Abstract
Background:
Besides the well-known effects of ghrelin on adiposity and food intake regulation, the ghrelin system has been shown to regulate aspects of behavior including anxiety and stress. However, the effect of virus-mediated overexpression of the ghrelin receptor in the amygdala has not previously been addressed directly.
Methods:
First, we examined the acute effect of peripheral ghrelin administration on anxiety- and depression-like behavior using the open field, elevated plus maze, forced swim, and tail suspension tests. Next, we examined the effect of peripheral ghrelin administration and ghrelin receptor deficiency on stress in a familiar and social environment using the Intellicage system. Importantly, we also used a novel approach to study ghrelin receptor signaling in the brain by overexpressing the ghrelin receptor in the amygdala. We examined the effect of ghrelin receptor overexpression on anxiety-related behavior before and after acute stress and measured the modulation of serotonin receptor expression.
Results:
We found that ghrelin caused an anxiolytic-like effect in both the open field and elevated plus maze tests. Additionally, it attenuated air-puff–induced stress in the social environment, while the opposite was shown in ghrelin receptor deficient mice. Finally, we found that overexpression of the ghrelin receptor in the basolateral division of the amygdala caused an anxiolytic-like effect and decreased the 5HT1a receptor expression.
Conclusions:
Ghrelin administration and overexpression of the ghrelin receptor in the amygdala induces anxiolytic-like behavior. Since the ghrelin receptor has high constitutive activity, ligand-independent signaling in vivo may be important for the observed anxiolytic-like effects. The anxiolytic effects seem to be mediated independently from the HPA axis, potentially engaging the central serotonin system.
Keywords: ghrelin, anxiety, amygdala, stress, rAAV
Introduction
The endocrine hormone ghrelin (Kojima et al., 1999) is an important regulator of food intake and metabolism. Ghrelin acts through its receptor (Ghr-R), previously known as the growth hormone secretagogue receptor (Howard et al., 1996), which is expressed in numerous brain regions, including the brainstem, hypothalamus, hippocampus, and amygdala (Guan et al., 1997; Zigman et al., 2006; Alvarez-Crespo et al., 2012; Mani et al., 2014). Ghrelin has been shown to increase food intake when injected into discrete brain regions, including the hypothalamus, hippocampus, and ventral tegmental area (Olszewski et al., 2003; Carlini et al., 2004; Abizaid et al., 2006). However, the central effects of ghrelin are diverse and extend beyond food intake, including effects on anxiety- and depression-related behavior as well as stress.
Adrenalin is a powerful stimulator of ghrelin release (Zhao et al., 2010; Engelstoft et al., 2013), and β1-adrenergic receptors are highly enriched in ghrelin-producing cells (Engelstoft et al., 2013), indicating an important role of stress in the regulation of ghrelin secretion. Secretion of ghrelin may be a counter regulatory mechanism to stress, as both calorie restriction and exogenous ghrelin injections have been shown to have anxiolytic- and antidepressant-like effects (Lutter et al., 2008; Yamamoto et al., 2009). In accordance with this, Ghr-R knockout (KO) mice displayed increased stress response in the chronic social defeat stress model (Lutter et al., 2008), and ghrelin KO mice showed increased anxiety-like behavior after acute restraint stress (Spencer et al., 2012). However, in apparent discrepancy with these findings, several other studies have reported anxiogenic- and depressant-like effects of ghrelin administration in rodents (Asakawa et al., 2001; Carlini et al., 2002, 2004; Currie et al., 2011; Hansson et al., 2011).
An important brain region in the regulation of emotional reactivity is the amygdala, which is involved in modulation of anxiety as well as memory, motivation, and learning (Murray, 2007; Forster et al., 2012). Interestingly, ghrelin has been shown to affect food intake, memory, and learning when injected into the amygdala (Carlini et al., 2004; Diano et al., 2006; Tóth et al., 2010). With regard to anxiety-like behavior, the basolateral division of the amygdala (BL-d), including the lateral (La) and basolateral (BLA) nuclei, was recently shown to be important for the anxiolytic-like effects of ghrelin (Alvarez-Crespo et al., 2012; Currie et al., 2014). Thus, ghrelin caused an anxiolytic-like effect when injected into the La in food-restricted rats (Alvarez-Crespo et al., 2012). However, the opposite has also been shown by different groups, which found that ghrelin had an anxiogenic-like effect when injected into the BLA (Currie et al., 2014; Meyer et al., 2014). This underlines the importance of the amygdala in the central effects of ghrelin, although the precise role with respect to anxiety remains unclear.
Here, we further study the ghrelin system in anxiety-like behavior and stress through different approaches. First, we examined the effect of acute peripheral ghrelin injections on these behaviors. Second, we used Ghr-R KO mice to study similar behaviors. Finally, we used a novel approach to study the effect of Ghr-R signaling on anxiolytic-like behavior and stress by overexpressing the Ghr-R in the BL-d of mice using a recombinant adeno-associated viral vector (rAAV). Importantly, this receptor is highly constitutively active, and the expression on its own may be sufficient to exert a function independent of ghrelin availability. In addition, we study potential Ghr-R overexpression-induced changes in expression levels of key serotonin receptors previously suggested to be involved in ghrelin-mediated signaling in the amygdala (Hansson et al., 2011, 2014).
MATERIALS AND METHODS
Animals and Drugs
For the Intellicage experiments, we used female C57BL/6 (Taconic, Denmark, age 8–10 weeks) and female Ghr-R KO mice and wild type (WT) littermate controls (C57BL/6 background, fully backcrossed, age 10 weeks) (Zigman et al., 2005). For all other experiments, male NMRI mice were used (Taconic, DK, age 8–11 weeks). All mice were socially housed in Macrolon cages (20x35x15cm) enriched with housing and nesting material and kept at a constant temperature (22±2°C) in a 12-hour-light/-dark cycle with free access to standard chow and water, unless otherwise specified. All experiments were conducted in accordance with guidelines from the Animal Experimentation Inspectorate, Denmark and were in accordance with the legal requirements of the European Community. Human ghrelin (Polypeptide Laboratories, DK) was dissolved in saline and injected i.p. or s.c.
Behavioral Testing
An observer blinded to the treatment of the animals scored all tests. Open field, elevated plus maze (EPM), forced swim, and tail suspension tests were run in the light phase in a room with low, indirect light.
Open Field and EPM Tests
The mice were placed in the center of the open field arena (40x40cm with 65-cm walls) (Olesen et al., 2012a) or the central zone of the plus maze (7x7cm, surrounded by 4 arms, 27x7cm, 2 opposing open arms and 2 opposing closed arms with 13.5-cm walls) (Olesen et al., 2012a). Each session was recorded and later scored for various behavioral parameters using Ethovision software (Noldus Information Technology, NL) via center-point detection. For the ghrelin administration experiments, open field was performed with 60-minute habituation to the open field followed by ghrelin or saline injections i.p., after which a 60-minute session was recorded. In the plus maze test, the mice received ghrelin or saline injections i.p. 10 minutes prior to start of a 10-minute session. For the rAAV-mediated Ghr-R overexpression experiments, open field was run in 15-minute sessions without habituation and the plus maze test was run in 5-minute sessions.
Forced Swim Test
The mice were placed individually in a Plexiglas cylinder (diameter 10cm, height 25cm) containing 10cm of water maintained at 22 to 25°C for 6 minutes (Olesen et al., 2012a). The mice were considered immobile when floating in an upright position, making only small movements to keep their head above water (visually rated from video recordings). Ghrelin or saline was injected i.p. 10 minutes prior to a 6-minute session.
Tail Suspension Test
The mice were fastened to a stainless-steel bar 50cm above the floor using adhesive tape for 6 minutes as previously described (Christiansen and Woldbye, 2010). The stainless-steel bar was secured in a 3-sided container with black walls to isolate the mice from visual distractions while leaving one side open for video recording of the sessions. The mice were considered immobile when hanging passively and motionless from the bar (visually rated from video recordings). Ghrelin or saline was injected i.p. 10 minutes prior to a 6-minute session.
Restraint Stress
The mice were placed for 15 minutes into a 50-mL tube (NUNC, DK) fitted with 60 evenly distributed holes (3mm diameter) to facilitate air circulation (Christiansen et al., 2011). A piece of rubber (1 cm×2 cm×6cm) was fixed in place just behind the mouse to prevent the mouse from rotating or moving back and forth in the tube while allowing movement of the head.
During all of the behavioral tests mentioned above, mice did not have access to food in the period from ghrelin injections until test initiation and during testing.
Intellicage System
The Intellicage system (New Behavior AG, CH) is a newly developed system for automated behavioral assessment in a social home-cage environment. The system was described previously (Knapska et al., 2006; Rudenko et al., 2009). Briefly, the system consisted of a cover plate with 4 operant behavioral chambers fitted into a standard cage (Techniplast 2000). Each chamber consisted of a central tubular opening that led to a platform with 2 nose-poke holes with programmable mechanical doors leading to a drinking-bottle nozzle. Upon entry into the platform, the mice were registered via transponders, which were implanted s.c. under brief isoflurane anesthesia prior to the start of the experiment. Each corner was also equipped with an air-puff device for punishment, infrared motion detectors, and lickometers. Mice had free access to food from a central food hopper in the cover plate. The mice were habituated to the system for a minimum of 5 days before testing was started. For the experiments testing the effect of ghrelin, ghrelin or saline was injected s.c. 5 minutes prior to the start of the dark phase on test days. For the experiment comparing Ghr-R KO mice with WT controls, the mice received no injections. Test sessions were initiated at the start of the dark phase, at which point the Intellicage protocol was altered from free access to water in all corners to a new protocol, where the mice received an air-puff punishment when entering 1 of the 4 corners, chosen at random for each individual mouse. For the more severe stress experiment, the protocol was changed from free access to water in all corners to air-puffs upon entry to all corners from onset of dark phase. The stress protocols were run for up to 6 hours (see Results for details). Mice had free access to food at all times during testing in Intellicage.
rAAV-Mediated Ghr-R Overexpression
The rAAV vectors (GeneDetect, Auckland, NZ) derived from a mixture of serotypes 1 and 2 encoding the full-length cDNA for the human Ghr-R (stock solution: rAAV-Ghr-R; 1x1012 genomic particles/mL) and an empty vector as control (rAAV-Ctrl; 1x1012 genomic particles/mL). The transgene was subcloned into an rAAV expression cassette consisting of the rat neuron-specific enolase promoter, the woodchuck posttranscriptional regulatory element, and a bovine growth hormone polyA signal flanked by rAAV inverted terminal repeats that was previously successful for achieving overexpression of G-protein coupled receptors (Woldbye et al., 2010). The injection method was described earlier (Olesen et al., 2012a, 2012b). The mice were pretreated with the analgesics Rimadyl (5mg/kg, Pfizer) and Temgesic (0.06mg/kg, Reckit Benckiser) and the antibiotic Baytril (10mg/kg, Bayer Healthcare) before they were anaesthetized using isoflurane gas anesthesia (Baxter) and placed into a stereotaxic frame (Kopf Instruments). A total volume of 1 μL viral vector suspension was injected through a glass pipette at 0.2 μL/min bilaterally at 2 sites into the amygdala (anterior-posterior: -1.4mm and medial-lateral: +/-3.2mm relative to Bregma; dorsal-ventral: 3.9 mm+4.2mm relative to dura, 0.5 μL at each injection site) (Paxinos and Franklin, 2001). The injection pipette was left in place for 3 minutes after injection to prevent backflow of viral particles through the injection track. The mice received daily postoperative treatment with analgesic (Rimadyl, 5mg/kg) and antibiotic (Baytril, 10mg/kg) for 2 days.
Verification of rAAV-Mediated Overexpression of the Ghrelin Receptor
Mice were terminated by decapitation and brains were rapidly removed and frozen in powdered dry ice. Brains were sectioned on a cryostat in 14-μm-thick coronal sections spanning the amygdala. Sections were mounted on Superfrost glass slides and kept at -80°C until use.
In Situ Hybridization
The in situ hybridization procedure was essentially performed as described earlier (Woldbye et al., 2005; Christensen et al., 2006). The slides were defrosted for 10 minutes at room temperature and subsequently fixed in 4% paraformaldehyde for 5 minutes and rinsed in phosphate-buffered saline for 2x5 minutes. The slides were then acetylated for 10 minutes (0.25% acetic anhydride, 0.9% NaCl, 1,4% triethanolamine), run through a series of dehydration steps (70% ethanol – 5 minutes, 95% ethanol – 1 minute, 99% ethanol – 1 minute, chloroform – 5 minutes, 99% ethanol – 1 minute, 95% ethanol – 1 minute) and left to dry. Using the following synthetic anti-sense oligonucleotide DNA probes: Ghr-R mRNA: 5’-GGA TGA AGG CAA ACA CCA CTA CAG CCA GCA TTT TCA CGG TTT GCT TG-3’ and c-fos mRNA: 5’-CGG GCA GTG GCA CGT CTG GAT GCC GGC TGC CTT GCC TTC TCT GAC TGC-3’, the oligoprobes were labeled at the 3’-end with [33P]dATP (3000 Ci/mmol; NEG312H250UC; PerkinElmer) using terminal deoxynucleotidyl transferase (Roche Diagnostics, Mannheim, Germany). The labeled probe was added to a hybridization buffer containing 50% formamide (vol/vol), 20% saline sodium citrate (x20), 10% dextransulphate, 2% Denhards (x50), 0.5% yeast t-RNA (50mg/mL), 5% salmon DNA-sperma (10mg/mL), and 10mM dithiotreitol. After adding a volume of 120 μL hybridization mixture to each slide, the slides were covered with Parafilm and left at 37°C in humidity boxes overnight. At room temperature, the slides were subsequently briefly rinsed in saline sodium citrate, washed for 4x15 minutes in saline sodium citrate at 60°C, and passed through a series of 1-minute rinses in saline sodium citrate, 70% ethanol, and 95% ethanol. Finally, the slides were air-dried and exposed together with 14C-microscales to Kodak BioMax MR films (Amersham Biosciences) for 2 to 4 weeks and developed in a Kodak GBX developer. The specificity of the antisense oligoprobe was confirmed by adding nonlabeled antisense probes (competitive control).
Laser Capture Microdissection (LCM)
Brain sections from Ghr-R overexpressing mice subjected to restraint stress were stained for 30 seconds with 0.1% cresyl violet acetate (Sigma-Aldrich) solution dissolved in 70% EtOH. Sections were subsequently dehydrated briefly in 96% and 99.9% EtOH and finally dried at room temperature for at least 2 minutes. Using a Zeiss P.A.L.M. Microdissection instrument, the BL-d was identified and captured on CapSure Macro LCM Caps (Life Technologies, Applied Biosystems, Carlsbad, CA). BL-d was collected bilaterally from two 14-μm coronal sections per animal.
RNA Extraction and cDNA Synthesis from LCM Dissected Samples
Cell lysis and RNA extraction were performed using the RNeasy Micro Kit with DNase digestion according to the manufacturer’s instructions (Qiagen). The RNA was eluded in 14 μL H2O, and cDNA for quantitative polymerase chain reaction (qPCR) analysis was produced using SuperScript III (Invitrogen).
qPCR Analysis
We used a fixed cDNA volume of 0.83 μL cDNA per sample, diluted 1:6 in H2O. qPCR analysis was performed on an LightCycler480 (Roche Applied Science, Penzberg, Germany) using PrecisionPLUS Mastermix premixed with SYBRgreen (Primerdesign). The expression levels of the genes of interest were normalized to the average expression of the housekeeping genes TATA-box binding protein (TBP) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta (YWHAZ), using the ΔΔCt method (Livak and Schmittgen, 2001). All runs consisted of a preincubation step at 95°C for 2 minutes followed by 45 cycles of (95°C, 15 seconds; 60°C, 45 seconds). The following forward (F) and reverse (R) primers were used: TBP: F: TCA AAC CCA GAA TTG TTC TCC; R: GGT AGA TGT TTT CAA ATG CTT CA; YWHAZ: F: AGA CGG AAG GTG CTG AGA AA; R: GAA GCA TTG GGG ATC AAG AA; corticotropin-releasing factor (CRF): F: TTC TTG CAG CCG GAG CAG CC; R: GCG TGG AGT TGG GGG ACA GC; 5-HT1a: F: ACA GGG TGA GGA CGA CA; R: CTC TCC AAG CAG GCG GGG AC; 5-HT2c: F: GCT CTC TAG TGC AGG AAA AGG CTG C; R: GCA GGT CCA CGA ATG AAA CAA TGG C; 5-HT5a: F: TGG TAC ATG AGC TGT CTG GGC G; R: AGG TGG CGC GTG ATT GAC CA; 5-HT7: F: ACA CAA GCG ATT CCC CGG AGC; R: GGT GGA AAG TCA CAA GGA CCC TGC. All analyses were performed in duplicates.
Hormone Measurements
Blood was sampled through the orbital vein into EDTA-coated tubes (Microvette 500 K3E, Sarstedt, Germany) on ice. Blood samples were centrifuged at 3000 x g for 15 minutes at 4°C and plasma was isolated, frozen, and stored at -80°C until use. Corticosterone and adrenocorticotropic hormone (ACTH) plasma levels were measured using ELISA according to the manufacturer’s guidelines (corticosterone ELISA, Demeditec; ACTH ELISA, Calbiotech, CA). Blood samples for ghrelin measurements were treated as above except for the addition of Pefabloc (Sigma-Aldrich) to a final concentration of 1mg/mL. After centrifugation, plasma was acidified with HCl to a final concentration of 0.05N. Total and active ghrelin were measured according to the manufacturer’s instructions (Human ghrelin [Total] and [Active], Millipore, Billerica, MA).
Statistics
All data were analyzed using Graphpad Prism 6.0. The level of significance was set at P≤.05, and all data are presented as mean ± SEM. See table and Figure legends for details on individual analyses.
RESULTS
Ghrelin Exerts Anxiolytic-Like Effects and Increases Activity but Does Not Affect Depression-Like Behavior
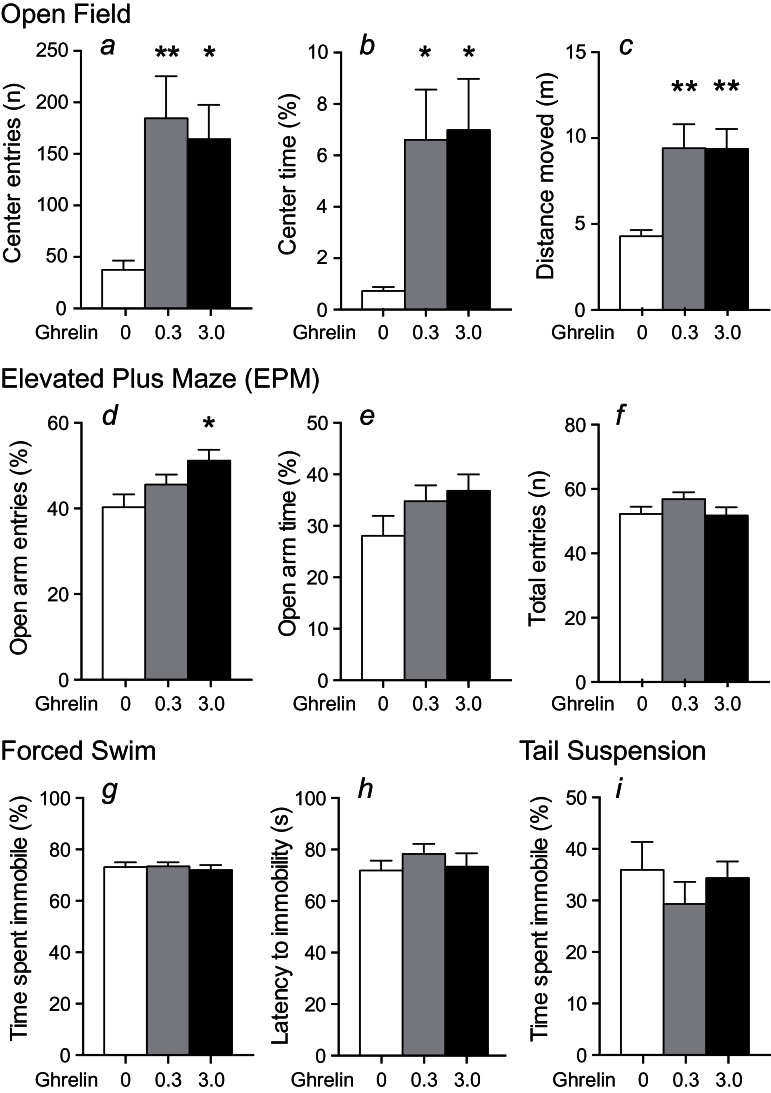
To determine the effect of acute ghrelin administration on anxiety-like behavior, we exposed male NMRI mice to the open field and EPM tests after ghrelin administration (0.3 and 3.0mg/kg, i.p.). Ghrelin increased center entries and total time spent in the center of the open field arena at both doses, indicating an anxiolytic-like effect (Figure 1a-b). Ghrelin also caused increased locomotor activity, seen as increased total distance moved in the open field arena (Figure 1c). The anxiolytic-like effect of ghrelin was confirmed in the EPM test, where we observed a dose-dependent response to ghrelin, seen as an increased number of entries into and time spent on the open arms (Figure 1d-e). This reached statistical significance on open arm entries with the highest dose of ghrelin. In the EPM test, we observed no effect on total number of entries to both open and closed arms (Figure 1f), indicating that the anxiolytic-like effect in this model is independent of effects on locomotor activity. In contrast, we observed no effect of ghrelin on depression-like behavior in male NMRI mice measured as time spent immobile and latency to first immobility in the forced swim test (Figure 1g-h) and time spent immobile in the tail suspension test (Figure 1i). Mice did not have access to food during testing.
Figure 1.
Acute effect of ghrelin (0, 0.3, and 3.0mg/kg, i.p.) on anxiety- and depression-like behavior in male NMRI mice. (a-c) Ghrelin increased center entries (a), center time (b), and distance moved (c) in the open field arena at both doses tested, indicating an anxiolytic-like effect of ghrelin (n=8). (d-f) Ghrelin dose-dependently increased open arm entries (d) and time (e) in the plus maze test, reaching statistical significance with the highest dose on open arm entries, while having no effect on total entries (f), supporting an anxiolytic-like effect of ghrelin (n=17–18). Open arm entries and time were calculated as open arm/(open+closed arms). (g-h) Ghrelin had no effect on time spent immobile (g) or latency to the first immobility (h) in the forced swim test (n=10). (i) Ghrelin had no effect on time spent immobile in the tail suspension test (n=10). Asterisks indicate statistical significance compared with the vehicle group in a Dunnett’s posthoc test after significant 1-way ANOVA (*P<.05, **P<.01).
Ghrelin Increases Activity during Acute Stress in a Social Home-Cage Environment
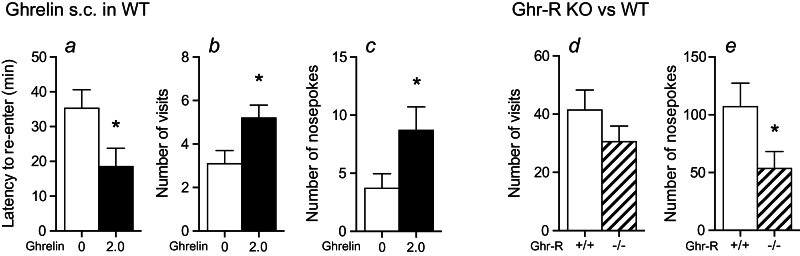
A limitation of most behavioral tests is that they are performed on mice in an isolated and novel environment even though mice are social animals. This potentially leads to elevated stress levels, resulting in altered behavior compared with the normal social condition. It is therefore interesting to evaluate behavioral parameters related to stress and anxiety in mice in a familiar home-cage social environment in order to minimize stress. This is especially true for C57BL/6 mice, which are used as background strain for most transgenic mouse models, since they are highly susceptible to stress. To minimize environmentally induced stress, we utilized the Intellicage system, in which various behavioral parameters can be evaluated in a familiar and social environment (Knapska et al., 2006; Endo et al., 2011). Using this system, we examined how ghrelin affected activity during acute stress, presented as an air-puff when visiting 1 of the 4 corners holding water bottles. In female C57BL/6 mice, acute ghrelin administration (2mg/kg, s.c.) decreased the latency to reenter any corner after a visit to the punished corner (Figure 2a). Also, reduced stress was seen as increased number of visits and nosepokes within the first hour after ghrelin administration (Figure 2b-c). These data support the notion that ghrelin has an anxiolytic-like effect during an acute stressful condition. Conversely, Ghr-R KO mice exposed to a similar test in the Intellicage system displayed a tendency to lower number of visits and significantly fewer nosepokes compared with WT mice, suggesting that Ghr-R deficiency is associated with anxiogenic-like effects (Figure 2d-e).
Figure 2.
Effect of ghrelin administration (2.0mg/kg, s.c.) and Ghrelin receptor (Ghr-R) knockout (KO) during mild stress (air-puff in one corner) in a social home-cage environment (Intellicage system). (a-c) Ghrelin decreased latency to reenter any corner after a visit to the air-puff punished corner (calculated as the mean time after first and second visit to punished corner to next visit to any corner) (a) and increased total number of visits (b) and nosepokes (c) to any corner during 1 hour of air-puff punishment in 1 of 4 corners in the Intellicage system, indicating a stress-attenuating effect of ghrelin (n=10). (d-e) Ghr-R KO decreased total number of visits (d) and nosepokes (e) during 6 hours of air-puff punishment in 1 of 4 corners in the Intellicage system, supporting that ghrelin protects against acute stress (n=7). We chose to analyze the effect of ghrelin during 1 hour after administration because of the short half-life of ghrelin. In contrast, since the Ghr-R KO and WT mice received no injections, we chose to analyze a longer period to see a baseline behavioral effect (6 hours). Asterisks indicate statistical significance in unpaired Student’s t test (*P≤.05).
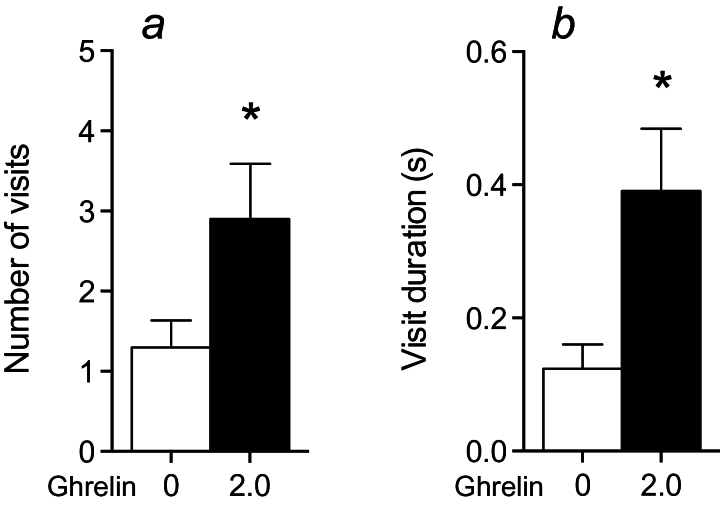
To further evaluate the effect of ghrelin during more severe stress, mice were subjected to air-puff punishment upon entry to any of the 4 corners holding water bottles. In accordance with results from the mild stress test, we observed that ghrelin administration increased both number and duration of visits to any corner even during this increased stress (Figure 3), although there was no effect on nosepokes (data not shown). Due to the high stress level, the number of reentries after punishment was low and latency to reenter was not evaluated. Mice had free access to food during all testing in Intellicage.
Figure 3.
Effect of ghrelin administration (2.0mg/kg, s.c.) during stronger stress (air-puff in all corners) in a social environment (Intellicage system). Ghrelin increased visit number (a) and visit duration (b) during 3 hours of air-puff punishment in all corners in the Intellicage system, indicating a stress-attenuating effect of ghrelin (n=10). Because of the stronger stress, the mice displayed rather few visits, and we consequently had to analyze data over a longer period (3 hours) in this experiment compared with the mild stress experiment (Figure 2 a-c). Asterisks indicate statistical significance in unpaired Student’s t test (*P≤.05).
rAAV-Mediated Intra-Amygdala Overexpression of the Ghr-R Causes Modest Anxiolytic-Like Behavior without Effect on Acute Stress Responses
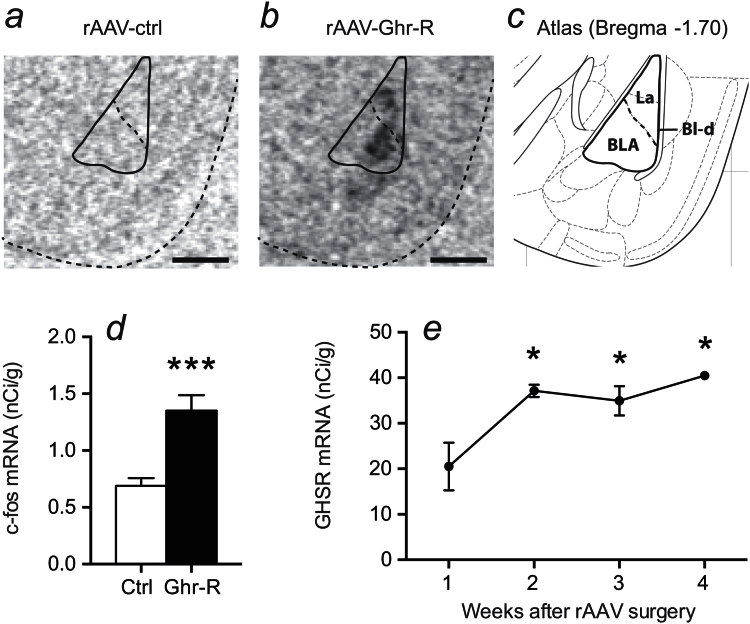
The amygdala, more specifically the BL-d, has been suggested as an important region for ghrelin-mediated regulation of anxiety-like behavior (Alvarez-Crespo et al., 2012; Currie et al., 2014). Therefore, we used a rAAV vector to overexpress the Ghr-R in the BL-d and examined the effect on anxiety-like behavior during basal conditions and after acute restraint stress. We confirmed overexpression of Ghr-R in the BL-d by in situ hybridization (Figure 4a-b). Additionally, we performed in situ hybridization for c-fos and showed that the rAAV-mediated Ghr-R overexpression was associated with significantly increased c-fos activity in the BL-d (Figure 4d), suggesting that the overexpressed Ghr-Rs are functionally active and cause increased activity in the region. To optimize the timing of our experiments, we performed Ghr-R in situ hybridization on mice terminated 1, 2, 3, and 4 weeks after rAAV injection surgery. We found Ghr-R mRNA expression to reach maximum levels 2 weeks after surgery and the expression level to be stable through weeks 3 and 4 (Figure 4e). Consequently, we evaluated the effect of Ghr-R overexpression on anxiety-like behavior starting 2 weeks after rAAV surgery. We did this by testing the mice under basal conditions and after 15 minutes of acute restraint stress in the open field and EPM tests, 4 tests in total with 1 week in between each test.
Figure 4.
Verification of recombinant adeno-associated viral vector (rAAV)-mediated overexpression of Ghrelin receptor (Ghr-R) in the basolateral division of the amygdala (BL-d). (a-c) In situ hybridization shows Ghr-R mRNA expression in the lateral (La) and basolateral (BLA) nuclei of Ghr-R-overexpressing mice. (d) c-fos mRNA expression is significantly increased in the BL-d in Ghr-R-overexpressing mice compared with controls (quantified from in situ hybridization, n=8–12). (e) Timeline of Ghr-R mRNA expression after rAAV surgery showing a peak after 2 weeks, which is stable until 4 weeks after surgery (n=3). Scale bars=0.5mm. Asterisks indicate statistical significance in unpaired Student’s t test (d) or compared with 1 week after rAAV surgery in Dunnett’s posthoc test following significant 1-way ANOVA (e) (*P<.05, ***P<.001).
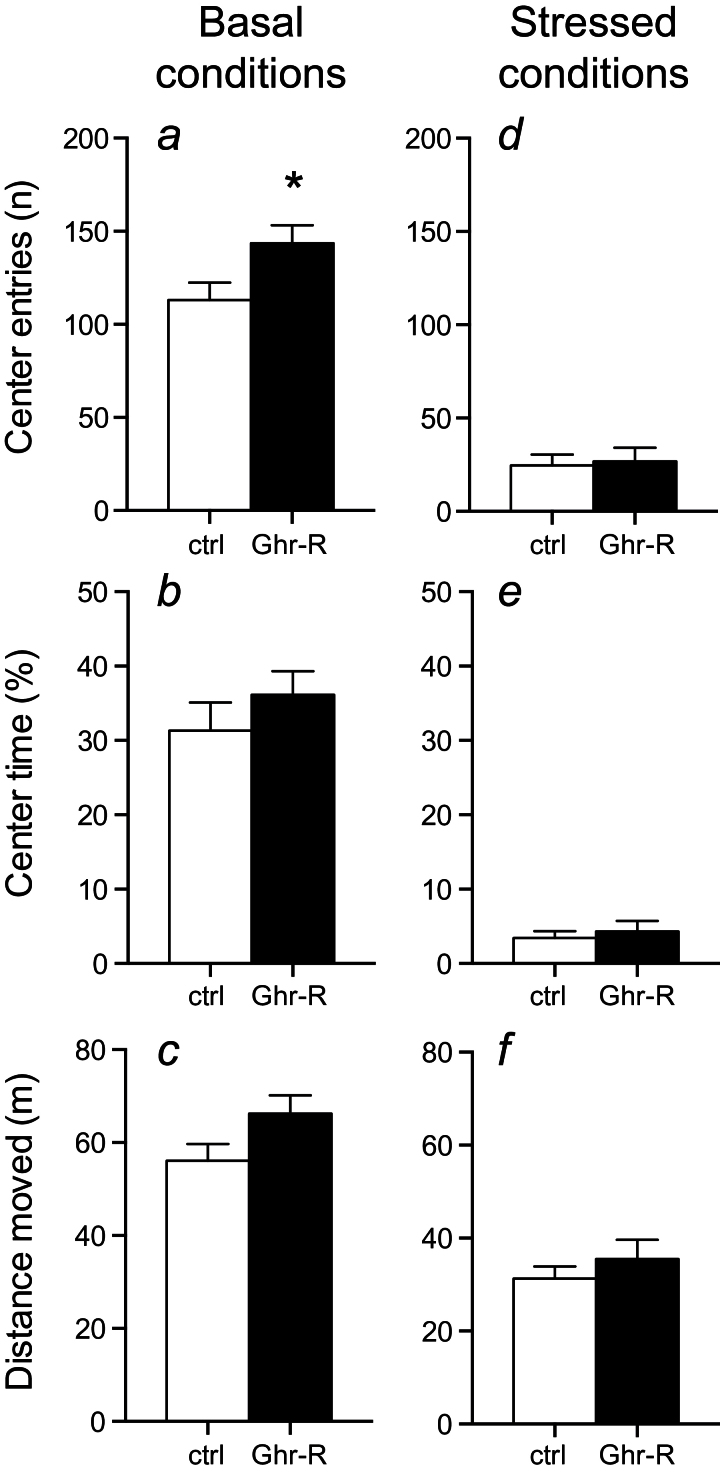
In open field, the Ghr-R overexpression caused a significant increase in center entries during the basal conditions (Figure 5a), while a trend was seen towards increased time spent in center and total distance moved (Figure 5b-c). Restraint stress prior to open field testing resulted in decreased distance moved and decreased center entries and time spent in the open field compared with baseline levels (Figure 5d-f), thus verifying the effectiveness of the stress in producing an anxiogenic-like effect. In the stressed condition, no difference was observed between Ghr-R overexpression and control mice. Mice did not have access to food during testing.
Figure 5.
Effect of intra-basolateral division of the amygdala (BL-d) recombinant adeno-associated viral vector (rAAV)-mediated Ghrelin receptor (Ghr-R) overexpression on behavior in the open field test during basal conditions and after 15-minute restraint stress (n=8–12). (a-c) Ghr-R overexpression caused significantly increased center entries and a trend towards increased center time and distance moved in the open field. (d-f) After 15 minutes of restraint stress, the mice had decreased center entries, spent decreased time in the center, and moved a shorter distance, but there was no difference between Ghr-R-overexpressing and empty vector control mice on either parameter tested. Asterisk indicates statistical significance in unpaired Student’s t test (*P<.05).
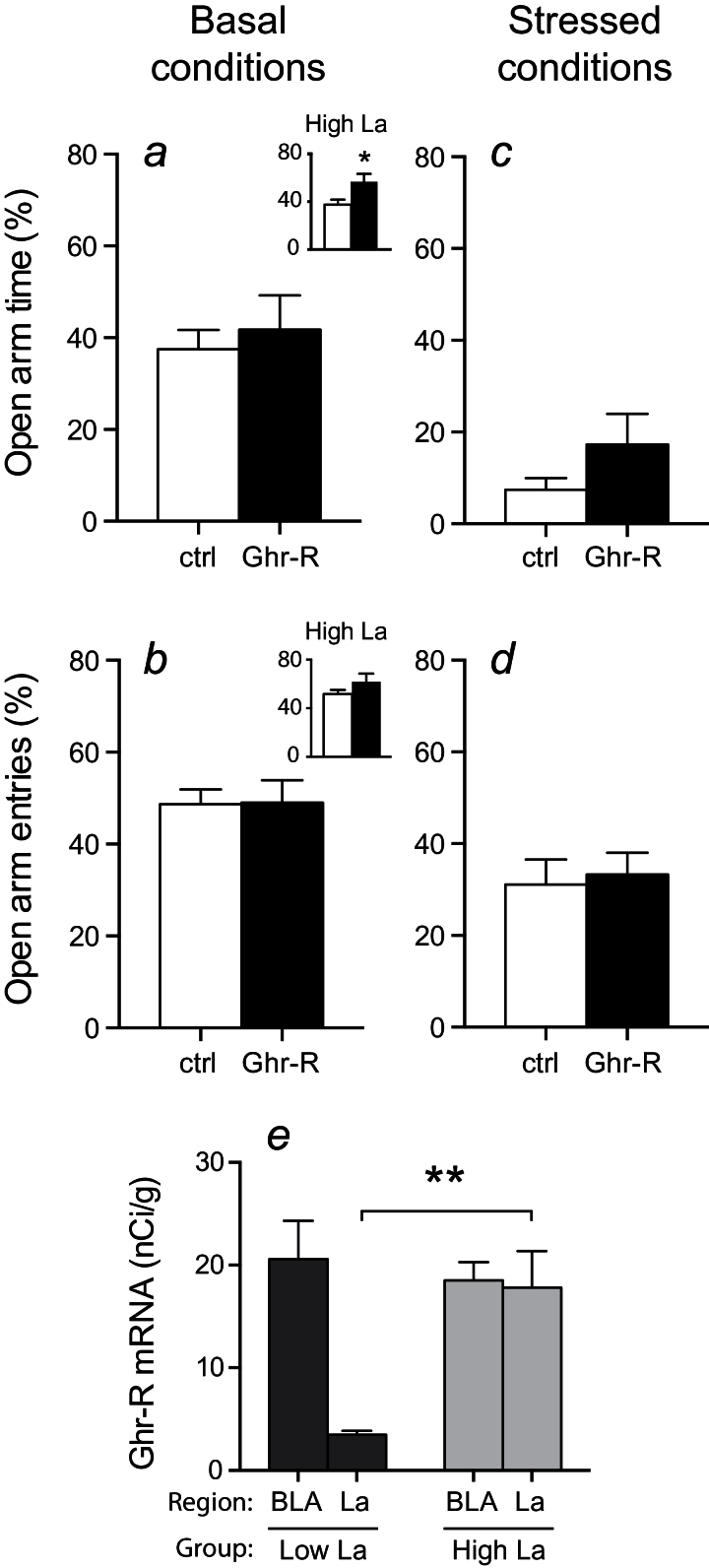
In the EPM test, Ghr-R overexpression caused no change in open arm time or entries compared with control mice under basal conditions (Figure 6a-b). Restraint stress was also verified in this behavioral test by an increase in anxiety-like behavior, seen as decreased time spent in open arms and decreased open arm entries (Figure 6c-d). However, there was no difference between Ghr-R overexpressing mice and controls after this type of acute stress, indicating a similar behavioral response to acute stress in the 2 groups. Mice did not have access to food during testing.
Figure 6.
Effect of intra-basolateral division of the amygdala (BL-d) recombinant adeno-associated viral vector (rAAV)-mediated Ghrelin receptor (Ghr-R) overexpression on behavior in the elevated plus maze (EPM) test during basal conditions and after 15-minute restraint stress. (a-b) Ghr-R overexpression had no significant effect on open arm time and open arm entries in the EPM (n=8–12). (c-d) After 15 minutes of restraint stress, the mice spent less time on and had fewer entries to the open arms of the plus maze, but there was no difference between Ghr-R-overexpressing and control mice for either parameter tested (n=8–12). (e) Ghr-R mRNA levels quantified using in situ hybridization in basolateral (BLA) and lateral (La) nuclei of the 2 subgroups with low and high La expression, respectively. (a-b inserts) The subgroup of mice, which displayed high Ghr-R overexpression in the La in addition to the BLA (n=4), spent significantly more time and had a tendency toward increased number of entries into the open arms of the EPM compared with the empty vector control group (n=12). Asterisks indicate statistical significance in unpaired Student’s t test (a) or in Bonferroni posthoc test after significant 2-way ANOVA (e) (*P<.05, **P<.01).
Interestingly, when we reanalyzed the Ghr-R in situ data with respect to subnuclear localization, we observed a difference in Ghr-R overexpression in the 2 BL-d subnuclei, BLA and La (Figure 4). We essentially observed high Ghr-R mRNA expression in the BLA in all mice, whereas the Ghr-R expression in the La was more varied. Because of this, we divided the mice into 2 subgroups having similar Ghr-R expression in the BLA and either low or high Ghr-R expression in the La (Figure 6e). We then reanalyzed the behavioral data looking specifically at the 2 subgroups vs the control group and found that the subgroup with high Ghr-R expression in the La showed a significant increase in open arm time in the EPM test and a trend toward open arm entries during basal conditions (Figure 6a-b, inserts). No differences between the subgroups were seen after acute stress (data not shown). This suggests that expression of Ghr-R in La may be specifically important for the effect of ghrelin in regulating anxiety-like behavior evaluated in the EPM.
In a different cohort of mice, we examined the effect of intra-BL-d Ghr-R overexpression on stress-induced hormone response by subjecting the mice to 15 minutes of restraint stress after which eye-blood was drawn. Ghr-R overexpression had no significant effect on corticosterone plasma concentration (rAAV-Ghr-R: 189.2±12.0ng/mL, control: 195.5±18.4ng/mL; P=.23) while causing a trend towards a decrease in ACTH plasma concentration (rAAV-Ghr-R: 1730±194 pg/mL, control: 2257±190 pg/mL; P=.066) (Table 1), although not reaching statistical significance.
Table 1.
Effect of rAAV-Mediated Overexpression of the Ghrelin Receptor on the HPA Axis
| Control | rAAV-Ghr-R | |
|---|---|---|
| ACTH (pg/mL) | 2257±190 | 1730±194 (P=.066) |
| Corticosterone (ng/mL) | 196±18 | 189±12 |
Abbreviations: ACTH: adrenocorticotropic hormone.
rAAV-mediated overexpression of the ghrelin receptor in NMRI mice (n=11–12). Data tested with unpaired 2-tailed t test.
We also assessed the effect of ghrelin treatment on the HPA axis and plasma ghrelin levels in separate cohorts of C57Bl/6 female and NMRI male mice in setups aimed to mimic behavioral testing. Male NMRI mice were injected with ghrelin (3mg/kg i.p.) in the early light phase and single-housed in novel cages. After 15 minutes, blood was sampled. Female C57Bl/6 mice were injected with ghrelin (2mg/kg s.c.) and group housed for 30 minutes before blood sampling. The ghrelin blood concentration in NMRI mice increased from 194±34.8 to 11801±1708 pg/mL (P<.0001) for total ghrelin, while active ghrelin levels increased from 52.1±10.2 to 1769±293 pg/mL (P<.001) relative to controls (Table 2). In C57Bl/6 female mice, total ghrelin increased from 338±31.4 to 3646±153 pg/mL (P<.0001) and active ghrelin increased from 97.6±17.9 to 708±81.5 pg/mL (P<.0001) relative to controls (Table 2). Ghrelin injections increased corticosterone levels in male NMRI mice from 95.7±9.8 to 160±8.5ng/mL (P<.001), whereas ACTH levels were unaltered (Table 2). Ghrelin treatment in C57Bl/6 female mice did not affect ACTH or corticosterone levels significantly (Table 2).
Table 2.
Effect of Ghrelin Injections on the HPA Axis and Ghrelin Plasma Levels
| C57BL/6 Females Saline | C57BL/6 Females Ghrelin (2mg/kg s.c.) | NMRI Males Saline | NMRI Males Ghrelin (3mg/kg i.p.) | |
|---|---|---|---|---|
| Ghrelin (active) (pg/mL) | 97.6±17.9 | 708 ±81.5#### | 52.1±10.2 | 1769±293*** |
| Ghrelin (total) (pg/mL) | 338±31.4 | 3646±153#### | 194±34.8 | 11801±1708**** |
| ACTH (pg/mL) | 455±14.0 | 440±10.9 | 465±43.9 | 494±23.7 |
| Corticosterone (ng/mL) | 256±7.74 | 281±9.2 | 95.7±9.8 | 160±8.5 *** |
Abbreviations: ACTH: adrenocorticotropic hormone.
Acute effect of ghrelin in C57Bl/6 female mice (2mg/kg s.c.) and in male NMRI mice (3mg/kg i.p.) on total and active ghrelin, ACTH and corticosterone levels measured 30min after injection (C57Bl/6 females) or 15 minutes after injection (NMRI males). n=8/group. Data tested with unpaired two-tailed t-test. (#### P<.0001 C57Bl/6 females saline vs ghrelin, ***P<.001, ****P<.0001 NMRI males saline versus ghrelin).
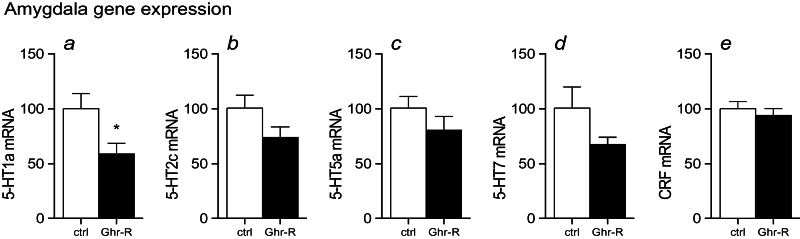
It has previously been suggested that the serotonin system plays an important role in mediating the ghrelin-induced change in anxiety-like behavior (Hansson et al., 2011, 2014). To explore the impact of the serotonin system for the behavioral effects observed in the Ghr-R overexpressing mice subjected to restraint stress, we performed laser capture microdissection of the BL-d and analyzed for expression of the serotonin receptors previously identified as being regulated by ghrelin administration, that is, 5HT1a, 5HT2c, 5HT5a, and 5HT7 receptors (Hansson et al., 2014). We found a decreased expression of the 5HT1a receptor in the Ghr-R overexpression group compared with the control group (Figure 7a), whereas none of the other 5HT receptors were significantly regulated (Figure 7b-d). Likewise, CRF expression was unaltered in the Ghr-R overexpression group compared with controls (Figure 7e).
Figure 7.
Effect of intra-basolateral division of the amygdala (BL-d) recombinant adeno-associated viral vector (rAAV)-mediated Ghrelin receptor (Ghr-R) overexpression on expression of corticotropin-releasing factor (CRF) and 5-HT1a, 5-HT2c, 5-HT5a, and 5-HT7 receptor mRNA in the BL-d in mice subjected to restraint stress. Ghr-R overexpression significantly decreased 5-HT1a expression (a) but had no significant effect on the expression of 5-HT2c, 5-HT5a, 5-HT7, or CRF (b-e) (n=11–12). Levels of mRNA were calculated relative to the mean CT value of the reference genes TATA-box binding protein (TBP) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta (YWHAZ); empty vector control levels were set to 100. Asterisk indicates statistical significance in unpaired Student’s t test (*P<.05).
Discussion
Studying the effect of ghrelin on anxiety- and depression-like behavior and stress, we found peripherally injected ghrelin to have anxiolytic-like and stress-attenuating effects, while no effect on depression-like behavior was observed. The opposite was observed in Ghr-R KO mice. We also found that overexpression of the constitutively active Ghr-R in the BL-d of the amygdala caused an anxiolytic-like effect, which was associated with decreased expression of the 5HT1a receptor in the BL-d. We examined both female and male mice and included both C57Bl/6 and NMRI mice in our analyses, as different strains have divergent behavioral responses to different tests (Griebel et al., 2000) and thus some strains are more suited for certain tests than others. However, even when using different strains and both genders, we found an overall anxiolytic-like effect of ghrelin treatment.
The ghrelin system was previously suggested to regulate anxiety- and depression-like behavior. Thus, in rodents, ghrelin was initially found to increase anxiety-like behavior when injected either peripherally or into discrete brain regions (Asakawa et al., 2001; Carlini et al., 2002, 2004; Currie et al., 2011; Hansson et al., 2011). Conversely, other studies indicate that ghrelin has anxiolytic- and anti-depression-like effects (Lutter et al., 2008; Yamamoto et al., 2009), and no consensus on the effect of ghrelin on these behaviors has been reached. We examined the acute effect of ghrelin on anxiety- and depression-like behavior and found that peripheral ghrelin administration increased time spent in and number of entries into the center of the open field. Furthermore, ghrelin dose-dependently increased open arms entries in the EPM test, indicating an anxiolytic-like effect of ghrelin.
Interaction between the ghrelin system and stress has been demonstrated in various ways. Thus, β1-adrenergic receptors have been found highly enriched on the ghrelin-producing cells and adrenergic agonists potently stimulate ghrelin release (Zhao et al., 2010; Engelstoft et al., 2013). Ghrelin plasma levels are increased by stress in rodents (Kristenssson et al., 2006; Lutter et al., 2008) and humans (Rouach et al., 2007), indicating that ghrelin might protect against the negative effects of stress. This is supported by observations from Ghr-R-deficient mice that show exacerbated stress-related symptoms in a model of chronic social defeat stress (Lutter et al., 2008). Additionally, ghrelin-deficient mice were found to be more anxious after acute restraint stress compared with WT controls (Spencer et al., 2012). To further study the effect of ghrelin on stress, we used the Intellicage system. In this system, behavioral parameters can be studied under socially housed conditions, thereby eliminating the isolation-induced stress occurring in most of the widely used behavioral tests. From these studies, we found that peripheral ghrelin administration attenuated air-puff–induced stress, seen as increased activity during air-puff punishment and shorter delay to approach the corners after receiving an air-puff punishment in the Intellicage system. In agreement with this, Ghr-R-deficient mice displayed decreased activity during air-puff punishment, indicating an increased stress response compared with WT mice. Taken together, these results support the view that increasing ghrelin receptor signaling results in anxiolytic-like effects during basal conditions and protects against the adverse effects of stress.
The amygdala lies at the center of emotional connectivity in the mammalian brain, and it is highly important for regulating anxiety-related behavior (Forster et al., 2012). The Ghr-R is expressed in numerous brain regions, including the amygdala (Guan et al., 1997; Zigman et al., 2006; Alvarez-Crespo et al., 2012; Mani et al., 2014), supporting the concept that the ghrelin system may be involved in the modulation of anxiety and related emotions in this region. Thus, one study found that ghrelin decreases anxiety-like behavior when injected directly into the La (Alvarez-Crespo et al., 2012), whereas other studies found ghrelin to have an anxiogenic-like effect when injected into the BLA (Currie et al., 2014; Meyer et al.,2014).
In the present study, we used a different approach to examine the role of ghrelin signaling in the amygdala on anxiety-like behavior and stress, overexpressing Ghr-Rs in the BL-d using a viral vector. The subsequent behavioral tests were performed in free fed mice to ensure low plasma levels of ghrelin and no exogenous ghrelin was administered. Under these conditions, we found that Ghr-R overexpression caused an anxiolytic-like effect both in the open field and elevated plus maze tests.
These results indicate that the level of Ghr-R expression and availability regulates the activity without pharmacological administration of ghrelin. However, even under basal conditions, a constant low level of ghrelin is secreted and it is possible that the observed effect is caused by an increased number of receptors available for the circulating ghrelin. Another explanation is that the ghrelin receptor is responsible for the observed effect via its inherent constitutive activity. Such constitutive activity allows the Ghr-R to transmit signals to the cell, also when no ligand is present (Holst et al., 2003, 2004), and simple changes in the expression of the receptor may alter the intracellular signaling. Increased signaling of the ghrelin receptor mediates an elevated activity of the cells, as this receptor is coupled to Gαq. The Gαqwill lead to accumulation of inositol phosphate and increase in the intracellular calcium level (Holst et al., 2004). The observed increase in cFos activity may reflect this elevated activation state. For instance, it has been shown that during fasting and other conditions associated with high appetite, where the plasma level of ghrelin is increased, the ghrelin receptor expression in the appetite-regulating arcuate nucleus of the hypothalamus is also increased (Nogueiras et al., 2004; Petersen et al., 2009). Since the typical pattern for G-protein coupled receptors is that the expression decreases in response to high levels of agonist exposure (Ferguson and Caron, 1998), the fact that the expression of the Ghr-R follows that of ghrelin is proposed to be a mechanism to further increase the appetite (Petersen et al., 2009). Only indirect evidence is available to support that the constitutive activity of the ghrelin receptor has important physiological functions. For example, it has been shown that administration of an inverse agonist of the Ghr-R decreases appetite and body weight and that humans born with a variant in their Ghr-R that eliminates the constitutive activity has short stature (Pantel et al., 2006; Petersen et al., 2009). The observations from the present study support that simple overexpression of the Ghr-R leads to increased signaling in the BL-d that attenuates anxiety-like behavior.
CRF expression in the amygdala and plasma levels of corticosterone and ACTH was not affected by Ghr-R overexpression in the amygdala, pointing to an anxiolytic-like effect mediated through an independent pathway of the HPA axis. Interestingly, the effect of intra-ventricular ghrelin has been associated with increased turnover of serotonin and increased expression of several serotonin receptors in the amygdala (Hansson et al., 2014). When we measured the gene expression level of the 4 5HT receptors previously shown to be regulated in the amygdala by intra-ventricular ghrelin (Hansson et al., 2014), we found a significant decrease in the expression of the 5HT1a receptor. The 5-HT1a receptor has previously been shown to be essential in modulation of mood and anxiety in BLA. Though the literature is somewhat contradictory, most studies indicate that decreased fear and anxiety is associated with enhanced serotonergic transmission primarily in the BLA (Inoue et al., 2004; Leite-Panissi et al., 2006). With regard to the 5HT1a receptor, antagonism was shown to prevent the anxiolytic effect of approved antidepressant treatment (Vicente and Zangrossi, 2014). Also, 5HT1a receptor KO mice have an anxiogenic phenotype, and 5HT1a overexpression is associated with anxiolytic behavior in mice (Bert et al., 2008). As previously mentioned, chronic stimulation of a nonconstitutively active receptor, such as the 5HT1a receptor, is frequently associated with a counter regulatory decrease in the receptor expression, which could explain the decreased 5HT1a receptor expression in the Ghr-R-overexpressing mice.
In conclusion, our findings support the view that ghrelin has acute anxiolytic-like effects and protects against the negative effects of acute stress. Moreover, we show that overexpression of the Ghr-R in the BL-d is associated with an anxiolytic-like effect. Interestingly, this was done without administration of ghrelin and under conditions where endogenous ghrelin plasma levels were low. This suggests that the effect we see is likely a result of constitutive activity of the receptor itself, although an effect through endogenous ghrelin cannot be ruled out in the present study. The anxiolytic-like effect after rAAV-Ghr-R administration is associated with decreased expression of the 5HT1a receptor, which is in agreement with previous studies, highlighting that the 5HT1a receptor is important for anxiolytic behavior.
Funding
This work was supported by funding from the Novo Nordisk Foundation and the University of Copenhagen BioScaRT Program of Excellence.
Interest Statement
None.
Acknowledgements
We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen for access.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Crespo M, Skibicka KP, Farkas I, Molnár CS, Egecioglu E, Hrabovszky E, Liposits Z, Dickson SL. (2012) The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS One 7:e46321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M. (2001) A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74:143–147. [DOI] [PubMed] [Google Scholar]
- Bert B, Fink H, Rothe J, Walstab J, Bönisch H. (2008) Learning and memory in 5-HT(1A)-receptor mutant mice. Behav Brain Res 195:78–85. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. (2002) Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun 299:739–743. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. (2004) Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun 313:635–641. [DOI] [PubMed] [Google Scholar]
- Christensen DZ, Olesen MV, Kristiansen H, Mikkelsen JD, Woldbye DP. (2006) Unaltered neuropeptide Y (NPY)-stimulated [35S]GTPgammaS binding suggests a net increase in NPY signalling after repeated electroconvulsive seizures in mice. J Neurosci Res 84:1282–1291. [DOI] [PubMed] [Google Scholar]
- Christiansen SH, Woldbye DP. (2010) Regulation of the galanin system by repeated electroconvulsive seizures in mice. J Neurosci Res 88:3635–3643. [DOI] [PubMed] [Google Scholar]
- Christiansen SH, Olesen MV, Wörtwein G, Woldbye DP. (2011) Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav Brain Res 216:585–591. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Khelemsky R, Rigsbee EM, Dono LM, Coiro CD, Chapman CD, Hinchcliff K. (2011) Ghrelin is an orexigenic peptide and elicits anxiety–like behaviors following administration into discrete regions of the hypothalamus. Behav Brain Res 226:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Schuette LM, Wauson SE, Voss WN, Angeles MJ. (2014) Activation of urocortin 1 and ghrelin signaling in the basolateral amygdala induces anxiogenesis. Neuroreport 25:60–64. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. (2006) Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 9:381–388. [DOI] [PubMed] [Google Scholar]
- Endo T, Maekawa F, Võikar V, Haijima A, Uemura Y, Zhang Y, Miyazaki W, Suyama S, Shimazaki K, Wolfer DP, Yada T, Tohyama C, Lipp HP, Kakeyama M. (2011) Automated test of behavioral flexibility in mice using a behavioral sequencing task in IntelliCage. Behav Brain Res 221:172–181. [DOI] [PubMed] [Google Scholar]
- Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2:376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Caron MG. (1998) G protein-coupled receptor adaptation mechanisms. Semin Cell Dev Biol 9:119–127. [DOI] [PubMed] [Google Scholar]
- Forster G, Novick A, Scholl J, Watt M. (2012) The role of the amygdala in anxiety disorders. In: The amygdala: a discrete multitasking manager. (Barbara Ferry, ed), ISBN: 978-953-51-0908-2, InTech, doi: 10.5772/50323. Available from: http://www.intechopen.com/books/the-amygdala-a-discrete-multitasking-manager/the-role-of-the-amygdala-in-anxiety-disorders. [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. (2000) Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology 148(2):164–170. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. (1997) Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48:23–29. [DOI] [PubMed] [Google Scholar]
- Hansson C, Haage D, Taube M, Egecioglu E, Salomé N, Dickson SL. (2011) Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience 180:201–211. [DOI] [PubMed] [Google Scholar]
- Hansson C, Alvarez-Crespo M, Taube M, Skibicka KP, Schmidt L, Karlsson-Lindahl L, Egecioglu E, Nissbrandt H, Dickson SL. (2014) Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology 79:498–505. [DOI] [PubMed] [Google Scholar]
- Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. (2003) High constitutive signaling of the ghrelin receptor-identification of a potent inverse agonist. Mol Endocrinol 17:2201–2210. [DOI] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. (2004) Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem 279:53806–53817. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974–977. [DOI] [PubMed] [Google Scholar]
- Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S, Koyama T. (2004) Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. Eur J Pharmacol 497:311–316. [DOI] [PubMed] [Google Scholar]
- Knapska E, Walasek G, Nikolaev E, Neuhäusser-Wespy F, Lipp HP, Kaczmarek L, Werka T. (2006) Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem 13:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660. [DOI] [PubMed] [Google Scholar]
- Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C, Håkanson R, Lindström E. (2006) Acute psychological stress raises plasma ghrelin in the rat. Regul Pept 134:114–117. [DOI] [PubMed] [Google Scholar]
- Leite-Panissi CR, Ferrarese AA, Terzian AL, Menescal-de-Oliveira L. (2006) Serotoninergic activation of the basolateral amygdala and modulation of tonic immobility in guinea pig. Brain Res Bull 69:356–364. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. (2008) The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11:752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perelló M, Andrews ZB, Zigman JM. (2014) Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RM, Burgos-Robles A, Liu E, Correla SS, Goosens KA. (2014) A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry 19(12):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. (2007) The amygdala, reward and emotion. Trends Cogn Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tovar S, Mitchell SE, Rayner DV, Archer ZA, Dieguez C, Williams LM. (2004) Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 53:2552–2558. [DOI] [PubMed] [Google Scholar]
- Olesen MV, Christiansen SH, Gøtzsche CR, Holst B, Kokaia M, Woldbye DP. (2012a) Y5 neuropeptide Y receptor overexpression in mice neither affects anxiety- and depression-like behaviours nor seizures but confers moderate hyperactivity. Neuropeptides 46:71–79. [DOI] [PubMed] [Google Scholar]
- Olesen MV, Christiansen SH, Gøtzsche CR, Nikitidou L, Kokaia M, Woldbye DP. (2012b) Neuropeptide Y Y1 receptor hippocampal overexpression via viral vectors is associated with modest anxiolytic-like and proconvulsant effects in mice. J Neurosci Res 90:498–507. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. (2003) Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides 24:597–602. [DOI] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie-Luton MP, Grouselle D, de Kerdanet M, Kadiri A, Epelbaum J, Le Bouc Y, Amselem S. (2006) Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 116:760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2001) The mouse brain in stereotaxic coordinates, 2nd ed New York: Academic Press. [Google Scholar]
- Petersen PS, Woldbye DP, Madsen AN, Egerod KL, Jin C, Lang M, Rasmussen M, Beck-Sickinger AG, Holst B. (2009) In vivo characterization of high basal signaling from the ghrelin receptor. Endocrinology 150:4920–4930. [DOI] [PubMed] [Google Scholar]
- Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y. (2007) The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 32:693–702. [DOI] [PubMed] [Google Scholar]
- Rudenko O, Tkach V, Berezin V, Bock E. (2009) Detection of early behavioral markers of Huntington’s disease in R6/2 mice employing an automated social home cage. Behav Brain Res 203:188–199. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, Andrews ZB. (2012) Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry 72:457–465. [DOI] [PubMed] [Google Scholar]
- Tóth K, László K, Lénárd L. (2010) Role of intraamygdaloid acylated-ghrelin in spatial learning. Brain Res Bull 81:33–37. [DOI] [PubMed] [Google Scholar]
- Vicente MA, Zangrossi H. (2014) Involvement of 5-HT2C and 5-HT1A receptors of the basolateral nucleus of the amygdala in the anxiolytic effect of chronic antidepressant treatment. Neuropharmacology 79:127–135. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Nanobashvili A, Sørensen AT, Husum H, Bolwig TG, Sørensen G, Ernfors P, Kokaia M. (2005) Differential suppression of seizures via Y2 and Y5 neuropeptide Y receptors. Neurobiol Dis 20:760–772. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Angehagen M, Gøtzsche CR, Elbrønd-Bek H, Sørensen AT, Christiansen SH, Olesen MV, Nikitidou L, Hansen TV, Kanter-Schlifke I, Kokaia M. (2010) Adeno-associated viral vector-induced overexpression of neuropeptide Y Y2 receptors in the hippocampus suppresses seizures. Brain 133:2778–2788. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Tanahashi T, Kawai T, Chikahisa S, Katsuura S, Nishida K, Teshima-Kondo S, Sei H, Rokutan K. (2009) Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics 39:227–235. [DOI] [PubMed] [Google Scholar]
- Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. (2010) Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S A 107:15868–15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. (2005) Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]