [DOI] [PubMed] [Google Scholar]
- 15.Patterson LJ, Patient R. The "ets" factor: Vessel formation in zebrafish--the missing link? PLoS Biol. 2006;4:e24. doi: 10.1371/journal.pbio.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee D, Park C, Lee H, et al. Er71 acts downstream of bmp, notch, and wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhaus H, Muller F, Hollemann T. Xenopus er71 is involved in vascular development. Dev Dyn. 2010;239:3436–3445. doi: 10.1002/dvdy.22487. [DOI] [PubMed] [Google Scholar]
- 18.Sumanas S, Gomez G, Zhao Y, et al. Interplay among etsrp/er71, scl, alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Val S, Chi NC, Meadows SM, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka H, Hayashi M, Nakagawa R, et al. Etv2/er71 induces vascular mesoderm from flk1+pdgfralpha+ primitive mesoderm. Blood. 2011;118:6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ets factors and tgfbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 23.Ma H, Calderon TM, Fallon JT, et al. Hepatocyte growth factor is a survival factor for endothelial cells and is expressed in human atherosclerotic plaques. Atherosclerosis. 2002;164:79–87. doi: 10.1016/s0021-9150(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 24.Romashkova JA, Makarov SS. Nf-kappab is a target of akt in anti-apoptotic pdgf signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 25.Holland EC, Celestino J, Dai C, et al. Combined activation of ras and akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 26.Jones RG, Parsons M, Bonnard M, et al. Protein kinase b regulates t lymphocyte survival, nuclear factor kappab activation, and bcl-x(l) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Cheng J, Hackett NR, et al. Adenovirus e4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/akt signaling pathway. J Biol Chem. 2004;279:11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Butler JM, O'Donnell R, et al. Angiocrine factors from akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goumans MJ, Liu Z, ten Dijke P. Tgf-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 30.Heldin CH, Miyazono K, ten Dijke P. Tgf-beta signalling from cell membrane to nucleus through smad proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Massague J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 32.Clifford RL, Deacon K, Knox AJ. Novel regulation of vascular endothelial growth factor-a (vegf-a) by transforming growth factor (beta)1: Requirement for smads, (beta)-catenin, and gsk3(beta) J Biol Chem. 2008;283:35337–35353. doi: 10.1074/jbc.M803342200. [DOI] [PubMed] [Google Scholar]
- 33.Mandriota SJ, Menoud PA, Pepper MS. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem. 1996;271:11500–11505. doi: 10.1074/jbc.271.19.11500. [DOI] [PubMed] [Google Scholar]
- 34.James D, Nam HS, Seandel M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by tgfbeta inhibition is id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watabe T, Nishihara A, Mishima K, et al. Tgf-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol. 2003;163:1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HC, Ema H, Osawa M, et al. Hematopoietic stem cells express tie-2 receptor in the murine fetal liver. Blood. 2000;96:3757–3762. [PubMed] [Google Scholar]
- 39.Gomez GA, Veldman MB, Zhao Y, et al. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS One. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong KS, Proulx K, Rost MS, et al. Identification of vasculature-specific genes by microarray analysis of etsrp/etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- 41.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein S. Replicative senescence: The human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 43.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conery AR, Cao Y, Thompson EA, et al. Akt interacts directly with smad3 to regulate the sensitivity to tgf-beta induced apoptosis. Nat Cell Biol. 2004;6:366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- 45.Remy I, Montmarquette A, Michnick SW. Pkb/akt modulates tgf-beta signalling through a direct interaction with smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan S, Bernal-Mizrachi E, Ohsugi M, et al. Glucose promotes pancreatic islet beta-cell survival through a pi 3-kinase/akt-signaling pathway. Am J Physiol Endocrinol Metab. 2002;283:E784–E793. doi: 10.1152/ajpendo.00177.2002. [DOI] [PubMed] [Google Scholar]
- 47.Fujio Y, Nguyen T, Wencker D, et al. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 49.Ferdous A, Caprioli A, Iacovino M, et al. Nkx2–5 transactivates the ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci U S A. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen RH, Su YH, Chuang RL, et al. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/akt-dependent pathway. Oncogene. 1998;17:1959–1968. doi: 10.1038/sj.onc.1202111. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Waddell DS, Wang W, et al. Ligand-dependent ubiquitination of smad3 is regulated by casein kinase 1 gamma 2, an inhibitor of tgf-beta signaling. Oncogene. 2008;27:7235–7247. doi: 10.1038/onc.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: A switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- 55.Arciniegas E, Sutton AB, Allen TD, et al. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 56.Hoot KE, Lighthall J, Han G, et al. Keratinocyte-specific smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–2732. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gratton JP, Morales-Ruiz M, Kureishi Y, et al. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 59.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.