Abstract
Nine new cannabinoids (1–9) were isolated from a high-potency variety of Cannabis sativa. Their structures were identified as (±)-4-acetoxycannabichromene (1), (±)-3″-hydroxy-Δ(4″,5″)-cannabichromene (2), (−)-7-hydroxycannabichromane (3), (−)-7R-cannabicoumarononic acid A (4), 5-acetyl-4-hydroxycannabigerol (5), 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol (6), 8-hydroxycannabinol (7), 8-hydroxycannabinolic acid A (8), and 2-geranyl-5-hydroxy-3-n-pentyl-1,4-benzoquinone (9) through 1D and 2D NMR spectroscopy, GC-MS, and HRESIMS. The known sterol β-sitosterol-3-O-β-d-glucopyranosyl-6′-acetate was isolated for the first time from cannabis. Compounds 6 and 7 displayed significant antibacterial and antifungal activities, respectively, while 5 displayed strong antileishmanial activity.
More than 525 constituents have been identified from Cannabis sativa L. (Cannabaceae).1–7 The best-known and most specific class of cannabis constituents are the C21 terpenophenolic cannabinoids. Other phenolic cannabis constituents include flavonoids, spiroindans, dihydrostilbenes, phenanthrenes, and dihydrophenanthrenes.1–6,8,9 As part of our program aimed at the discovery of new cannabinoids and other metabolites with significant biological activity from high-potency cannabis (Δ9-THC > 10%, w/w), we have reported 25 new metabolites.2–5 In this paper, we report the isolation and identification of nine additional new cannabinoids (1–9), including three cannabichromene derivatives (1–3), (−)-7R-cannabicoumarononic acid A (4), two cannabigerol derivatives (5 and 6), two cannabinol derivatives (7 and 8), and a C21 benzoquinone derivative (9). The known sterol β-sitosterol-3-O-β-d-glucopyranosyl-6′-acetate was also isolated and identified for the first time from cannabis. The antifungal, antibacterial, antimalarial, antileishmanial, and cytotoxic activities of the isolates are also presented.
Results and Discussion
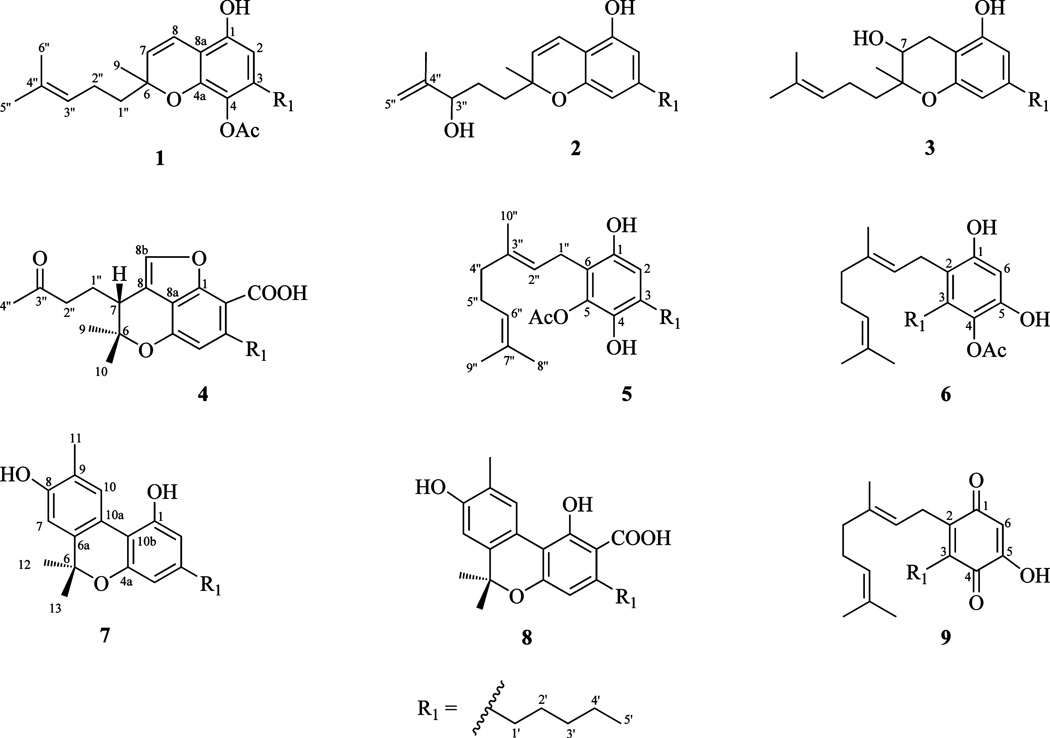
Compound 1 was isolated as an optically inactive yellow oil. Its molecular formula was determined to be C23H32O4 from GC-MS (m/z 372, [M]+) and HRESIMS (m/z 373.2409, [M + H]+), indicating eight degrees of unsaturation. The 1H NMR spectrum of 1 (Table 1) displayed an AB olefinic spin system [δH 5.48 (d, J = 10.0 Hz, H-7), 6.57 (d, J = 10.0 Hz, H-8)], an isolated olefinic proton [δH 5.10 (t, J = 7.2 Hz, H-3″)], a sharp aromatic singlet [(δH 6.07 (s, H-2)], six methylenes (δH 1.30–2.35), two olefinic methyls [δH 1.58 (s, H3-5″), 1.66 (s, H3-6″)], a tertiary methyl [δH 1.33 (s, H3-9)], and an acetoxy methyl resonance [δH 2.29 (s, OCOCH3)]. The small coupling constant between vicinal protons H-7 and H-8 (10.0 Hz) indicated a cis double bond.11 The 13C and APT NMR experiments (Table 1) revealed 23 carbons, including five methyl, six methylene, four methine, and eight quaternary carbon resonances. The quaternary carbons included one ester carbonyl (δC 169.7), three oxyaryl (δC 131.3, 145.3, 148.8), and one oxygenated sp3 carbon (δC 79.1, C-6). The 1H and 13C NMR, IR, and UV spectroscopic data were similar to those reported for cannabichromene,12–14 except for the substitution of an aromatic proton by an acetoxy group at C-4. The location of the acetoxy group was established by the observed deshielding of C-4 and the shielding of C-4a and C-3 relative to cannabichromene.14 Thus, the structure of 1 was determined to be (±)-4-acetoxycannabichromene.
Table 1.
1H (400 MHz) and 13C NMR (100 MHz) Spectroscopic Data of 1–4 (CDCl3)a
| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| carbon | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) |
| 1 | 145.3 | 154.1 | 161.4 | 154.8 | ||||
| 2 | 107.6 | 6.07, s | 108.0 | 6.12, s | 102.3 | 6.21, s | 106.7 | |
| 3 | 135.9 | 145.1 | 145.1 | 148.6 | ||||
| 4 | 131.3 | 109.2 | 6.23, s | 108.2 | 6.14, s | 111.3 | 6.55, s | |
| 4a | 148.8 | 151.3 | 152.3 | 153.2 | ||||
| 6 | 79.1 | 78.3 | 74.1 | 83.5 | ||||
| 7 | 127.7 | 5.48, d (10.0) | 127.1 | 5.46, d (10.0) | 89.5 | 4.68, t (6.8) | 41.4 | 2.89, dd (3.6, 10.8) |
| 8 | 117.1 | 6.57, d (10.0) | 117.3 | 6.62, d (10.0) | 27.5 | 3.03, d (6.8) | 115.3 | |
| 8a | 108.4 | 108.0 | 110.1 | 115.4 | ||||
| 8b | 138.5 | 7.37, s | ||||||
| 9 | 26.3 | 1.33, s | 17.9 | 1.37, s | 23.1 | 1.28 | 25.0 | 1.48, s |
| 10 | 27.2 | 1.29, s | ||||||
| 11 | ||||||||
| 1′ | 30.4 | 2.35, t (7.2) | 36.9 | 2.49, t (7.2) | 36.1 | 2.53, t (7.2) | 35.5 | 3.01 t (7.2) |
| 2′ | 29.7 | 1.54, m | 31.4 | 1.59, m | 31.3 | 1.54, m | 32.4 | 1.63 m |
| 3′ | 31.8 | 1.30, m | 32.1 | 1.35, m | 31.7 | 1.28, m | 32.1 | 1.34, m |
| 4′ | 22.6 | 1.31, m | 22.7 | 1.35, m | 22.7 | 1.28, m | 22.8 | 1.34, m |
| 5′ | 14.2 | 0.87, t (6.8) | 14.2 | 0.87, t (7.2) | 14.3 | 0.89, t (7.2) | 14.3 | 0.88, t (7.2) |
| 1″ | 41.4 | 1.65, m | 37.2 | 2.57, m | 37.1 | 2.62, m | 23.8 | 2.15, m |
| 2″ | 22.8 | 2.06, m | 29.6 | 1.68, m | 22.8 | 2.05, m | 41.3 | 2.55, m |
| 3″ | 124.4 | 5.10, t (7.2) | 76.2 | 4.07, t (6.0) | 124.3 | 5.08, t (7.2) | 208.6 | |
| 4″ | 131.9 | 147.5 | 132.2 | 30.8 | 2.08, s | |||
| 5″ | 17.8 | 1.58, s | 110.0 | 4.83, bs/4.92, bs | 17.8 | 1.58, s | ||
| 6″ | 25.9 | 1.66, s | 26.7 | 1.70 | 25.9 | 1.66, s | ||
| OCOCH3 | 20.7 | 2.29, s | ||||||
| OCOCH3 | 169.7 | |||||||
| COOH | 170.6 | |||||||
Assignments confirmed by DEPT-135, HMQC, COSY, and HMBC NMR experiments.
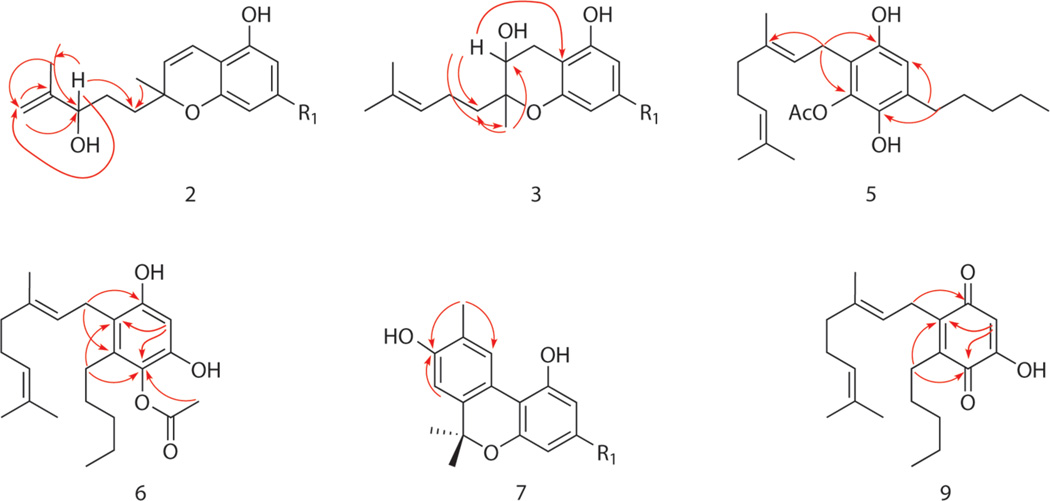
Compound 2 was obtained as an optically inactive brown oil. The HRESIMS exhibited an ion at m/z 331.2193 [M + H]+ corresponding to the molecular formula C21H30O3 (seven degrees of unsaturation). The UV and IR spectra of 2 exhibited patterns similar to those of cannabichromene.12–14 The 1H NMR spectrum of 2 (Table 1) included an AB olefinic spin system [δH 5.46 (d, J = 10.0 Hz, H-7), 6.62 (d, J = 10.0 Hz, H-8)], two aromatic protons [δH 6.12 (s, H-2), 6.23 (s, H-4)], and six methylene resonances (δH 1.35–2.57), confirming the cannabichromene skeleton.12–14 The 1H, 13C, and DEPT NMR spectra displayed additional hydroxymethine [δH 4.07 (t, J = 6.0 Hz), δC 76.2] and exomethylene [δH 4.83 (bs), 4.92 (bs), δC 110.0] functionalities, which, in conjunction with the absence of the C-3″/C-4″ double bond, indicated a migration of the double bond to C-4″/C-5″. This was confirmed by HMBC correlations (H2-5″/C-6″, C-4″, C-3″; H3-6″/C-5″, C-3″) (Figure 1). The oxymethine proton was assigned at C-3″ on the basis of its downfield chemical shift and HMBC correlations with C-5″, C-1″, and C-6″ (Figure 1). Accordingly, 2 was identified as (±)-3″-hydroxy-Δ(4″,5″)-cannabichromene.
Figure 1.
Key HMBC correlations for 2, 3, 5, 6, 7, and 9.
Compound 3 was obtained as an optically active pale yellow oil. The molecular formula was determined to be C21H32O3 from its HRESIMS [M – H]− ion at m/z 331.2254, indicating six degrees of unsaturation. The 13C, DEPT, and HMQC NMR spectra revealed 21 carbons (Table 1), including four methyl, seven methylene, four methine, and six quaternary resonances. The 1H and 13C NMR spectroscopic data of 3 (Table 1) were similar to those of cannabichromene,12–14 except for the absence of the olefinic protons at C-7 and C-8 and the presence of a hydroxy group at C-7 [δH 4.68 (t, J= 6.8 Hz, H-7), δC 89.5], which was established by a COSY correlation between H-7 and H-8 and confirmed by HMBC correlations (H-7/C-9, C-1″, C-8a; H3-9/C-7, C-1″) (Figure 1). The GC-MS analysis of the trimethylsilyl derivative of 3 displayed a molecular ion at m/z 476, confirming the HRESIMS result as well as the presence of two hydroxy groups. The relative configuration at C-7 could not be determined due to insufficient material. Therefore, the structure of 3 was assigned as (−)-7-hydroxycannabichromane.
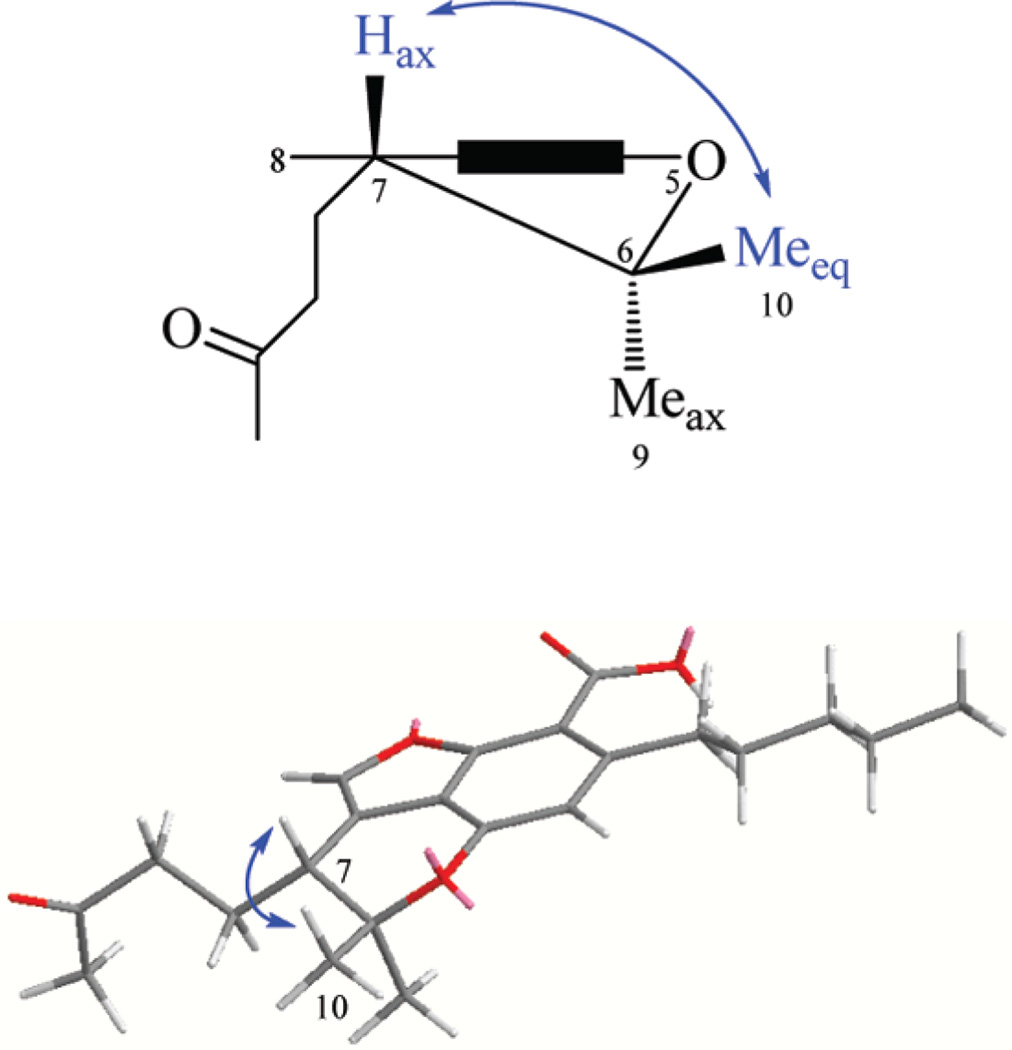
Compound 4 was isolated as a brown oil. Its molecular formula was found to be C22H28O5 by HRESIMS (m/z 395.1847, [M + Na]+) and GC-MS (m/z 372, [M]+). The IR spectrum of 4 indicated the presence of two carbonyl groups (νmax 1716, 1700 cm−1). The 1H, 13C, and DEPT NMR spectroscopic data (Table 1) showed the presence of four methyl, six methylene, three methine, and nine quaternary carbons. The IR, UV, GC-MS, and 1H and 13C NMR data of 4 were in good agreement with those reported for cannabicoumaronone,15 except for the substitution of the aromatic proton at C-2 by a carboxylic acid group, which was confirmed by the additional 44 amu in the GC-MS and HRESIMS analyses, by the GC-MS analysis of the trimethylsilyl derivative of 4 (m/z 444, [M]+), and by the 13C NMR carbonyl resonance at δC 170.6. The ROESY correlation between H-7 (δH 2.89) and pseudoequatorial H3-10 (δH 1.29, δC 27.2) indicated a 7R absolute configuration (Figure 2). The conformation of the C-6 methyl substituents is based on published NMR values for (−)-Δ9-THC, (−)-Δ9-THC acid A, (−)-Δ8-THC, (−)-hexahydrocannabinol, and a series of cannabichromanone derivatives.5 The 13C NMR chemical shift of the β-pseudoequatorial C-6 methyl is downfield from the α-pseudoaxial C-6 methyl for these compounds.5 The CD spectrum of 4 (0.1 mg/mL, MeOH) displayed a positive CE at 246 nm (π→π*) and a negative CE at 295 nm (n→π*), indicating a 7R absolute configuration. Also, the negative specific rotation and the 1H NMR chemical shift of H-7 of 4 were in agreement with the cannabichromanone derivatives that have H-7β configurations.5a Thus, the structure of 4 was established as (−)-7R-cannabicoumarononic acid A.
Figure 2.
Key ROESY correlation between H-7 and pseudoequatorial H3-10 of 4.
The molecular formula of 5 (C23H34O4) was established from HRESIMS (m/z 375.2530, [M + H]+) and 13C NMR data. The 1H, 13C, and DEPT NMR spectroscopic data (Table 2) showed the presence of one aromatic methine, a geranyl moiety,2 an n-pentyl group,2 and an acetoxy group [δH 2.33 (s), δC 20.8, 170.1]. The presence of the acetoxy group was supported by the IR absorption band at νmax 1735 cm−1. The spectroscopic data of 5 were similar to those reported for cannabigerol,16 except for the presence of the acetyl and hydroxy groups at C-5 and C-4, respectively, based on their chemical shifts and HMBC correlations (H2-1″/C-1, C-5; H2-1′/C-4, C-2) (Figure 1). Thus, 5 was established as 5-acetyl-4-hydroxycannabigerol.
Table 2.
1H (400 MHz) and 13C NMR (100 MHz) Spectroscopic Data of 5, 6, and 9 (CDCl3)a
| 5 | 6 | 9 | ||||
|---|---|---|---|---|---|---|
| carbon | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) |
| 1 | 152.6 | 152.9 | 187.7 | |||
| 2 | 108.6 | 6.27, s | 118.6 | 141.2 | ||
| 3 | 133.8 | 135.1 | 146.3 | |||
| 4 | 131.4 | 131.2 | 184.7 | |||
| 5 | 146.4 | 146.1 | 154.3 | |||
| 6 | 113.0 | 102.8 | 6.28, s | 107.8 | 6.04, s | |
| 1′ | 30.4 | 2.40, t (7.6) | 27.7 | 2.42, t (7.2) | 26.7 | 2.48, t (7.8) |
| 2′ | 29.7 | 1.52, m | 30.0 | 1.40, m | 29.0 | 1.49, m |
| 3′ | 31.8 | 1.30, m | 32.4 | 1.31, m | 32.4 | 1.33, m |
| 4′ | 22.6 | 1.30, m | 22.6 | 1.31, m | 22.6 | 1.33, m |
| 5′ | 14.2 | 0.88, t (6.8) | 14.3 | 0.88, t (6.4) | 14.1 | 0.89, t (6.8) |
| 1″ | 23.1 | 3.40, d (7.6) | 25.3 | 3.26, d (6.0) | 25.8 | 3.21, d (6.8) |
| 2″ | 123.9 | 5.04, t (7.6) | 123.3 | 5.09, t (6.0) | 119.9 | 4.93, t (6.8) |
| 3″ | 139.5 | 136.5 | 137.5 | |||
| 4″ | 39.9 | 2.05, m | 39.9 | 1.98, m | 40.0 | 1.97, m |
| 5″ | 26.5 | 2.10, m | 26.7 | 2.06, m | 26.5 | 2.05, m |
| 6″ | 121.6 | 5.27, t (6.4) | 124.3 | 5.04, t (6.4) | 124.2 | 5.03, t (6.8) |
| 7″ | 132.4 | 131.8 | 131.7 | |||
| 8″ | 17.9 | 1.59, s | 17.9 | 1.57, s | 17.9 | 1.57, s |
| 9″ | 25.9 | 1.67, s | 25.9 | 1.65, s | 25.8 | 1.65, s |
| 10″ | 16.4 | 1.79, s | 16.4 | 1.75, s | 16.6 | 1.73, s |
| OCOCH3 | 20.8 | 2.33, s | 20.8 | 2.28, s | ||
| OCOCH3 | 170.1 | 170.0 | ||||
Assignments confirmed by DEPT-135, HMQC, COSY, and HMBC NMR experiments.
Compound 6 was isolated as a yellow oil with molecular formula C23H34O4 (HRESIMS: m/z 375.2528, [M + H]+; GC-MS: m/z 374, [M]+). The 13C, DEPT, and HMQC NMR spectra (Table 2) revealed 23 carbons, including five methyl, seven methylene, three methine, and eight quaternary resonances. The spectroscopic data of 6 (Table 2) resembled those of 5, except for the chemical shifts of the aromatic carbons, indicating a different substitution pattern of the functional groups. HMBC correlations fixed the n-pentyl moiety at C-3 (H2-1″/C-3, C-1; H2-1′/C-2, C-4), the acetoxy group at C-4, and the second hydroxy group at C-5 (H-6/C-4, C-2; OCOCH3/C-4) (Figure 1). Thus, the structure of 6 was established as 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol.
Compound 7 was assigned the molecular formula C21H26O3 from its HRESIMS (m/z 349.1781, [M + Na]+) and 13C NMR data. 1H NMR data showed three methyl singlets, a primary methyl group, and four aromatic and four methylene protons (Table 3). The 13C and DEPT NMR data revealed four methyl, four methylene, four methine, and nine quaternary carbons. The NMR and GC-MS data (m/z 326, [M]+) suggested 7 to be a hydroxylated cannabinol derivative,16 while HMBC correlations (H3-11/C-8, C-10; H-7/C-8) (Figure 1) fixed the structure as 8-hydroxycannabinol. This is the first report of 7 from a natural source; however, it has been prepared synthetically.17
Table 3.
1H (400 MHz) and 13C NMR (100 MHz) Spectroscopic Data of 7 and 8 (CDCl3)a
| 7 | 8 | |||
|---|---|---|---|---|
| carbon | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) |
| 1 | 153.9 | 162.9 | ||
| 2 | 110.1 | 6.27, s | 104.3 | |
| 3 | 143.7 | 148.1 | ||
| 4 | 111.0 | 6.42, s | 113.2 | 6.42, s |
| 4a | 152.6 | 153.3 | ||
| 6 | 77.2 | 78.3 | ||
| 6a | 120.7 | 119.8 | ||
| 7 | 109.8 | 6.68, s | 109.6 | 6.68, s |
| 8 | 152.9 | 158.7 | ||
| 9 | 139.6 | 138.6 | ||
| 10 | 129.0 | 8.14, s | 129.8 | 8.41, s |
| 10a | 122.5 | 122.6 | ||
| 10b | 110.0 | 109.1 | ||
| 11 | 16.0 | 2.23, s | 15.9 | 2.29, s |
| 12 | 27.3 | 1.60, s | 27.6 | 1.59, s |
| 13 | 27.3 | 1.60, s | 27.6 | 1.59, s |
| 1′ | 35.8 | 2.48, t (7.6) | 36.9 | 2.93, t (7.2) |
| 2′ | 30.8 | 1.60, m | 31.4 | 1.59, m |
| 3′ | 31.7 | 1.30, m | 32.1 | 1.35, m |
| 4′ | 22.8 | 1.31, m | 22.7 | 1.35, m |
| 5′ | 14.3 | 0.88, t (7.2) | 14.2 | 0.87, t (7.2) |
| COOH | 176.0 | |||
| 1-OH | 12.6 | |||
Assignments confirmed by DEPT-135, HMQC, COSY, and HMBC NMR experiments.
The molecular formula of 8 was found to be C22H26O5 by HRESIMS (m/z 369.1731, [M – H]−), and its IR spectrum showed hydroxy and carbonyl absorption bands at νmax 3400 and 1650 cm1, respectively. The 13C NMR spectroscopic data of 8 (Table 3) were similar to those of 7, with the addition of a carboxylic group (δC 176.0) located at C-2, as confirmed in the 1H NMR spectrum by the presence of a downfield shifted hydrogen-bonded hydroxy proton (δH 12.6) and the absence of the H-2 proton resonance observed in 7. The GC-MS data of 8 and 7 were identical due to the in situ decarboxylation of 8 that occurs upon injection at 250 °C. On the basis of the above, 8 was elucidated as 8-hydroxycannabinolic acid A.
Compound 9 was isolated as an orange, amorphous powder. The molecular formula C21H30O3 was established by HRESIMS (m/z 353.2066, [M + Na]+). The IR spectrum of 9 indicated the presence of an α,β-unsaturated ketone moiety (νmax 1663 cm−1). The 13C NMR, DEPT, and HMQC spectra of 9 revealed 21 resonances, including four methyl, seven methylene, three olefinic methine, and seven quaternary carbons (Table 2). The two carbonyl carbons resonating at δC 187.7 and 184.7 (Table 2) are characteristic for a benzoquinone skeleton, while NMR analysis suggested geranyl, n-pentyl, and hydroxy substituents, indicating a trisubstituted-1,4-benzoquinone derivative.3,18 The HMBC correlations placed the geranyl moiety at C-2 (H-1″/C-1), the n-pentyl moiety at C-3 (H-1′/C-2, C-4), and the hydroxy group at C-5 (H-6/C-2, C-4) (Figure 1), confirming 9 to be 2-geranyl-5-hydroxy-3-n-pentyl-1,4-benzoquinone. Compound 9 is the second reported 1,4-benzoquinone derivative isolated from cannabis.3
The known compound β-sitosterol-3-O-β-d-glucopyranosyl-6′-acetate was identified by comparison of its spectroscopic data with literature values.19
Biological Activity
The isolated compounds were evaluated for their antimicrobial (Table 4), antiprotozoal (Table 5), and cytotoxic activities. Compound 7 exhibited good antifungal activity against Candida albicans (IC50 4.6 µM), while 2, 6, and 8 showed weak anticandidal activity. Compounds 2 and 6 possessed mild anti-MRSa activity (IC50 24.4 and 6.7 µM, respectively), and 8 showed good anti-Staphylococcus aureus activity (IC50 3.5 µM). Compound 7 exhibited moderate antibacterial activity against Mycobacterium intracellulare (IC50 30.6 µM) (Table 4). Compound 5 showed strong antileishmanial activity (IC50 10.7, IC90 18.7 µM), while 1, 2, and 6 possessed moderate antileishmanial activity. Compounds 1 and 5 had mild antimalarial activities (Table 5). All the isolates lacked cytotoxicity against Vero cells (African green monkey kidney fibroblast).
Table 4.
In Vitro Antimicrobial Activities of 2, 5, 6, 7, and 8 (IC50 in µM)a
| antifungal | antibacterial | |||||
|---|---|---|---|---|---|---|
| compound |
C. albicans |
C. krusei |
MRSa |
S. aureus |
E. coli |
M. intracellulare |
| 2 | 60.5 | 60.5 | 24.4 | 29.6 | na | na |
| 5 | na | nt | 53.4 | na | na | na |
| 6 | na | 53.4 | 6.7 | 12.2 | na | na |
| 7 | 4.6 | nt | nt | nt | na | 30.6 |
| 8 | na | 54.0 | nt | 3.5 | 54.0 | na |
| amphotericin B | 0.3 | 0.7 | ||||
| ciprofloxacin | 0.4 | 0.4 | 0.0 | 1.5 | ||
IC50 = the test concentration that affords 50% inhibition of growth. MRSa = methicillin-resistant Staphylococcus aureus. na = not active. nt = not tested.
Table 5.
In Vitro Antiprotozoal Activities of 1, 2, 5, and 6 (IC50 and IC90 in µM)a
| antileishmanial | antimalarial | |||
|---|---|---|---|---|
| L. donovani | P. falciparum | |||
| compound | IC50 | IC90 | D6 | W2 |
| 1 | 40.3 | 91.3 | 7.2 | 4.0 |
| 2 | 57.5 | 96.8 | na | na |
| 5 | 10.7 | 18.7 | 7.2 | 6.7 |
| 6 | 42.7 | 85.4 | na | na |
| pentamidine | 3.8 | 19.1 | ||
| chloroquine | 0.1 | 0.5 | ||
IC50 = the test concentration that kills 50% cells compared to the solvent controls. IC90 = the test concentration that kills 90% cells compared to the solvent controls.
Experimental Section
General Experimental Procedures
1D and 2D NMR spectra were recorded in CDCl3 on a Varian AS 400 spectrometer. IR spectra were recorded on a Bruker Tensor 27 spectrophotometer. UV spectra were obtained on a Varian Cary 50 Bio UV–visible spectrophotometer. Specific rotations were measured at ambient temperature using a Rudolph Research Analytical Autopol IV automatic polarimeter. HRESIMS were obtained using a Bruker Bioapex FTMS in ESI mode. TLC was carried out on aluminum-backed plates precoated with silica gel F254 (20 × 20 cm, 200 µm, 60 Å, Merck). Visualization was accomplished by spraying with Fast Blue B salt (0.5% w/w in water) or p-anisaldehyde [0.5 mL in glacial acetic acid (50 mL) and sulfuric acid (97%, 1 mL)] spray reagent followed by heating. Flash silica gel (40–63 µm, 60 Å, SiliCycle) and SiliaBond C18 silica gel (40–63 µm, 60 Å, 17% carbon loading, SiliCycle) were used for column chromatography. Analytical HPLC was performed on a Waters 2695 separations module connected to a Waters 2996 photodiode array (PDA) detector (190–500 nm) and a Sedere Sedex 75 evaporative light scattering (ELS) detector (3.5 psi N2, 50 °C) using a Phenomenex Luna C18 HPLC column (150 × 4.6 mm, 5 µm, 100 Å). Semipreparative HPLC was performed on a Waters Delta Prep 4000 preparative chromatography system connected to a Waters 486 tunable absorbance detector (206 nm) using Phenomenex Luna Silica and C18 HPLC columns (250 × 21.2 mm, 5 µm, 100 Å). GC-MS analysis was carried out on a HP 6890 series GC, equipped with a split/splitless capillary injector, a HP 6890 Series injector autosampler, and an Agilent DB-5 ms column (30 m × 0.25 mm × 0.25 µm). The GC was interfaced to a HP 5973 quadrupole mass selective detector through a transfer line set at 280 °C. The injector temperature was 250 °C, and 1 µL injections were performed in the split (1:10) mode. Column flow was set at a constant pressure of 20 psi, giving an initial flow of 2.2 mL/min, using helium as carrier gas. The oven temperature was raised from 70 to 300 °C (hold 8.5 min) at a rate of 20 °C/min, for a total run time of 20 min. The filament was operated at 70 eV, with an emission current of 35 µA. The multiplier voltage was automatically set to 2247 V. The ion source and quadrupole temperatures were 230 and 150 °C, respectively. The acquisition range was m/z 30–800 at 1.95 scans per second, starting 3.5 min after injection.
Plant Material
Plants were grown from high-potency Mexican C. sativa seeds (variety code CHPF-01). The seeds and plants were authenticated by Dr. Suman Chandra, The University of Mississippi, and a specimen (S1310V1) was deposited at the Coy Waller Complex, The University of Mississippi. Whole buds of mature female plants were harvested, air-dried, packed in barrels, and stored at low temperature (−24 °C).
Biological Assays
The isolated compounds were evaluated for in vitro antifungal (Candida albicans ATCC 90028, Candida krusei ATCC 6258, and Aspergillus fumigatus ATCC 90906), antibacterial (methicillin-resistant Staphylococcus aureus ATCC 33591, Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068), antileishmanial (culture of Leishmania donovani), antimalarial [Plasmodium falciparum (D6 clone) and P. falciparum (W2 clone)], and cytotoxic activity [Vero cells (African green monkey kidney fibroblast)].2,21–23
Extraction and Isolation
The plant material (9.0 kg) was sequentially extracted with hexanes (2 × 60 L), CH2Cl2 (48 L), EtOAc (40 L), EtOH (37.5 L), EtOH/H2O (36 L, 1:1), and H2O (40 L) at room temperature. The extracts were evaporated under reduced pressure at 40 °C to afford hexanes (1.48 kg), CH2Cl2 (0.15 kg), EtOAc (0.13 kg), EtOH (0.09 kg), EtOH/H2O (0.77 kg), and H2O (0.54 kg) extracts for a total extract of 3.16 kg (35.1%). Portions of the CH2Cl2, EtOAc, and EtOH extracts were combined (191.0 g) based on similar TLC profiles (EtOAc/n-hexane, 4:6) and were subjected to silica gel VLC, eluting with EtOAc/n-hexane [0:100, 10:90, 20:80, 30:70, 40:60, 50: 50, 75:25, 100:0 (2 L of each mixture)] followed by EtOH (4 L), yielding nine fractions (A–I). Fraction A (13.1 g) was fractionated over a silica gel column eluted with EtOAc/n-hexane (0:100 to 5:95, 5% stepwise) to afford 22 subfractions. Subfraction A17–20 (106 mg) was purified on silica gel HPLC eluting with EtOH/n-hexane (5:95) to yield 1 (2.8 mg), 3 (0.8 mg), 5 (8.9 mg), and 6 (4.0 mg). Fraction C (16.7 g) was applied to a silica gel column using EtOAc/n-hexane (0: 100 to 20:80) to give 10 subfractions. Subfraction C6 (565 mg) was further chromatographed over a C18 SPE column (10 g), eluting with MeOH/H2O (75:25), to afford 4 (170 mg), 9 (13.1 mg), and 7 (6.6 mg). Subfraction C9 (3.2 g) was chromatographed over Sephadex LH-20 eluting with MeOH followed by C18 HPLC purification using MeCN/H2O (55:45), yielding 2 (2.4 mg) and 8 (6 mg). Fraction E (5.7 g) was chromatographed on a silica gel column using EtOAc/n-hexane (20: 80) as a mobile phase to afford β-sitosterol-3-O-β-d-glucopyranosyl-6′-acetate (208 mg).
Trimethylsilyl Derivatization
Dried samples (ca. 100 µg) were treated with pyridine (5 µL, silylation grade, Pierce) and BSTFA [N,O-bis(trimethylsilyl)trifluoroacetamide] (100 µL, 98+%, Acros Organics), followed by heating at 75 °C for 1 h. After cooling to room temperature, methylene chloride (0.9 mL) was added to the reaction mixture and the solution analyzed by GC-MS.
(±)-4-Acetoxycannabichromene (1): yellow oil; UV (MeOH) λmax 227, 280 nm; IR (neat) νmax 3415, 2930, 1735 cm−1; 1H and 13C NMR, see Table 1; EIMS m/z 372 [M]+ (11), 357 (9), 331 (90), 289 (100), 247 (85), 190 (17), 69 (8), 43 (8); HRESIMS m/z 373.2409 [M + H]+ (calcd for C23H33O4, 373.2380).
(±)-3″-Hydroxy-Δ(4″,5″)-cannabichromene (2): brown oil; UV (MeOH) λmax 227, 280 nm; IR (neat) νmax 3405, 3310, 2920, 1590 cm−1; 1H and 13C NMR, see Table 1; EIMS m/z 330 [M]+ (3), 312 (5), 231 (100), 187 (5), 174 (16); HRESIMS m/z 331.2193 [M + H]+ (calcd for C21H31O3, 331.2273).
(−)-7-Hydroxycannabichromane (3): pale yellow oil; [α]25D −66.2 (c 0.15, MeOH); UV (MeOH) λmax 227, 252 nm; IR (neat) νmax 3410, 3310, 2920, 1590 cm−1; 1H and 13C NMR, see Table 1; EIMS m/z 332 [M]+ (30), 314 (5), 299 (7), 271 (5), 247 (30), 231 (24), 206 (65), 193 (20), 164 (20), 150 (100), 135 (62), 109 (60), 69 (35), 43 (33); HRESIMS m/z 331.2254 [M – H]− (calcd for C21H31O3, 331.2273).
(−)-7R-Cannabicoumarononic acid A (4): brown oil; [α]25D −15.0 (c 0.10, MeOH); UV (MeOH) λmax 225, 280 nm; IR (neat) νmax 2910, 1716, 1700, 1640, 1570 cm−1; 1H and 13C NMR, see Table 1; EIMS m/z 372 [M]+ (15), 354 (8), 329 (10), 311 (100), 297 (8), 284 (14), 258 (20), 213 (9); HRESIMS m/z 395.1847 [M + Na]+ (calcd for C22H28O5Na, 395.1835).
5-Acetyl-4-hydroxycannabigerol (5): brown oil; UV (MeOH) λmax 215, 255, 300 nm; IR (neat) νmax 3402, 1735, 1610 cm−1; 1H and 13C NMR, see Table 2; EIMS m/z 374 [M]+ (14), 332 (87), 289 (10), 263 (10), 247 (50), 209 (100), 190 (10), 152 (35), 123 (22), 69 (26), 43 (20); HRESIMS m/z 375.2530 [M + H]+ (calcd for C23H35O4, 375.2535).
4-Acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol (6): yellow oil; UV (MeOH) λmax 215, 255, 300 nm; IR (neat) νmax 3402, 1735, 1610 cm−1; 1H and 13C NMR, see Table 2; EIMS m/z 374 [M]+ (11), 332 (57), 317 (4), 263 (6), 247 (75), 209 (60), 191 (37), 153 (100), 123 (14), 91 (10), 69 (35), 43 (30); HRESIMS m/z 375.2528 [M + H]+ (calcd for C23H35O4, 375.2535).
8-Hydroxycannabinol (7): brown, amorphous powder; UV (MeOH) λmax 220, 267, 330 nm; IR (neat) νmax 3400, 1641, 1610, 873 cm−1; 1H and 13C NMR, see Table 3; EIMS m/z 326 [M]+ (25), 311 (100), 254 (20), 239 (18); HRESIMS m/z 349.1781 [M + Na]+ (calcd for C21H26O3Na, 349.1780).
8-Hydroxycannabinolic acid A (8): brown oil; UV (MeOH) λmax 220, 267, 330 nm; IR (neat) νmax 3400, 1650, 1610, 873 cm−1; 1H and 13C NMR, see Table 3; EIMS (decarboxylated compound) m/z 326 [M]+ (25), 311 (100), 254 (20), 239 (18); HRESIMS m/z 369.1731 [M – H]− (calcd for C22H25O5, 369.1702).
2-Geranyl-5-hydroxy-3-n-pentyl-1,4-benzoquinone (9): orange, amorphous powder; UV (MeOH) λmax 205, 270, 385 nm; IR (neat) νmax 1663, 1613 cm−1; 1H and 13C NMR, see Table 2; EIMS m/z 330 [M]+ (3), 274 (5), 261 (14), 247 (25), 231 (5), 191 (14), 163 (14), 119 (16), 91 (16), 69 (100), 41 (65); HRESIMS m/z 353.2066 [M + Na]+ (calcd for C21H30O3Na, 353.2092).
Supplementary Material
Chart 1.
Acknowledgments
The project described was supported by Grant Number 5P20RR021929-02 from the National Center for Research Resources and in part by the National Institute on Drug Abuse, contract #N01DA-5-7746. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We are grateful to Dr. B. Avula for assistance with the HRESIMS, and to Dr. M. Jacob, Ms. M. Wright, Dr. B. Tekwani, and Dr. S. Khan for conducting the antimicrobial and antiprotozoal testing.
Footnotes
Supporting Information Available: 1H and 13C NMR spectroscopic data for compounds 1–9. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.ElSohly MA, Slade D. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Radwan MM, Ross SA, Slade D, Ahmed SA, Zulfiqar F, ElSohly MA. Planta Med. 2008;74:267–272. doi: 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radwan MM, ElSohly MA, Slade D, Ahmed SA, Wilson L, El-Alfy AT, Khan IA, Ross SA. Phytochemistry. 2008;69:2627–2633. doi: 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed SA, Ross SA, Slade D, Radwan MM, Zulfiqar F, ElSohly MA. J. Nat. Prod. 2008;71:536–542. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Ahmed SA, Ross SA, Slade D, Radwan MM, Ikhlas AK, ElSohly MA. Tetrahedron Lett. 2008;49:6050–6053. doi: 10.1016/j.tetlet.2008.07.178. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choi YH, Hazekamp A, Peltenburg-Looman AMG, Frederich M, Erkelens C, Lefeber AWM, Verpoorte R. Phytochem. Anal. 2004;15:345–354. doi: 10.1002/pca.787. [DOI] [PubMed] [Google Scholar]; (c) Archer RA, Johnson DW, Hagaman EW, Moreno LN, Wenkert E. J. Org. Chem. 1977;42:490–495. doi: 10.1021/jo00423a021. [DOI] [PubMed] [Google Scholar]; (d) Archer RA, Boyd DB, Demarco PV, Tyminski IJ, Allinger NL. J. Am. Chem. Soc. 1970;92:5200–5206. doi: 10.1021/ja00720a033. [DOI] [PubMed] [Google Scholar]
- 6.Ross SA, ElSohly MA. Zagazig J. Pharm. Sci. 1995;4:1–10. [Google Scholar]
- 7.Appendino G, Giana A, Gibbons S, Maffie M, Gnavi G, Grassi G, Sterner O. Nat. Prod. Commun. 2008;3:1977–1980. [Google Scholar]
- 8.Pate DW. In: Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential. Grotenhermen F, Russo E, editors. Chapter 2. Binghamton, NY: The Haworth Press, Inc.; 2002. pp. 15–26. Taxonomy of Cannabinoids. [Google Scholar]
- 9.ElSohly MA. In: Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential. Grotenhermen F, Russo E, editors. Chapter 3. Binghamton, NY: The Haworth Press, Inc.; 2002. pp. 27–36. Chemical Constituents of Cannabis. [Google Scholar]
- 10.Brenneisen R. In: Marijuana and the Cannabinoids. ElSohly MA, editor. Chapter 2. Totowa, NJ: Humana Press Inc.; 2007. pp. 17–50. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. [Google Scholar]
- 11.Crews P, Rodriguez J, Jaspars M. Organic Structure Analysis. New York: Oxford University Press; 1998. p. 135. [Google Scholar]
- 12.Claussen U, Spulak F, Korte F. Tetrahedron. 1966;22:1477–1479. [Google Scholar]
- 13.Gaoni Y, Mechoulam R. Chem. Commun. 1966;1:20–21. [Google Scholar]
- 14.Kane VV, Martin AR, Peters JA, Crew P. J. Org. Chem. 1984;49:1793–1796. [Google Scholar]
- 15.Grote H, Spiteller G. Tetrahedron. 1978;34:3207–3213. [Google Scholar]
- 16.Choi YH, Hazekamp A, Peltenburg-Looman AG, Frederich M, Erkelens C, Lefeber AM, Verpoorte R. Phytochem. Anal. 2004;15:345–354. doi: 10.1002/pca.787. [DOI] [PubMed] [Google Scholar]
- 17.Novak J, Salemink CA. J. Chem. Soc., Perkin Trans. 1983;12:2867–2871. [Google Scholar]
- 18.Mossa JS, Muhammad I, Ramadan AF, Mirza HH, El-Feraly FS, Hufford CD. Phytochemistry. 1999;50:1063–1068. [Google Scholar]
- 19.Liu G, Ma S, Zheng J, Zhang J, Lin R. Zhongcaoyao. 2005;36:814–817. [Google Scholar]
- 20.Insufficient amount of 3 (0.8 mg) was available for bioassays.
- 21.Babu KS, Li XC, Jacob MR, Zhang Q, Khan SI, Ferreira D, Clark AM. J. Med. Chem. 2006;49:7877–7886. doi: 10.1021/jm061123i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharate SB, Khan SI, Yunus NAM, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP. Bioorg. Med. Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Ross SA, Rodríguez-Guzmán R, Radwan MM, Jacob M, Ding Y, Li XC, Ferreira D, Manly SP. J. Nat. Prod. 2008;71:1764–1767. doi: 10.1021/np800446g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.