Summary
Background
Paediatric renal cell carcinoma (RCC) is a rare neoplasm which differs significantly in its clinico-pathological behaviour from the adult variant. The clear cell variant constitutes a relatively small histological subset of this neoplasm.
Case Report
We present a very unusual, pathologically proven case of clear cell variety of pediatric RCC which showed invasion into the pelvicalyceal system with contiguous extension up to the urinary bladder. Such a novel manifestation of paediatric RCC has not been described previously in literature. A relevant review of literature is included.
Conclusions
The aggressive biological behaviour of the paediatric RCC in our case and the consequent atypical imaging findings are distinctly unusual. These findings may represent a new aggressive variant of this rare neoplasm. The radiologist and clinician should be aware of these novel manifestations of paediatric clear cell RCC.
MeSH Keywords: Carcinoma, Renal Cell; Kidney Neoplasms; Pediatrics; Rare Diseases
Background
RCC is a rare malignancy of childhood and most of our knowledge of this neoplasm stems from a few population based-studies or case series. Based on the previously published literature, the clinical behaviour of paediatric RCC is believed to be fundamentally different from the adult variant. These tumours are more commonly found to be symptomatic; localized disease is more prevalent and histopathologically there is over-representation of papillary architecture. The clear cell variant constitutes a relatively small histological subset of this neoplasm. In this case we describe the CT findings, clinical behaviour and histopathological correlation of a case of clear cell paediatric RCC which had invaded into the renal collecting system.
Case Report
A 13-year-old male child presented at the emergency of a peripheral hospital with acute retention of urine, pain on the left side of the abdomen and history of passing blood clots in urine. Patient was catheterised and was referred to the department of surgery of our institution. Abdominal examination revealed a ballotable hard mass in the left lumbar region. The patient was admitted and initial routine laboratory tests were significant for mild anaemia (Hb 9.3 g/dL) and presence of red blood corpuscles in the urine examination. Ultrasound of the abdomen revealed a large, lobulated, heterogeneous echotexture mass replacing almost the entire left kidney. Nodular soft tissue mass was also present in the urinary bladder on the left side. An X-ray of the chest carried out on the same day was found to be normal. A CT of the abdomen was performed on the following day and plain CT sections were acquired followed by contrast-enhanced CT in the corticomedullary and delayed phases. The CT revealed a markedly enlarged left kidney due to replacement by an ill-marginated, heterogeneously enhancing mass of approximate size – 15×6.5×7.0 cm, with CT attenuation ranging 60–75 HU in solid portions and 25–35 HU in necrotic portions. Few punctiform and linear foci of calcification were also identified within the tumour (better appreciated on plain CT images). The corticomedullary demarcation was lost with extension of the mass into the dilated pelvicalyceal system. The tumour was extending through the dilated ureter up to the left vesicoureteric junction with a focal intraluminal projection into the bladder (Figures 1–4). Additionally, there were multiple enlarged lymph nodes in the retroperitoneal and renal hilar locations with the largest node measuring 1.7 cm (Figure 3). There was no evidence of renal vein or inferior vena cava thrombosis. Keeping in consideration the age of the patient, clinical presentation and radiological findings, we kept a possibility of Wilms’ tumour (stage T4N1M0) or atypical paediatric RCC. A decision was taken by the surgeon to perform radical nephrectomy with partial ureterectomy and to tackle the deposits in the bladder in the second sitting. No neoadjuvant chemotherapy was given. Patient was transfused one unit of blood before surgery. Diseased left kidney was approached by transabdominal subcostal incision. The whole kidney was found to be replaced by nodular tumour and there were multiple lymph nodes in the renal hilum and para-aortic region. There was no extension of tumour in the renal vein. The left ureter was dilated and was filled with tumour. Nephrectomy was performed along with removal of the upper two-third of the ureter and some para-aortic lymph nodes.
Figure 1.
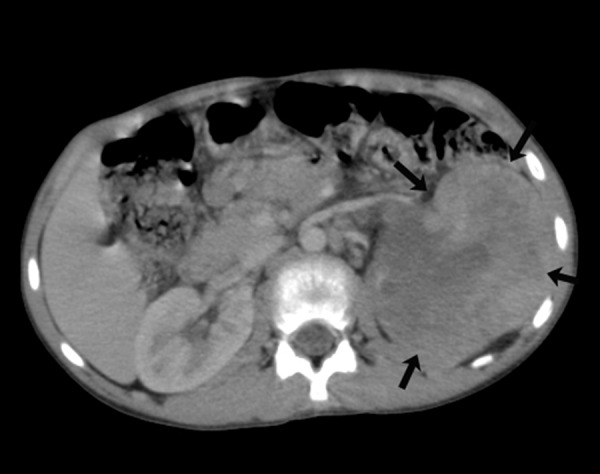
Axial contrast-enhanced CT image showing a heterogeneous tumour mass replacing the left kidney (arrows)
Figure 2.
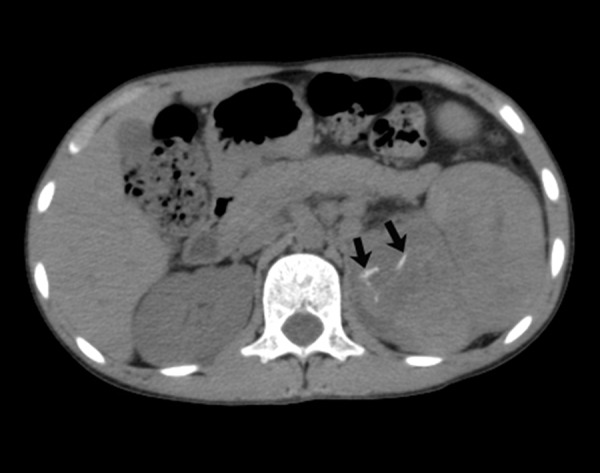
Axial non-contrast CT image showing linear foci of calcifications in the renal mass (arrows).
Figure 3.
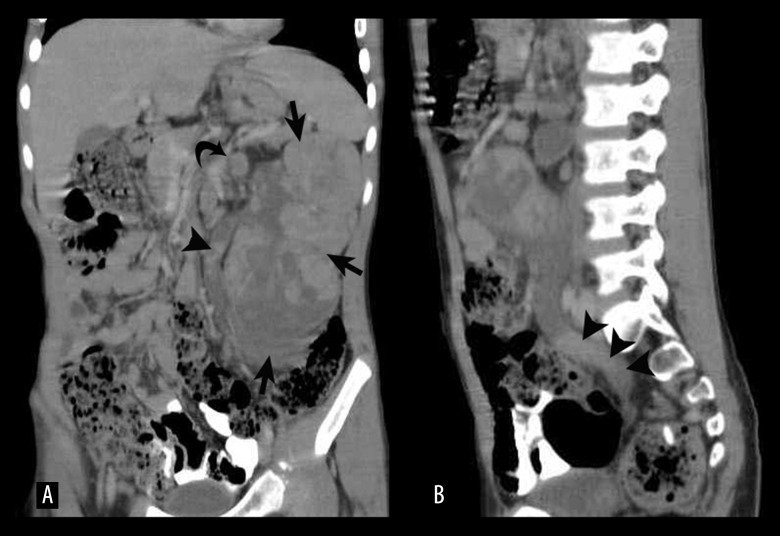
(A) Coronal MPR image showing the tumour replacing the entire left kidney (arrows) along with a dilated ureter filled with multiple nodular deposits (arrowheads). Enlarged hilar nodes are also visualized (curved arrow). (B) Sagittal MPR image showing ureteric and renal pelvis dilatation with multiple tumour deposits (arrowheads).
Figure 4.
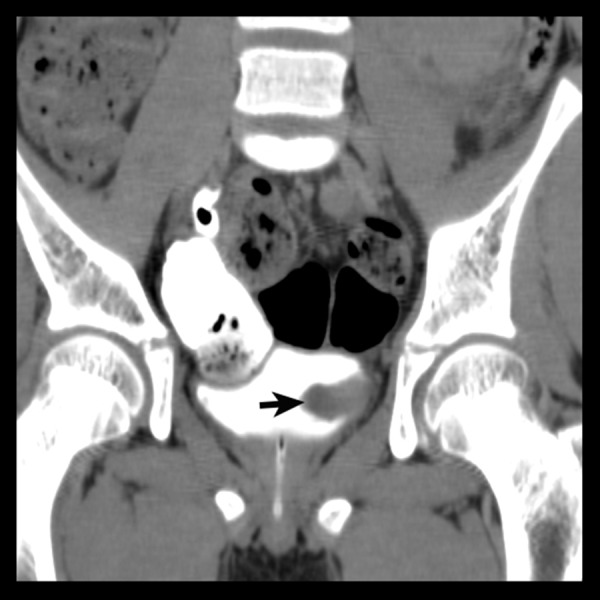
Coronal MIP image in the delayed phase showing the intraluminal tumour projection into the urinary bladder through the left vesicoureteric junction (arrow).
On histopathological examination of the specimen, the entire kidney was replaced by a solid tumour with areas of necrosis and haemorrhages. The attached ureter was dilated and filled with tumour. Sections examined showed features consistent with clear cell carcinoma of Fuhrman grade II. The tumour was invading the perinephric fat and lymph nodes. Sections examined from hilar vessels were free from tumour (Figures 5, 6).
Figure 5.
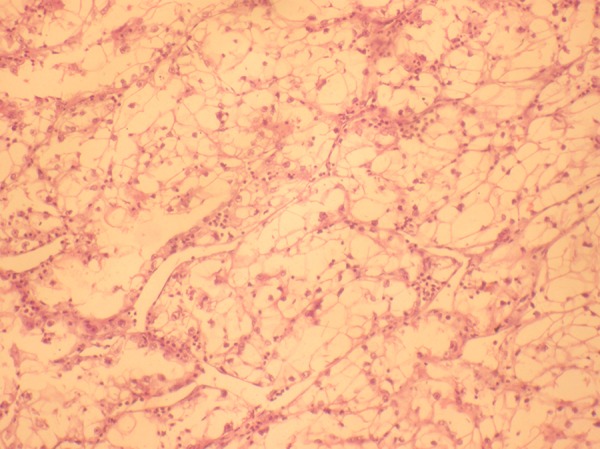
Haematoxylin and Eosin stain (10×) shows tumour composed of cells with abundant clear cytoplasm and nuclei with moderate pleomorphism. Neutrophils are seen between the tumour cell lobules.
Figure 6.
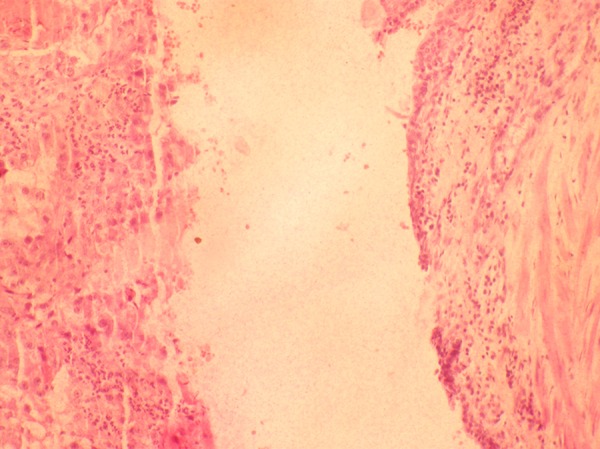
Haematoxylin and Eosin stain (10×) of a section from the resected margin of the ureter shows luminal dilatation, attenuated transitional cell lining epithelium and presence of tumour in the lumen.
Discussion
Childhood RCC is a rare malignancy constituting 5.9% of malignant renal neoplasms in children and adolescents [1]. Overall, the incidence is estimated to be 0.01 per 100,000 population [2]. There is some variance in the median age of presentation with two of the largest studies reporting the mean age as 10.6 years [3] and 17 years [2] respectively. Nonetheless, it is generally seen to occur in late childhood. Gender distribution is almost equal [3] which is different from the predominant male prevalence in adult RCC [1]. Some comorbid conditions or diseases such as: tuberous sclerosis, urogenital malformations, neuroblastoma or teratoma with chemotherapy, chronic renal failure etc., are believed to be causally associated with paediatric RCC. Selle et al. in their study found every third child or their families to be harbouring these conditions [3]. They also reported some new conditions like Saethre-Chotzen syndrome, XYY syndrome, and accessory nipple which they believe to be causally linked with paediatric RCC. Paediatric RCC is also known to occur as a second malignancy in children already suffering from other cancers. Of these cancers, neuroblastoma-associated RCC is a distinct subgroup which is now classified as a separate entity under the new WHO classification of 2004. Although RCC is more commonly identified in patients with neuroblastoma who receive some form of chemotherapy [4], it is believed that a specific independent relationship between NB and subsequent RCC also exists [5]. Chemotheraputic treatment of some other tumours is also reported to be complicated with the occurrence of RCCs later on. Among those reported are: supratentorial PNET, acute promyelocytic leukemia, cardiac leiomyosarcoma and Wilms’ tumour [6–9]. Cyclophosphamide was an agent which was most commonly implicated followed by topoisomerase II [8,10]. It is however not clear if the renal tumour development is a result of particular chemotherapeutic agent or due to underlying constitutional tumoural predisposition. Our patient did not have any comorbid conditions or intake of chemotherapy.
WHO (2004 classification) classified the RCCs as clear cell, papillary, chromophobe, Xp 11-translocation RCC, RCC associated with NB, and unclassified RCC. The paediatric RCCs are typically characterized by the predominance of papillary and translocation subtypes and the clear cell variety is distinctly uncommon. Selle et al. reported the incidence of various pathological subtypes of RCC: papillary 33% (n-16), translocation type 22% (n-11), unclassified 16% (n-8), and rarely clear cell (n-3) [3]. Our patient showed the very rare clear cell histological subtype. Of particular interest in our case was the non-occurrence of the VHL syndrome. It was pointed out by Renshaw et al. that the occurrence of clear cell paediatric RCC is exceedingly rare in patients without VHL [11]. On the other hand, Bruder et al. and Geller et al. argued that clear cell RCC can be seen in paediatric age groups even without VHL [1,12]. It is difficult to speculate whether there is no association or otherwise of RCC with VHL in our study as it is a report of just a single case.
Another remarkable feature of our case was its deviant and novel biological behaviour which was characterized by the invasion of the renal pelvis and ureter with extension into the bladder. We extensively searched the literature but did not come across such a bizarre tumour behaviour in a paediatric RCC. According to the previously published literature, paediatric RCC is characterized by localized disease (69%), regional lymph node metastasis (16%) or distant metastasis (8%) [3] but none of these studies reported on such a contiguous extension of tumour from the kidney through the ureter up to the bladder, as was seen in our case. The histopathological examination of this creeping tumour also revealed similar clear cell subtype as seen in the dominant renal mass. The clinical presentation of the abdominal mass, hematuria and urinary retention in our patient is however in keeping with the commonly described clinical features of this tumour [1,3].
There is scarcity of literature pertaining to the imaging features of paediatric RCCs which follows from the relative rarity of the disease. Recently Downey et al. described the CT/MRI appearances of 10 paediatric RCCs. In their study, 90% of the tumours showed heterogeneous post-contrast enhancement, 50% had associated haemorrhage and 40% contained internal calcifications [13]. Imaging plays an important role in paediatric renal masses mainly through distinguishing RCC from other more common renal neoplasms like Wilms’ tumour [6]. Miniati et al. reported 82% accuracy in radiological correlation with tumour histology [14]. Of particular value on imaging is the presence of tumoural calcifications which may suggest RCC. The calcifications on imaging may correspond on pathological examination to psammomatous calcification seen in Xp11.2 translocation RCCs [6]. The imaging features in our case included large tumour size, heterogeneous post-contrast enhancement with solid areas interspersed with necrotic areas, relatively maintained reniform shape (despite large tumour size), multiple foci of small calcifications, loss of demarcation between the renal sinus and medulla with invasion of the collecting system, and multiple urothelial deposits in the ureter and bladder. In addition, multiple enlarged loco-regional nodes were also identified. Of the CT findings listed above, the heterogeneous enhancement and foci of calcifications conform to the findings described by Miniati et al. [14]. Rest of the findings, i.e. loss of demarcation between the pelvis and medulla, invasion of the pelvicalyceal system and multiple urothelial deposits, are unique to our case and to the best of our knowledge were reported for the first time. These radiological findings were subsequently validated on surgery and histopathological examination of the resected specimen.
Surgical resection is the sine qua non for therapeutic approach to paediatric RCCs with radical or partial nephrectomy being the procedure of choice depending on the stage of the disease at presentation. We performed radical nephrectomy because of the advanced stage (T3a) of the disease along with partial ureterectomy (to remove urothelial deposits) and partial lymphadenectomy. Full lymph node dissection was not feasible in view of the extensive lymphadenopathy adherent to the aorta and IVC. In fact, the role of lymphadenectomy is still being debated. In a study by Galler et al., the majority of children with node-positive RCC survived without undergoing lymph node dissections. They proposed that the second-look lymph node dissections were not required in children who do not undergo lymph node dissection in the first procedure [1]. A recent study by Indolfi et al. however favours aggressive surgical management with radical retroperitoneal lymph node dissection at the time of nephrectomy, citing improved outcome in their study [15]. Most researchers however do agree that while lymph node metastasis is associated with adverse outcomes in paediatric RCC, the prognostic implications are not as grave as in adult RCC.
The role of adjuvant chemotherapy in node-positive RCC (N plus M0) is peppered with some criticism. Galler et al. questioned the significance of adjuvant therapy in this group as far as the disease outcome is concerned. There is however a role for adjuvant therapy in distant metastases and unresectable primary tumours. Ismail et al. described encouraging results with whole-body hyperthermia and chemotherapy in advanced pediatric RCCs [16]. We planned partial cystectomy along with ureterectomy of the lower ureter and lymphadenectomy (of the remaining nodes) as a second-stage operation after a month. A trial of immunotherapy is also being considered.
Conclusions
The biological behaviour of the tumour in our case and the radiological findings are distinct from the known or established knowledge and may represent a new variant of this rare neoplasm. The reasons for this atypical and aggressive biological behaviour seen in our case are not clear and difficult to conjecture. It could represent a sporadic case of hitherto unknown behaviour or a case with a new genetic mutation resulting in a more aggressive tumour phenotype. Further research and documentation of more cases with similar findings is needed before they can be labelled as a new aggressive variant of clear cell paediatric RCC.
References
- 1.Geller JI, Dome JS. Local lymph node involvement does not predict poor outcome in pediatric renal cell carcinoma. Cancer. 2004;101:1575–83. doi: 10.1002/cncr.20548. [DOI] [PubMed] [Google Scholar]
- 2.Silberstein J, Grabowski J, Saltzstein SL, Kane CJ. Renal cell carcinoma in the pediatric population: results from the California cancer registry. Pediatr Blood Cancer. 2009;52:237–41. doi: 10.1002/pbc.21779. [DOI] [PubMed] [Google Scholar]
- 3.Selle B, Furtwangler R, Graf N, et al. Population-based study of renal cell carcinoma in children in Germany, 1980–2005. More frequently localized tumors and underlying disorders compared with adult counterparts. Cancer. 2006;107:2906–14. doi: 10.1002/cncr.22346. [DOI] [PubMed] [Google Scholar]
- 4.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report fromn the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–83. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros LJ, Palmedo G, Krigman HR, et al. Oncocytoid renal cell carcinoma after neuroblastoma: A report of four cases of a distinct clincopathological entity. Am J Surg Pathol. 1999;23:772–80. doi: 10.1097/00000478-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sausville JE, Hernandez DJ, Argani P, Gearhart JP. Pediatric renal cell carcinoma. J Pediatr Urol. 2009;5:308–14. doi: 10.1016/j.jpurol.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Schafernak KT, Yang XJ, Hsueh W, et al. Pediatric renal cell carcinoma as second malignancy: Reports of two cases and a review of the literature. Can J Urol. 2007;14:3739–44. [PubMed] [Google Scholar]
- 8.Argani P, Lae M, Ballard ET, et al. Translocation carcinomas of kidney after chemotherapy in childhood. J Clin Oncol. 2006;24:1530–34. doi: 10.1200/JCO.2005.04.4693. [DOI] [PubMed] [Google Scholar]
- 9.Dhall D, Al-Ahmadie HA, Dhall G, et al. Pediatric renal cell carcinoma with oncocytoid features occurring in a child after chemotherapy for cardiac leiomyosarcoma. Urology. 2007;70:178.e13–15. doi: 10.1016/j.urology.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Fleitz JM, Wootton-Gorges SL, Wyatt-Ashmead J, et al. Renal cell carcinoma in long-term survivors of advanced stage neuroblastoma in early childhood. Pediatr Radiol. 2003;33:540–45. doi: 10.1007/s00247-003-0913-x. [DOI] [PubMed] [Google Scholar]
- 11.Renshaw AA, Granter SR, Fletcher JA, et al. Renal cell carcinomas in children and young adults. Am J Surg Pathol. 1999;23:795–802. doi: 10.1097/00000478-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Bruder E, Passera O, Harms D, et al. Morphologic and molecular characterization of renal cell carcinoma in children and young adults. Am J Surg Pathol. 2004;28:1117–32. doi: 10.1097/01.pas.0000131558.32412.40. [DOI] [PubMed] [Google Scholar]
- 13.Downey RT, Dillman JR, Ladino-Torres MF, et al. CT and MRI appearances and radiologic staging of pediatric renal cell carcinoma. Pediatr Radiol. 2012;42:410–17. doi: 10.1007/s00247-011-2319-5. [DOI] [PubMed] [Google Scholar]
- 14.Miniati D, Gay AN, Parks KV, et al. Imaging accuracy and incidence of Wilms’ and non-Wilms’ renal tumors in children. J Pediatr Surg. 2008;43:1301–7. doi: 10.1016/j.jpedsurg.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 15.Indolfi P, Bisogno G, Cecchetto G, et al. Local lymph node involvement in pediatric renal cell carcinoma: a report from Italian TREP project. Pediatr Blood Cancer. 2008;51:475–78. doi: 10.1002/pbc.21652. [DOI] [PubMed] [Google Scholar]
- 16.Ismail-Zade RS, Zhavrid EA, Potapnev P. Whole body hyperthermia in adjuvant therapy of children with renal cell carcinoma. Pediatr Blood Cancer. 2005;44:679–81. doi: 10.1002/pbc.20299. [DOI] [PubMed] [Google Scholar]