Abstract
Background:
Major depressive disorder is characterized as persistent low mood. A chronically stressful life in genetically susceptible individuals is presumably the major etiology that leads to dysfunctions of monoamine and hypothalamus-pituitary-adrenal axis. These pathogenic factors cause neuron atrophy in the limbic system for major depressive disorder. Cell-specific pathophysiology is unclear, so we investigated prelimbic cortical GABAergic neurons and their interaction with glutamatergic neurons in depression-like mice.
Methods:
Mice were treated with chronic unpredictable mild stress for 3 weeks until they expressed depression-like behaviors confirmed by sucrose preference, Y-maze, and forced swimming tests. The structures and functions of GABAergic and glutamatergic units in prelimbic cortices were studied by cell imaging and electrophysiology in chronic unpredictable mild stress-induced depression mice vs controls.
Results:
In depression-like mice, prelimbic cortical GABAergic neurons show incoordination among the subcellular compartments, such as decreased excitability and synaptic outputs as well as increased reception from excitatory inputs. GABAergic synapses on glutamatergic cells demonstrate decreased presynaptic innervation and increased postsynaptic responsiveness.
Conclusions:
Chronic unpredictable mild stress-induced incoordination in prelimbic cortical GABAergic and glutamatergic neurons dysregulates their target neurons, which may be the pathological basis for depressive mood. The rebalance of compatibility among subcellular compartments would be an ideal strategy to treat neural disorders.
Keywords: depression, GABA, glutamate, neuron, prefrontal cortex, stress
Introduction
Major depressive disorder is characterized as anhedonia, low self-esteem, and suicide. Its etiology is thought to be stressful environments plus genetic susceptibility (Camp and Cannon-Albright, 2005; Jabbi et al., 2008; Lohoff, 2010; Keers and Uher, 2012; Hamilton et al., 2013; Klengel and Binder, 2013; Moylan et al., 2013; Wilde et al., 2013). The sustained stress to genetically vulnerable individuals leads to dysfunctions of monoamine, brain-derived neurotrophic factor, and hypothalamus-pituitary-adrenal axis (Elhwuegi, 2004; Brunoni et al., 2008; Rohleder et al., 2010; Strekalova et al., 2011; Berton et al., 2012; Guo and Lu, 2014), which induce neuron atrophy in brain reward circuits such as the prefrontal cortex, amygdala, and hippocampus in depressive patients and stress animals (Bennett et al., 2008; Elizalde et al., 2008; Pittenger and Duman, 2008; C. H. Duman, 2010; Banasr et al., 2011; Lin and Sibille, 2013; Sandi and Haller, 2015). The brain includes the excitatory and inhibitory neurons. Their physiological coordination is critical for neuron encoding to manage well-organized cognitions (Freund, 2003; Buzsaki et al., 2004; Ascoli et al., 2008). Cell-specific pathophysiology in major depressive disorder remains unclear (R. S. Duman and Aghajanian, 2012; Thompson et al., 2015).
In terms of the role of GABAergic neurons in major depressive disorders, immunocytochemistry in postmortem brain tissues from major depression subjects demonstrates the decrease of neuronal density in the prefrontal cortices (Sanacora et al., 2004; Rajkowska et al., 2007; Karolewicz et al., 2010; Maciag et al., 2010; Khundakar et al., 2011). Studies by imaging, biochemistry, and gene analyses from the depression subjects indicate low GABAergic tone in the brain (Oruc et al., 1997; Torrey et al., 2005; Bajbouj et al., 2006; Hettema et al., 2006; Hasler et al., 2007; Price et al., 2009; Levinson et al., 2010; Croarkin et al., 2011; Plante et al., 2012; Veeraiah et al., 2014), despite argument (Godlewska et al., 2015). Therefore, the enhancers of GABAA receptors are used as antidepressants, but there is controversy regarding therapeutic outcome (Petty et al., 1995; Smith et al., 2002; Kendell et al., 2005; Morishita, 2009; Luscher et al., 2011; Mohler, 2012). As for the inconsistences, we hypothesize that there are incompatible changes in the subcompartments of GABAergic neurons and synapses, such as presynaptic GABA release vs postsynaptic GABA receptors and/or the outputs of GABAergic neurons vs their reception from excitatory inputs. We aimed to examine depression-related pathology in the subcellular compartment of GABAergic neurons and their interaction with glutamatergic neurons in the medial prefrontal cortex. The elucidation of these issues provides new ideas for developing antidepressants in the manner of type-specific neurons and their subcellular compartments.
Pathophysiological changes in the prelimbic cortical GABAergic and glutamatergic neurons were examined in the mice expressing depression-like behavior induced by chronic unpredictable mild stress (CUMS). Cortical GABAergic neurons and glutamatergic neurons in the mice were genetically labeled by green fluorescent protein (GFP) and yellow fluorescent protein (YFP), respectively (G. Zhang et al., 2013). With their identification, we were able to study mutual innervation between GABAergic and glutamatergic neurons by confocal cell imaging as well as their spike encoding and synapse dynamics by whole-cell recordings. With these analyses, we expect to reveal cell-specific pathology of major depressive disorders, especially subcellular incoordination.
METHODS AND MATERIALS
All experiments were done in accordance with the guidelines and regulations by the Administration Office of Laboratory Animals at Beijing, China. All experimental protocols were approved by the Institutional Animal Care Unit Committee in Administration Office of Laboratory Animals at Beijing, China (B10831).
The Mouse Model of Major Depressive Disorder Induced by CUMS
To examine neuron-specific pathophysiology associated with major depressive disorders, we applied C57 Thy1-YFP/GAD-GFP mice whose GABAergic neurons and glutamatergic neurons were genetically labeled by GFP and YFP, respectively (G. Zhang et al., 2013). The male mice were used starting at postnatal day 21. In week 1 for their adaptation to the experiments, their body weight, locomotion, sucrose preference, and Y-maze test were measured to collect self-control data. The mice showing consistent values in these measurements were separated into 2 groups, CUMS and control, to reduce the variations among them. The control mice lived without the following stresses.
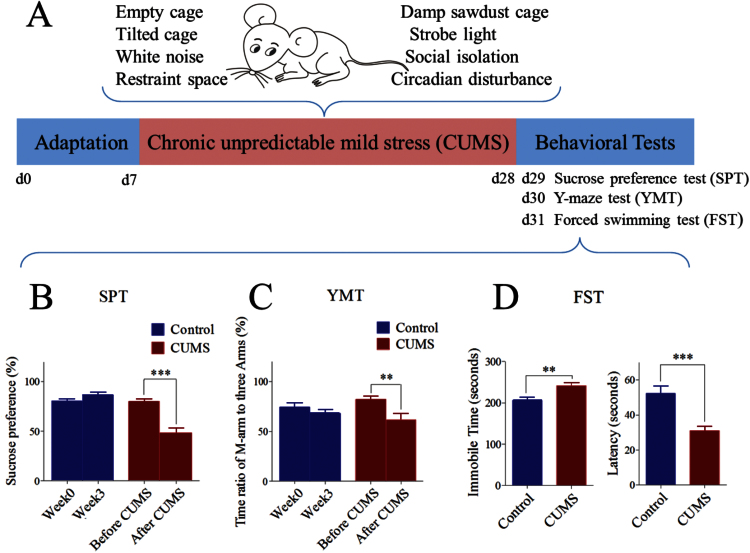
Based on depression risk factors, such as weaknesses in cognitive function, emotional regulation, social interaction skill, circadian and stress response (Southwick and Charney, 2012), we used chronic stress to produce depression-like mice in the following manner. The mice lived in a stressful environment, made efforts to challenge these conditions, and experienced defeat outcomes, which then drove them to feel cognitive and emotional inabilities and in turn to have anhedonia and low self-esteem. The procedures for the CUMS mice include their adaptation, the CUMS, and the behavioral tests (Figure 1A).
Figure 1.
Chronic unpredictable mild stress (CUMS) leads the mice to express depression-like behaviors. (A) The procedures produced depression-like mice including the adaptation for 1 week, the CUMS for 3 weeks, and the behavioral tests in 3 days. (B) The sucrose preference test (SPT) values (%) in the mice from the CUMS (red bar) and control group (blue). (C) The ratios of stay time in M-arm to stay time in 3 arms by the Y-maze test (YMT) in mice from the CUMS (red bar) and control (blue) groups. (D) Immobile time (left) and latency (right) of staying in the water cylinder in the forced swim test (FST) in mice from the CUMS (red bar) and control (blue) groups. **P < .01 and ***P < .001. 1-way ANOVA was used for the comparisons between the CUMS and control, while paired-t test was for the comparisons before and after the CUMS.
The stressful environments included social isolation, tilted cage, empty cage, damp sawdust cage, restraint space, white noise, strobe light, and circadian disturbance (Willner, 2005; Schweizer et al., 2009; Strekalova et al., 2011; Berton et al., 2012; Hill et al., 2012). Except for the social isolation, these conditions were randomly selected to treat the mice in the manner of their separations or combinations every day. These treatments were applied about 1 to 14 hours in duration and at 1- to 12-hour intervals (Table 1). The durations and intervals were unpredictable to the mice. This CUMS was sustained for 3 weeks until some of the mice expressed anhedonia and low self-esteem. We did not use extreme stress in a single pattern, such as electrical shock, social defeat, and tail clamp, since these protocols might induce the outcome similar to posttraumatic stress disorder.
Table 1.
Mild Stresses and Their Applications to Induce Major Depressive Disorder in Mice
| Mild Stimulations | Definition | Durations | Intervals | Intensity | ||||
|---|---|---|---|---|---|---|---|---|
| Social isolation | Living in a cage alone | 3 weeks | ||||||
| Empty cage | Staying in a cage without sawdust | 7–14 hours per time | 1–2 days | |||||
| Tilted cage | Staying in 45°-slanted cage with 50ml water in its low end | 6–12 hours per time | 2–3 days | |||||
| Damp sawdust cage | Staying in wet sawdust-filled cage | 5–12 hours per time | 1–3 days | |||||
| Restraint space | Staying in a body-fitted container | 1–3 hours per time | 1–2 days | |||||
| White noise | Audible hissing sound | 4–6 hours per time | 1–3 days | 70 dB | ||||
| Strobe light | Flashes at 2.5 Hz | 6–14 hours per time | 2–3 days | 500 lux | ||||
| Circadian disturbance | Living with irregular illumination | 3 weeks | ||||||
| weeks Days |
One | Two | Three | Four | Five | Six | Seven | |
| One | SI (0–24), CD SL (21-10) EC (21-10) |
SI (0–24), CD RS (13–14) |
SI (0–24), CD DSC (9–15) RS (15–18) |
SI (0–24), CD WN (11–15) TC (16–22) |
SI (0–24), CD RS (9–11) EC (14–21) SL (23–9) |
SI (0–24), CD DSC (10–16) |
SI (0–24), CD TC (20-14) SPT |
|
| Two | SI (0–24), CD RS (15–16) WN (15–20) |
SI (0–24), CD RS (14–16) TC (20–9) SL (20–9) |
SI (0–24), CD RS (12–14) WN (18–23) |
SI (0–24), CD DSC (10–20) |
SI (0–24), CD RS (9–10) SL (22-10) WN (22–3) |
SI (0–24), CD DSC (9–18) |
SI (0–24), CD EC (21-13) SPT |
|
| Three | SI (0–24), CD WN (13–19) EC (21-11) SL (21-11) |
SI (0–24), CD RS (13–15) |
SI (0–24), CD DSC (13–21) SL (15–21) |
SI (0–24), CD DSC (9–17) WN (20–2) |
SI (0–24), CD RS (10–13) TC (21–9) |
SI (0–24), CD WN (10–16) |
SI (0–24), CD RS (18–20) SPT |
|
| Four for the tests | YMT | FST | ||||||
Abbreviations: CD, circadian disturbance; dB, decibel; DSC, damp sawdust cage; EC, empty cage; FST, forced swim test; RS, restraint space; SI, social isolation; SL, strobe light; SPT, sucrose preference test; TC, tilted cage; WN, white noise; YMT, Y-maze test.
Whether the CUMS-treated mice in 3 weeks fell into anhedonia and low self-esteem was tested at days 29 to 31. The sucrose preference test (SPT) and Y-maze test (YMT) were used to assess the anhedonia, and the forced swim test (FST) was used to estimate their self-esteem (Porsolt et al., 1978; Willner et al., 1987; Dellu et al., 1992; C. H. Duman, 2010; Overstreet, 2012). The SPT was conducted with 1% sucrose water vs water for 4 hours. The SPT value was presented as a ratio of the ingested sucrose water to the ingested sucrose water plus water. The YMT was performed by monitoring a mouse staying in a special arm and the other 2 arms for 2 minutes. The end of this special arm included a female mouse (referred to as the M-arm). M-arm stay time was presented as a ratio of stay time in M-arm to that in the other 3 arms. The FST was done by recording immobile time in a water cylinder (10cm in diameter and 19cm deep at 25±1°C). To quantify the FST, immobile time and latency (a period to mouse immobility for the first time) were presented. In these tests, the SPT was given once per week, the YMT was given before and after the CUMS, and the FST was given one time after the CUMS. Before the SPT, the mice in the CUMS and control were deprived of food and water for 3 hours to drive their motivation to drink water. In the YMT, these arms were cleaned with 70% ethanol and then water after each test to reduce the effect of odor on the test. Care was taken in these tests by performing them in a quiet room with no additional stresses, the same circadian circle for all mice, and an adaptation period in the test environment.
An expression of depression-like behaviors was accepted if the mice in the CUMS group showed decreases in sucrose preference (twice at the end of weeks 2 and 3) and M-maze stay time and latency, as well as an increase in immobile time, compared with the respective values during their self-control period (the first week) and in the control group of mice. The mice with significant changes in all of 3 tests were defined as CUMS-induced depression-like mice or depression-like mice. About 30% of CUMS-treated mice in 3 weeks met this criterion, implying their vulnerability to the stress. These mice were used as depression-like mice to study cell imaging and electrophysiology. It is noteworthy that some mice without any change in these 3 tests were considered as resilient, that is, they were invulnerable to stress situations, in our study. The mechanism underlying stress vulnerability and invulnerability is not our current topic but will be addressed in the future. Because 30% of CUMS-treated mice met the depression criteria and all CUMS-treated mice did not show a change of the SPT at the end of week 1, the stressful situations in our study were considered to be mild stress.
Brain Slices and Neurons
To have more healthy brain cells for whole-cell recordings, we prepared cortical slices using the following procedures. The mice were anesthetized by isoflurane inhaling and were infused by the artificial cerebrospinal fluid (ACSF) and oxygenated (95% O2 and 5% CO2) at 4°C into their left ventricles until the bodies became cold; the concentrations (mM) of the chemicals were 124 NaCl, 3 KCl, 1.2 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 4 MgSO4, 10 dextrose, and 220 sucrose at pH 7.35. The mouse heads were immediately decapitated by guillotine and placed into this cold oxygenated ACSF with the brain isolation. The cortical slices (300 μm) in coronal direction were cut by Vibratome in this cold oxygenated ACSF. They were held in another oxygenated ACSF (124 NaCl, 3 KCl, 1.2 NaH2PO4, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 10 dextrose, and 5 HEPES, pH 7.35) at 25°C for 2 hours. Each slice was placed into a submersion chamber (Warner RC-26G) that was perfused by the oxygenated ACSF at 31°C for electrophysiological recordings (J.-H. Wang and Kelly, 2001; Chen et al., 2008; Ge et al., 2014). The chemical reagents were from Sigma.
Whole-cell recording was done on GFP-labeled GABAergic and YFP-labeled glutamate neurons in layer III-IV of the prelimbic cortices under DIC-fluorescent microscope (Nikon FN-E600, Japan). The wavelength at 488nm excited the fluorescence of GFP-labeled neurons, and that at 575nm excited the fluorescence of YFP-labeled neurons. GABAergic neurons expressed fast spikes with less adaptation in their amplitude and frequency, the typical properties for the interneurons (Freund and Buzsaki, 1996; McKay and Turner, 2005; J.-H. Wang et al., 2008; Lu et al., 2014). Glutamatergic neurons demonstrated the pyramidal somata and spike adaptation.
Whole-Cell Recording and Neuronal Functions
The neurons were recorded by MultiClamp-700B amplifier under voltage-clamp for their synaptic activity and the current-clamp for their intrinsic property. Electrical signals were inputted to pClamp-10 (Axon Instrument Inc.) for data acquisition and analysis. An output bandwidth of the amplifier was set at 3kHz. The pipette solution for recording excitatory events included (mM) 150 K-gluconate, 5 NaCl, 5 HEPES, 0.4 EGTA, 4 Mg-ATP, 0.5 Tris-GTP, and 5 phosphocreatine (pH 7.35; Ge et al., 2011; Yang et al., 2014). The solution for studying inhibitory synapses contained (mM) 130 K-gluconate, 20 KCl, 5 NaCl, 5 HEPES, 0.5 EGTA, 4 Mg-ATP, 0.5 Tris–GTP, and 5 phosphocreatine (F. Zhang et al., 2012). These pipette solutions were freshly made and filtered (0.1 μm). The osmolarity was 295 to 305 mOsmol and pipette resistance was 5 to 6 MΩ.
The functions of GABAergic neurons were assessed including their active intrinsic properties and inhibitory outputs (J.-H. Wang, 2003). The inhibitory outputs were assessed by recording spontaneous inhibitory postsynaptic currents (sIPSC) on glutamatergic neurons in the presence of 10 μM 6-Cyano-7-nitroquinoxaline-2,3-dione and 40 µM D-amino-5-phosphonovanolenic acid in the ACSF to block ionotropic glutamatergic receptors. A total of 10 µM bicuculline was washed onto the slices at the end of experiments for blocking sIPSCs to test that synaptic responses were mediated by GABAAR. The pipette solution with a high concentration of chloride ions makes the reversal potential -42 mV. sIPSCs are inward when membrane potential is held at -65 mV (Wei et al., 2004; F. Zhang et al., 2012).
The functions of excitatory neurons were evaluated based on their active intrinsic properties and excitatory output (J.-H. Wang, 2003). The excitatory outputs were assessed by recording spontaneous excitatory postsynaptic currents (sEPSC) on GABAergic neurons in the presence of 10 µM bicuculline in the ACSF to block GABAAR (J.-H. Wang, 2003; Yu et al., 2012). A total of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 40 µM D-amino-5-phosphonovanolenic acid were added into the ACSF at the end of experiments to test whether synaptic responses were mediated by glutamate receptor, which blocked sEPSCs in our studies.
The recording of spontaneous synaptic currents, instead of evoked synaptic currents, is based on the following reasons. sEPSC and sIPSC amplitudes represent the responsiveness and densities of postsynaptic receptors. The frequencies imply the probability of transmitter release from an axon terminal and the number of presynaptic axons innervated on the recorded neuron (Zucker and Regehr, 2002; Stevens, 2004). Such parameters can be used to analyze presynaptic and postsynaptic mechanisms as well as to compare them with morphological data about neuronal interaction. The evoked postsynaptic currents cannot separate these mechanisms. We did not use tetrodotoxin in the ACSF to record miniature postsynaptic currents, since we had to record neuronal excitability. As the frequency of synaptic activities was less than those of sequential spikes (Figures 2, 4–5) and spontaneous spikes were never recorded on the neurons in our cortical slices, sIPSCs and sEPSCs were not generated from spontaneous action potentials. Synaptic events in our recording are presumably miniature postsynaptic currents. This point is granted by a single peak of postsynaptic currents in our study.
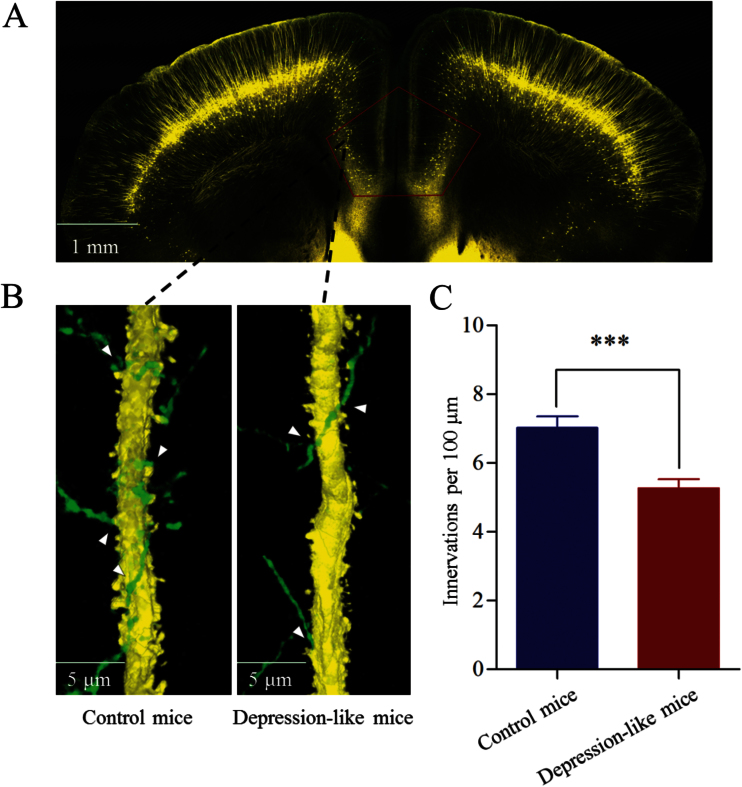
Figure 2.
Inhibitory axon innervations onto the glutamatergic neurons are downregulated in the prelimbic cortex from depression-like mice. (A) The area studied by morphology of neurons and synapses in the prelimic cortex (dashed line). (B) The innervations of GABAergic axons (green) onto the apical dendrites of glutamatergic neurons (yellow) in control (left) and depression-like mice (right). (C) The comparisons of innervations per 100-µm dendrite from depression-like mice (red bar, n = 64 apical dendrites) and controls (blue, n = 60 apical dendrites; ***P < .001).
Action potentials at the cortical neurons were induced by injecting the depolarization pulse. Their excitability was assessed by input-outputs (spikes vs normalized stimuli) when various stimuli were given (Chen et al., 2006). We did not measure rheobase to show cellular excitability, as this strength-duration relationship was used to assess the ability to fire single spike. We measured the ability of firing sequential spikes (J. H. Wang et al., 2008).
Data were analyzed if the recorded neurons had the resting membrane potentials negatively more than -60 mV and action potential amplitudes more than 90 mV. The criteria for the acceptance of each experiment also included <5% changes in resting membrane potential, spike magnitude, and input resistance throughout each recording. The series and input resistances in all neurons were monitored by injecting hyperpolarization pulses (5 mV/50ms) and calculated by voltage pulses vs instantaneous and steady-state currents.
Cell Imaging in the Prelimbic Cortex
The mice were anesthetized by i.p. injection of urethane (1.5g/kg) and perfused by 4% paraformaldehyde in 0.1M phosphate-buffered saline into their left ventricle until their bodies were rigid. The brains were fixed in 4% paraformaldehyde for an additional 24 hours. The cortical tissues were sliced in a series of coronal sections (100 μm). The images for glutamatergic neurons and GABAergic neurons in layers III to IV were photographed under confocal microscopy with oil lens (Plan Apo VC 60X, 1.4NA; Nikon A1R plus, Tokyo, Japan). Although the peaks of GFP and YFP emission wavelength were 510 and 525nm, respectively, we scanned GFP by setting the optical grate at 510nm and YFP by the grate at 540nm, respectively, to separate their images.
The processes of glutamatergic and GABAergic neurons were measured in each of the sections (Ni et al., 2010) by using ImageJ (version 1.47; National Institute of Health). In terms of the structural interaction between excitatory and inhibitory neurons, we analyzed their mutual innervations by counting the contacts of presynaptic boutons on postsynaptic neurons. These contacts were counted from the layer-by-layer confocal cell imaging, that is, they were not the overlaps of 3-dimensional imaging. YFP-labeled glutamatergic axon boutons on GFP-labeled GABAergic neurons in contacts per neuron and GFP-labeled GABAergic axon terminals on YFP-labeled glutamatergic dendrites in contacts per 100-μm length were counted (G. Zhang et al., 2013). It is noteworthy that fluorescent proteins are not labeled to all neurons due to low-efficiency promoters. These low densities of neuronal contacts are parallel in control and depression-like mice.
Statistical Analyses
The data of behavior tests, electrophysiology, and morphology are presented as mean ± SE. Paired t test was used in the comparisons of experimental data before and after the CUMS in each of the mice. One-way ANOVA was used to make statistical comparisons in neuronal activity and morphology between control and depression-like groups.
RESULTS
CUMS Induces Mice to Express Depression-Like Behaviors
The mice were treated by CUMS or control for 3 weeks. Their mood states were assessed by SPT, YMT, and FST. In the mice showing the significant changes in all of these tests, the SPT values are 48.58 ± 5.1% in CUMS-treated mice (n = 10), 80.1 ± 2.2% before their CUMS treatment (self-control), and 86.62 ± 2.8% in control mice (n = 11) (Figure 1B). The SPT values in CUMS-treated mice vs their self-control and control mice were statistically different (P < .001). The ratios of stay time in M-arm to stay time in total arms were 61.6 ± 6.2% in CUMS-treated mice (n = 10), 82.1 ± 3.4% in their self-control, and 68.2 ± 3.7% in control mice (n = 11) (Figure 1C). These values are different for CUMS-treated mice vs their self-control and control mice (P < .01). In addition, the values of immobile time in the FST were 241.6 ± 8 seconds in CUMS-treated mice (n = 10) and 206.2 ± 8.2 seconds in controls (P < .01, n = 11) (Figure 1D, left). Latencies in the FST were 31.1 ± 2.6 seconds in CUMS-treated mice (n = 10) and 52.45 ± 3.9 seconds in controls (P < .001, n = 11) (Figure 1D, right). The mice that showed significant changes in all of these parameters are thought to be depression-like mice.
The CUMS led the mice to express depression-like behavior. Pathophysiological interaction between the excitatory and inhibitory neurons in the prelimbic cortex was investigated in depression-like mice and controls. The outputs of GABAergic neurons were studied by analyzing sIPSCs and axon innervations on their targeted glutamatergic neurons. The intrinsic property of GABAergic neurons was assessed by measuring their input-output curves. The receptions of GABAergic neurons were evaluated by analyzing their processes and glutamatergic terminals.
The Outputs Decrease in the GABAergic Neuron of the Prelimbic Cortex of Depression-Like Mice
The innervations from GABAergic axons to glutamatergic neurons were counted by GFP-labeled axonal terminals on YFP-labeled glutamatergic neuron in the prelimbic cortex (Figure 2A). Their contacts appear decreased in depression-like mice (Figure 2B). GFP-labeled axonal terminals per 100 μm of YFP-labeled apical dendrites from glutamatergic neurons were 5.26 ± 0.3 in depression-like mice (n = 64; Figure 2C, red bar) and 7.02 ± 0.34 in control mice (P < .001, n = 60 apical dendrites; Figure 2C, blue). Depression-like behavior is associated with the decreased innervation from GABAergic axons onto glutamatergic neurons in the prelimbic cortex.
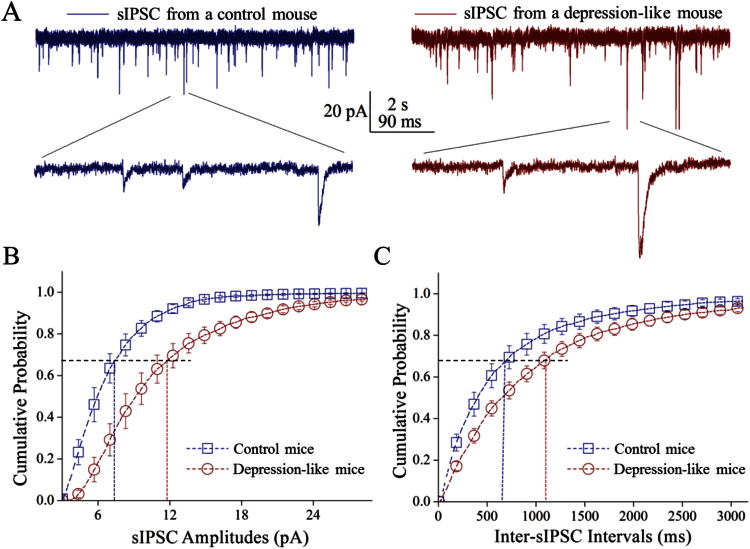
Lower sIPSC frequency and higher sIPSC amplitude appeared in depression-like mice (Figure 3). Figure 3B shows cumulative probability vs sIPSC amplitude from depression-like mice and control. Figure 3C shows cumulative probability vs inter-sIPSC interval from 2 groups of mice. The values of sIPSC amplitudes at 67% cumulative probability were 11.7 ± 1.1 pA in depression-like mice (n = 10 cells) and 7.28 ± 0.6 pA in controls (n = 10 cells; P = .004). Inter-sIPSC intervals at 67% cumulative probability were 1115 ± 111ms in depression-like mice (n = 10) and 737 ± 128ms in control (n = 10; P = .03). Depression-like behavior is associated with an incompatible change in inhibitory synaptic efficacy, that is, the decreased GABA release and increased receptor responsiveness in the prelimbic cortex. The decreases in both GABA release and presynaptic GABAergic innervations strengthen the reliability of our data.
Figure 3.
Inhibitory synaptic transmission is downregulated in the glutamatergic neurons of the prelimbic cortex from depression-like mice. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded under voltage-clamp in the brain slices from control and depression-like mice in the presence of 10 μM 6-Cyano-7-nitroquinoxaline-2,3-dione and 40 µM D-amino-5-phosphonovanolenic acid. (A) Left panels show sIPSCs from a control mouse (blue traces), and right panels illustrate sIPSCs from depression-like mouse (reds). Calibration bars: vertical bar=20 pA; horizontal=2 seconds (top) and 90 milliseconds (bottom). (B) Cumulative probability vs sIPSC amplitudes from the depression-like mice (red circles) and controls (blue squares). Dashed lines indicate sIPSC amplitudes at cumulative probability to 67% (CP67) in the control (blue line; n = 10) and in depression-like mice (red; n = 10, P < .01). (C) Cumulative probability vs inter-sIPSC intervals from the depression-like mice (red circle symbols) and control (blue square symbols). Dash-lines indicate inter-sIPSC intervals at cumulative probability to 67% (CP67) in control (blue line; n = 10) and depression-like mice (red; n = 10, P < .05).
The Excitability Decreases in GABAergic Neurons of the Prelimbic Cortex of Depression-Like Mice
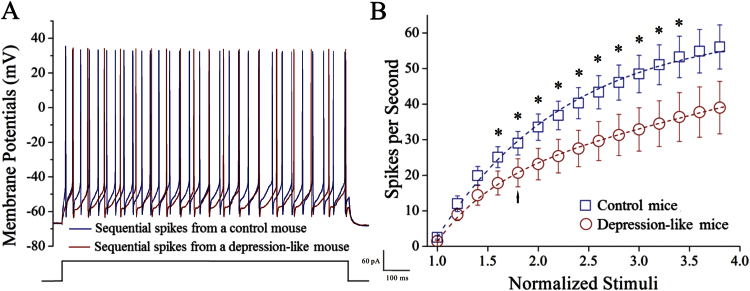
Figure 4 shows neuronal abilities to convert excitatory inputs into spikes. GABAergic neurons in depression-like mice appear to have a lower capacity to encode spikes (Figure 4A, red trace) compared with the control (blue trace). Figure 4B illustrates spikes vs normalized stimuli in these GABAergic neurons from control mice (blue symbols) and depression-like mice (red). The input-output curve in GABAergic neurons (n = 16) of depression-like mice shifts right-low compared with that in controls (n = 15; P < .05). Depression-like behavior is associated with the decreased capability to convert excitatory inputs into digital spikes in the GABAergic neurons of the prelimbic cortex.
Figure 4.
The ability to produce the sequential spikes on the GABAergic neurons of the prelimbic cortices decreases in depression-like mice. The sequential spikes induced by various stimulus intensities were recorded on the GABAergic neurons in cortical slices under current-clamp. (A) Depolarization induced the sequential spikes on the GABAergic neurons in depression-like (red trace) and control mice (blue trace). (B) Spikes per second vs normalized stimuli in GABAergic neurons from the depression-like mice (red circles, n = 16 neurons) and the controls (blue squares, n = 15 neurons). *P < .05. Arrow indicates spikes vs stimulus intensity taken for A.
Glutamatergic Innervations Increase in Prelimbic Cortical GABAergic Neurons of Depression-Like Mice
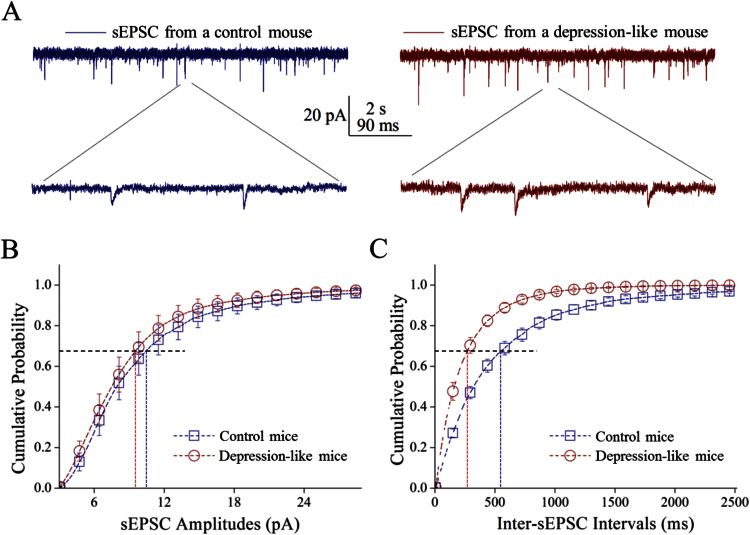
Excitatory synaptic activity was recorded on GABAergic neurons (Figure 5). sEPSC frequencies appear higher in depression-like mice than controls (Figure 5A). Figure 5B shows cumulative probability vs sEPSC amplitudes in depression-like mice and controls. Figure 5C shows cumulative probability vs inter-sEPSC intervals in 2 groups of mice. sEPSC amplitudes at 67% cumulative probability were 9.81 ± 1.3 pA from depression-like mice (n = 11 neurons) and 10.58 ± 1.4 pA from controls (n = 9 neurons; p = 0.69). Inter-sEPSC intervals at 67% cumulative probability were 280 ± 31ms from depression-like mice (n = 11) and 563 ± 55ms in control (n = 9; P < .001). Depression-like behavior is associated with the increased release of glutamates onto GABAergic neurons.
Figure 5.
The frequency of excitatory synaptic transmission is upregulated in GABAergic neurons of the prelimbic cortex from depression-like mice. Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded under voltage-clamp from control and depression-like mice in the presence of 10 μM bicuculline. (A) Left panels illustrate sEPSCs from control mice (blue traces) and right panels show sEPSCs from depression-like mice (reds). Calibration bars are 20 pA as well as 2 seconds (top traces) and 90ms (bottoms). (B) Cumulative probability vs sEPSC amplitudes from the depression-like mice (red circles) and control (blue squares). Dashed lines indicate sEPSC amplitudes at cumulative probability to 67% (CP67) in the control (blue line; n = 9) and depression-like mice (red; n = 11, P = .69). (C) Cumulative probability vs inter-sEPSC intervals from depression-like (red circles) and control mice (blue squares). Dashed lines are inter-sEPSC intervals at the cumulative probability to 67% (CP67) in the control (blue line; n = 9) and depression-like mice (red; n = 11, P < .001).
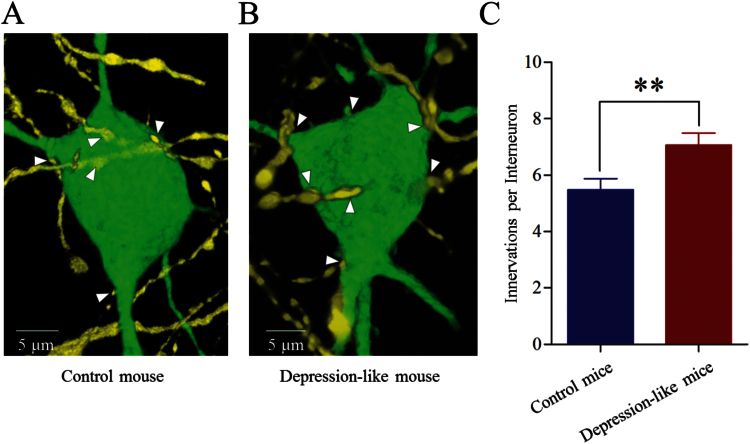
The innervation from glutamatergic axons onto GABAergic neurons was counted by YFP-labeled axonal terminals on GFP-labeled GABAergic neurons. As shown in Figure 6A-B, their contacts appear increased in depression-like mice. YFP-labeled axonal terminals on each GABAergic neuron were 7.07 ± 0.4 from depression-like mice (n = 43 neurons; Figure 6C, red bar) and 5.48 ± 0.4 from controls (P < .01, n = 43 neurons; Figure 6C, blue bar). Depression-like behavior is associated with the increased terminations from glutamatergic axons onto GABAergic neurons.
Figure 6.
Excitatory axon innervation onto the GABAergic neurons is upregulated in the prelimbic cortex from depression-like mice. (A) Innervations of glutamatergic axons (yellow) onto the soma of GABAergic neuron (green) in control mice. (B) Innervations of the glutamatergic axons (yellow) onto the soma of the GABAergic neuron (green) in depression-like mice. (C) The comparisons of innervations per neuron from depression-like mice (red bar, n = 43 neurons) and controls (blue, n = 43 neurons; **P < .01).
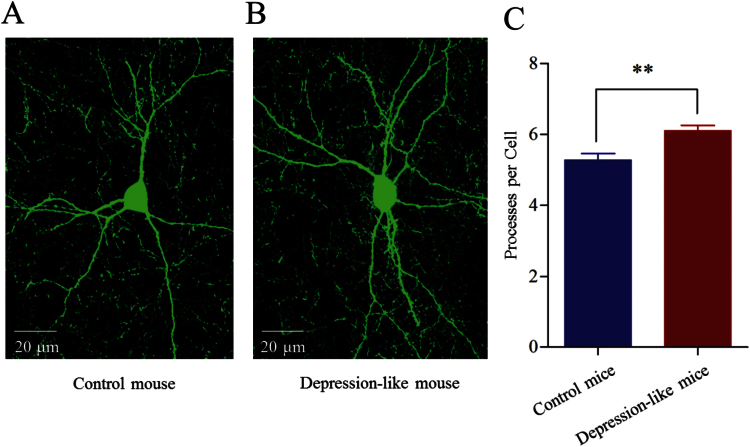
The reception of GABAergic neurons from synaptic inputs was also examined by measuring their processes. The number of their dendritic processes appeared greater in depression-like mice (Figure 7A-B). Processes per GABAergic neuron are 6.1 ± 0.15 in the depression-like mice (n = 70 cells; Figure 7C, red bar) and 5.27 ± 0.18 in controls (P < .001, n = 76 cells; Figure 7C, blue bar). Depression-like behavior is associated with an increased receptive field in prelimbic cortical GABAergic neurons. The consistent changes in sEPSC frequency, presynaptic glutamatergic innervation, and receptive field at GABAergic cells from depression-like mice strengthen our conclusion.
Figure 7.
The dendritic processes on the GABAergic neurons are upregulated in the prelimbic cortex from depression-like mice. (A) A GABAergic neuron and its processes from control mice. (B) A GABAergic neuron and its processes from a depression-like mouse. (C) The comparison of processes per GABAergic neuron from depression-like mice (red bar, n = 70 neurons) and controls (blue, n = 76 neurons; **P < .001).
No Change in Excitability in the Prelimbic Cortical Glutamatergic Neurons of Depression-Like Mice
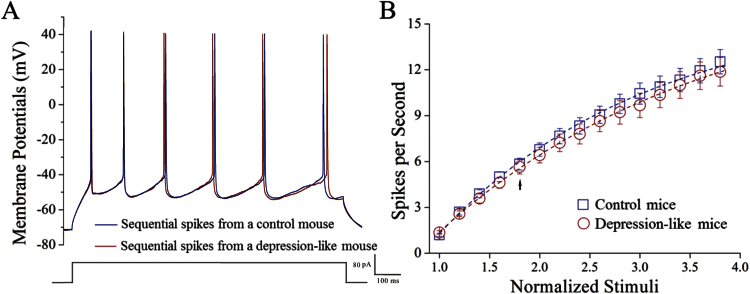
Figure 8 illustrates the ability of glutamatergic neurons to convert excitatory inputs into spikes in the prelimbic cortex. Glutamatergic neurons in depression-like mice did not appear to change in excitability (Figure 8A, red trace), compared with controls (Figure 8A, blue trace). Figure 8B shows spikes vs normalized stimuli in glutamatergic neurons from depression-like mice (red circles, n = 28 neurons) and controls (blue squares, n = 37). The CUMS does not influence the capability of glutamatergic neurons to convert excitatory inputs into spikes in the prelimbic cortex.
Figure 8.
The ability to fire the sequential spikes on the glutamatergic neurons of the prelimbic cortices does not change in depression-like mice. The sequential spikes induced by various stimulus intensities were recorded on GABAergic neurons in cortical slices under current-clamp. (A) Depolarization-induced sequential spikes on glutamatergic neurons from control (blue trace) and depression-like mice (red trace). (B) Spikes per second vs normalized stimuli in glutamatergic neurons from depression-like mice (red circles, n = 28 neurons) and controls (blue squares, n = 37 neurons). Arrow indicates spikes vs stimulus intensity taken for panel A.
Discussion
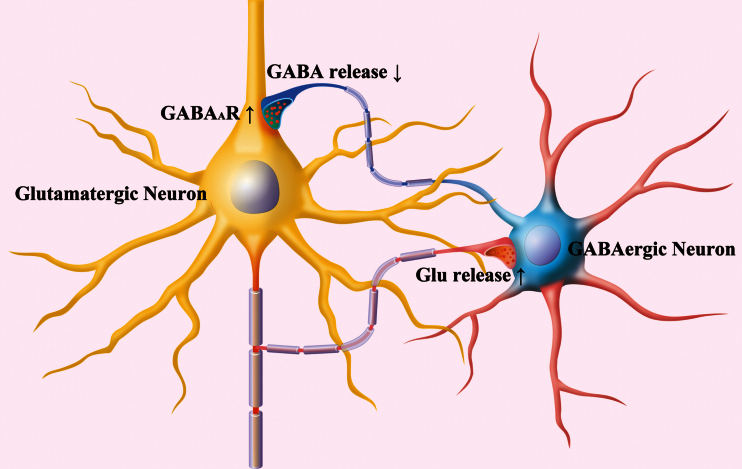
The GABAergic neurons in the prelimbic cortex from depression-like mice possess decreased inhibitory synapse output and excitability (Figure 2–4) as well as increased reception from excitatory synapses (Figures 5–7). Although the bidirectional change in their outputs vs receptions appears to be compensatory homeostasis, this lack of coordination among subcellular compartments of GABAergic neurons may reduce their ability to regulate the downstream neurons. On the other hand, the increased sensitivity to inhibitory inputs (Figure 3) and the unchanged excitability in the glutamatergic neurons (Figure 8) may depress their excitatory output to drive the target cells. The incompatibility among subcellular compartments and incoordination between inhibitory and excitatory neurons may cause the dysfunction of the prelimbic cortex in major depressive disorder (Figure 9).
Figure 9.
Incompatible alternations occur in the GABAergic neurons and glutamatergic neurons of the prelimbic cortices from depression-like mice. In GABAergic neurons (round), their intrinsic property and synaptic outputs decrease (blue). Their receptions from excitatory synaptic transmission and innervations as well as their receptive fields increase (red). The incompatibility among the subcellular compartments of GABAergic neurons reduces their efficiency to coordinate their downstream neurons. In glutamatergic neurons (pyramidal), their responses to GABAergic inputs increase, their spiking capability does not change, and their excitatory outputs increase. These incompatible changes among the subcellular compartments of glutamatergic neurons attenuate their efficiency to program the neural codes. Together, these changes, the interactions between GABAergic and glutamatergic neurons, are deteriorated.
The dysfunction of GABAergic neurons is hypothetically a primary change to induce subcellular compartment incompatibility as well as inhibitory vs excitatory neuron incoordination, since they are vulnerable to the pathological factors (Akaike, 1995; J.-H. Wang, 2003; Luscher et al., 2011; J. H. Wang et al., 2015). For instance, the stress hormones affect the function of GABAA receptors (Hu et al., 2010; Skilbeck et al., 2010; Gunn et al., 2011; Mody and Maguire, 2011) and reduce the density of GABAergic neurons in the prenatal period (Uchida et al., 2014). Chronic stress impairs the reversal potential and density of GABA receptor-channels (Quintero et al., 2011; Wislowska-Stanek et al., 2013; MacKenzie and Maguire, 2015) and lowers GABAergic tones (Torrey et al., 2005; Hasler et al., 2007; Plante et al., 2012; Seney et al., 2014). In these studies and our data in the prelimbic cortex, a testable hypothesis is that stress-impaired GABAergic neurons lead to subcellular unit incompatibility and neuronal incoordination in major depressive disorder. Securing the GABAergic neurons in the limbic system to reverse the subcellular changes remains to be examined.
Studies from depression patients indicate low GABAergic tone in the central nervous system (Oruc et al., 1997; Torrey et al., 2005; Bajbouj et al., 2006; Hettema et al., 2006; Hasler et al., 2007; Price et al., 2009; Levinson et al., 2010; Croarkin et al., 2011; Plante et al., 2012). By analyzing GABAergic cells and synapses in the prelimbic cortex from depression-like mice, we observed the decreases in GABAergic axon output and soma excitability (Figures 2–4). Interestingly, the reception from glutamatergic axonal inputs increases in GABAergic neurons (Figures 5–7). These changes may be due to the fact that stress-induced primary dysfunction in GABAergic neurons initiates an unknown mechanism to enhance their sensitivity and reception from excitatory synapses, that is, a compensatory homeostasis among subcellular compartments for neuron survival (Chen et al., 2008). Moreover, a decrease in presynaptic GABA release and an increase in postsynaptic GABAA-receptor responses (Figure 3) imply homeostasis within GABAergic synapses, which may explain the controversy over the use of GABA-receptor enhancers as an antidepressant. The compensatory changes among subcellular compartments tend to maintain functional homeostasis in the GABAergic neurons and synapses. However, the incompatibility among subcellular compartments and the incoordination between presynaptic and postsynaptic compartments make neuronal interaction and synaptic transmission inefficient.
The increases in glutamatergic axonal terminations and actions in prelimbic cortical GABAergic neurons are seen in depression-like mice treated with CUMS for 3 weeks (Figures 5–6). This result is consistent with recent reports that the potentiation of excitatory synapses on the activated neurons in the prelimbic cortex are associated with learned helplessness (M. Wang et al., 2014) as well as that the stress induces increases in the number of hippocampal pyramidal neurons (Stockmeier et al., 2004) and in the densities of postsynaptic glutamate NR2A-receptors and PSD-95 in lateral amygdala (Karolewicz et al., 2009). This excitatory effect may act on prelimbic cortical GABAergic neurons to induce their excitotoxicity for cell pathophysiology associated with major depressive disorder. This point is supportive for recent findings that a low dose of NMDA-receptor antagonist ketamine improves depression patients resistant to typical antidepressants and reverses synaptic deficits in depression-like mice (R. S. Duman and Aghajanian, 2012; Zarate et al., 2013).
On the other hand, a few studies present different conclusions. The depression of excitatory synapses is seen on the parvalbumin neurons of the prefrontal cortex in learned helplessness mice evoked by extreme stress (Perova et al., 2015). The weakness of excitatory synapses mediated by AMPAR in the nucleus accumbens is associated with the depression in mice induced by stress in 5 days (Lim et al., 2012). Restraint stress in 7 days induces a decreased excitatory synaptic activity in the prefrontal cortical pyramidal neurons, including both NMDAR and AMPAR components (Yuen et al., 2012). To explain these differences in these studies from animal models, we consider the following reasons. The different types of neurons, neuronal circuits, and brain areas (such as positive vs negative circuits) may express different cellular changes to make the complicated signs in major depressive disorders. The procedures in the different stress patterns and periods may lead to the inconsistent pathological changes. Different from the studies that applied learned helplessness or restraint in 5 to 7 days, our CUMS procedure included mild stresses for 3 weeks; the mice in the first week did not show depression-like behaviors. Their mood and cognition are likely based on neuronal plasticity mixed from learning, working memory, and emotion deficiency, similar to stressful social life in depression patients.
Our studies demonstrate the incompatibility among subcellular compartments and incoordination between GABAergic and glutamatergic neurons in the prefrontal cortex from depression-like mice. These data imply that the incoordination among subcellular compartments constitutes neural substrates for major depressive disorder and the rebalance of their coordination should be considered as a therapeutic strategy, since coordination and compatibility among subcellular compartments are present under physiological conditions (Chen et al., 2008; J. H. Wang et al., 2013). It is noteworthy that other diseases in the central nervous system, such as anxiety and epilepsy, are associated with subcellular incoordination (Liu et al., 2014; Lei et al., 2015; J. H. Wang et al., 2015; Wen et al., 2015). The reset of neuronal homeostasis by rebalancing subcellular compatibility and coordination should be ideal strategies for the treatment of neural disorders.
By using the mice with YFP-labeled glutamatergic and GFP-labeled GABAergic neurons, we were able to analyze type-specific cell pathology in their subcellular compartments and mutual interaction. The analyses by morphology and electrophysiology indicate that the changes in cell structures and functions are consistent. The studies with neuronal identification and mutual supportive data make us confident in our conclusions. In the prelimbic cortices from depression-like mice induced by chronic mild stress, the synaptic outputs and excitability of GABAergic neurons decrease and the reception of GABAergic neurons from excitatory synapses increases. Stress-induced incompatibility among the subcellular compartments of the GABAergic neurons as well as the incoordination between GABAergic and glutamatergic neurons lead to imbalanced neural networks in the prelimbic cortex, which are the bases of depressive mood.
Interest Statement
None.
Acknowledgements
This study is funded by the National Basic Research Program (2013CB531304 and 2011CB504405) and Natural Science Foundation China (30990261, 81171033 and 81471123) to J.-H.W.
References
- Akaike N. (1995) Time-dependent rundown of GABA response in mammalian CNS neuron during experimental anoxia. Obes Res 3:769S–777S. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, et al (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9:557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P. (2006) Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59:395–400. [DOI] [PubMed] [Google Scholar]
- Banasr M, Dwyer JM, Duman RS. (2011) Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol 23:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P, Wilkinson C, Turner J, Brain K, Edwards RT, Griffith G, France B, Gray J. (2008) Psychological factors associated with emotional responses to receiving genetic risk information. Journal of genetic counseling 17:234–241. [DOI] [PubMed] [Google Scholar]
- Berton O, Hahn CG, Thase ME. (2012) Are we getting closer to valid translational models for major depression? Science 338:75–79. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11:1169–1180. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Geisler C, Henze DA, Wang XJ. (2004) Interneuron diversity series: circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci 27:186–193. [DOI] [PubMed] [Google Scholar]
- Camp NJ, Cannon-Albright LA. (2005) Dissecting the genetic etiology of major depressive disorder using linkage analysis. Trends Mol Med 11:138–144. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhu Y, Gao X, Guan S, Wang J-H. (2006) Sodium channel-mediated intrinsic mechanisms underlying the differences of spike programming among GABAergic neurons. Biochem Biophys Res Comm 346:281–287. [DOI] [PubMed] [Google Scholar]
- Chen N, Chen X, Wang J-H. (2008) Homeostasis established by coordination of subcellular compartment plasticity improves spike encoding. J Cell Sci 121:2961–2971. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ. (2011) Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev 35:818–825. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. (1992) A two-trial memory task with automated recording: study in young and aged rats. Brain Res 588:132–139. [DOI] [PubMed] [Google Scholar]
- Duman CH. (2010) Models of depression. Vitam Horm 82:1–21. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhwuegi AS. (2004) Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry 28:435–451. [DOI] [PubMed] [Google Scholar]
- Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, Del Rio J, Tordera RM. (2008) Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology (Berl) 199:1–14. [DOI] [PubMed] [Google Scholar]
- Freund TF. (2003) Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci 26:489–495. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. (1996) Interneurons of the hippocampus. Hippocampus 6:347–470. [DOI] [PubMed] [Google Scholar]
- Ge R, Qian H, Wang JH. (2011) Physiological synaptic signals initiate sequential spikes at soma of cortical pyramidal neurons. Mol Brain 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Qian H, Chen N, Wang JH. (2014) Input-dependent subcellular localization of spike initiation between soma and axon at cortical pyramidal neurons. Mol Brain 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Near J, Cowen PJ. (2015) Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 232:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. (2011) Neurosteroids and GABA(A) Receptor interactions: a focus on stress. Front Neurosci 5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Lu XY. (2014) Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl Psychiatry 4:e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. (2013) Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis 52:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. (2007) Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJ, Kendler KS, Chen X. (2006) Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry 11:752–762. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. (2012) Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36:2085–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Flugge G, Zhang W. (2010) Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 35:1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Ormel J, Kema IP, den Boer JA. (2008) Investigating the molecular basis of major depressive disorder etiology: a functional convergent genetic approach. Ann N Y Acad Sci 1148:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. (2009) Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol 12:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. (2010) Reduced level of glutamic acid decarboxylase-67kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 13:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Uher R. (2012) Gene-environment interaction in major depression and antidepressant treatment response. Curr Psychiatry Rep 14:129–137. [DOI] [PubMed] [Google Scholar]
- Kendell SF, Krystal JH, Sanacora G. (2005) GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets 9:153–168. [DOI] [PubMed] [Google Scholar]
- Khundakar A, Morris C, Thomas AJ. (2011) The immunohistochemical examination of GABAergic interneuron markers in the dorsolateral prefrontal cortex of patients with late-life depression. Int Psychogeriatr 23:644–653. [DOI] [PubMed] [Google Scholar]
- Klengel T, Binder EB. (2013) Gene-environment interactions in major depressive disorder. Can J Psychiatry 58:76–83. [DOI] [PubMed] [Google Scholar]
- Lei Z, Liu B, Wang J-H. (2015) Reward memory relieves anxiety-related behavior through synaptic strengthening and protein kinase C in dentate gyrus. Hippocampus. In press. [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. (2010) Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 67:458–464. [DOI] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. (2012) Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Sibille E. (2013) Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front Pharmacol 4:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Feng J, Wang J-H. (2014) Protein kinase C is essential for kainate-induced anxiety-related behavior and glutamatergic synapse upregulation in prelimbic cortex. CNS Neurosci Therapeut. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff FW. (2010) Overview of the genetics of major depressive disorder. Curr Psychiatry Rep 12:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Wen B, Zhang F, Wang JH. (2014) Voltage-independent sodium channels emerge for an expression of activity-induced spontaneous spikes in GABAergic neurons. Mol Brain 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. (2011) The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Hughes J, O’Dwyer G, Pride Y, Stockmeier CA, Sanacora G, Rajkowska G. (2010) Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry 67:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G, Maguire J. (2015) Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res 109:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Turner RW. (2005) Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol (London) 567(Pt3):829–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Maguire J. (2011) The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H. (2012) The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62:42–53. [DOI] [PubMed] [Google Scholar]
- Morishita S. (2009) Clonazepam as a therapeutic adjunct to improve the management of depression: a brief review. Hum Psychopharmacol 24:191–198. [DOI] [PubMed] [Google Scholar]
- Moylan S, Maes M, Wray NR, Berk M. (2013) The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606. [DOI] [PubMed] [Google Scholar]
- Ni H, Huang L, Chen N, Zhang F, Liu D, Ge M, Guan S, Zhu Y, Wang JH. (2010) Upregulation of barrel GABAergic neurons is associated with cross-modal plasticity in olfactory deficit. PLoS ONE 5:e13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oruc L, Verheyen GR, Furac I, Ivezic S, Jakovljevic M, Raeymaekers P, Van Broeckhoven C. (1997) Positive association between the GABRA5 gene and unipolar recurrent major depression. Neuropsychobiology 36:62–64. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. (2012) Modeling depression in animal models. Methods Mol Biol 829:125–144. [DOI] [PubMed] [Google Scholar]
- Perova Z, Delevich K, Li B. (2015) Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci 35:3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F, Trivedi MH, Fulton M, Rush AJ. (1995) Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry 38:578–591. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109. [DOI] [PubMed] [Google Scholar]
- Plante DT, Jensen JE, Schoerning L, Winkelman JW. (2012) Reduced gamma-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: a link to major depressive disorder? Neuropsychopharmacology 37:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. (2009) Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero L, Cardenas R, Suarez-Roca H. (2011) Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain 152:1909–1922. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. (2007) GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Wolf OT. (2010) Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci Biobehav Rev 35:104–114. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. (2004) Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61:705–713. [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J. (2015) Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16:290–304. [DOI] [PubMed] [Google Scholar]
- Schweizer MC, Henniger MS, Sillaber I. (2009) Chronic mild stress (CMS) in mice: of anhedonia, ‘anomalous anxiolysis’ and activity. PLoS One 4:e4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Tripp A, McCune S, Lewis D, Sibille E. (2014) Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis 73C:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilbeck KJ, Johnston GA, Hinton T. (2010) Stress and GABA receptors. J Neurochem 112:1115–1130. [DOI] [PubMed] [Google Scholar]
- Smith WT, Londborg PD, Glaudin V, Painter JR, Summit Research N. (2002) Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression? J Affect Disord 70:251–259. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. (2012) The science of resilience: implications for the prevention and treatment of depression. Science 338:79–82. [DOI] [PubMed] [Google Scholar]
- Stevens CF. (2004) Presynaptic function. Curr Opin Neurobiol 14:341–345. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Couch Y, Kholod N, Boyks M, Malin D, Leprince P, Steinbusch HM. (2011) Update in the methodology of the chronic stress paradigm: internal control matters. Behav Brain Funct BBF 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. (2015) An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. (2005) Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry 57:252–260. [DOI] [PubMed] [Google Scholar]
- Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A. (2014) Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry 4:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraiah P, Noronha JM, Maitra S, Bagga P, Khandelwal N, Chakravarty S, Kumar A, Patel AB. (2014) Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry 76:231–238. [DOI] [PubMed] [Google Scholar]
- Wang J-H. (2003) Short-term cerebral ischemia causes the dysfunction of interneurons and more excitation of pyramidal neurons. Brain Research Bulletin 60:53–58. [DOI] [PubMed] [Google Scholar]
- Wang J-H, Kelly PT. (2001) Ca2+/CaM signalling pathway up-regulates glutamatergic synaptic function in non-pyramidal fast-spiking neurons of hippocampal CA1. J Physiol (Lond) 533:407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Wei J, Chen X, Yu J, Chen N, Shi J. (2008) The gain and fidelity of transmission patterns at cortical excitatory unitary synapses improve spike encoding. J Cell Sci 121:2951–2960. [DOI] [PubMed] [Google Scholar]
- Wang JH, Yang Z, Qian H, Chen N. (2013) Functional compatibility between Purkinje cell axon branches and their target neurons in the cerebellum. Biophys J 104:330a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Lu W, Wen B. (2015) Neuron-specific mechanisms for epilepsy self-termination. Mol Cellular Epilep 2:e716. [Google Scholar]
- Wang M, Perova Z, Arenkiel BR, Li B. (2014) Synaptic modifications in the medial prefrontal cortex in susceptibility and resilience to stress. J Neurosci 34:7485–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Zhang M, Zhu Y, Wang JH. (2004) Ca2+-calmodulin signalling pathway upregulates GABA synaptic transmission through cytoskeleton-mediated mechanisms. Neuroscience 127:637–647. [DOI] [PubMed] [Google Scholar]
- Wen B, Qian H, Feng J, Ge RJ, Xu X, Cui ZQ, Zhu RY, Pan LS, Lin ZP, Wang JH. (2015) A portion of inhibitory neurons in human temporal lobe epilepsy are functionally upregulated: an endogenous mechanism for seizure termination. CNS Neurosci Ther 21:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Mitchell PB, Meiser B, Schofield PR. (2013) Implications of the use of genetic tests in psychiatry, with a focus on major depressive disorder: a review. Depress Anxiety 30:267–275. [DOI] [PubMed] [Google Scholar]
- Willner P. (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93:358–364. [DOI] [PubMed] [Google Scholar]
- Wislowska-Stanek A, Lehner M, Skorzewska A, Krzascik P, Maciejak P, Szyndler J, Ziemba A, Plaznik A. (2013) Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in low- and high-anxiety rats. Behav Brain Res 253:337–345. [DOI] [PubMed] [Google Scholar]
- Yang Z, Gu E, Lu X, Wang JH. (2014) Essential role of axonal VGSC inactivation in time-dependent deceleration and unreliability of spike propagation at cerebellar Purkinje cells. Mol Brain 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Qian H, Wang JH. (2012) Upregulation of transmitter release probability improves a conversion of synaptic analogue signals into neuronal digital spikes. Mol Brain 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. (2012) Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C, Duman RS, Liu G, Sartori S, Quiroz J, Murck H. (2013) New paradigms for treatment-resistant depression. Ann N Y Acad Sci 1292:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Liu B, Lei Z, Wang J. (2012) mGluR1,5 activation improves network asynchrony and GABAergic synapse attenuation in the amygdala: implication for anxiety-like behavior in DBA/2 mice. Mol Brain 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Gao Z, Guan S, Zhu Y, Wang JH. (2013) Upregulation of excitatory neurons and downregulation of inhibitory neurons in barrel cortex are associated with loss of whisker inputs. Mol Brain 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. (2002) Short-term synaptic plasticity. Ann Rev Physiol 25:355–405. [DOI] [PubMed] [Google Scholar]