Abstract
Background:
Individuals with substance-use disorders exhibit emotional problems, including deficits in emotion recognition and processing, and this class of disorders also has been linked to deficits in dopaminergic markers in the brain. Because associations between these phenomena have not been explored, we compared a group of recently abstinent methamphetamine-dependent individuals (n=23) with a healthy-control group (n=17) on dopamine D2-type receptor availability, measured using positron emission tomography with [18F]fallypride.
Methods:
The anterior cingulate and anterior insular cortices were selected as the brain regions of interest, because they receive dopaminergic innervation and are thought to be involved in emotion awareness and processing. The Toronto Alexithymia Scale, which includes items that assess difficulty in identifying and describing feelings as well as externally oriented thinking, was administered, and the scores were tested for association with D2-type receptor availability.
Results:
Relative to controls, methamphetamine-dependent individuals showed higher alexithymia scores, reporting difficulty in identifying feelings. The groups did not differ in D2-type receptor availability in the anterior cingulate or anterior insular cortices, but a significant interaction between group and D2-type receptor availability in both regions, on self-report score, reflected significant positive correlations in the control group (higher receptor availability linked to higher alexithymia) but nonsignificant, negative correlations (lower receptor availability linked to higher alexithymia) in methamphetamine-dependent subjects.
Conclusions:
The results suggest that neurotransmission through D2-type receptors in the anterior cingulate and anterior insular cortices influences capacity of emotion processing in healthy people but that this association is absent in individuals with methamphetamine dependence.
Keywords: Methamphetamine dependence, emotion processing, alexithymia, dopamine, PET
Introduction
Individuals with substance use disorders exhibit emotional problems, including impaired emotional awareness (Goldstein et al., 2009; Fernández-Serrano et al., 2010) and alexithymia, which is considered to be a set of trait-like deficits in emotion processing (Rybakowski et al., 1988; G. J. Taylor et al., 1990; Pinard et al., 1996; de Haan et al., 2014). Features of alexithymia are thought to influence the emotional disposition to substance use (Bonnet et al., 2013), possibly with substance abuse serving as a self-medication for alexithymia (G. J. Taylor et al., 1999). Helping to clarify the neurobiological basis of abnormalities in emotion processing associated with substance use disorders, therefore, ultimately may contribute to improved treatment strategies for individuals with these individuals.
Neuroimaging findings provide evidence that the combined action of the anterior cingulate cortex (ACC) and the anterior insular cortex (AIC) contributes to a facet of emotion processing associated with alexithymia. Those regions are conjointly activated in paradigms involving emotion processing (Medford and Critchley, 2010). Change in cerebral blood flow induced in ACC by presentation of exteroceptively and interoceptively emotional cues, and assessed using positron emission tomography (PET), is positively correlated with scores on the Levels of Emotional Awareness Scale (Lane et al., 1998; McRae et al., 2008), and the capacity for emotional experience (Schafer et al., 2007) is blunted in patients with traumatic injury or cerebrovascular insults in the frontal cortex, including ACC. Functional magnetic resonance imaging (fMRI) has been used to show AIC activation when subjects reexperience emotions by recalling personal emotional experiences (Damasio et al., 2000) and that self-reports of alexithymia and empathy are associated with activation in the AIC induced by viewing pleasant and unpleasant pictures (Silani et al., 2008). Studies of brain structure have shown an association of alexithymia with smaller gray-matter volume in the ACC and AIC (Borsci et al., 2009; Grabe et al., 2014) and associations of cingulate-cortex and posterior-insula volumes with cognitive and affective dimensions of alexithymia (Goerlich-Dobre et al., 2014).
Individuals who have low emotional clarity, a latent construct of alexithymia, perform worse in tests of several executive domains, including self-monitoring and error recognition, than subjects with greater emotional clarity (Koven and Thomas, 2010). Therefore, impaired emotional processing and error monitoring would be expected to have some commonality in neuroanatomical substrates. ACC and AIC are key regions contributing to emotional awareness as well as sensitivity to errors in research participants with addictions (for review, see Goldstein et al., 2009; Moeller and Goldstein, 2014). In one fMRI study, blunted sensitivity to punishment in a go/no-go task was associated with less activation in ACC and right AIC of cocaine users than in healthy controls (Hester et al., 2013). Cocaine users have less capacity for inhibitory control and reduced error awareness than healthy-control subjects, as well as less task-related fMRI activity elicited in these regions by a go/no-go task (Kaufman et al., 2003; Hester et al., 2007). Notably, however, reduction in task-related ACC activity during an inhibitory-control task was greater in cocaine users who had impaired insight than in those without the impairment (Moeller et al., 2014). Moreover, a recent fMRI study demonstrated that intramodule connectivity in the salience network, including ACC and AIC, is negatively related to the Difficulty Describing Feeling scale in TAS-20 in cocaine users (Liang et al., 2015).
Methamphetamine (MA) use disorder (ie, amphetamine-type substance use disorder, DSM-5) represents a substantial worldwide public health problem (Rawson, 2013). Compared with healthy controls, MA-dependent subjects exhibit structural and functional abnormalities in the ACC and insula (London et al., 2004; Morales et al., 2012; Gowin et al., 2013) and evidence of deficits in central dopaminergic transmission (Chang et al., 2007). As postmortem studies of the human brain have shown dopaminergic projections to the ACC and insula and D2-type receptors in ACC and insula (Gaspar et al., 1989; Hall et al., 1996), it is plausible that dopaminergic dysfunction linked to MA use is linked to emotional-processing deficits associated with alexithymia. This view is supported by a high incidence of alexithymia in patients with neurological diseases that feature dopaminergic dysfunction, such as Parkinson’s disease (Costa et al., 2010).
In prior studies comparing MA-dependent subjects and healthy-control subjects, the MA group exhibited higher scores on one TAS-20 subscale, which indexes difficulty in identifying feelings (Payer et al., 2011). MA-dependent subjects also show lower striatal dopamine D2-type receptor availability (Volkow et al., 2001; B. Lee et al., 2009), raising the possibility that the two phenomena are related. Nonetheless, the role of dopaminergic signaling in emotion identification or processing had not been definitively tested in healthy control subjects or in those with addictions. We therefore tested the relationship between emotion processing, as measured by self-report on the TAS-20, and dopamine D2-type receptor availability in the ACC and AIC, comparing healthy control with MA-dependent participants using PET with [18F]fallypride, a radiotracer with sufficient affinity for D2-type receptors to allow measurement in extrastriatal regions (Mukherjee et al., 1995; Mukherjee et al., 2002).
Methods
Participants
All procedures were approved by the University of California Los Angeles (UCLA) Office for the Protection of Research Subjects. Participants, 18 to 55 years of age were healthy volunteers or individuals with MA dependence who were not seeking treatment. They were recruited using Internet and local newspaper advertisements, were assigned to 2 groups (control and MA), and received a detailed description of the study protocol. All participants provided written informed consent. The sample studied here overlaps with a previous study of dopamine D2-type receptors and impulsiveness (16 of 17 controls and 16 of 23 MA-group participants in this study) (B. Lee et al., 2009) and another previous study of affect processing in MA-dependent individuals, in which the TAS-20 scale was administered (15 of 17 controls and 20 of 23 MA-group participants in this study) (Payer et al., 2011).
Eligibility was determined using questionnaires, including the Structured Clinical Interview for DSM-IV Axis-I Disorders, to determine psychiatric diagnoses and a medical examination. Inclusion criteria for the MA group were current MA dependence, as indicated by DSM-IV criteria, and a positive urine test for MA at entry to the study. Exclusion criteria were any current Axis-I diagnosis (other than MA dependence or substance-induced mood or anxiety disorder in the MA group, or nicotine dependence in either group); use of psychotropic medications or illicit drugs other than MA or nicotine as indicated above, although light use of marijuana and alcohol was allowed (see below); central nervous system, cardiovascular, pulmonary, or systemic disease; human immunodeficiency virus seropositive status; severe hepatic impairment; chronic inflammation; pregnancy; and lack of English fluency. Use of marijuana or alcohol was allowed in both groups as long as it was below the threshold for a diagnosis of abuse or dependence as defined by DSM-IV.
All of the MA group participants were admitted to the UCLA General Clinical Research Center, where they maintained abstinence from MA and other drugs of abuse other than nicotine (in cigarettes) and caffeine (in beverages), as verified by urine screening. The control group participants were asked to visit the research sites on different days for psychological testing and imaging. On the days when brain-imaging or self-report data on the TAS-20 were collected, participants also provided urine samples negative for MA and other drugs. On completion of the study, participants were compensated with cash, gift certificates, and vouchers.
MRI and PET Imaging
Structural MRI scans were acquired on a 1.5-T Siemens Sonata tomograph for coregistration with PET images to define volumes of interest (VOI) (described below). A high-resolution sagittal T1-weighted 3D volumetric scan was acquired using a whole-brain magnetization-prepared rapid acquisition with gradient echo sequence (TR=1900ms, TE=4.38ms, flip angle=15, field of view=256×256×160, 160 slices, thickness=1mm).
PET scans were acquired using [18F]fallypride. The tomograph, a Siemens ECAT EXACT HR+ scanner, has an in-plane resolution full-width at half-maximum of 4.6mm, axial resolution full-width at half-maximum of 3.5mm, and axial field of view of 15.52cm in the 3-dimensional scanning mode. Participants were placed in the supine position, and their heads were immobilized using a thermoplastic mask that was marked for repositioning. A transmission scan for attenuation correction was conducted using a rotating 68Ga/68Ge rod source. Emission data were collected for 80 minutes after the radiotracer injection. Participants then were removed from the scanner for a 20-minute break, during which they were instructed to void their bladders to reduce radiation exposure. They returned to the scanner and were repositioned. After another transmission scan, additional emission data were collected for an 80-minute period to achieve the 180-minute scan duration required for an accurate measurement of binding potential using [18F]fallypride (details in PET data processing) (Vernaleken et al., 2011).
Self-Report Assessment
Capacity for emotional processing was assessed using the TAS-20 (Bagby et al., 1994), a widely used questionnaire that has been validated for testing of substance abusers (Cleland et al., 2005). The TAS-20 consists of 3 subscales that provide scores for corresponding factors: factor 1: difficulty identifying feelings; factor 2: difficulty describing feelings; and factor 3: externally oriented thinking. TAS-20 total score (the sum of the 3 subscale scores) is interpreted as follows: 0 to 51: no alexithymia; 52 to 60: possible alexithymia; 61 and higher: alexithymia (G. J. Taylor et al., 1999). The Beck Depression Inventory (BDI) (Beck et al., 1996) was administered to verify depressive symptoms and potentially control for its confounding effects on TAS-20 scores because of indications that depression contributes to TAS-20 score (Honkalampi et al., 2000).
PET Data Processing
Reconstructed PET data were combined into 16 images, each reflecting data averaged over 10 minutes. FSL MCFLIRT (FMRIB Centre, Department of Clinical Neurology, University of Oxford, Oxford, UK) was used to correct for head motion. The PET images were coregistered to the structural MRI images using a 6-parameter, rigid-body spatial transformation (FSL FLIRT [Jenkinson et al., 2002]). A single VOI, defining ACC bilaterally in Montreal Neurological Institute (MNI) space, was based on the Harvard-Oxford Atlas. Separate VOIs for the anterior insulae were defined in MNI space using the definition specified by the functional connectivity analyses of Deen et al. (2011). These VOIs were transformed to native space using FSL FNIRT. The primary regions investigated were the ACC and AIC because of their known roles in emotional self-awareness, but adhoc exploratory analysis was performed on the other regions, which have relatively high densities of dopamine D2-type receptors, including amygdala, hippocampus, globus pallidus, thalamus, and striatum. Bilateral VOIs for the exploratory regions were anatomically defined on each participant’s MRI images using auto-segmentation procedures in FSL FIRST software (Patenaude et al., 2011). Cerebellar VOIs were manually drawn on both hemispheres in MNI space, then transformed to native space. Striatum (caudate and putamen) and cerebellum VOIs were used in PET modeling (see below).
Time-activity data were extracted from motion-corrected, coregistered PET data and imported into PMOD Kinetic Modeling (PMOD Technologies Ltd., Zurich, Switzerland). Time-activity curves were first fit for the striatum, which has a high density of D2-type receptors. The simplified reference tissue model (Lammertsma and Hume, 1996) was used to provide an estimate of the rate constant k2′ for the transfer of the radiotracer from the reference-region tissue compartment to the plasma. The cerebellum was used as the reference region because of its low specific binding for [18F]fallypride (Mukherjee et al., 2002). The VOI time-activity curves were refit using the simplified reference tissue model 2 model (Wu and Carson, 2002) using PMOD Kinetic Modeling, with the k2′value derived from the striatum applied to all VOIs. Binding potential (BPnd), an index of receptor availability, was then calculated as R1*k2′/k2a –1, where R1=K1/K1′ is the ratio of tracer-delivery parameters from plasma to the target region and reference region, and k2a is the single-compartment rate constant for transfer from the target-region tissue compartment to plasma.
Statistical Analysis
Group differences in age, education, BDI, and TAS-20 scores were tested by unpaired Student’s t tests, and group differences in sex and smoking status were evaluated by Fisher’s exact test. Group differences in BPnd were tested by ANCOVA, controlling for age, sex, and smoking status, because of strong evidence for age-related decline of D2-type receptors in brain (Rinne et al., 1993; Kaasinen et al., 2000), for a sex difference in D2-type BPnd in cortical regions (Kaasinen et al., 2001) and for lower striatal D2-type BPnd among smokers than nonsmokers (Fehr et al., 2008; Brown et al., 2012). The interaction between group and BPnd on emotional self-awareness was tested using ANCOVA, with TAS-20 total score as the dependent variable and age, sex, smoking status, the interval (in days) between the PET scan and TAS-20 assessment (which was not balanced between groups; see Results), subject group, BPnd, and the interaction of group and BPnd as the independent variables. Relationships between the TAS-20 scores and D2-type BPnd were tested using partial correlations controlling for age, sex, smoking status, and the interval between PET and TAS-20 assessment for the reasons given above. The criterion for statistical significance was P<.05, 2-tailed. All statistical analyses were conducted using SPSS IBM 19 (IBM, Armonk, NY).
Results
Participants
Forty participants were recruited and assigned to a control group (n=17) and a MA group (n=23). The groups did not differ significantly in age or in sex distribution (Table 1). The control group included almost equal numbers of men and women, whereas there were twice as many men as women in the MA group. Cigarette smokers were more prevalent in the MA group. MA group participants reported using MA for 10.4 years (SD: 7.33; range: 2–24) and age of first MA use at 22.8 years (SD: 7.43; range: 13–38) and were abstinent from MA for 7.2 days (SD: 3.11; range: 4–15) before PET scanning and for 8.0 days (SD: 2.91; range: 4–17) before administration of the TAS-20. MA-group participants reported having used MA for 20.2 days (SD: 7.48; range: 5–30) in the month before entering the study, and the mean amount of MA use in the week before enrolling the study was 2.90g (SD: 3.36; range: 0–14.5). Fourteen MA group participants but none of the control subjects reported marijuana use within 1 month of entering the study, reporting use for 0.6 days (SD: 1.09; range: 0–4) in the last month. Due to scheduling needs of the participants and the imaging facility, the TAS-20 administrations and PET scans were conducted on different days. The interval between the PET scan and TAS-20 administration was 1.8 days on average for the MA group participants and significantly longer for the control group (32.5 days; unpaired Student’s t tests P<.001).
Table 1.
Demographic and Psychological Measures of Research Participants
| Control Group (n = 17) | MA groupa (n = 23) | Group difference: P value | |
|---|---|---|---|
| Age (years) | 32.1 (7.60) | 35.6 (9.12) | 0.21 b |
| Sex (male/female) | 9/8 | 15/8 | 0.52 c |
| Education (years) | 13.4 (1.75) | 12.2 (3.42) | 0.23 b |
| Cigarette smokers | 5 | 20 | < 0.001 c |
| TAS-20 total score | 32.41 (8.27) | 39.79 (10.32) | 0.02 b |
| Factor 1: Difficulty Identifying Feelings | 7.94 (1.20) | 11.30 (4.53) | 0.01 b |
| Factor 2: Difficulty Describing Feelings | 8.24 (3.78) | 10.42 (5.45) | 0.20 b |
| Factor 3: Externally Oriented Thinking | 16.24 (5.08) | 18.08 (5.20) | 0.16 b |
| BDI | 3.65 (3.90) | 6.09 (5.62) | 0.13 b |
Abbreviations: BDI, Beck Depression Inventory; TAS-20, 20-item Toronto Alexithymia Scale.
All data are shown as means (SD).
aMA use: age of first use was 22.8 (7.43); 20.2 (7.48) days of use in month before enrolling in study; 2.90 (3.36) g used in week before enrolling.
bGroup difference evaluated by unpaired Student’s t test.
cGroup difference in distribution evaluated by Fisher’s exact test.
Self-Report Assessments
All control group participants and all but 5 of the MA group participants gave TAS-20 total scores consistent with no alexithymia (ie, ≤51); the remaining 5 MA group participants met the criteria for possible alexithymia. TAS-20 total scores were significantly lower for the control group than the MA group (t 38=-2.268, P=.03), with significantly lower scores for factor 1: difficulty identifying feelings (t 35.884=-3.255, P=.003: equal variances not assumed), but not for either of the other 2 factors (factor 2: t 38=-1.177, P=.25; factor 3: t 38=-1.109, P=.27) (Table 1), consistent with the findings obtained with the larger sample from which these participants were drawn (Payer et al., 2011).
The TAS-20 total score was not significantly correlated with BDI score (r=-0.093, P=.57 by Pearson’s correlation). The mean BDI scores, obtained close to the time of TAS-20 measurement, were 3.65 (SD: 3.90) and 6.09 (SD: 3.65) for the control and MA groups, respectively, and did not differ significantly between groups (t 38=-1.534, P=.133).
Dopamine D2-Type Receptor Availability and Association with TAS-20 Total Score
In all analyses, left and right AIC VOI data were volume-weight averaged, because they were strongly correlated (r=0.884, P < .001 by Pearson’s correlation). There was no significant group difference in BPnd in the ACC (control: 0.53 [SD: 0.13]; MA: 0.51 [SD: 0.13]; F1, 35=0.136, P=.715), AIC (control: 0.75 [SD: 0.15]; MA: 0.69 [SD: 0.19]; F1, 35=1.616, P=.21), or other regions tested except for the striatum. In the striatum, the control group had a significantly higher BPnd than the MA group (control: 16.32 [SD: 1.80]; MA: 14.99 [SD: 1.87]; F1, 35=5.405, P=.03).
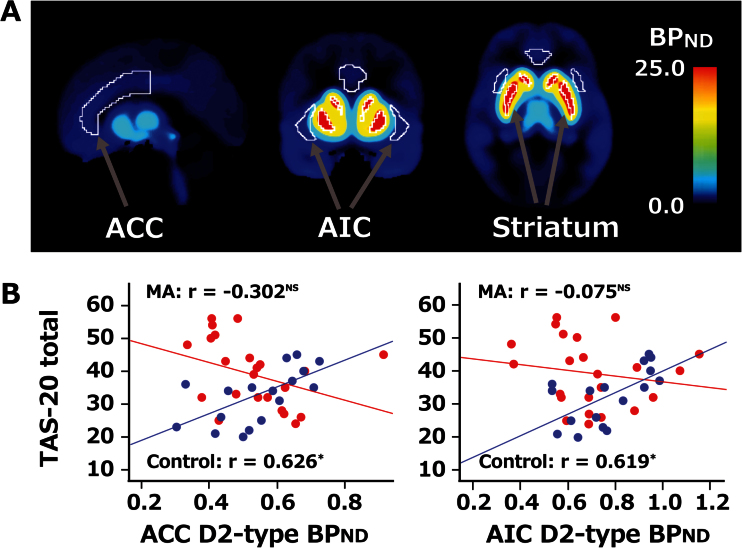
Taking TAS-20 total score as the dependent variable in the ANCOVA, there was a significant interaction between group (control vs MA) and BPnd in both the ACC (F1, 31=9.716, P=.004) and AIC (F1, 31=5.508, P=.03) (Figure 1; Table 2). For the control group, significant positive partial correlations were found between TAS-20 total score and BPnd in both the ACC (r=0.626, P=.02) and AIC (r=0.619, P=.02), with these effects retaining significance after Bonferroni correction for multiple comparisons (ie, for the 2 VOIs). In the MA group, D2-type BPnd in the ACC (r=-0.302, P=.21) and AIC (r=-0.075, P=.76) showed nonsignificant negative partial correlations with the TAS-20 total score. Exploratory analyses of additional VOIs, with the TAS-20 total score as the dependent variable in ANOVA, indicated insignificant interactions between group and BPnd in the amygdala (F1, 31=2.950, P=.09), hippocampus (F1, 31=2.060, P=.16), globus pallidus (F1, 31=2.703, P=.11), thalamus (F1, 31=2.910, P=.10), and striatum (F1, 31=3.042, P=.09) (Table 2). Partial correlations between BPnd and the TAS-20 score were in the same directions as in primary regions: positive in the control group and negative in the MA group, although those correlations did not reach significance.
Figure 1.
D2-type receptor binding potential (BPnd) and association with Toronto Alexithymia Scale (TAS-20) total score. (a) Volumes of interest (VOIs) of the anterior cingulate cortex (ACC), anterior insular cortex (AIC), and striatum are shown on an averaged BPnd map of the control group (n=17). VOIs outlined in white. MNI coordinates: x=90, y=135, z=75 (mm). (b) Scatterplots showing individual data for the TAS-20 total score and BPnd in ACC and AIC. Blue circles represent data from individual participants in the control group, and red circles represent data from the MA group. BPnd was corrected for age, sex, smoking. Partial correlation analysis was used to determine r-values (*P < .05, NS nonsignificant, with P values not corrected for multiple-region comparisons).
Table 2.
Association of Dopamine D2-type BPnd with the TAS-20 Total Score
| Group-by-BPnd Interaction: P value | Correlation Coefficients | ||
|---|---|---|---|
| Control Group (n = 17) | MA Group (n = 23) | ||
| ACC | 0.004 a | 0.626 b | -0.302 |
| AIC | 0.03 a | 0.619 b | -0.075 |
| Amygdala | 0.08 | 0.191 | -0.335 |
| Hippocampus | 0.11 | 0.276 | -0.400 |
| Globus pallidus | 0.07 | 0.536 | -0.269 |
| Thalamus | 0.09 | 0.410 | -0.264 |
| Striatum | 0.09 | 0.307 | -0.191 |
Abbreviations: ACC, anterior cingulate cortex; AIC, anterior insular cortex; TAS-20, the 20-item of Toronto Alexithymia Scale.
The P values shown are not corrected for multiple comparisons (ie, tests in 2 regions: ACC and AIC).
aSignificant interaction by ANCOVA controlling for age, sex, smoking status, and days of interval between PET and TAS-20.
bSignificant correlation by partial correlation analysis, controlling for age, sex, smoking status, and days of interval between PET and TAS-20.
Discussion
The goal of this study was to determine whether dopamine D2-type receptors are involved in the processing of emotion and whether they may play a different role in MA-dependent individuals compared with healthy controls. The focus was on the ACC and AIC, which have been linked to emotion processing and awareness of internal states (Critchley et al., 2004; Silani et al., 2008; Medford and Critchley, 2010) and which also show structural and functional deficits in individuals with MA use disorder (London et al., 2004; Morales et al., 2012; Gowin et al., 2013). A significant group difference in D2-type receptor availability, control group > MA group, was found in striatum in agreement with previous studies (Volkow et al., 2001; B. Lee et al., 2009) but not in any other regions tested, including ACC and AIC. While positive associations of D2-type receptor availability in ACC and AIC with the TAS-20 total score were observed for healthy control subjects, this relationship was not found among MA-dependent subjects, who exhibited higher TAS-20 total scores than control subjects. These findings suggest a difference in the contributions of dopaminergic transmission through D2-type receptors to the capacity of MA-dependent subjects in early abstinence to process feelings.
The positive relationship between D2-type BPnd and TAS-20 score in healthy subjects, implying that high receptor availability is associated with problems in emotion processing, is not immediately consistent with a report that L-dopa administration improves emotion perception in patients with Parkinson’s disease (Fleury et al., 2014). This pharmacological finding suggests that in individuals with impaired dopaminergic neurotransmission, such as the MA-dependent subjects in our study, dopamine-enhancing interventions may help ameliorate deficits in emotion processing associated with identifying/describing feelings. On the other hand, if one considers D2-type BPnd as a neurochemical marker of potential for signaling through D2-type receptors, it would appear that high levels of D2-type BPnd contribute to alexithymia and less capacity for processing emotion. It is possible that optimal capacity for emotion processing is conferred by an optimal level of signaling through D2-type dopamine receptors in ACC and AIC, with exceedingly high levels linked to problems with emotion regulation. As none of the control participants met the criteria for alexithymia, however, the relationship may simply reflect a physiological correlation within the range of normality.
Given the lack of group differences in D2-type BPnd in the cortical regions assayed and the fact that the effects of MA use are not limited to the dopamine system, a disruption in the association of D2-type receptors with the TAS-20 score can reflect effects on other neurotransmitter systems, such as the GABAergic and glutamatergic systems, which interact with dopamine D2-type signaling (Jayanthi et al., 2004, 2014). As positive associations of both GABA and glutamate concentrations in both the ACC and left AIC with the TAS-20 score have been observed (Ernst et al., 2014), MA effects on these neurotransmitter systems may contribute to the observations made here.
The lack of association between D2-type BPnd and the TAS-20 score in MA-dependent subjects stands in contrast to observation of an inverse association between intramodule connectivity in the salience-network with the Difficulty Describing Feelings subscale score on the TAS-20 in cocaine-dependent individuals but not healthy controls (Liang et al., 2015). Obvious differences between the studies are the type of stimulant and the fact that the primary self-report measure in the present study was the TAS-20 total score and not the Difficulty Describing Feeling subscale. In addition, the cocaine users were not required to maintain abstinence for several days prior to testing, and almost one-half of the cocaine group subjects had positive urine tests for cocaine on the testing day, whereas the MA-dependent subjects maintained supervised abstinence from all illicit drugs of abuse and alcohol for at least 4 days prior to testing. Aside from differences in potential effects of residual drug directly on behavior, there also might have been difference in the state of withdrawal in the two studies. Whether TAS-20 scores in this study were affected by acute withdrawal needs to be considered. Nevertheless, the TAS-20 score has been shown to be robust to substance withdrawal, especially for individuals without clinically significant alexithymia (de Haan et al., 2014). MA group participants actually were abstinent from MA for 8.0 days on average when the TAS-20 was administered; acute withdrawal symptoms from MA usually resolve within 1 week of initiating drug abstinence (Zorick et al., 2010). Nonetheless, future studies are warranted to determine how associations between D2-type receptor BPnd and measures of emotion processing vary with time of abstinence from MA.
There are limitations to this study, one of which is that TAS-20 scores were relatively low compared with those in previous studies (Saladin et al., 2012; Ernst et al., 2014), and most of the participants did not meet the criterion for clinical alexithymia. The range in TAS-20 scores, however, was similar to that in a previous study showing a correlation between a neurochemical marker and the TAS-20 total score (Ernst et al., 2014). In addition, for logistical reasons, there were some gaps in time between the self-report and PET measures, which ideally would have been collected on the same day. However, the time interval was controlled for statistically; and given the temporal stability of TAS-20 (Tolmunen et al., 2011), it is likely that this limitation is minimal. Another limitation is the small number of participants; necessitating the replication of our findings in a larger sample to demonstrate its reproducibility.
Finally, there are limitations associated with the use of [18F]fallypride and PET. Although [18F]fallypride has sufficient affinity for dopamine D2-type receptors to allow measurement in striatum and extrastriatal regions (Mukherjee et al., 2002), it does not distinguish between D2 and D3 receptor subtypes and does not resolve binding to receptors in different compartments, such as pre- vs postsynaptic receptors on different cellular elements in a region. Another factor to consider is that receptor availability, measured by BPnd, represents the receptor pool accessible to radiotracer, and as such it can be influenced by both receptor density and the concentration of intrasynaptic endogenous dopamine (Ito et al., 2011). Indeed, [18F]fallypride binding in human cortex is responsive to change in intrasynaptic dopamine level induced by administration of amphetamine (Riccardi et al., 2006; Cropley et al., 2008). However, on the basis of observations on the effect of dopamine depletion with α-methyltyrosine on the binding of [11C]raclopride, another D2-type receptor ligand (Martinez et al., 2009), the extent to which BPnd measured using this tracer would be affected by individual differences in intrasynaptic dopamine in the absence of a challenge would be expected to be small.
Despite these limitations, this study is the first to suggest a role of dopaminergic neurotransmission through D2-type receptors in capacity for identifying/describing feelings and to show that this relationship is disrupted in individuals with MA dependence. It has been hypothesized that substance-dependent individuals use drugs in an attempt to medicate themselves for unpleasant emotional states, which may be experienced as overwhelming due to impaired capacity to identify emotions (Khantzian, 1985). To the extent that this assertion is correct, further studies are warranted to help clarify the neurobiological basis for the problems in emotional processing in individuals with MA-related and other substance use disorders.
Statement of Interest
None.
Acknowledgments
The authors thank Dr. Milky Kohno for her advice regarding statistical analysis.
This research was supported by grants from the National Institute on Drug Abuse (R01 DA015179, R01 DA020726, P20 DA022539, T32 DA024635); the National Center for Research Resources (M01 RR00865 [UCLA GCRC]); an endowment from the Thomas P and Katherine K Pike Chair in Addiction Studies; and a gift from the Marjorie M Greene Trust.
The study was presented in part at 76th Annual Meeting of College on Problems of Drug Dependence; June 14–19, 2014; San Juan, Puerto Rico.
References
- Bagby RM, Parker JD, Taylor GJ. (1994) The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res 38:23–32. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. (1996) Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67:588–597. [DOI] [PubMed] [Google Scholar]
- Bonnet A, BrÉJard V, Pedinielli J-L. (2013) Emotional dispositions and substance use: mediating effect of alexithymia. Psychol Rep 112:289–302. [DOI] [PubMed] [Google Scholar]
- Borsci G, Boccardi M, Rossi R, Rossi G, Perez J, Bonetti M, Frisoni GB. (2009) Alexithymia in healthy women: a brain morphology study. J Affect Disord 114:208–215. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED. (2012) Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol 15:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction (Abingdon, England) 102:16–32. [DOI] [PubMed] [Google Scholar]
- Cleland C, Magura S, Foote J, Rosenblum A, Kosanke N. (2005) Psychometric properties of the Toronto Alexithymia Scale (TAS-20) for substance users. J Psychosom Res 58:299–306. [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Carlesimo GA, Salamone G, Caltagirone C. (2010) Prevalence and characteristics of alexithymia in Parkinson’s disease. Psychosomatics 51:22–28. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. (2004) Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, Ryu YH, Sprague KE, Pike VW, Fujita M. (2008) Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse (New York, NY) 62:399–408. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. (2000) Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci 3:1049–1056. [DOI] [PubMed] [Google Scholar]
- de Haan HA, van der Palen J, Wijdeveld TG, Buitelaar JK, De Jong CA. (2014) Alexithymia in patients with substance use disorders: state or trait? Psychiatry Res 216:137–145. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. (2011) Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex (New York, NY: 1991) 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Boker H, Hattenschwiler J, Schupbach D, Northoff G, Seifritz E, Grimm S. (2014) The association of interoceptive awareness and alexithymia with neurotransmitter concentrations in insula and anterior cingulate. Soc Cogn Affect Neurosci 9:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. (2008) Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry 165:507–514. [DOI] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Lozano T, Pérez-García M, Verdejo-García A. (2010) Impact of severity of drug use on discrete emotions recognition in polysubstance abusers. Drug Alcohol Depend 109:57–64. [DOI] [PubMed] [Google Scholar]
- Fleury V, Cousin E, Czernecki V, Schmitt E, Lhommee E, Poncet A, Fraix V, Tropres I, Pollak P, Krainik A, Krack P. (2014) Dopaminergic modulation of emotional conflict in Parkinson’s disease. Front Aging Neurosci 6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. (1989) Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 279:249–271. [DOI] [PubMed] [Google Scholar]
- Goerlich-Dobre KS, Bruce L, Martens S, Aleman A, Hooker CI. (2014) Distinct associations of insula and cingulate volume with the cognitive and affective dimensions of alexithymia. Neuropsychologia 53:284–292. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. (2009) The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci 13:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Stewart JL, May AC, Ball TM, Wittmann M, Tapert SF, Paulus MP. (2013) Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: losses lose impact. Addiction (Abingdon, England). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe HJ, Wittfeld K, Hegenscheid K, Hosten N, Lotze M, Janowitz D, Volzke H, John U, Barnow S, Freyberger HJ. (2014) Alexithymia and brain gray matter volumes in a general population sample. Hum Brain Mapp 35:5932–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G. (1996) Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse 23:115–123. [DOI] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. (2007) Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology 32:1974–1984. [DOI] [PubMed] [Google Scholar]
- Hester R, Bell RP, Foxe JJ, Garavan H. (2013) The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend 133:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nandam LS, O’Connell RG, Wagner J, Strudwick M, Nathan PJ, Mattingley JB, Bellgrove MA. (2012) Neurochemical enhancement of conscious error awareness. J Neurosci 32:2619–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkalampi K, Hintikka J, Tanskanen A, Lehtonen J, Viinamaki H. (2000) Depression is strongly associated with alexithymia in the general population. J Psychosom Res 48:99–104. [DOI] [PubMed] [Google Scholar]
- Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, Suhara T. (2011) Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. J Neurosci 31:7886–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. (2004) Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J 18:238–251. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL. (2014) Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry 76:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. (2001) Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiatry 158:308–311. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J. (2000) Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 21:683–688. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. (2003) Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci 23:7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. (1985) The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 142:1259–1264. [DOI] [PubMed] [Google Scholar]
- Koven NS, Thomas W. (2010) Mapping facets of alexithymia to executive dysfunction in daily life. Pers Individ Dif 49:24–28. [Google Scholar]
- Lammertsma AA, Hume SP. (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. (1998) Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci 10:525–535. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. (2009) Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29:14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Rawson RA. (2008) A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev 27:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y. (2015) Interactions between the salience and default-mode networks are disrupted in cocaine addiction. J Neurosci 35:8081–8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. (2004) Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61:73–84. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. (2009) Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry 166:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. (2008) Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. Neuroimage 41:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. (2010) Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. (2014) Impaired self–awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci 18:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, Goldstein RZ. (2014) Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry 71:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O’Neill J, London ED. (2012) Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend 125:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Das MK, Brown T. (1995) Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol 22:283–296. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. (2002) Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse (New York, NY) 46:170–188. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED. (2011) Neural correlates of affect processing and aggression in methamphetamine dependence. Arch Gen Psychiatry 68:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard L, Negrete JC, Annable L, Audet N. (1996) Alexithymia in substance abusers. Am J Addict 5:32–39. [Google Scholar]
- Rawson RA. (2013) Current research on the epidemiology, medical and psychiatric effects, and treatment of methamphetamine use. J Food Drug Anal 21:S77–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, Anderson S, Doop M, Woodward N, Schoenberg E, Schmidt D, Baldwin R, Kessler R. (2006) Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 31:1016–1026. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, Lehikoinen P, Oikonen V, Syvalahti E. (1993) Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab 13:310–314. [DOI] [PubMed] [Google Scholar]
- Rybakowski J, Ziolkowski M, Zasadzka T, Brzezinski R. (1988) High prevalence of alexithymia in male patients with alcohol dependence. Drug Alcohol Depend 21:133–136. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Santa Ana EJ, LaRowe SD, Simpson AN, Tolliver BK, Price KL, McRae-Clark AL, Brady KT. (2012) Does alexithymia explain variation in cue-elicited craving reported by methamphetamine-dependent individuals? Am J Addict 21:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer R, Popp K, Jorgens S, Lindenberg R, Franz M, Seitz RJ. (2007) Alexithymia-like disorder in right anterior cingulate infarction. Neurocase 13:201–208. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. (2005) Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend 78:125–134. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. (2008) Levels of emotional awareness and autism: an fMRI study. Soc Neuroscience 3:97–112. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Parker JD, Bagby RM. (1990) A preliminary investigation of alexithymia in men with psychoactive substance dependence. Am J Psychiatry 147:1228–1230. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA. (1999) Disorders of affect regulation: alexithymia in medical and psychiatric illness. Cambridge: Cambridge University Press. [Google Scholar]
- Tolmunen T, Heliste M, Lehto SM, Hintikka J, Honkalampi K, Kauhanen J. (2011) Stability of alexithymia in the general population: an 11-year follow-up. Compr Psychiatry 52:536–541. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Peters L, Raptis M, Lin R, Buchholz HG, Zhou Y, Winz O, Rosch F, Bartenstein P, Wong DF, Schafer WM, Grunder G. (2011) The applicability of SRTM in [(18)F]fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab 31:1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. (2001) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. (2002) Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22:1440–1452. [DOI] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. (2010) Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction (Abingdon, England) 105:1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]