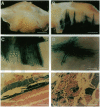
Abstract
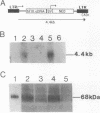
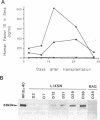
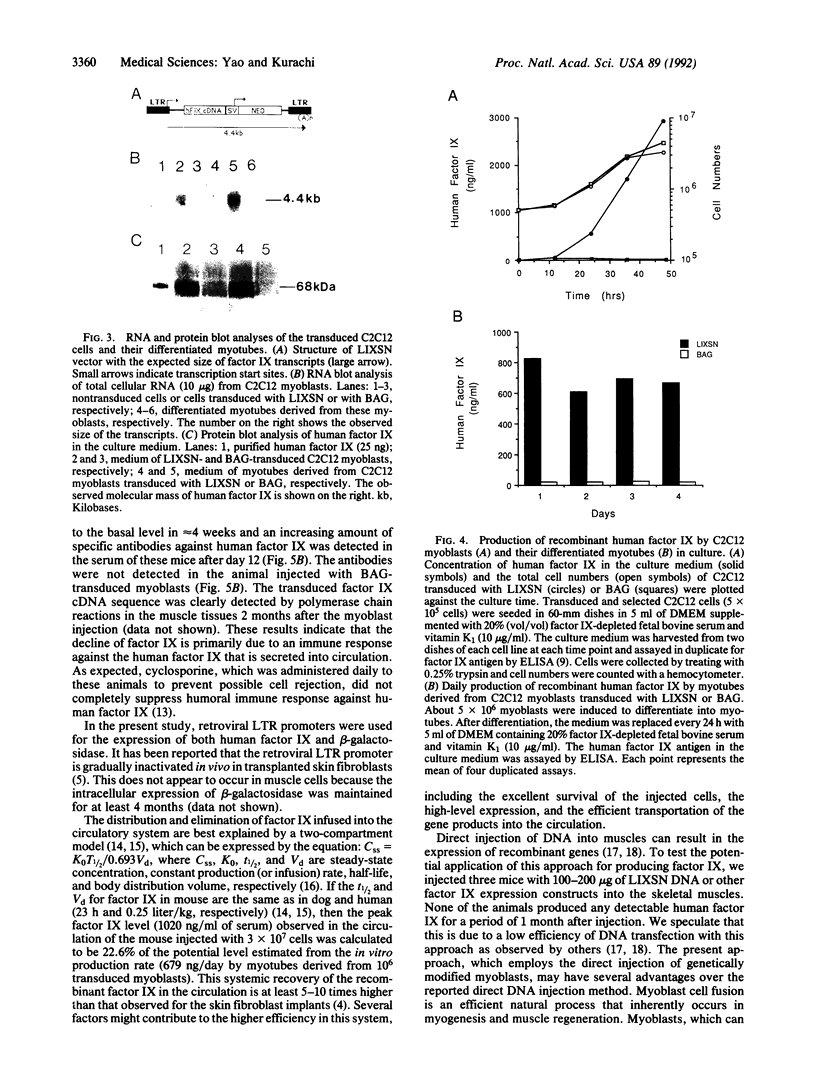
Hemophilia B is an X chromosome-linked recessive bleeding disorder. To develop a somatic gene therapy for this disease, we have examined whether mouse skeletal myoblasts can serve as efficient vehicles for systemic delivery of recombinant factor IX. When mouse myoblasts (C2C12) transduced with a Moloney murine leukemia virus-based vector containing the bacterial beta-galactosidase gene were injected into mouse skeletal muscles, they fused with the existing and regenerating myofibers and continued to express beta-galactosidase. C2C12 myoblasts that were infected with recombinant retroviruses containing a human factor IX cDNA secreted biologically active human factor IX cDNA secreted biologically active human factor IX into the culture medium at a rate of 2.6 micrograms per 10(6) cells per day. Myotubes derived from these cells in culture continued to express human factor IX (0.68 micrograms/day from myotubes derived from 10(6) C2C12 cells). After injection of the transduced C2C12 myoblasts into skeletal muscles of mice, the systemic level of recombinant human factor IX was found to be as high as approximately 1 microgram/ml of serum. These results provide the rationale for using skeletal myoblasts as an efficient gene delivery vehicle in the somatic gene therapy for hemophilia B.
Full text
PDF


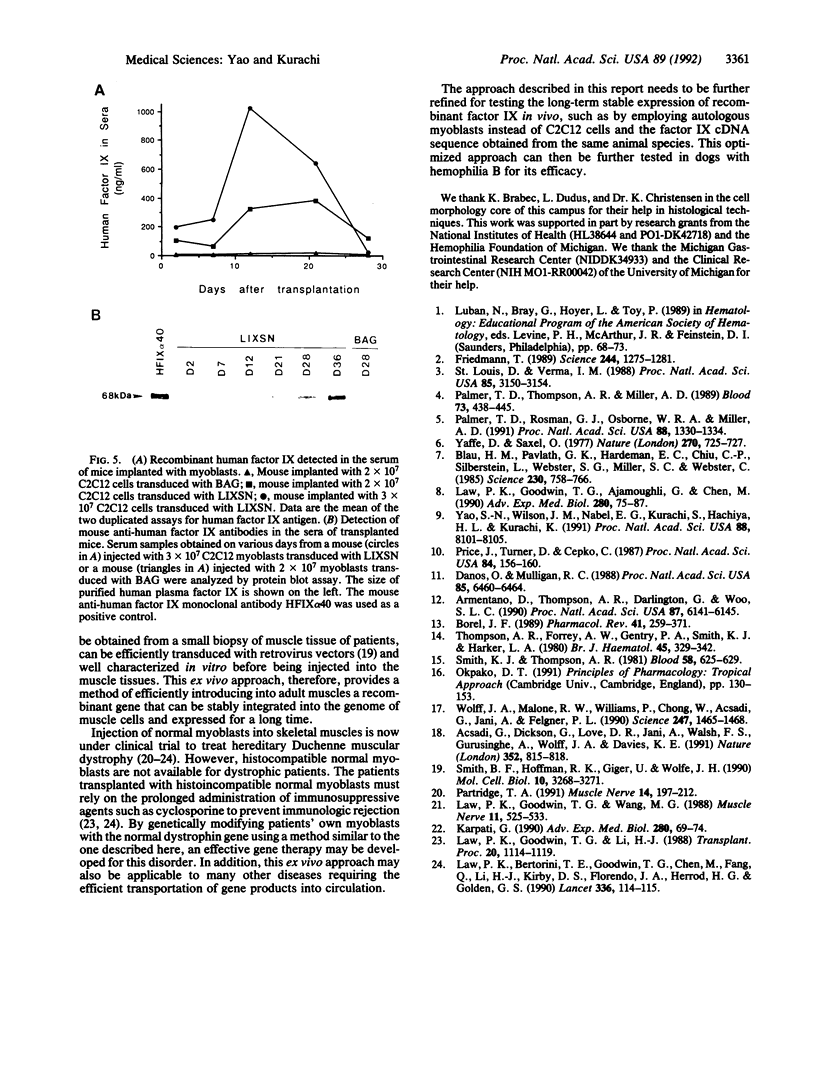
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acsadi G., Dickson G., Love D. R., Jani A., Walsh F. S., Gurusinghe A., Wolff J. A., Davies K. E. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991 Aug 29;352(6338):815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- Armentano D., Thompson A. R., Darlington G., Woo S. L. Expression of human factor IX in rabbit hepatocytes by retrovirus-mediated gene transfer: potential for gene therapy of hemophilia B. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6141–6145. doi: 10.1073/pnas.87.16.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Borel J. F. Pharmacology of cyclosporine (sandimmune). IV. Pharmacological properties in vivo. Pharmacol Rev. 1990 Sep;41(3):259–371. [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. Progress toward human gene therapy. Science. 1989 Jun 16;244(4910):1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- Karpati G. The principles and practice of myoblast transfer. Adv Exp Med Biol. 1990;280:69–74. doi: 10.1007/978-1-4684-5865-7_9. [DOI] [PubMed] [Google Scholar]
- Law P. K., Bertorini T. E., Goodwin T. G., Chen M., Fang Q. W., Li H. J., Kirby D. S., Florendo J. A., Herrod H. G., Golden G. S. Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy. Lancet. 1990 Jul 14;336(8707):114–115. doi: 10.1016/0140-6736(90)91628-n. [DOI] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Li H. J., Ajamoughli G., Chen M. Myoblast transfer improves muscle genetics/structure/function and normalizes the behavior and life-span of dystrophic mice. Adv Exp Med Biol. 1990;280:75–87. doi: 10.1007/978-1-4684-5865-7_10. [DOI] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Li H. J. Histoincompatible myoblast injection improves muscle structure and function of dystrophic mice. Transplant Proc. 1988 Jun;20(3 Suppl 3):1114–1119. [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Wang M. G. Normal myoblast injections provide genetic treatment for murine dystrophy. Muscle Nerve. 1988 Jun;11(6):525–533. doi: 10.1002/mus.880110602. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Rosman G. J., Osborne W. R., Miller A. D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Thompson A. R., Miller A. D. Production of human factor IX in animals by genetically modified skin fibroblasts: potential therapy for hemophilia B. Blood. 1989 Feb;73(2):438–445. [PubMed] [Google Scholar]
- Partridge T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve. 1991 Mar;14(3):197–212. doi: 10.1002/mus.880140302. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. F., Hoffman R. K., Giger U., Wolfe J. H. Genes transferred by retroviral vectors into normal and mutant myoblasts in primary cultures are expressed in myotubes. Mol Cell Biol. 1990 Jun;10(6):3268–3271. doi: 10.1128/mcb.10.6.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Thompson A. R. Labeled factor IX kinetics in patients with hemophilia-B. Blood. 1981 Sep;58(3):625–629. [PubMed] [Google Scholar]
- St Louis D., Verma I. M. An alternative approach to somatic cell gene therapy. Proc Natl Acad Sci U S A. 1988 May;85(9):3150–3154. doi: 10.1073/pnas.85.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. R., Forrey A. W., Gentry P. A., Smith K. J., Harker L. A. Human factor IX in animals: kinetics from isolated, radiolabelled protein and platelet destruction following crude concentrate infusions. Br J Haematol. 1980 Jun;45(2):329–342. doi: 10.1111/j.1365-2141.1980.tb07152.x. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yao S. N., Wilson J. M., Nabel E. G., Kurachi S., Hachiya H. L., Kurachi K. Expression of human factor IX in rat capillary endothelial cells: toward somatic gene therapy for hemophilia B. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8101–8105. doi: 10.1073/pnas.88.18.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]