Abstract
Background
Infections are common following stroke and associated with worse outcome. Using an animal model of pneumonia, we assessed the effect of infection and its treatment on the immune response and stroke outcome.
Methods
Lewis rats were subjected to transient cerebral ischemia and survived for 4 weeks. One day after stroke animals were exposed to aerosolized Staphylococcus aureus, Pseudomonas aeruginosa or saline. Antibiotics (ceftiofur or enrofloxacin) were started immediately after exposure or delayed for 3 days. Behavioral tests were performed weekly. ELISPOT assays were done on lymphocytes from spleen and brain to assess autoimmune responses to myelin basic protein (MBP).
Results
Among animals that received immediate antibiotic therapy, infection was associated with worse outcome in ceftiofur but not enrofloxacin treated animals. (The outcome with immediate enrofloxacin therapy was so impaired that further worsening may have been difficult to detect.) A delay in antibiotic therapy was associated with better outcomes in both ceftiofur and enrofloxacin treated animals. Infection was associated with an increased likelihood of developing Th1(+) responses to MBP in non-infarcted brain (OR=2.94 [1.07, 8.12]; P=0.04), and Th1(+) responses to MBP in spleen and non-infarcted brain were independently associated with a decreased likelihood of stroke recovery (OR=0.16 [0.05, 0.51; P=0.002 and OR=0.32 [0.12, 0.84]; P=0.02, respectively).
Conclusions
Infection worsens stroke outcome in ceftiofur treated animals and increases Th1 responses to MBP. These data may help explain how infection worsens stroke outcome and suggest that treatment of infection may contribute to this outcome.
Keywords: pneumonia, antibiotics, lymphocytes, Th1, Th17, stroke, outcome
Graphical abstract
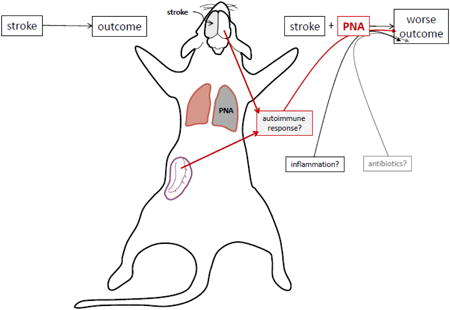
1.1 Introduction
Patients who become infected in the immediate post-stroke period have increased morbidity and mortality in comparison to patients who remain infection free (Westendorp et al., 2011). We previously showed that exposure to lipopolysaccharide (LPS) during experimental stroke increases Th1 immune responses to myelin basic protein (MBP) and worsens outcome (Becker et al., 2005). In an observational study, individuals with post-stroke pneumonia were at similar risk for developing Th1 responses to MBP and worse clinical outcome (Becker et al., 2011). Whether early antibiotic therapy might prevent Th1 responses and improve outcome is unknown, and trials of prophylactic antibiotics to prevent post-stroke infection have had mixed results (Chamorro et al., 2005; Harms et al., 2008; Kalra et al., 2015; Schwarz et al., 2008; Westendorp et al., 2015). In uninfected animals, treatment with enrofloxacin (a fluoroquinolone antibiotic), but not ceftiofur (a β-lactam antibiotic), leads to worse outcome after stroke (Zierath et al., 2015a). In this study we examined the effects of pneumonia on the immune response to MBP and stroke outcome. Staphylococcus aureus and Pseudomonas aeruginosa are common respiratory pathogens after stroke and chosen here as prototypes of Gram-positive and Gram-negative infections (Hassan et al., 2006; Hilker et al., 2003; Tanzi et al., 2011; Walter et al., 2007; Yan et al., 2015). Broad spectrum antibiotics used for empiric treatment of infection include β-lactams and fluoroquinolones; we chose ceftiofur and enrofloxacin as representatives of each class. The effects of pathogen, antibiotic and antibiotic timing on the immune response and stroke outcome were explored.
1.2 Materials and Methods
Animals
Male Lewis rats (275-325 grams) were purchased from Taconic Farms. All experiments were approved by the University of Washington Institutional Animal Care and Use Committee.
Middle Cerebral Artery Occlusion (MCAO)
Anesthesia was induced with 5% and maintained with 1.5% isoflurane. After midline neck incision, the right common carotid, internal carotid and pterygopalantine arteries were ligated. A monofilament suture (Doccol©; 4.0) was inserted into the common carotid artery and advanced into the internal carotid artery to block the origin of the middle cerebral artery (MCA). Animals were maintained at normothermia during surgery and reperfused 2 hours after MCA occlusion (MCAO). Rectal temperature and body weight were assessed at set times. Animals were sacrificed 4 weeks after surgery.
Pneumonia Induction
Twenty-four hours after MCAO, animals were exposed to aerosolized Staphylococcus aureus (Newman strain), Pseudomonas aeruginosa (PAK strain), or saline in a whole animal exposure chamber with a computer interface to control pressures and flows (Biaera Technologies, Hagerstown, MD). Bacteria were prepared as described (Skerrett et al., 1999) and suspended in PBS at 4 × 1011 CFU/ml (S. aureus) or 3 × 1010 CFU/ml (P. aeruginosa). Bacterial suspensions were aerosolized using a Mini-Heart Hi-Flo nebulizer driven at 44 psi, with airflow through the chamber maintained at 19.5 L/min during the 10 minute exposure. Pilot studies demonstrated that these conditions resulted in bacterial depositions of approximately 5×107 CFU/lung (S. aureus) and 2×106 CFU/lung (P. aeruginosa) as determined by culture of homogenized lung tissue harvested immediately after exposure. Infected rats developed transient hypothermia and neutrophilic lung inflammation.
Antibiotic Administration
Antibiotics were started immediately after exposure to bacteria (or saline) or delayed for 3 days and dosed according to protocol. Ceftiofur was given subcutaneously daily (10 mg/kg) for 7 days and enrofloxacin was given subcutaneously in 2 doses 3 days apart (20 mg/kg per dose).
Behavioral Outcomes
Animals were trained on the rotarod prior to MCAO and performance assessed weekly thereafter (Kunze et al., 2014). Only animals with a neurological score ≥3 at 24 hours after MCAO were randomized to infection/antibiotic therapy (Bederson et al., 1986). The experimental protocol is detailed in Figure 1. Behavioral testing was done by an investigator masked to treatment status.
Figure 1.
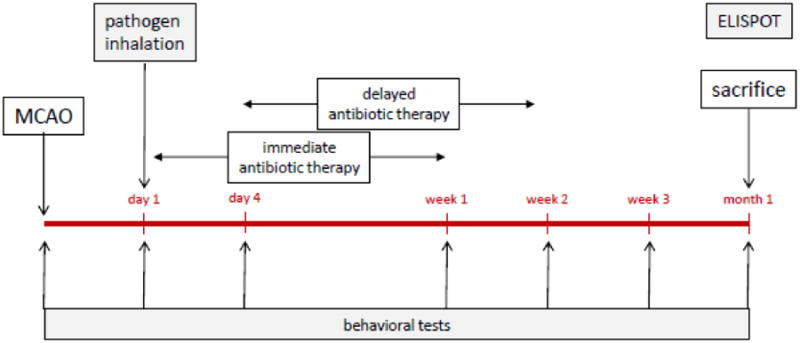
Experimental protocol.
ELISPOT Assays
At the time of sacrifice, lymphocytes were isolated from the brain and spleen (Becker et al., 2005; Zierath et al., 2015b). ELISPOT assays were used to detect MBP and ovalbumin (OVA) specific secretion of interferon (IFN)-γ, interleukin (IL)-17 and transforming growth factor (TGF)-β1. Rat MBP was manufactured by NeoBioSci™. OVA was purchased from Sigma. Antigens were used at a concentration of 50 μg/mL; responses were assessed in triplicate.
Lymphocytes (1×105 cells/well) were cultured in media alone or in media supplemented with antigen for 48 hours in 96 well plates (Multiscreen®-IP, Millipore). Plates were developed using standard protocols (R & D Systems). Spots were counted with the aid of a semi-automated system (AID iSPOT®) and expressed as the ratio of the relative increase in antigen-specific IFN-γ secreting cells to that of TGF-β1 secreting cells (Th1 response) or as the ratio of the relative increase in antigen-specific IL-17 secreting cells to that of TGF-β1 secreting cells (Th17 response). For the purposes of this study, animals were considered to be Th1 (+) or Th17(+) if the Th1 or Th17 response to the antigen (MBP or OVA) was greater than the 75th percentile of uninfected animals treated with the same antibiotic. Analyses of ELISPOT plates was done by an investigator masked to treatment status.
Statistics
Parametric data are displayed as mean ± standard deviation (sd) and compared using the t-test. Non-parametric data are displayed as median (interquartile range [IQR]) and compared using the Mann-Whitney U test or Kruskall-Wallis H test. Categorical data are compared using the likelihood ratio. Multivariate logistic regression was used to determine the effect of infection (pathogen), antibiotic therapy and the immune response on return to baseline rotarod performance at 1 month. Significance was set at P<0.05.
1.3 Results
Effect of Infection and Antibiotics on Outcome
Mortality was 4/150 (3%) and did not differ by treatment group. Infection was associated with relative hypothermia from days 2-6 after pathogen exposure, but change in body weight did not differ between infected and uninfected animals (Figure 2). Since we previously showed that antibiotics affected outcome in uninfected animals (Zierath et al., 2015a), these data are stratified by antibiotic. In ceftiofur treated animals, infection was associated with worse performance on the rotarod when antibiotics were given concomitant with infection but not when antibiotics were delayed for 3 days after infection (Figure 3a). In enrofloxacin treated animals, infection did not worsen rotarod performance when the antibiotics were given concomitant with infection, but delay of antibiotic administration for 3 days was associated with better outcomes (Figure 3b). In ceftiofur treated animals, a delay in antibiotic initiation was consistently associated with better outcomes in S. aureus infected animals (c). For enrofloxacin treated animals (d), a delay in antibiotic initiation was associated with better outcomes in P. aeruginosa infected animals, and for animals that received immediate enrofloxacin therapy, P. aeruginosa infection was associated with worse outcomes than S. aureus infection.
Figure 2.
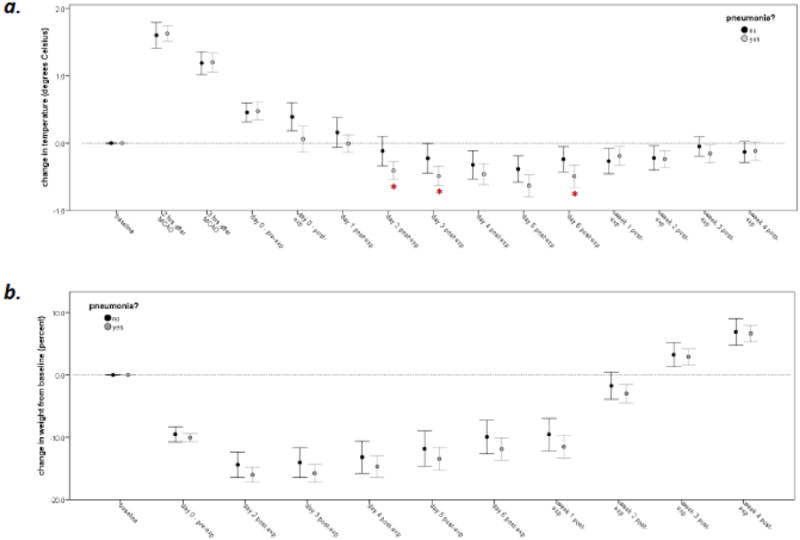
Changes in temperature (a) and body weight (b) in infected (n=100) and uninfected (n=46) animals. Data represent mean (SEM); statistics are by t-test; *P<0.05.
Figure 3.

Performance on the rotarod (as a percentage of the pre-stroke baseline) is depicted on the Y-axis and data presented as the median (IQR). Infection worsened outcomes in animals treated with ceftiofur, but only when the antibiotic was given concomitant with infection (a). Infection did not worsen outcomes in enrofloxacin treated animals, and delay of enrofloxacin was associated with better outcomes (b). Among ceftiofur treated animals, a delay in antibiotics was associated with a better outcome in Staphylococcus aureus (SA) infected animals (c). For enrofloxacin treated animals, a delay in antibiotics was associated with a better outcome in Pseudomonas aeruginosa (PA) infected animals (d), PA infection was generally associated with worse outcomes than SA infections (d). Statistics are by Kruskal-Wallis H test (a and b) and differences among the three groups noted by *(P<0.05) or **(P<0.01). Pathogen related differences for either immediate or delayed antibiotics are assessed by Mann-Whitney U-test (c and d) and differences indicated by †(P<0.05) or ‡ (P<0.01). For a given pathogen, differences based on antibiotic timing are assessed by Mann-Whitney U-test and indicated by *(P<0.05) or **(P<0.01).
The Immune Response and Outcome
Delayed antibiotic therapy was associated with decreased cellularity in the spleen in both ceftiofur and enrofloxacin treated animals, as well as increased lymphocyte infiltration into the non-ischemic hemisphere of ceftiofur treated animals and into the ischemic hemisphere of enrofloxacin treated animals (Table 1). Among ceftiofur treated animals, infection did not increase the proportion of animals that developed Th1(+) or Th17(+) responses (Table 2). In fact, infection was associated with fewer animals developing Th17(+) responses to MBP in the non-infarcted hemisphere of the brain. In enrofloxacin treated animals, on the other hand, infection with P. aeruginosa led to an increase in the proportion of animals developing Th1(+) responses to MBP in the infarcted hemisphere of the brain while both pathogens increased the proportion of animals developing Th1(+) responses to MBP in the non-infarcted hemisphere. The timing of antibiotic therapy did not have a significant effect on the pathogen related immune responses (data not shown). Controlling for the antibiotic, infection increased the proportion of animals developing a Th1(+) response to MBP in the non-infarcted hemisphere (OR=2.94 [1.07, 8.12]; P=0.04), but not in the infarcted hemisphere or spleen. Infection did not increase the proportion of animals developing a Th17(+) response to MBP in any of the immune compartments.
Table 1.
Cell counts in the spleen and brain 4 weeks after MCAO/infection.
| antibiotic: | ceftiofur | enrofloxacin | ||||||
|---|---|---|---|---|---|---|---|---|
| infection: | INF(-) | INF(+) | INF(+) | INF(-) | INF(+) | INF(+) | ||
| Timing of antibiotics: | Immediate N=22 | Immediate N=35 | Delayed N=23 | P | Immediate N=25 | Immediate N=22 | Delayed N=20 | P |
| Spleen (× 108) | 4.33±1.69 | 4.60±2.05 | 2.80±1.50 | 0.001 | 5.02±1.52 | 4.18±1.43 | 3.34±0.90 | <0.001 |
| Infarcted hemisphere (× 106) | 6.35±2.65 | 6.86±2.52 | 7.88±2.39 | NS | 6.57±2.65 | 5.65±2.48 | 7.79±2.48 | 0.03 |
| Non-infarcted hemisphere (× 106) | 5.25±2.09 | 6.24±2.95 | 7.47±2.28 | 0.04 | 6.79±2.82 | 6.54±3.30 | 7.18±1.70 | NS |
MCAO=middle cerebral artery occlusion, INF(-)=no infection, INF(+)=infected, NS=P≥0.10
Table 2.
Proportion of animals with Th1(+) or Th17(+) responses to MBP or OVA (response >75th percentile seen in non-infected animals treated with the same antibiotic).
| ceftiofur | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| infection: | spleen | infarcted brain | non-infarcted brain | |||||||||
| no | SA | PA | P | no | SA | PA | P | no | SA | PA | P | |
| Th1 MBP | 5/22 (23%) | 6/25 (24%) | 10/33 (30%) | NS | 3/13 (23%) | 6/25 (24%) | 8/33 (24%) | NS | 3/13 (23%) | 7/25 (28%) | 9/33 (27%) | NS |
| Th1 OVA | 5/22 (23%) | 8/25 (32%) | 11/33 (33%) | NS | 3/13 (23%) | 10/24 (42%) | 12/33 (36%) | NS | 3/13 (23%) | 3/25 (12%) | 4/33 (12%) | NS |
| Th17 MBP | 5/22 (23%) | 6/25 (24%) | 5/33 (15%) | NS | 3/13 (23%) | 3/25 (12%) | 2/33 (6%) | NS | 3/13 (23%) | 0/25 | 0/33 | 0.004 |
| Th17 OVA | 5/22 (23%) | 2/25 (8%) | 8/33 (24%) | NS | 3/13 (23%) | 3/23 (13%) | 1/33 (3%) | NS | 3/13 (23%) | 4/25 (16%) | 5/33 (15%) | NS |
| enrofloxacin | ||||||||||||
| infection: | spleen | infarcted brain | non-infarcted brain | |||||||||
| no | SA | PA | P | no | SA | PA | P | no | SA | PA | P | |
| Th1 MBP | 6/25 (24%) | 3/20 (15%) | 1/22 (4%) | NS | 6/20 (30%) | 3/20 (15%) | 13/22 (59%) | 0.008 | 5/19 (16%) | 11/20 (55%) | 14/22 (64%) | 0.04 |
| Th1 OVA | 5/24 (21%) | 5/20 (25%) | 4/22 (18%) | NS | 4/18 (22%) | 5/15 (33%) | 8/22 (36%) | NS | 4/17 (24%) | 9/17 (53%) | 13/22 (59%) | 0.06 |
| Th17 MBP | 6/25 (24%) | 2/20 (10%) | 1/22 (4%) | NS | 5/20 (25%) | 5/20 (25%) | 10/22 (45%) | NS | 5/19 (26%) | 4/20 (20%) | 7/22 (32%) | NS |
| Th17 OVA | 5/25 (20%) | 4/20 (20%) | 4/22 (18%) | NS | 4/18 (22%) | 3/15 (20%) | 10/22 (45%) | NS | 4/17 (24%) | 4/16 (25%) | 6/22 (27%) | NS |
MBP=myelin basic protein, OVA=ovalbumin, SA=Staphylococcus aureus, PA=Pseudomonas aeruginosa, NS=not significant (P≥0.10)
Ceftiofur treated animals that developed Th1(+) responses to MBP in spleen or non-infarcted brain were less likely to return to baseline performance on the rotarod (Table 3). Among enrofloxacin treated animals, Th17(+) responses to MBP in the non-infarcted brain were associated with worse performance on the rotarod. Multivariate analyses were done to assess the independent effect of the immune response to MBP on outcome (ie. return to baseline rotarod performance), controlling for the antibiotic, timing of antibiotic therapy, infection/pathogen and non-specific responses to OVA (Table 4). A Th1(+) response to MBP in spleen was independently associated with greater than an 80% decrease in the odds of returning to baseline rotarod performance while a Th1(+) response to MBP in the non-infarcted hemisphere of the brain was independently associated with a 70% decrease in the odds of returning to baseline rotarod performance. Th17(+) responses to MBP were not independently associated with outcome at 1 month, but enrofloxacin (as opposed to ceftiofur) was uniformly associated with worse outcomes and a delay in antibiotic therapy was associated with better outcomes.
Table 3.
Return to baseline rotarod performance among animals with Th1(+) or Th17(+) immune response to MBP or OVA (response >75th percentile of that seen in non-infected animals treated with the same antibiotic).
| ceftiofur treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| return to baseline rotarod performance: | spleen | infarcted brain | non-infarcted brain | ||||||
| no | yes | P | no | yes | P | no | yes | P | |
| Th1 MBP | 14/32 (44%) | 7/48 (15%) | 0.004 | 9/31 (29%) | 8/40 (20%) | NS | 13/31 (42%) | 6/40 (15%) | 0.01 |
| Th1 OVA | 12/32 (38%) | 12/48 (26%) | NS | 9/30 (30%) | 16/40 (40%) | NS | 5/31 (16%) | 5/40 (12%) | NS |
| Th17 MBP | 8/32 (25%) | 8/48 (17%) | NS | 4/31 (13%) | 4/40 (10%) | NS | 1/31 (3%) | 2/40 (5%) | NS |
| Th17 OVA | 8/32 (25%) | 7/48 (15%) | NS | 3/30 (10%) | 4/39 (10%) | NS | 8/31 (26%) | 4/40 (10%) | 0.08 |
| enrofloxacin treated | |||||||||
| return to baseline rotarod performance: | spleen | infarcted brain | non-infarcted brain | ||||||
| no | yes | P | no | yes | P | no | yes | P | |
| Th1 MBP | 8/40 (20%) | 2/27 (7%) | NS | 14/40 (35%) | 8/22 (36%) | NS | 19/39 (49%) | 11/22 (50%) | NS |
| Th1 OVA | 9/40 (22%) | 5/26 (19%) | NS | 10/36 (28%) | 7/19 (37%) | NS | 14/36 (39%) | 12/20 (60%) | NS |
| Th17 MBP | 5/40 (12%) | 4/27 (15%) | NS | 13/40 (32%) | 7/22 (32%) | NS | 14/39 (36%) | 2/22 (9%) | 0.02 |
| Th17 OVA | 6/40 (15%) | 7/27 (26%) | NS | 9/36 (25%) | 8/19 (42%) | NS | 11/35 (31%) | 3/20 (15%) | NS |
MBP=myelin basic protein, OVA=ovalbumin, NS=not significant (P≥0.10)
Table 4.
Predictors of return to baseline rotarod performance based on antibiotic (and timing), infection and Th1(+) or Th17(+) immune responses in each organ. Data are presented as the odds ratio (OR) and 95% confidence interval (CI).
| spleen | infarcted brain | non-infarcted brain | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Th1 MBP(+) | 0.16 (0.05, 0.51) | 0.002 | 0.57 (0.22, 1.43) | NS | 0.32 (0.12, 0.84) | 0.02 |
| Th1 OVA(+) | 1.56 (0.58, 4.24) | NS | 2.76 (1.08, 7.07) | 0.03 | 2.42 (0.86, 6.79) | 0.09 |
| enrofloxacin | 0.30 (0.14, 0.64) | 0.002 | 0.34 (0.14, 0.79) | 0.01 | 0.34 (0.14, 0.83) | 0.02 |
| antibiotic delay | 5.41 (2.11, 13.87) | <0.001 | 6.66 (2.34, 19.00) | <0.001 | 4.94 (1.91, 12.79) | 0.001 |
| S. aureus | 0.38 (0.13, 1.10) | 0.07 | 0.65 (0.18, 2.32) | NS | 1.04 (0.32, 3.46) | NS |
| P. aeruginosa | 0.59 (0.23, 1.50) | NS | 1.28 (0.43, 3.81) | NS | 1.67 (0.56, 4.94) | NS |
| spleen | infarcted brain | non-infarcted brain | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Th17 MBP(+) | 0.98 (0.39, 2.46) | NS | 0.68 (0.24, 1.91) | NS | 0.47 (0.13, 1.72) | NS |
| Th17 OVA(+) | 0.69 (0.31, 1.55) | NS | 2.34 (0.97, 5.64) | 0.06 | 1.48 (0.60, 3.69) | NS |
| enrofloxacin | 0.36 (0.18, 0.75) | 0.006 | 0.33 (0.14, 0.79) | 0.01 | 0.37 (0.15, 0.91) | 0.03 |
| antibiotic delay | 4.40 (1.79, 10.81) | 0.001 | 7.02 (2.45, 20.11) | <0.001 | 4.60 (1.82, 11.63) | 0.001 |
| S. aureus | 0.48 (0.18, 1.29) | NS | 0.62 (0.17, 2.25) | NS | 0.80 (0.25, 2.60) | NS |
| P. aeruginosa | 0.70 (0.29, 1.70) | NS | 1.16 (0.40, 3.43) | NS | 1.27 (0.44, 3.69) | NS |
MBP=myelin basic protein, OVA=ovalbumin, NS=not significant (P≥0.10)
1.4 Discussion
Abundant data show that post-stroke infection, especially pneumonia, is associated with worse outcome (Westendorp et al., 2011; Westendorp et al., 2015). Given that a systemic inflammatory insult (ie. injection of LPS) at the time of stroke increases the likelihood of developing detrimental Th1 responses to brain antigens (specifically, MBP)(Becker et al., 2005), we hypothesized that post-stroke infection leads to Th1 immune responses to MBP, and that these immune responses contribute to the worse outcome. An observational study confirmed that patients with post-stroke infection, especially pneumonia, were more likely to develop Th1 responses to MBP and experience poor outcome 3 months after stroke (Becker et al., 2011). Post-stroke pneumonia may be caused by a variety of pathogens, and Gram-positive organisms like S. aureus and Gram-negative organisms like P. aeruginosa are well represented in observational studies (Hassan et al., 2006; Hilker et al., 2003; Tanzi et al., 2011; Walter et al., 2007). We aimed to recreate the clinical scenario of post-stroke pneumonia in a rodent model, and to explore the interactions between Gram-positive and Gram-negative pathogens and different classes of antibiotics on the development of immune responses to MBP and stroke outcome. Additionally, we evaluated the effect of timing of antibiotic delivery on outcome, with immediate administration following pathogen exposure mirroring the strategy of prophylactic antibiotics (ie. many patients will have aspirated prior to presentation with stroke) and delayed administration reflecting the usual clinical practice of initiating antibiotics only after infection is suspected, which is often several days after the inciting event (ie. aspiration).
The antibiotics for this study were chosen to represent the most commonly used classes in clinical practice – cephalosporins/β-lactams (ceftiofur) and fluoroquinolones (enrofloxacin). Both antibiotics have broad spectrum activity and are appropriate for treating animals infected with S. aureus or P. aeruginosa (Lopez-Cadenas et al., 2013; Yancey et al., 1987). Because the first randomized controlled trial of antibiotic prophylaxis showed harm associated with levofloxacin when started within 24 hours of stroke onset (Chamorro et al., 2005), we hypothesized that fluoroquinolones may have direct neurotoxic effects on the brain. And in a study that evaluated the effect of ceftiofur and enrofloxacin treatment in uninfected animals subjected to MCAO, we showed that enrofloxacin treatment was associated with worse outcomes than control and ceftiofur treatment (Zierath et al., 2015a). For the current study, we again assumed that enrofloxacin would negatively impact outcome in infected animals. We also assumed that a delay in antibiotic therapy would be associated with worse infections, and thus worse outcomes.
Infection leads to inflammation through activation of the innate immune system. Pathogen associated molecular patterns (PAMPs), which are highly conserved molecular motifs in bacteria (and other pathogens), activate the innate immune response through toll-like receptors (TLRs). LPS is a component of the Gram-negative bacterial cell wall and is released during infection with Gram-negative organisms. Lipoteichoic acid (LTA) is a component of the Gram-positive bacterial cell wall and released during Gram-positive infections. We previously showed that systemic administration of LPS, but not LTA, at the time of stroke increased Th1(+) responses to brain antigens (Becker et al., 2005; Zierath et al., 2010). In the current study we found that infection led to worse outcomes in animals treated immediately with ceftiofur, but not enrofloxacin. While there are several potential explanations for this disparity, it seems most likely that immediate administration of enrofloxacin worsened outcome sufficiently in uninfected animals such that it was difficult to detect further worsening associated with infection.
Perhaps most surprising was the observation that a delay in antibiotic administration was associated with better outcomes. Administration of enrofloxacin to uninfected animals was associated with a worse outcome, likely because of a direct negative impact from the drug (Zierath et al., 2015a). For enrofloxacin, the benefit in delaying antibiotic therapy could thus be related to delaying the delivery of a putative neurotoxin to an acutely vulnerable brain (ie. the antibiotic/neurotoxin is more detrimental when given 1 day after stroke as opposed to 4 days after stroke). For ceftiofur, where treatment in uninfected animals was not associated with any untoward effects, it could be that the interaction of the antibiotic with bacterial pathogens led to a Jarisch-Herxheimer like reaction, with an exaggerated inflammatory response that was detrimental to the brain.(Hurley, 1995) Cephalosporin therapy can lead to rapid cell lysis (Shahid et al., 2009). Release of LTA and peptidoglycan (PGN) from Gram-positive bacteria (Lotz et al., 2006; van Langevelde et al., 1998) activates the innate immune system, primarily through TLR2 (Zeytun et al., 2007), and release of LPS from Gram-negative bacteria activates the innate immune system through TLR4 (Trautmann et al., 1999; Zeytun et al., 2007). The bactericidal effects of ceftiofur may have led to release of LTA, PGN (from S. aureus) as well as LPS (from P. aeruginosa), increasing the systemic inflammatory response, and potentially worsening outcome. Because many rodents are able to clear bacteria from their lungs effectively without antibiotic therapy (Johansen et al., 1994; Olszewski et al., 2007), delaying the initiation of therapy may have allowed the infections to be partially controlled by the animals' innate immune system with less bacterial cell lysis/inflammation occurring after initiation of antibiotics. The amount of LTA/LPS needed to elicit a response differs significantly between rodents and humans, with the immune system of rodents favoring “tolerance” to immunologic threats and the immune system of humans favoring “resistance” to such threats (Warren et al., 2010; Zschaler et al., 2014). For instance, intravenous administration of endotoxin at doses of 15 μg/kg leads to a severe systemic response with shock in humans (Sauter and Wolfensberger, 1980; Taveira da Silva et al., 1993), while the median lethal dose of endotoxin/LPS in mice is approximately 10-12 mg/kg (Glode et al., 1976; Rose and Bradley, 1971). Further, it takes about 200× more endotoxin/LPS in mice to elicit systemic levels of IL-6 similar to that seen in humans (Copeland et al., 2005). The contribution of antibiotic induced bacterial death to post-stroke inflammation requires further study.
Because infection causes worse outcome, strategies to prevent post-stroke infection may be beneficial. To date, however, studies of prophylactic antibiotics to prevent infection (and thus improve outcome) after stroke have not shown definitive benefit (Chamorro et al., 2005; Harms et al., 2008; Kalra et al., 2015; Schwarz et al., 2008; Westendorp et al., 2015). Our study shows that very early antibiotic therapy is not effective in improving outcome in rodents, and in fact, is detrimental. Differences in the immune systems of rodents and humans aside, our findings argue that early treatment with fluoroquinolone antibiotics after stroke onset, either prophylactically or to treat an established infection, should probably be avoided.
We previously showed that both Th1 and Th17 immune responses to MBP following stroke influenced outcome (Becker et al., 2003; Zierath et al., 2013). In this study we again show that Th1(+) responses to MBP within the spleen and non-infarcted brain were independently associated with worse outcome. The effect of Th17(+) responses on outcome in this pneumonia/antibiotic therapy model, however, were not as robust as those seen previously. In these prior studies we found that LPS administration increased Th1 but not Th17 responses to MBP39, and in this study we found that infection increases Th1+ but not Th17 responses to MBP. Of note, it was the immune response in the spleen and non-infarcted brain that best predicted outcome, suggesting that systemic immune responses to brain occur after stroke and that lymphocytes with antigen specificity are able to gain access to normal brain (ie. the non-infarcted hemisphere) through an intact blood-brain barrier.
Limitations of this study include the fact that the animals did not become severely ill following infection. In pilot studies, as well as in this study, infection led to hypothermia, suggesting that there was a pathogen related effect on the immune response. We hypothesized that delaying antibiotic therapy would worsen infection, but instead we observed no change in infection severity and better stroke outcomes. It remains possible that induction of more severe infections or infection with different pathogens would alter both the clinical and immunological outcomes. Assessment of outcomes in this study was also limited (ie. rotarod performance); future studies should incorporate additional outcome measures, including cognitive outcomes, to determine the effect of infection and its treatment on more than a motor task.
In summary, this study showed that pneumonia was associated with an increase in Th1 (but not Th17) responses to MBP after stroke. Further, these responses were independently associated with worse outcomes, especially when found in the spleen and non-infarcted brain. Enrofloxacin robustly worsened outcome when administered 24 hours after MCAO, such that an additional detrimental effect of infection was difficult to detect; this negative impact, however, was attenuated by delaying antibiotic therapy. These data argue that certain classes of antibiotics (ie. fluoroquinolones) should be avoided in acute phase of brain injury. Additionally, the data show that an immune response to brain antigens is detected in the periphery as well as in the non-infarcted hemisphere of the brain following stroke, suggesting that strategies to prevent the development of an immune response to brain antigens might be of benefit.
Highlights.
Pneumonia is associated with worse stroke outcome in ceftiofur treated animals.
Enrofloxacin is associated with worse stroke outcome in animals with pneumonia.
A delay in antibiotic therapy is associated with better outcomes from stroke.
Infection increases Th1(+) responses to MBP.
Th1(+) response to MBP are independently associated with worse stroke outcome.
Acknowledgments
None.
Sources of Funding: NINDS 5R01NS056457
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, Hadwin J, Carter KT, Shibata D, Cain KC. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Horcajada JP, Obach V, Vargas M, Revilla M, Torres F, Cervera A, Planas AM, Mensa J. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke. 2005;36:1495–1500. doi: 10.1161/01.STR.0000170644.15504.49. [DOI] [PubMed] [Google Scholar]
- Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode LM, Mergenhagen SE, Rosenstreich DL. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976;14:626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms H, Prass K, Meisel C, Klehmet J, Rogge W, Drenckhahn C, Gohler J, Bereswill S, Gobel U, Wernecke KD, Wolf T, Arnold G, Halle E, Volk HD, Dirnagl U, Meisel A. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE. 2008;3:e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, Khealani BA, Shafqat S, Aslam M, Salahuddin N, Syed NA, Baig SM, Wasay M. Stroke-associated pneumonia: microbiological data and outcome. Singapore Med J. 2006;47:204–207. [PubMed] [Google Scholar]
- Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- Hurley JC. Antibiotic-induced release of endotoxin. A therapeutic paradox. Drug Saf. 1995;12:183–195. doi: 10.2165/00002018-199512030-00004. [DOI] [PubMed] [Google Scholar]
- Johansen HK, Cryz SJ, Jr, Hoiby N. Clearance of Pseudomonas aeruginosa from normal rat lungs after immunization with somatic antigens or toxin A. APMIS. 1994;102:545–553. [PubMed] [Google Scholar]
- Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, Patel A, Rebollo-Mesa I. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00126-9. [DOI] [PubMed] [Google Scholar]
- Kunze A, Zierath D, Drogomiretskiy O, Becker K. Variation in behavioral deficits and patterns of recovery after stroke among different rat strains. Transl Stroke Res. 2014;5:569–576. doi: 10.1007/s12975-014-0337-y. [DOI] [PubMed] [Google Scholar]
- Lopez-Cadenas C, Sierra-Vega M, Garcia-Vieitez JJ, Diez-Liebana MJ, Sahagun-Prieto A, Fernandez-Martinez N. Enrofloxacin: pharmacokinetics and metabolism in domestic animal species. Curr Drug Metab. 2013;14:1042–1058. doi: 10.2174/1389200214666131118234935. [DOI] [PubMed] [Google Scholar]
- Lotz S, Starke A, Ziemann C, Morath S, Hartung T, Solbach W, Laskay T. Beta-lactam antibiotic-induced release of lipoteichoic acid from Staphylococcus aureus leads to activation of neutrophil granulocytes. Ann Clin Microbiol Antimicrob. 2006;5:15. doi: 10.1186/1476-0711-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski MA, Falkowski NR, Surana R, Sonstein J, Hartman A, Moore BB, Huffnagle GB, Toews GB. Effect of laparotomy on clearance and cytokine induction in Staphylococcus aureus infected lungs. Am J Respir Crit Care Med. 2007;176:921–929. doi: 10.1164/rccm.200606-763OC. [DOI] [PubMed] [Google Scholar]
- Rose WC, Bradley SG. Enhanced toxicity for mice of combinations of antibiotics with Escherichia coli cells or Salmonella typhosa endotoxin. Infect Immun. 1971;4:550–555. doi: 10.1128/iai.4.5.550-555.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter C, Wolfensberger C. Interferon in human serum after injection of endotoxin. Lancet. 1980;2:852–853. doi: 10.1016/s0140-6736(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Al-Shajlawi F, Sick C, Meairs S, Hennerici MG. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke: the Mannheim infection in stroke study (MISS) Stroke. 2008;39:1220–1227. doi: 10.1161/STROKEAHA.107.499533. [DOI] [PubMed] [Google Scholar]
- Shahid M, Sobia F, Singh A, Malik A, Khan HM, Jonas D, Hawkey PM. Beta-lactams and beta-lactamase-inhibitors in current- or potential-clinical practice: a comprehensive update. Crit Rev Microbiol. 2009;35:81–108. doi: 10.1080/10408410902733979. [DOI] [PubMed] [Google Scholar]
- Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol. 1999;276:L715–727. doi: 10.1152/ajplung.1999.276.5.L715. [DOI] [PubMed] [Google Scholar]
- Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, Shibata D, Hadwin J, Carter K, Becker K. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14:244–252. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveira da Silva AM, Kaulbach HC, Chuidian FS, Lambert DR, Suffredini AF, Danner RL. Brief report: shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N Engl J Med. 1993;328:1457–1460. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- Trautmann M, Heinemann M, Moricke A, Seidelmann M, Lorenz I, Berger D, Steinbach G, Schneider M. Endotoxin release due to ciprofloxacin measured by three different methods. J Chemother. 1999;11:248–254. doi: 10.1179/joc.1999.11.4.248. [DOI] [PubMed] [Google Scholar]
- van Langevelde P, van Dissel JT, Ravensbergen E, Appelmelk BJ, Schrijver IA, Groeneveld PH. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, Kloth A. Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J Neurol. 2007;254:1323–1329. doi: 10.1007/s00415-007-0520-0. [DOI] [PubMed] [Google Scholar]
- Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, Cavaillon JM. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201:223–232. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, Kwa VI, Weisfelt M, Remmers MJ, ten Houten R, Schreuder AH, Vermeer SE, van Dijk EJ, Dippel DW, Dijkgraaf MG, Spanjaard L, Vermeulen M, van der Poll T, Prins JM, Vermeij FH, Roos YB, Kleyweg RP, Kerkhoff H, Brouwer MC, Zwinderman AH, van de Beek D, Nederkoorn PJ. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385:1519–1526. doi: 10.1016/S0140-6736(14)62456-9. [DOI] [PubMed] [Google Scholar]
- Yan L, Qing Y, Xingyi J, Hongbo Q. Etiologic diagnosis and clinical treatment of multiple drug-resistant bacteria infection in elderly patients with stroke-associated pneumonia after neurosurgery. Cell Biochem Biophys. 2015;71:731–734. doi: 10.1007/s12013-014-0256-2. [DOI] [PubMed] [Google Scholar]
- Yancey RJ, Jr, Kinney ML, Roberts BJ, Goodenough KR, Hamel JC, Ford CW. Ceftiofur sodium, a broad-spectrum cephalosporin: evaluation in vitro and in vivo in mice. Am J Vet Res. 1987;48:1050–1053. [PubMed] [Google Scholar]
- Zeytun A, van Velkinburgh JC, Pardington PE, Cary RR, Gupta G. Pathogen-specific innate immune response. Adv Exp Med Biol. 2007;598:342–357. doi: 10.1007/978-0-387-71767-8_24. [DOI] [PubMed] [Google Scholar]
- Zierath D, Kunze A, Fecteau L, Becker K. Effect of antibiotic class on stroke outcome. Stroke. 2015a;46:2287–2292. doi: 10.1161/STROKEAHA.115.008663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath D, Kunze A, Fecteau L, Becker K. Promiscuity of autoimmune responses to MBP after stroke. J Neuroimmunol. 2015b;285:101–105. doi: 10.1016/j.jneuroim.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath D, Schulze J, Kunze A, Drogomiretskiy O, Nhan D, Jaspers B, Dressel A, Becker K. The immunologic profile of adoptively transferred lymphocytes influences stroke outcome of recipients. J Neuroimmunol. 2013;263:28–34. doi: 10.1016/j.jneuroim.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, Becker KJ. CNS immune responses following experimental stroke. Neurocrit Care. 2010;12:274–284. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34:433–454. [PubMed] [Google Scholar]


