Abstract
Background:
Chronic ethanol exposure reduces dopamine transmission in the nucleus accumbens, which may contribute to the negative affective symptoms associated with ethanol withdrawal. Kappa opioid receptors have been implicated in withdrawal-induced excessive drinking and anxiety-like behaviors and are known to inhibit dopamine release in the nucleus accumbens. The effects of chronic ethanol exposure on kappa opioid receptor-mediated changes in dopamine transmission at the level of the dopamine terminal and withdrawal-related behaviors were examined.
Methods:
Five weeks of chronic intermittent ethanol exposure in male C57BL/6 mice were used to examine the role of kappa opioid receptors in chronic ethanol-induced increases in ethanol intake and marble burying, a measure of anxiety/compulsive-like behavior. Drinking and marble burying were evaluated before and after chronic intermittent ethanol exposure, with and without kappa opioid receptor blockade by nor-binaltorphimine (10mg/kg i.p.). Functional alterations in kappa opioid receptors were assessed using fast scan cyclic voltammetry in brain slices containing the nucleus accumbens.
Results:
Chronic intermittent ethanol-exposed mice showed increased ethanol drinking and marble burying compared with controls, which was attenuated with kappa opioid receptor blockade. Chronic intermittent ethanol-induced increases in behavior were replicated with kappa opioid receptor activation in naïve mice. Fast scan cyclic voltammetry revealed that chronic intermittent ethanol reduced accumbal dopamine release and increased uptake rates, promoting a hypodopaminergic state of this region. Kappa opioid receptor activation with U50,488H concentration-dependently decreased dopamine release in both groups; however, this effect was greater in chronic intermittent ethanol-treated mice, indicating kappa opioid receptor supersensitivity in this group.
Conclusions:
These data suggest that the chronic intermittent ethanol-induced increase in ethanol intake and anxiety/compulsive-like behaviors may be driven by greater kappa opioid receptor sensitivity and a hypodopaminergic state of the nucleus accumbens.
Keywords: anxiety, compulsion, voltammetry, release, uptake
Introduction
Alcoholism is a disease of chronic relapse, consisting of repeated bouts of excessive intake and abstinence, resulting in withdrawal symptoms that contribute to the resumption of heavy drinking. For example, human alcoholics report increased anxiety (Wetterling and Junghanns, 2000) and compulsive behaviors (Suzuki et al., 2002) during abstinence periods, which correlate with escalated ethanol consumption (Wetterling et al., 2006; American Psychological Association, 2013). Examination of these symptoms in a mouse model of alcohol use disorders showed that C57BL/6 (C57) mice exposed to ethanol vapor in a chronic, intermittent (chronic intermittent ethanol [CIE]) pattern demonstrate escalated ethanol intake during abstinence (Griffin et al., 2009). Traditional measures of anxiety-like behavior, such as the elevated plus maze and light-dark box, have not found ethanol withdrawal-induced anxiogenic phenotypes in C57 mice (Ghozland et al., 2005; McCool and Chappell, 2015). However, data supporting ethanol withdrawal-related increases in nontraditional anxiety/compulsive-like behaviors, such as marble burying, are emerging. For example, recent work utilizing C57 mice exposed to chronic ethanol injections or an ethanol-containing liquid diet (Perez and DeBiasi, 2015) showed increased marble burying during ethanol withdrawal. Marble burying is used to model compulsive- and anxiety-like behaviors in rodents, and pharmacotherapies for anxiety and obsessive-compulsive disorder reduce this behavior (Nicolas et al., 2006). Thus, marble burying may provide a useful behavioral measure of ethanol withdrawal-associated negative affect in this mouse strain.
Dopamine transmission is markedly attenuated after chronic ethanol exposure (Karkhanis et al., 2015), which may be driving CIE-induced changes in behavior. A previous study reported reduced dopamine terminal function in the nucleus accumbens (NAc) after 3 cycles of CIE and 72 hours of abstinence (Karkhanis et al., 2015), indicating a hypodopaminergic state of this region during abstinence. The time-course of these findings is similar to CIE-induced increases in ethanol drinking using a similar protocol (Griffin et al., 2009), suggesting a link between attenuated accumbal function and augmented ethanol intake. As the dopamine system has been implicated as an important regulator of ethanol drinking (Nealey et al., 2011) and anxiety/compulsive-like behaviors (Ballester-Gonzáles et al., 2015), it is possible that dysregulated dopamine transmission may underlie chronic ethanol-induced increases in these behaviors.
Although dopamine terminal function is attenuated after CIE, the precise mechanism(s) underlying this change are not well understood. Dopamine transmission in the NAc is regulated by a variety of receptors, including kappa opioid receptors (KORs). Intra-accumbal pharmacological activation of KORs reduces dopamine release in this region, while blockade transiently increases extracellular levels of dopamine (Spanagel et al., 1992). Consistent with these data, administration of a KOR agonist in naïve rats (Todtenkopf et al., 2004) increases brain reward thresholds, as measured by intra-cranial self-stimulation, suggesting that KOR activation reduces mesocorticolimbic signaling and produces negative affect. Notably, KORs on mesolimbic dopamine neurons mediate place aversion to KOR agonists (Chefer et al., 2012), supporting a modulatory role of KORs on mesolimbic dopamine system function that influences hedonic state. Further, withdrawal from ethanol (Schulteis et al., 1995) and cocaine (Chartoff et al., 2012) increases brain reward thresholds, an effect that is blocked with KOR antagonists (Chartoff et al., 2012). Thus, KOR blockade may attenuate the negative reinforcing effects of ethanol by regulating dopamine transmission.
Of interest to this particular study are data demonstrating the utility of KORs as a potential pharmacotherapeutic target to alleviate the symptoms of ethanol withdrawal. CIE-exposed rats routinely demonstrate increased ethanol self-administration after a period of abstinence compared with air-exposed controls, an effect that is reduced to control levels with systemic (Walker et al., 2011), intracerebroventricular (Walker and Koob, 2008), or intra-NAc (Nealey et al., 2011) administration of nor-binaltorphimine (norBNI), a KOR-specific antagonist. As KOR manipulation alters dopamine system function (Todtenkopf et al., 2004; Zapata and Shippenberg, 2006; Chartoff et al., 2012) and KOR blockade reduces ethanol self-administration in dependent rats (Walker and Koob, 2008; Walker et al, 2011; Nealey et al., 2011), it is possible that changes in KOR function and dopamine transmission drive CIE-induced behavioral changes observed during ethanol abstinence. The present study used repeated cycles of CIE and air exposure to examine the role of KORs in ethanol consumption and marble burying in C57 mice. Additionally, ex vivo fast scan cyclic voltammetry (FSCV) in brain slices of the NAc was used to identify CIE-induced changes in dopamine terminal and KOR function.
Methods and Materials
Subjects
Male C57 mice (65–75 days old, Jackson Laboratories) were used for all experiments. Mice were individually housed and maintained on a 12-:12-hour-light cycle (lights off at 2:00 pm) with a red room light illuminated during the dark cycle. Mice habituated to housing conditions for 1 week before experiment start and were provided with standard chow and water ad libitum, unless otherwise noted. The Institutional Animal Care and Use Committee at Wake Forest School of Medicine approved all experimental protocols. Animals were cared for according to National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities.
CIE Exposure: Back-to-Back CIE and Back-to-Back CIE+Drinking Protocol
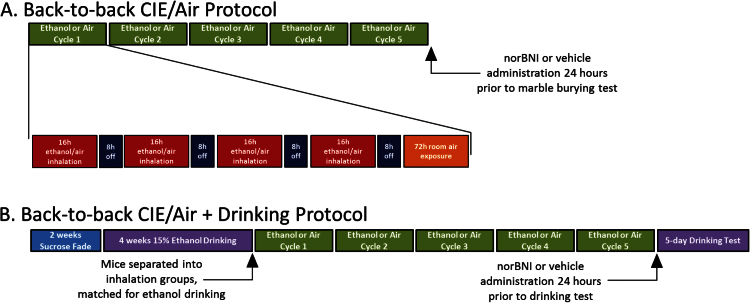
Mice were exposed to ethanol vapor or room air for 4 days (16 hours of exposure,8 hours of abstinence), followed by 72 hours of room air exposure (one cycle). Cycles were repeated 5 times. Mice were treated (i.p.) with a solution containing 1.6g/kg ethanol (CIE) or saline (air) and 1.0 mmol pyrazole (Sigma Aldrich), an ethanol dehydrogenase inhibitor, 30 minutes prior to inhalation treatment. Blood ethanol concentrations were tested after the first and fourth inhalation exposure to ensure physiologically relevant blood ethanol concentrations (Griffin et al., 2009) (Supplementary Figure 2). The back-to-back CIE protocol (Figure 1A) was modified (back-to-back CIE+drinking protocol, Figure 1B) to examine CIE-induced changes in ethanol drinking.
Figure 1.
Back-to-back chronic intermittent ethanol (CIE) protocols. (A) Visual depiction of the back-to-back CIE protocol. Mice were exposed to 5 cycles of ethanol vapor or room air (green bars). Each cycle consisted of 4 days of 16 hours of exposure (red bars), 8 hours of abstinence (dark blue bars), followed by 72 hours of room air exposure (orange bar). (B) Visual depiction of the back-to-back CIE + drinking protocol. Mice underwent a 2-bottle choice drinking procedure (2 weeks of sucrose fade [blue bar] followed by 4 additional weeks of access to 15% (vol/vol) ethanol [left purple bar]). Both CIE and air groups underwent 5 cycles of inhalation exposure (green bars), and ethanol drinking was examined following all CIE exposure cycles (right purple bar).
Ethanol Drinking Tests
Animals in ethanol drinking groups underwent a 2-bottle choice procedure that included 2 weeks of sucrose fade (Samson, 1986) consisting of 2 days each: 10% ethanol/5% sucrose, 12%/5%, 15%/5%, 15%/2%, and 15%/1% (wt/vol), followed by 4 weeks of access to 15% (vol/vol) ethanol. Experimental solutions were provided for 2h/d (1:30–3:30 pm) 5d/wk and paired with an identical bottle of water. Bottle placement was switched daily. Mice were assigned to inhalation groups, counterbalanced for intake. Ethanol drinking was examined for 5 days after all cycles (Figure 1B). Separate groups of animals were injected with norBNI (10mg/kg, i.p.; NIDA) or vehicle (injectable water) 24 hours prior to the postinhalation ethanol drinking test.
A separate group of mice was not exposed to the inhalation treatment, but during the final (fourth) week of baseline (15% vs water) drinking, they were injected with saline (0.1mL, i.p.) 30 minutes prior to drinking start time (1:30 pm). During the following several weeks, mice were injected with saline (0.1mL) on Mondays, Wednesdays, and Fridays. In a Latin-square design, mice were injected i.p. with 1.0, 3.0, 6.0, and 10mg/kg U50,488 on Tuesdays and Thursdays. Drinking data were collapsed across saline and each dose of U50,488.
Marble Burying
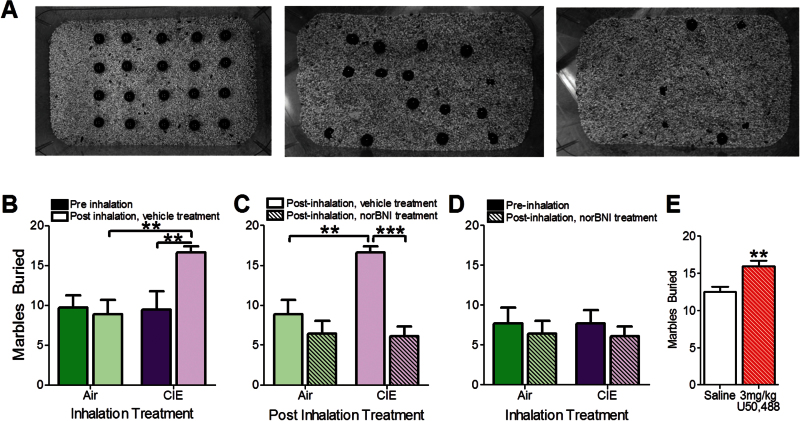
Preinhalation marble burying tests occurred on the morning of the first day of CIE cycle 1. Mice were placed in a standard polycarbonate cage lined with ~4cm of cob bedding (The Andersons) for 120 minutes before the addition of 20 clean, black glass marbles (14mm, Rainbow Turtle). A template was used to ensure marble placement (Figure 4A, left). After the 30-minute testing session, the number of marbles >75% buried was counted. Water was provided during habituation, but both food and water were unavailable during testing. Animals were placed into inhalation groups counterbalanced for marbles buried during the pretest. CIE-induced changes in marble burying were assessed 72 hours after the fifth CIE cycle. Separate groups of mice were systemically injected with norBNI (10mg/kg) or vehicle 24 hours prior to postinhalation testing. A separate group of mice was injected with saline (0.1mL) or 3.0mg/kg U50,488 30 minutes prior to testing at approximately 90 minutes into their habituation session.
Figure 4.
Chronic intermittent ethanol (CIE) exposure increased marble burying behavior, which was nor-binaltorphimine (norBNI)-sensitive and was replicated with kappa opioid receptor (KOR) agonist administration. (A) Representative pictures of marble configuration: before testing (left), after 5 cycles of air (middle), and CIE (right) exposure. (B) CIE exposure significantly augmented marble burying compared with air-exposed mice and preinhalation marble-burying behavior. (C) CIE augmented marble burying in CIE-exposed compared with air-exposed mice, which was reduced with norBNI treatment. (D) Marble burying following norBNI treatment is similar to preinhalation exposure marble-burying behavior. (A-D) n (air)=7; n (CIE)=9 (E) KOR activation with a 3.0-mg/kg dose of U50,488 significantly augmented marble-burying behavior in naïve mice compared with mice treated with a saline challenge. N (saline)=7; n (3.0mg/kg U50,488)=7. **P<.01; ***P<.001.
Ex Vivo FSCV
Ex vivo FSCV was used to characterize dopamine kinetics in the NAc core (Karkhanis et al., 2015). Mice were sacrificed 72 hours after the fifth inhalation exposure to coincide with behavioral testing. Mice were anesthetized with isoflurane, rapidly decapitated, and brains removed. Brain slices containing the NAc (300 µm thick) were prepared with a vibrating tissue slicer and incubated in oxygenated artificial cerebrospinal fluid (32°C). A carbon fiber recording electrode (≈50–100 µm length, 7 µM radius; Goodfellow Corporation) and a bipolar stimulating electrode (Plastics One) were placed (~100 µm apart) on the surface of the slice. Dopamine efflux was induced with a single, rectangular, 4.0-ms duration electrical pulse (350 µA, monophasic, inter-stimulus interval: 180 seconds). To detect dopamine release, a triangular waveform (-0.4 to +1.2 to -0.4V vs silver/silver chloride, 400V/sec) was applied every 100ms to the recording electrode. To assess the effects of inhalation treatment on KOR function, the KOR agonist, U50,488 (0.01–1.0 µM, Tocris Bioscience), was added cumulatively to artificial cerebrospinal fluid after baseline collections were stable. To obtain clear current vs time plots, background current subtraction methods were utilized. Electrode calibration was performed following each experiment using a flow-injection system to a known concentration of dopamine (3.0 µM).
Data Analysis
Ethanol consumption was calculated by weighing ethanol and water bottles before and after drinking tests. Preference was calculated as the volume of ethanol consumed divided by total intake (ethanol+water) consumed in each session. Marble burying was scored by visual inspection of marbles.
Demon Voltammetry and Analysis software (Yorgason et al., 2011) was used to collect and analyze all voltammetry data. Representative signals were analyzed before drug application to determine dopamine release per electrical stimulation (μM) and uptake (Vmax, μM/sec). Representative traces after drug application were similarly analyzed. The effects of U50,488 on dopamine release across the concentration response curve are presented as a percent of pre-drug dopamine release.
Graphs were created and statistical tests were applied using GraphPad Prism, Version 5. Two-way repeated measures (RM) ANOVA was used to compare the effects of CIE exposure and drug treatment (norBNI vs vehicle) on ethanol drinking, preference, and marble burying as well as the U50,488 concentration response curve, with drug concentration and inhalation treatment as factors. All drinking data were collapsed across the 5-day exposure or drug dose (saline or U50,488). When a significant main effect was found, Bonferroni posthoc analysis was performed. A 1-way ANOVA was used to analyze the effects of acute U50,488 on ethanol drinking and preference. When a significant main effect was detected, Tukey’s posthoc analysis was applied. Two-tailed Student’s t tests were used to reveal preinhalation ethanol drinking and preference differences between inhalation groups, the effects of CIE exposure on baseline dopamine kinetics, the effect of 3.0mg/kg U50,488 on marble burying, and U50,488 IC50.
Results
CIE Increased Ethanol Intake and Preference without Inter-Cycle Ethanol Drinking
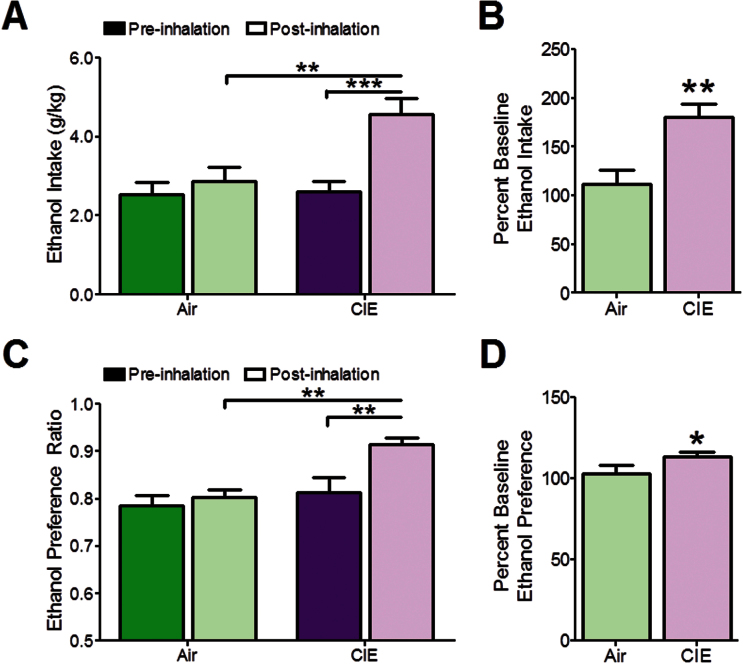
To confirm that the CIE exposure protocol used here increased ethanol drinking similarly to previously used protocols, separate groups of mice underwent a CIE+drinking protocol, (Griffin et al., 2009) (Supplementary Figure 1). Blood ethanol concentrations were collapsed across CIE exposure protocols and were within the behaviorally relevant range (Griffin et al., 2009) (Supplementary Figure 2, 204.9±58.47mg/dL). Two-way ANOVA revealed a main effect of inhalation exposure (Figure 2A, F1,236.599, P<.05) and time (pre- vs postinhalation exposure, F1,23=11.16, P<.01). Poshoc analysis confirmed similar preinhalation ethanol consumption between groups (air: 2.53±0.33, CIE: 2.60±0.28g/kg, P>.05), but an increase in ethanol intake in CIE-exposed mice was observed (P<.001). An interaction between inhalation exposure and time was also detected (F1,23=5.543, P<.05). As hypothesized, CIE-exposed mice exhibited increased ethanol intake, as a percent of preexposure intake levels, compared with controls as examined with a 2-tailed Student’s t test (Figure 2B: air: 112.00±14.30%, CIE: 180.30±13.15%, t10=4.49, P<.01). The amount of ethanol consumed on each day is depicted in Supplementary Figure 3.
Figure 2.
Chronic intermittent ethanol (CIE)-exposed mice demonstrated increased ethanol consumption and preference without inter-cycle ethanol drinking tests. (A) Preinhalation ethanol intake was similar between groups. CIE significantly augmented ethanol drinking in CIE-exposed mice compared with air-exposed control animals and pre-CIE exposure ethanol intake. (B) Ethanol intake was significantly greater in CIE-exposed compared with air-exposed mice when expressed as a percent of preinhalation intake. (C) CIE increased ethanol preference in CIE-exposed mice compared with air-exposed control mice and preinhalation ethanol preference. (D) Ethanol preference was significantly greater in CIE-exposed mice compared with air-exposed animals. n (air)=6; n (CIE)=7. *P<.05; **P<.01; ***P<.001.
Analysis of ethanol preference revealed a main effect of inhalation treatment (Figure 2C, F1,22=9.38, P<.05) and time (pre- vs postinhalation, F1,22=7.03, P<.05). Augmented ethanol preference in CIE-exposed mice compared with pre-CIE (P<.01) and air-exposed animals (P<.01) was observed with posthoc analysis levels (air: 0.81±0.02, CIE: 0.913±0.04). A 2-tailed Student’s t test revealed increased ethanol preference in CIE-exposed mice compared with controls when expressed as a percent of preinhalation preference (Figure 2D: air: 103.00±4.69%, CIE: 113.30±2.30%, t11=3.51, P<.05).
Kor Blockade Reduced Ethanol Consumption and Preference in CIE-Treated Mice
To examine the role of KORs in CIE-induced changes in ethanol drinking, norBNI or vehicle was systemically administered 24 hours prior to the 5-day drinking test (Broadbear et al., 1994). Two-way ANOVA revealed a main effect of inhalation (Figure 3A; F1,28=9.20, P<.01) and drug treatment (norBNI vs vehicle) on ethanol intake (F1,28=28.60, P<.001). An interaction between inhalation and drug treatment was also found (F1,28=10.01, P<.01). Posthoc analysis confirmed a significant increase in ethanol drinking in CIE-exposed mice treated with vehicle (4.57±0.40g/kg, P<.001) compared with CIE-exposed mice treated with norBNI (2.23±0.19g/kg). Ethanol drinking in norBNI treated/CIE-exposed mice was similar to intake of norBNI-treated/air-exposed (2.27±0.19g/kg, P>.05).
Figure 3.
Systemic nor-binaltorphimine (norBNI) administration reduced ethanol intake and preference chronic intermittent ethanol (CIE)-treated mice that was replicated with kappa opioid receptor (KOR) activation. (A) CIE significantly augmented ethanol drinking compared with air-exposed control mice, which was reduced with norBNI treatment. (B) Ethanol preference of CIE-exposed mice was significantly greater than air-exposed controls, which was reduced to control levels with norBNI treatment. (A-B) n (air/vehicle)=6; n (air/norBNI)=9; n (CIE/vehicle)=7n (CIE/norBNI)=10. (C) U50,488 increased ethanol intake and (D) preference, which returned to baseline at 10mg/kg. (C-D) n (saline)=14; n (1.0mg/kg U50,488)=9; n (3.0mg/kg U50,488)=10; n (6.0mg/kg U50,488)=9; n (10.0mg/kg U50,488)=9. *P<.05; **P<.01; ***P<.001.
Examination of ethanol preference after CIE/air exposure revealed a main effect of norBNI treatment (Figure 3B; F1,27=26.21, P<.001). Although no main effect of inhalation treatment was detected (F1,27=1.22, P=.28), potentially reflecting a ceiling effect due to high baseline preference, an interaction between inhalation and drug treatment (norBNI vs vehicle) was observed (F1,27=11.25, P<.01). Reduced ethanol preference in CIE-exposed mice treated with norBNI (preference ratio: 0.70±0.03) compared with CIE-exposed vehicle-treated animals (preference ratio: 0.92±0.015, P<.001) was found using posthoc analysis, but norBNI-treated/CIE-exposed mice had similar preferences to air-exposed mice treated with vehicle (preference ratio: 0.79±0.02, P>.05) or norBNI (preference ratio: 0.76±0.02, P>.03). The effects of inhalation and drug treatment were maintained for the 5-day drinking test (Supplementary Figure 3).
U50,488 Increased Ethanol Consumption and Preference
NorBNI reduced ethanol intake in CIE-exposed animals (Figure 3A-B), suggesting that KORs positively modulate drinking. Thus, we hypothesized that KOR activation would augment ethanol consumption and preference in naïve mice. A on1e-way ANOVA revealed a significant effect of U50,488 on ethanol intake (Figure 3C, F5,46=18.86, P<.0001) and preference (Figure 3D, F5,46=5.903, P<.001). Tukey’s posthoc analysis revealed a significant difference increase at the 1.0- (P<.001), 3.0- (P<.001), and 6.0- (P<.5) mg/kg doses of U50,488 in ethanol drinking compared with saline drinking. Further, Tukey’s posthoc analysis revealed a significant increase in ethanol preference at the 1.0- (P<.01), 3.0- (P<.05), and 6.0- (P<.5) mg/kg doses of U50,488 compared with control (saline). In both datasets (Figure 3C-D), posthoc analysis showed that drinking in mice given saline or the 10-mg/kg dose of U50,488 was similar (P>.05).
CIE Increased Marble Burying Behavior
Marble burying was used to examine the effects of CIE or air exposure on anxiety/compulsive-like behaviors. Representative pictures of testing chambers are shown in Figure 4A: before testing (left), after 5 cycles of air exposure (middle), and CIE exposure (right). A 2-way RM ANOVA revealed a main effect of time (pre- vs postinhalation; Figure 4B; F1,14=4.69, P<.05). Posthoc analysis showed an increase in marble-burying behavior between pre- (9.44±2.32 marbles buried) and post (16.67±0.73 marbles buried) CIE exposure (P<.01). A significant increase in marble burying between post-CIE and post-air exposure inhalation groups (8.86±1.82 marbles buried, P<.05) and an interaction between inhalation exposure and time (F1,28=5.42, P<.05) were observed.
KOR Blockade Reduced CIE-Induced Increases in Marble Burying
Mice were systemically injected with norBNI or vehicle 24 hours prior to the postinhalation marble-burying test. Two-way ANOVA revealed a main effect of inhalation condition (Figure 4C, F1,26=7.35, P<.05) and drug treatment (norBNI vs vehicle, F1,26=21.74, P<.001) on marble burying. Posthoc analysis confirmed an increase in marble burying in vehicle-treated CIE- vs air-exposed mice (P<.01). Additionally, marble-burying behavior of vehicle-treated/CIE-exposed mice was greater than that of CIE-exposed mice treated with norBNI (P<.001). An interaction between CIE exposure and drug treatment was detected (F1,16=8.59, P<.001). NorBNI reduced marble burying in CIE-exposed mice (6.13±1.22 marbles buried) to norBNI-treated air (6.43±1.60 marbles buried) and pre-CIE levels (Figure 4C; F1,26=0.01, P=.93). Additional experiments in naïve mice show that the effects of norBNI on ethanol drinking and marble burying are likely not attributable to changes in locomotion (Supplementary Figure 5).
To confirm that KOR activation would also augment marble burying, naive mice were injected with 3.0mg/kg U50,488 30 minutes prior to testing. A 2-tailed Student’s t test revealed augmented marbles buried in response to U50,488 administration (Figure 4E, saline: 12.57±0.649 marbles buried; 3.0mg/kg U50, 488: 16.00±0.723 marbles buried; t12=3.526, P<.01).
CIE Attenuated Dopamine Terminal Function via Reduced Dopamine Release and Increased Uptake
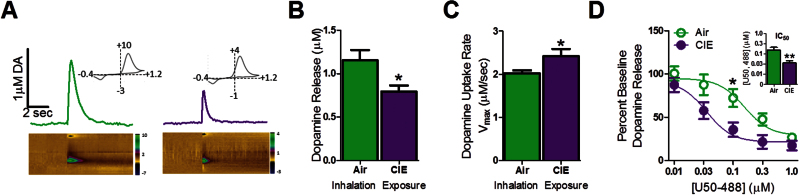
To examine changes in dopamine terminal function after CIE exposure, ex vivo FSCV was used. Figure 5A shows representative dopamine release and uptake traces with false color plots (air: left; CIE: right). A 2-tailed Student’s t test revealed decreased dopamine release (Figure 5B; air: 1.15±0.12 µM; CIE: 0.80±0.07 µM, t18=2.687, P<.05) and augmented uptake rate (Figure 5C; air: 2.02±0.07 µM/sec; CIE: 2.42±0.17 µM/sec, t21=2.20, P<.05) in CIE-exposed mice vs controls.
Figure 5.
Chronic intermittent ethanol (CIE) exposure attenuated accumbal dopamine terminal function and increased kappa opioid receptor (KOR) sensitivity. (A) Representative voltammetric traces after electrical stimulation of dopamine and associated false color plots (air, left, green; CIE, right, purple). False color plots demonstrate changes in current over time and in response to electrically evoked dopamine release (x axis: time, y axis: command voltage [−0.4 to +1.2 to -0.4 V], z axis: current). Cyclic voltammograms (insets) identify the peak oxidation (~+0.6V) and reduction voltages (~−0.2V) of dopamine. (B) CIE reduced electrically evoked dopamine release compared with air-exposed mice. (C) CIE augmented dopamine uptake in brain compared with controls. -(BC) n (air)=9; n (CIE)=11. (D) U50,488 dose-dependently reduced dopamine release across increasing concentrations, an effect was more pronounced in CIE-treated brain slices, reducing its IC50 in CIE-exposed mice (inset). n (air)=6; n (CIE)=7. *P<.05; **P<.01.
CIE Increases KOR Sensitivity in the NAc Core
Ex vivo FSCV was used to examine CIE-induced changes in KOR sensitivity. Two-way RM ANOVA revealed a main effect of CIE treatment on KOR sensitivity, suggesting increased KOR function in CIE-exposed mice compared with controls (Figure 5D; F1,11=5.00, P<.05). A main effect of U50,488 on dopamine release (F4,11=80.87, P<.001) and an interaction between inhalation treatment and U50,488 concentration were detected (F4,11=3.22, P<.05). A reduction in the U50,488 IC50 in CIE- compared with air-exposed mice was found (Figure 5D inset: air: 3.38±0.27 µM U50,488, CIE: 2.10±0.22 µM U50,488, t7=3.72, P<.01).
Discussion
The present work showed that CIE-induced behavioral changes in C57 mice were associated with augmented sensitivity of KORs on dopamine terminals and reduced dopamine transmission in the NAc. Ethanol drinking and preference were increased following a 5-week CIE protocol that also increased anxiety/compulsive-like marble-burying behavior. FSCV in the NAc showed that CIE reduced dopamine release, increased rates of dopamine uptake, and augmented the inhibitory effects of KORs on dopamine release, promoting a hypodopaminergic state of the NAc. These data suggest that accumbal hypodopaminergia may be partially driven by increased KOR function, leading, at least in part, to the potentiation of ethanol intake and anxiety/compulsive-like behaviors during ethanol abstinence.
CIE Exposure Increased Ethanol Consumption, Preference, and Marble-Burying Behavior
To examine the effects of CIE exposure on ethanol intake, both ethanol consumption and preference were examined using 2 distinct CIE exposure protocols. Experiments that included 5 days of ethanol drinking between each CIE cycle (Supplementary Figure 1) replicated previous findings that showed escalated ethanol consumption with sequential bouts of CIE and abstinence (Griffin et al., 2009). These data were similar to the abbreviated back-to-back CIE+drinking protocol, suggesting that CIE exposure alone is sufficient to increase ethanol drinking and preference. Thus, the back-to-back CIE exposure protocol was utilized for the remainder of the experiments.
Escalation of ethanol intake is a hallmark of chronic alcohol exposure that has been documented in humans (Wetterling et al., 2006; American Psychological Association, 2013) as well as in monkey (Grant et al., 2008), rat (Walker and Koob, 2008), and C57 mouse (Griffin et al., 2009; present work) models of alcoholism. Because abstinent alcoholics also report increased anxiety (Wetterling and Junghanns, 2000) and compulsive behaviors (Suzuki et al., 2002), a marble-burying assay was used to examine CIE-induced changes in anxiety/compulsive-like behaviors in C57 mice. This task is a nontraditional test for anxiety-like behavior and appears to measure some of the negative affective components of ethanol withdrawal, including anxiety-like and compulsive-like behaviors (Perez and DeBiasi, 2015; Nicolas et al., 2006). During ethanol abstinence, CIE-exposed mice exhibited increased marble burying compared with air-exposed controls. Since this CIE procedure also augmented ethanol drinking and preference, it is possible that CIE-exposed mice increase their ethanol intake to reduce the negative reinforcing effects of CIE exposure and withdrawal.
KOR Blockade Attenuated CIE-Induced Increases in Ethanol Consumption, Preference, and Marble Burying
After finding CIE-induced elevations in ethanol intake and marble burying, the role of KORs in both behaviors was tested. A single systemic administration of norBNI ameliorated CIE-induced increases in ethanol consumption and preference. This effect was maintained during the 5-day test, consistent with reports documenting a long-lasting effect of norBNI (Kishioka et al, 2013). Further, norBNI treatment attenuated CIE-induced increases in marble burying. Additional work in the present study showed that KOR activation with U50,488 increased ethanol drinking and preference in CIE-naïve mice. Moreover, a single dose of U50,488 increased the number of marbles buried, showing increased anxiety/compulsive-like behavior following KOR activation. Together, these data suggest that KORs play a role in ethanol withdrawal-associated negative affective symptoms and augmented drinking and suggest that pharmacological blockade of KORs could potentially serve a therapeutic role in withdrawn alcoholics.
Increased anxiety/compulsive-like behavior, opioid receptor signaling, and dopamine transmission following prolonged ethanol exposure are strikingly similar to studies examining different sources of negative affective states. In particular, negative affective states engendered by chronic stress or drug exposure have been tied to elevated KOR activity. For example, symptoms of withdrawal from ethanol (Walker et al., 2011; Nealey et al., 2011) or cocaine (Chartoff et al., 2012), social isolation rearing (Karkhanis et al., unpublished observations), social defeat stress (McLaughlin et al., 2006), and separation of pair-bonded prairie voles (Resendez et al., 2012) are rescued with KOR blockade. It is likely that KOR activity may be a ubiquitous mediator of negative affect and stress, although elevated KOR activity is a part of an extensive stress response system that also includes elevated levels of cortocotropin releasing factor (CRF; Britton et al., 2000). In fact, the CRF and KOR systems often play similar roles in stress responses (Britton et al., 2000; Lu et al., 2003), which has led to the hypothesis that the CRF and KOR systems are functionally linked (Van’t Veer et al., 2012).
Ethanol and Opioids
Acute ethanol administration increases both endorphin (Olive et al., 2001) and dynorphin levels (Marinelli et al., 2006; Lindholm et al., 2000), activating both mu opioid receptors (MORs) and KORs simultaneously. This would result in opposing effects on the mesolimbic dopamine system, as MOR activation increases and KOR activation reduces accumbal dopamine transmission (Spanagel et al., 1992, current study). Augmented accumbal dopamine levels in response to acute ethanol are due, in part, to increased inhibitory MOR activity on ventral tegmental area GABAergic interneurons, which causes a disinhibition of dopaminergic neurons in this region (Bergevin et al., 2002). Conversely, chronic ethanol exposure reduces MOR activity (Chen and Lawrence, 2000) while augmenting KOR signaling (Kissler et al., 2014; Siciliano et al., 2015; present work). As a G-protein coupled receptor, there are multiple signaling cascades associated with KORs (Bruchas and Chavkin, 2010), and changes in the function of one or more of these downstream effectors may be driving augmented KOR function. The use of agents that specifically target pathways downstream of KORs (Zhou et al., 2013; Lovell et al., 2015) may provide insight into potential intracellular signaling changes involved in altered KOR activity after ethanol.
Increased KOR function and reduced dopamine transmission may promote an overall deficiency in reward system function (Koob et al., 2013). The neurochemical changes associated with chronic ethanol exposure may produce negative affective symptoms specifically by increasing KOR sensitivity and reducing dopamine system function. The present data support this hypothesis, as reduced dopamine transmission and increased KOR signaling are associated with augmented anxiety/compulsive-like behavior and ethanol intake after a period of abstinence and further because KOR blockade reduces CIE-induced changes in behavior to control levels.
Currently, only one opioid receptor antagonist, naltrexone, is approved for alcohol use disorders by the Food and Drug Administration (Center for Substance Abuse Treatment, 2009). Naltrexone is an antagonist of all 3 classical opioid receptors (MOR, KOR, delta [DOR]). Because of the opposing functional effects of MORs, DORs, and KORs, the utility of a pan-opioid antagonist in treating alcoholics may be reduced, as inhibition of all opioid receptors may decrease the positive reinforcing effects of natural rewards as well as ethanol, thus decreasing compliance. For example, in humans, naltrexone decreased reward-related brain activation in response to palatable foods (Murray et al., 2014) as well as overall food (Yeomans and Wright, 1991) and sucrose (Fantino et al., 1986) intake. Additionally, naltrexone produces an aversive state in monkeys (Williams and Woods, 1999), healthy humans, and alcoholics (Swift et al., 1994). Since the present data show that KOR blockade is sufficient to reduce anxiety/compulsive-like behavior and ethanol drinking in CIE-exposed mice to nondependent levels, a KOR-specific antagonist may be fully efficacious at reducing relapse drinking in the human population while sparing the positive reinforcing salience of natural rewards (Fantino et al., 1986; Murray et al., 2014).
The Effects of CIE Exposure on Accumbal Dopamine Transmission
Chronic ethanol exposure consistently reduces dopamine system function. For example, previous work from our laboratory showed that CIE exposure of mice and rats attenuates dopamine transmission by reducing dopamine release and increasing the rate of dopamine uptake (Budygin et al., 2007; Karkhanis et al., 2015; current work), which is congruent with findings of reduced limbic system function in human alcoholics compared with control subjects (Volkow et al., 2002). These functional changes in dopamine transmission may reflect changes in the expression levels of proteins such as dopamine transporters, tyrosine hydroxylase, vesicular monoamine transporters, or other components of the machinery regulating dopamine release and reuptake. Further studies will be needed to define the underlying mechanisms of the functional changes documented here.
Chronic Ethanol Exposure and Dynorphin Levels
Despite consistent results across laboratories showing increased KOR-system function after chronic ethanol exposure and the present work demonstrating augmented KOR function at the level of the dopamine terminal, there is controversy regarding the status of dynorphin levels after ethanol exposure. Some studies suggest that acute ethanol increases dynorphin in the NAc (Lindholm et al., 2000; Marinelli et al., 2006) and that these effects may be long-lasting (Lindholm et al., 2000). Consistent with reduced dopamine system function following CIE, it has been hypothesized that extended exposure to ethanol may desensitize tryrosine receptor kinase B on D1-containing accumbal GABAergic medium spiny neurons (Logrip et al., 2008), one of the postsynaptic receptors that regulate dynorphin release. This would result in reduced dynorphin levels and supersensitive KORs, as reported here. However, one manuscript demonstrated a transient elevation of prodynorphin levels in the NAc after chronic ethanol exposure (Przewlocka et al., 1997) and another suggested that CIE exposure increased KOR sensitivity and dynorphin levels simultaneously in the central nucleus of the amygdala (Kissler et al., 2014). These data show that the effects of ethanol exposure and withdrawal on dynorphin levels are time dependent, as Kissler and colleagues (2014) examined dynorphin and KOR levels 6 hours into withdrawal in rats, while Przewlocka et al. (1997) found elevated prodynorphin levels 24 to 48 hours after the final ethanol exposure that returned to control levels by 96 hours (Przewlocka et al., 1997). We report increased KOR function 72 hours into ethanol withdrawal in mice, which may or may not coincide with augmented dynorphin levels. These findings suggest that the temporal profile of dynorphin and KOR changes may be important in understanding how chronic ethanol alters KOR signaling.
Conclusions
Human alcoholics report relapse to alcohol drinking in an effort to reduce withdrawal symptoms experienced during abstinence (Wetterling et al., 2006). As mounting evidence supports the involvement of accumbal KORs in the development of anxiety/compulsive-like behaviors and dependence-induced ethanol drinking (Nealey et al., 2011; Ballester-Gonzáles et al., 2015), chronic ethanol-induced reductions in dopamine transmission and increased KOR sensitivity in the NAc may promote the development of negative affective behavioral phenotypes associated with ethanol withdrawal. As such, our work supports a growing body of literature suggesting the potential utility of a KOR-antagonist to rescue chronic ethanol-induced changes in behavior and neurobiology.
Statement of Interest
None.
J.H.R., S.R.J., B.A.M., R.C., H.C.B., and M.F.L. developed experiments. J.H.R. and D.G. executed experiments. J.H.R. analyzed and graphed all data. J.H.R., A.N.K., and S.R.J. wrote the paper. All authors listed sufficiently contributed to and edited the manuscript before submission.
Supplementary Material
Acknowledgments
Nor-binaltorphimine was generously provided by the National Institute of Drug Abuse.
This work was supported by the National Institutes of Health (grant nos.: F31 DA03558 to J.H.R., T32 AA007565 21 to D.G. and A.N.K., DA006634 to R.C., UO1 AA020929 to M.F.L., P50AA010761 to H.C.B., P01 AA021099 to B.A.M. and S.R.J., R01 AA014445 and U01 AA020942 to B.A.M., and U01 AA014091 to S.R.J.).
References
- American Psychological Association (2013). Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Ballester González J, Dvorkin-Gheva A, Silva C, Foster JA, Szechtman H. (2015) Nucleus accumbens core and pathogenesis of compulsive checking. Behav Pharmacol 26:200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergevin A, Girardot D, Bourque MJ, Trudeau LE. (2002) Presynaptic mu-opioid receptors regulate a late step of the secretory process in rat ventral tegmental area GABAergic neurons. Neuropharmacology 42:1065–1078. [DOI] [PubMed] [Google Scholar]
- Britton KT, Akwa Y, Spina MG, Koob GF. (2000) Neuropeptide Y blocks anxiogenic-like behavioral action of corticotropin-releasing factor in an operant conflict test and elevated plus maze. Peptides 21:37–44. [DOI] [PubMed] [Google Scholar]
- Bruchas MC, Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 210:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Läck AK, Diaz MR, McCool BA, Jones SR. (2007) Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen Psychopharmacology 193:495–501. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment (2009) Incorporating alcohol pharmacotherapies into medical practice. Treatment Improvement Protocol Series 49. HHS Publication No. 09–4380. Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. (2012) Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology 62:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Bäckman CM, Gigante ED, Shippenberg TS. (2013) Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacology 38:2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Lawrence AJ. (2000) Effect of chronic ethanol and withdrawal on the mu-opioid receptor- and 5-Hydroxytryptamine(1A) receptor-stimulated binding of [(35)S]Guanosine-5’-O-(3-thio)triphosphate in the fawn-hooded rat brain: a quantitative autoradiography study. J Pharmacol Exp Ther 293:159–165. [PubMed] [Google Scholar]
- Fantino M, Hosotte J, Apfelbaum M. (1986) An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol 251:R91–96. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Chu K, Kieffer BL, Roberts AJ. (2005) Lack of stimulant and anxiolytic-like effects of ethanol and accelerated development of ethanol dependence in mu-opioid receptor knockout mice. Neuropharmacology 49:493–501. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. (2009). Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 33:1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, Jones SR. (2015) Early social isolation stress sensitizes kappa opioid receptors and downregulates dopamine in the nucleus accumbens. Neuropsychopharmacology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. (2015) Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishioka S, Kiguchi N, Kobayashi Y, Yamamoto C, Saika F, Wakida N, Ko MC, Woods JH. (2013) Pharmacokinetic evidence for the long-lasting effect of nor-binaltorphimine, a potent kappa opioid receptor antagonist, in mice. Neurosci Lett 552:98–102. [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. (2014) The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2013) Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. (2000) Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol 22:165–171. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. (2008) Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J 22:2393–2404. [DOI] [PubMed] [Google Scholar]
- Lovell KM, Frankowski KJ, Stahl EL, Slauson SR, Yoo E, Prisinzano TE, Aubé J, Bohn LM. (2015) Structure-activity relationship studies of functionally selective kappa opioid receptor agonists that modulate ERK 1/2 phosphorylation while preserving G protein over βarrestin2 signaling bias. ACS Chem Neurosci 6:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. (2003) Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem 84:1378–1386. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. (2006) A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res 30:982–990. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. (2006) Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. (2015) Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol 49:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Brouwer S, McCutcheon R, Harmer CJ, Cowen PJ, McCabe C. (2014) Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology 231:4323–43. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. (2011) κ-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EP. (2006) A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol 547:106–115. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. (2001) Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 21:RC184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EE, De Biasi M. (2015) Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol doi: 10.1016/j.alcohol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewłocka B, Turchan J, Lasoń W, Przewłocki R. (1997) Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett 238:13–16. [DOI] [PubMed] [Google Scholar]
- Resendez SL1, Kuhnmuench M, Krzywosinski T, Aragona BJ. (2012) κ-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci 32:6771–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10:436–442. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. (1995) Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A 92:5880–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR. (2015) Voluntary ethanol intake predicts κ-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci 35:5959–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89:2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K1, Muramatsu T, Takeda A, Shirakura K. (2002) Co-occurrence of obsessive-compulsive personality traits in young and middle-aged Japanese alcohol-dependent men. Alcohol Clin Exp Res 26:1223–1227. [DOI] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. (1994) Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry 151:1463–1467. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS1, Marcus JF, Portoghese PS, Carlezon WA., Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172:463–470. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA., Jr (2012) Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the κ-opioid receptor antagonist JDTic. Neuropsychopharmacology 37:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. (2002) Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res 116:163–172. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. (2008) Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. (2011) Systemic κ-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol 16:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterling T, Junghanns K. (2000) Psychopathology of alcoholics during withdrawal and early abstinence. Eur Psychiatry 15:483–488. [DOI] [PubMed] [Google Scholar]
- Wetterling T, Weber B, Depfenhart M, Schneider B, Junghanns K. (2006) Development of a rating scale to predict the severity of alcohol withdrawal syndrome. Alcohol 41:611–615. [DOI] [PubMed] [Google Scholar]
- Williams KL, Woods JH. (1999) Naltrexone reduces ethanol- and/or water-reinforced responding in rhesus monkeys: effect depends upon ethanol concentration. Alcohol Clin Exp Res 23:1462–1467. [PubMed] [Google Scholar]
- Yeomans MR, Wright P. (1991) Lower pleasantness of palatable foods in nalmefene-treated human volunteers. Appetite 16:249–259. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Shippenberg TS. (2006) Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol. Alcohol Clin Exp Res 30:592–597. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aubé J, Bohn LM. (2013) Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem 288:36703–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.