Abstract
Background
Sepsis is the result of the interaction between inflammatory mediators and coagulation pathway. Unfractionated heparin may play a role as an anti-inflammatory agent beyond its anticoagulatory effect in sepsis. As a result, it may cause reduction in organ failure rate in patients with sepsis due to its impact on both inflammatory and coagulation process.
Objectives
The aim of this study was to evaluate the anti-inflammatory effects of heparin in sepsis. Plasma plasminogen activator inhibitor-1 (PAI-1) as an inflammatory mediator and urinary necoutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney injury were investigated.
Patients and Methods
This prospective, randomized controlled trial was conducted in a 32-bed intensive care unit. Thirty patients with sepsis were randomized to receive heparin infusion of 500 units/hour or 5000 units of heparin three times a day, subcutaneously. The plasma level of PAI-1 and urinary level of NGAL were determined at day 0, 2 and 7.
Results
The infusion group had a lower plasma PAI-1 level compared to the subcutaneous group at day 7 (11.3 ± 1.6 vs. 16.5 ± 4.2; P = 0.003). The urinary NGAL level was lower in the infusion group at day 2 (131.3 ± 11.9 vs. 151.2 ± 20.6; P = 0.014); however, at day 7 the NGAL level was decreased in the subcutaneous group as much as the infusion group and there was no significant difference between the two groups. There was no significant difference in the acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA) scores between the two groups at day 0, 2 and 7.
Conclusions
Low-dose heparin infusion compared to subcutaneous heparin can decrease the plasma PAI-1 and urinary NGAL levels more rapidly. It can be related to anti-inflammatory effects of heparin, which may be more prominent in infusion route.
Keywords: Heparin, Plasminogen Activator Inhibitor 1, Neutrophil Gelatinase-Associated Lipocalin, Sepsis, Anti-Inflammatory Agents
1. Background
Sepsis is a consequence of systemic inflammatory response to an infection, which is characterized by release of pro- and anti-inflammatory mediators (1).
The coagulation and inflammation systems affect on each other directly and both are involved in sepsis; the inflammation provokes coagulation and conversely, coagulation promotes an inflammation process (2).
Inflammatory mediators such as tumor necrosis factor, interleukin 1, and interleukin 6 can damage the endothelium and initiate procoagulant and antifibrinolytic response. Activation of coagulation leads in consumption of anticoagulants such as antithrombin and protein C in blood circulation and release of plasminogen activator inhibitor-1 (PAI-1) (1). Plasminogen activator inhibitor-1 is a serine protease inhibitor, which inhibits the tissue plasminogen activator resulting in fibrinolysis (2).
The complex of activated protein C (APC) with PAI-1 precedes fibrinolysis; so, depletion of the APC in sepsis results in increased plasma PAI-1 level (2).
Plasminogen activator inhibitor-1 has a pivotal role in inhibition of fibrinolysis during sepsis. Multiple organ dysfunction syndrome (MODS) can occur as a result of high plasma levels of PAI-1, which can lead to increase in mortality in septic patients (3). Earlier studies have reported that an elevated PAI-1 level is associated with poor prognosis and higher disease severity and mortality in sepsis (2, 4). Around 30% of septic patients have multiple organs failure (5). The multiple organ dysfunction syndrome is one of the most fatal complications in septic patients which is the consequence of extensive thrombosis in blood vessels and reduction in perfusion of different organs (5).
Acute kidney injury (AKI) is common in critically ill patients, particularly in the presence of sepsis. Despite recent achievements in managing sepsis, AKI remains an obstacle in treatment of septic patients. It is partly due to the lack of a reliable marker for the assessment of kidney function. It has been well clarified in earlier studies, that serum creatinine has limitations, which make it an inaccurate marker of kidney function. Recent studies confirm that there is an increase in plasma and urine necoutrophil gelatinase-associated lipocalin (NGAL) in septic patients. The plasma NGAL can rise in patients with sepsis due to inflammation, independent of kidney failure but the urinary NGAL is more reliable in detecting kidney dysfunction in sepsis (6).
Unfractionated heparin (UFH) has well-known antithrombotic effects by inhibiting thrombin formation, which potentiates the activity of antithrombin. A less known property of heparin is its potential to act as an anti-inflammatory agent that appears to be independent of its role as an anticoagulant. Although the exact mechanism is not clear, it has been hypothesized it is partly due to the link between inflammation and thrombin activation; however, it seems that there are mechanisms other than anticoagulant effects of heparin. Earlier studies proposed that due to interconnection between thrombosis and inflammation, thrombin inhibition may result in limitation of inflammatory process (7, 8).
2. Objectives
Subcutaneous (SC) heparin is commonly used for prophylaxis of thrombosis. Since increased survival has been reported by use of therapeutic intravenous heparin in sepsis (9), we hypothesized that using low-dose heparin infusion for thromboprophylaxis may have anti-inflammatory properties and reduce MODS in these patients.
The aim of the current study was to compare the anti-inflammatory effects of intravenous heparin versus subcutaneous heparin in patients with sepsis. To compare this effect, the PAI-1 level as an inflammatory factor was used. Also, given that organ failure is a consequence of thrombosis and inflammation, NGAL, as a marker of kidney function, was compared.
3. Patients and Methods
This study was approved by the ethics committee of Tehran University of Medical Sciences and a written informed consent was obtained from the patients or from the family members (IRCT2015071122668N1). This prospective, randomized controlled trial was conducted in a 32-bed intensive care unit (ICU). Patients aged 18 years old or greater who were diagnosed with sepsis were enrolled. The diagnosis of sepsis was made according to “surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock” (10). Exclusion criteria were pregnancy, increased risk for bleeding, requirement of anticoagulation, transplantation, PLT < 50 × 106 per mL, history of HIT, allergy to heparin, and acute and chronic kidney injury. Thirty patients were included in the study and assigned randomly to the infusion group to receive 500 units of heparin per hour or the subcutaneous group to receive 5,000 units every eight hours. The total daily dose of heparin in the infusion group was 12,500 units and in the subcutaneous group was 15000 units and both continued for 7 days or until discharge or death if these occurred before. Other managements were based on latest guidelines and clinical judgment.
Variables that were recorded for each patient included sex, age, past medical history and site of infection. We also recorded vital signs, intake, output, blood urea nitrogen (BUN), serum creatinine, platelet count, lactic acid, the international normalized ratio (INR), activated partial thromboplastin time (aPTT), acute physiology and chronic health evaluation (APACHE)-II score, and sequential organ failure assessment (SOFA) score. The plasma PAI-1 and urinary NGAL levels were assessed on day 0, 2 and 7 of ICU admission.
Urine and venous blood samples were centrifuged within 30 minutes at 5,000 g for 7 minutes. Samples were kept in -80°C until further assay.
The PAI-1 and NGAL antigens were determined by the enzyme-linked immunosorbent assay (ELISA) (Crystal Day Biotech CO., LTD, Shanghai, China) as stated by manufacturer's instructions.
In the event of bleeding, heparin treatment was interrupted and continued only after normal coagulation tests were reported. In cases of prolonged aPTT (more than 70 seconds) infusion was paused and test was repeated and after reduction to less than 60 seconds, infusion was resumed. Infusion was stopped if the platelet counts fell below 50 × 106 per mL any time during study.
All variable data were tested for normality using the Kolmogorov-Smirnov test. Categorical variables were compared with the chi-square test. Statistical analyses were performed using Student’s t-test for comparison between the two groups. Differences between the two groups for each variable were analyzed using the analysis of variance for repeated measurements with one grouping factor (infusion versus subcutaneous) and one within-subject factor (day 0, 2, 7). A P value of less than 0.05 was considered statistically significant. Statistical analyses were performed with IBM SPSS statistics for windows, version 22.0 (Armonk, NY: IBM Corp).
4. Results
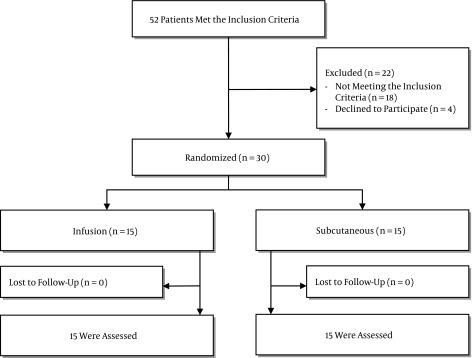
In total, 52 patients were assessed for eligibility and 30 patients met the inclusion criteria. Patients were randomly assigned to receive heparin 5,000 units three times a day, subcutaneously or heparin infusion 500 units per hour. Patient’s baseline characteristics are shown in Table 1. There were no significant differences regarding age, sex, preexisting conditions, source of infection, INR, aPTT, platelets count, SIRS criteria, serum creatinine, urinary output and procalcitonin on day 0.
Table 1. Baseline Characteristics of the Patients.
| Infusion (N = 15) | Subcutaneous (N = 15) | P Value | |
|---|---|---|---|
| Age, y a | 57.4 (19.3) | 59.6 (22.8) | 0.77 |
| Male sex b | 7 (46) | 8 (53) | 0.75 |
| Preexisting conditions b | |||
| CAD | 5 (33.3) | 6 (40) | 0.51 |
| CHF | 4 (26.6) | 2 (13.3) | 0.43 |
| HTN | 9 (60) | 6 (40) | 0.37 |
| COPD | 2 (13.3) | 1 (6.6) | 0.21 |
| CKD | 0 | 0 | 1 |
| Diabetes | 2 (13.3) | 3 (20) | 0.67 |
| Cancer | 3 (20) | 2 (13.3) | 0.15 |
| The source of infection b | |||
| Lung | 7 (46.6) | 5 (33.3) | 0.41 |
| Abdomen | 3 (20) | 2 (13.3) | 0.37 |
| Urinary | 2 (13.3) | 4 (26.6) | 0.27 |
| Other | 3 (20) | 4 (26.6) | 0.60 |
| APACHE II a | 24.9 (5.5) | 22.4 (4.2) | 0.17 |
| SOFA a | 7.0 (1.7) | 6.0 (2.2) | 0.15 |
| Procalcitonin, mcg/L a | 6.4 (11.8) | 4.3 (7.4) | 0.50 |
| SIRS criteria a | |||
| Temperature, °C | 38.0 (1.2) | 37.7 (1.02) | 0.41 |
| Heart rate, bpm | 102.9 (25.1) | 94.2 (16.6) | 0.27 |
| Mechanically ventilatedb | 13 (86.6) | 12 (80) | 0.7 |
| Leucocyte count, × 109/L | 14.0 | 12.6 (6.8) | 0.52 |
| ESR, mm/h a | 61.6 (31.9) | 61.4 (21.5) | 0.20 |
| CRP, mg/L a | 112.6 (62.9) | 90.2 (45.2) | 0.28 |
| Cr, mg/dL a | 1.2 (0.5) | 0.98 (0.5) | 0.32 |
| Urinary output, mL/24 h a | 2047 (1265) | 1862 (1316) | 0.64 |
| INR a | 1.5 (0.87) | 1.2 (0.2) | 0.25 |
| aPTT, s a | 48.9 (24.8) | 45.0 (23.6) | 0.66 |
| Platelet counts, × 10 9 /L a | 226.2 (104) | 174.7 (81.3) | 0.14 |
aValues are expressed as mean (SD).
bValues are expressed as No. (%).
At baseline, the APACHE II and SOFA scores were higher in the heparin infusion group, but they did not reach statistical significance (24.9 ± 5.5, 22.4 ± 4.2, P = 0.17). The APACHE II and SOFA scores were reduced during the study in both groups although there was no significant difference between these groups. No major bleeding was reported in none of these groups. Although there were cases of minor bleeding in both groups, no difference was observed between them.
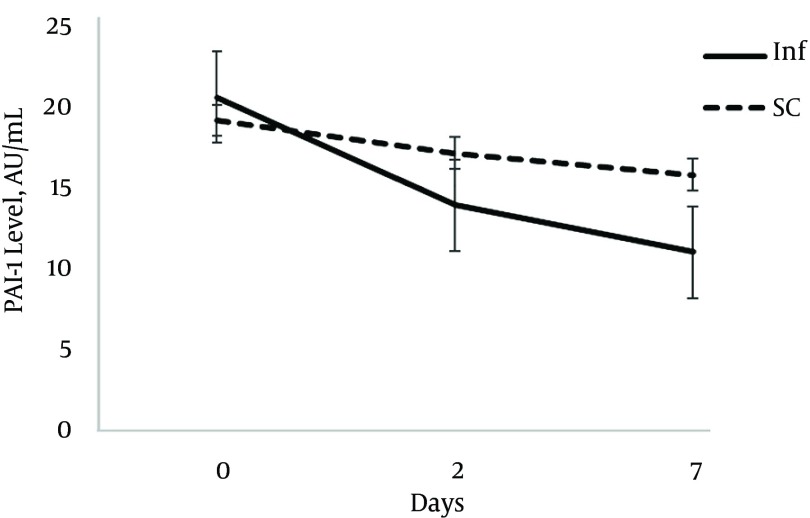
Table 2 shows the PAI-1 and urinary NGAL levels at day 0, 2 and 7. There was no difference regarding the PAI-1 level at baseline (P = 0.66); however, after 2 days the PAI-1 level was reduced in the infusion group, and at day 7 the PAI-1 level in the infusion group was significantly lower than that after 2 days (11.3 ± 1.6 vs. 16.5 ± 4.2; P = 0.003).
Table 2. The Plasminogen Activator Inhibitor-1 and Necoutrophil Gelatinase-Associated Lipocalin Levels at Baseline, Day 2 and Day 7a.
| Baseline | 2 Days | 7 Days | |
|---|---|---|---|
| PAI-1, AU/mL | |||
| Infusion | 21.86 (11.5) | 14.9 (5.0) | 11.3 (1.6) |
| Subcutaneous | 20.3 (7.0) | 18.3 (6.4) | 16.5 (4.2) |
| P value | 0.668 | 0.177 | 0.003 |
| NGAL, ng/mL | |||
| Infusion | 140.4 (20.2) | 131.3 (11.9) | 130.7 (10.1) |
| Subcutaneous | 142.6 (19.5) | 151.2 (20.6) | 136.7 (13.7) |
| P value | 0.757 | 0.014 | 0.258 |
aValues are expressed as mean (SD).
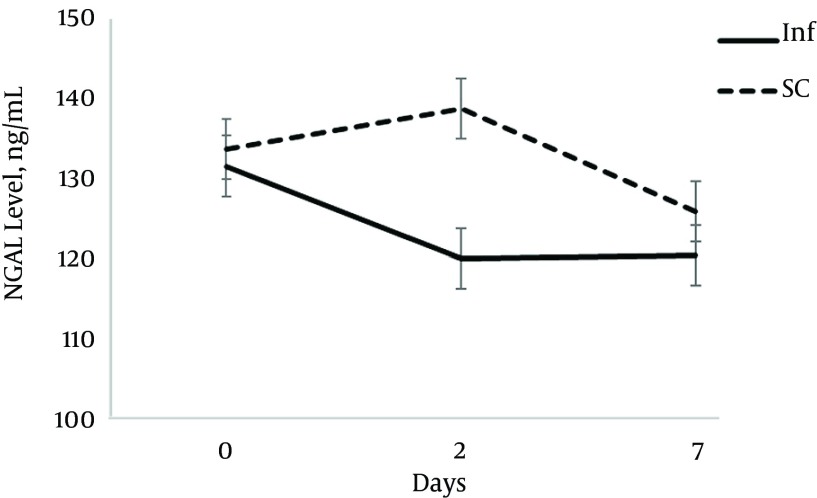
The urinary NGAL level had different pattern. Although two groups were similar at baseline, after 2 days the urinary NGAL level was significantly lower in the infusion group (131.3 ± 11.9 vs. 151.2 ± 20.6; P = 0.014). However, at day 7 the urinary NGAL level was similar in the two groups.
The ICU mortality (subcutaneous vs. infusion: 33.3% vs. 46.7% s, P = 0.456), and length of ICU stay (subcutaneous vs. infusion: 19.1 ± 5.9 vs. 16.3 ± 6.1 days, P = 0.14) were not significantly different between the two groups (Table 3, Figures 1 - 3).
Table 3. The Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment Scores at Baseline, Day 2 and Day 7a.
| Baseline | 2 Days | 7 Days | |
|---|---|---|---|
| APACHE II | |||
| Infusion | 24.9 (5.5) | 22.3 (5.7) | 16.0 (5.6) |
| Subcutaneous | 22.4 (4.2) | 19.2 (5.6) | 16.6 (5.6) |
| P value | 0.17 | 0.17 | 0.77 |
| SOFA | |||
| Infusion | 7.0 (1.7) | 7.1 (2.4) | 5.4 (2.0) |
| Subcutaneous | 6.0 (2.2) | 5.3 (3.1) | 5.8 (3.1) |
| P value | 0.15 | 0.13 | 0.7 |
aValues are expressed as mean (SD).
Figure 1. Flow Diagram for Patient Enrollment or Exclusion in the Study.
Figure 3. The Necoutrophil Gelatinase-Associated Lipocalin Level at Baseline, Day 2 and Day 7.
Figure 2. The Plasminogen Activator Inhibitor-1 Level at Baseline, Day 2 and Day 7.
5. Discussion
Our study found that low-dose heparin infusion in sepsis could have desirable effects on the plasma PAI-1 and urinary NGAL levels, while the routine dose of subcutaneous heparin for thromboprophylaxis was unable to show any favorable effect on the PAI-1 or NGAL level. There was no significant difference in levels of these factors at baseline. After 2 days the urinary NGAL level was significantly lower in the infusion group compared to the subcutaneous group but interestingly this difference abated on day 7. The plasma PAI-1 level showed different pattern. Although there was no difference in the PAI-1 level at baseline, gradually the difference between the two groups increased and it became statistically significant on day 7. The ICU mortality and ICU stay were not different between the two groups.
Heparin is commonly used in critically ill patients as an antithrombotic agent. However, recently the anti-inflammatory effect of heparin has been investigated in some studies (7, 11).
Heparin alters production and function of inflammatory mediators, which are released during sepsis (12).
Heparin reduces morality in sepsis, septic shock, and septic disseminated intravascular coagulation (DIC) (13). Earlier studies compared heparin with placebo or usual care. These studies reported the risk ratio of 0.88 for death. This reduction in mortality is believed to be associated with the effect of heparin on coagulation and inflammation pathways (13).
Administration of heparin via subcutaneous route decreases its bioavailability to a great extent compared to intravenous delivery of drug. Heparin binds highly to proteins and surfaces. Also, the administration of vasopressors reduces absorption in subcutaneous route (1, 14). Sepsis causes disruption in microcirculation, which decreases absorption of drugs to a greater extent (14). Due to these barriers patients may need to receive higher doses of heparin, subcutaneously.
Plasma concentration of heparin is reduced notably when low to moderate doses are administered subcutaneously compared to intravenous administration. However, in high doses plasma concentration is comparable in these two routes (15, 16).
Low-dose heparin infusion of 500 units/hour has been studied and confirmed efficacy versus placebo in patients with sepsis (17). This dose was preferred according to the best risk/benefit ratio to produce optimum heparin plasma concentration to activate antithrombin and decrease fibrin production without full anticoagulation and increased risk of bleeding (17). It is recommended to administer 10,000 - 15,000 units heparin per day as thromboprophylaxis in high-risk patients (10). By infusion of 500 units/hour, total daily dose would be equivalent to 12,500 units, which is according to the recommended dose for prophylaxis of theromboembolism.
Endotoxin production in sepsis can cause a rise in the plasma PAI- level (17). The plasminogen activator inhibitor-1 acts as a proinflammatory mediator and up-regulates inflammatory state during sepsis (18). In another study, heparin was unable to demonstrate any significant effect on the PAI-1 level; however, the APACHE II score, ICU stay, incidence of DIC and MODS were decreased much more in the heparin group compared to placebo (19). Heparin is inexpensive and safe in such low-doses and it seems reasonable to use early in sepsis.
Severity of sepsis, which is associated with SOFA score, lactic acid levels and coagulation abnormalities, is correlated with the plasma PAI-1 level (2).
The plasminogen activator inhibitor-1 plays an important role in inhibition of fibrinolysis and generating hypercoagulable state in sepsis, and causes formation of microthrombi in capillaries which results in MODS and increased mortality (18).
In a study of sepsis induced DIC, an elevated plasma PAI-1 level can predict poor prognosis. Patients with plasma PAI-1 levels higher than 90 ng/mL had lower 28-day survival compared to subgroup with lower than 90 ng/mL. Multivariate logistic regression analyses in this study confirmed that the PAI-1 level is an independent risk factor for 28-day mortality (5). In another study, patients with PAI-1 levels greater than 90 ng/mL had higher MODS than those with the PAI-1 level below 30 ng/mL (5). Shapiro et al. proved that the more sever sepsis, the higher plasma PAI-1 level (20).
In our study, the plasma PAI-1 level decreased in both study groups; however, it was more rapid in the infusion group. The difference between the two groups increased gradually and it became significant at day 7. This result is consistent with findings of other studies in which the PAI-1 level increased early in sepsis and decreased afterward (5, 18). The sustained level of serum heparin provided by continuous infusion may lead to more prominent decrease in PAI-1 as an inflammatory marker. Considering the link between inflammation and coagulation in sepsis, heparin may affect both of these pathways and alleviate the process.
Acute kidney injury is a consequence of progressive inflammation and coagulation in sepsis. Necoutrophil gelatinase-associated lipocalin is a biomarker synthesized by epithelial cells during inflammation. It is removed from the blood by glomerular filtration but reabsorbed completely in the proximal tubules by megalin-mediated endocytosis. The presence of NGAL in urine is an evidence for renal tubular injury and has been used as an early marker of AKI (6). However, some studies argued that urine NGAL could increase during sepsis without presence of AKI, as a result of saturation of megalin receptors in tubules. Even so, this increase is much higher in AKI patients (21).
Severity of sepsis associates with the plasma and urinary NGAL levels. Higher levels of NGAL have been reported in more sever sepsis (22).
In this study, the urinary NGAL level increased slightly in the SC group at day 2 and it decreased again to the level lower than baseline at day 7. In the infusion group, it decreased rapidly in first 48 hours of sepsis diagnosis to the level lower than even day 7 in the SC group. This rapid reduction could be related to heparin effect on coagulation pathway and decrease in production of microthrombi during sepsis. There is a higher rate of organ damage during the early phase of sepsis; therefore, the outcome of any intervention may be apparent in the early days. An increased urinary NGAL level in the SC group on day 2 could be related to poor perfusion of subcutaneous tissue and delayed absorption of drugs via this route of administration, especially in hemodynamically unstable patients. The SC injection may not be an acceptable way of drug delivery in these patients. Serum creatinine and urinary output were not different in the two groups during the study. It is in accordance with the finding of other studies in which the urinary NGAL is more sensitive than serum creatinine in diagnosis of kidney failure (21).
There are some limitations in the present study. First, this study was a prospective randomized controlled trial conducted in a single center; so, the findings in such studies could not be easily generalized to other centers. Second, the small number of patients enrolled in the study brings a need for larger validation studies to confirm our findings. Third, we did not exclude the majority of patients who were receiving steroids which may affect on proinflammatory mediators such as PAI-1.
Heparin infusion were associated with rapidly decrease of the PAI-1 and NGAL levels early in sepsis. Low-dose heparin infusion can be an inexpensive and relatively safe way in treatment of sepsis and may reduce DIC and organ damage in these patients.
Acknowledgments
We would like to express our gratitude to all nurses in Sina hospital critical care unit.
Footnotes
Authors’ Contribution:Study concept and design: Mojtaba Mojtahedzadeh, Arezoo Ahmadi; analysis and interpretation of data: Masoumeh Nouri, Elchin Barzegar; drafting of the manuscript: Masoumeh Nouri, Elchin Barzegar; critical revision of the manuscript for important intellectual content: Etezadi; statistical analysis: Masoumeh Nouri, Elchin Barzegar.
Funding/Support:This study was supported by Tehran University of Medical Sciences and Health Services grant NO. 93-01-33-25245.
References
- 1.Cornet AD, Smit EG, Beishuizen A, Groeneveld AB. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007;98(3):579–86. [PubMed] [Google Scholar]
- 2.Lorente L, Martin MM, Borreguero-Leon JM, Sole-Violan J, Ferreres J, Labarta L, et al. Sustained high plasma plasminogen activator inhibitor-1 levels are associated with severity and mortality in septic patients. Thromb Res. 2014;134(1):182–6. doi: 10.1016/j.thromres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Peres Wingeyer SD, Cunto ER, Nogueras CM, San Juan JA, Gomez N, de Larranaga GF. Biomarkers in sepsis at time zero: intensive care unit scores, plasma measurements and polymorphisms in Argentina. J Infect Dev Ctries. 2012;6(7):555–62. doi: 10.3855/jidc.2108. [DOI] [PubMed] [Google Scholar]
- 4.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129(3):290–5. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Madoiwa S, Nunomiya S, Ono T, Shintani Y, Ohmori T, Mimuro J, et al. Plasminogen activator inhibitor 1 promotes a poor prognosis in sepsis-induced disseminated intravascular coagulation. Int J Hematol. 2006;84(5):398–405. doi: 10.1532/IJH97.05190. [DOI] [PubMed] [Google Scholar]
- 6.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22(6):999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 7.Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 2009;102(5):823–8. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig RJ. Therapeutic use of heparin beyond anticoagulation. Curr Drug Discov Technol. 2009;6(4):281–9. doi: 10.2174/157016309789869001. [DOI] [PubMed] [Google Scholar]
- 9.Zarychanski R, Doucette S, Fergusson D, Roberts D, Houston DS, Sharma S, et al. Early intravenous unfractionated heparin and mortality in septic shock. Crit Care Med. 2008;36(11):2973–9. doi: 10.1097/CCM.0b013e31818b8c6b. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 11.Anastase-Ravion S, Blondin C, Cholley B, Haeffner-Cavaillon N, Castellot JJ, Letourneur D. Heparin inhibits lipopolysaccharide (LPS) binding to leukocytes and LPS-induced cytokine production. J Biomed Mater Res A. 2003;66(2):376–84. doi: 10.1002/jbm.a.10604. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Chi C, Guo L, Wang X, Guo L, Sun J, et al. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: a systematic review and meta-analysis. Crit Care. 2014;18(5):563. doi: 10.1186/s13054-014-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarychanski R, Abou-Setta AM, Kanji S, Turgeon AF, Kumar A, Houston DS, et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43(3):511–8. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 14.Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh J, Anand SS, Halperin JL, Fuster V, American Heart A. Guide to anticoagulant therapy: Heparin : a statement for healthcare professionals from the American Heart Association. Circulation. 2001;103(24):2994–3018. doi: 10.1161/01.cir.103.24.2994. [DOI] [PubMed] [Google Scholar]
- 16.Garcia DA, Baglin TP, Weitz JI, Samama MM, American College of Chest P. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S–43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaimes F, De la Rosa G, Arango C, Fortich F, Morales C, Aguirre D, et al. A randomized clinical trial of unfractioned heparin for treatment of sepsis (the HETRASE study): design and rationale [ NCT00100308]. Trials. 2006;7:19. doi: 10.1186/1745-6215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014;18(1):R13. doi: 10.1186/cc13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F, et al. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: A prospective clinical study. Exp Ther Med. 2014;7(3):604–8. doi: 10.3892/etm.2013.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro NI, Schuetz P, Yano K, Sorasaki M, Parikh SM, Jones AE, et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care. 2010;14(5):R182. doi: 10.1186/cc9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydogdu M, Gursel G, Sancak B, Yeni S, Sari G, Tasyurek S, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013;34(4):237–46. doi: 10.3233/DMA-130966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanmassenhove J, Glorieux G, Lameire N, Hoste E, Dhondt A, Vanholder R, et al. Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol. 2015;16:18. doi: 10.1186/s12882-015-0003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]