Abstract
Exposure to cocaine, and likely other drugs of abuse, generates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-silent glutamatergic synapses in the nucleus accumbens. These immature synaptic contacts evolve after drug withdrawal to redefine the neurocircuital properties. These results raise at least three critical questions: (1) what are the molecular and cellular mechanisms that mediate drug-induced generation of silent synapses; (2) how are neurocircuits remodeled upon generation and evolution of drug-generated silent synapses; and (3) what behavioral consequences are produced by silent synapse-based circuitry remodeling? This short review analyzes related experimental results, and extends them to some speculations.
Keywords: accumbens, anti-addictive, circuitry remodeling, cocaine, incubation, silent synapse
Drug addiction is a complex brain disease, partially mediated by drug-induced adaptive changes in multiple brain regions as well as within the projections among these brain regions. Whereas extensive experimental results have been generated depicting drug-induced adaptations at the molecular and cellular levels, it remains poorly understood how exposure to drugs of abuse reshapes neurocircuits. This brief review summarizes recent findings related to drug-induced circuitry consequences with the emphasis on silent synapse-based circuitry remodeling after exposure to cocaine.
Excitatory Synaptic Transmission in the Nucleus Accumbens
The forebrain region known as the nucleus accumbens (NAc) functions to prioritize emotional and motivational arousals for behavioral output. These emotional and motivational arousals are thought to be carried out and regulated by excitatory projections from several limbic and paralimbic regions, including the ventral tegmental area, prefrontal cortex, amygdala, hippocampus, thalamus, hypothalamus, and other regions (Wise, 1987). These excitatory projections undergo adaptive changes after exposure to cocaine or other drugs of abuse, and many of these drug-induced alterations in NAc glutamatergic transmission are essential for the expression of addiction-associated behaviors (Wolf, 1998, 2010).
Generation of Silent Synapses after Drug Experience
A prominent form of cocaine-induced circuitry remodeling is achieved via generation and subsequent evolution of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-silent excitatory synapses. Silent excitatory synapses are synapses that express stable N-methyl-D-aspartate (NMDA) receptor (NMDAR)-mediated postsynaptic responses, while AMPAR-mediated responses are minimal (Kerchner and Nicoll, 2008; Hanse et al., 2013). At the resting membrane potential, because NMDARs conduct little current, these synapses are largely silent. Electrophysiologically, AMPAR-silent, NMDAR-only synapses are demonstrated as synapses that are inactive at near-resting membrane potentials due to Mg2+-mediated blockade of NMDARs, and active at depolarized membrane potentials, at which Mg2+-mediated blockade of NMDARs is relieved. The percentage of silent synapses among all examined synapses can also be estimated using several electrophysiological approaches (Isaac et al., 1995; Liao et al., 1995; Huang et al., 2009).
Silent synapses can be generated by insertion of NMDARs to new synaptic contacts via a synaptogenesis process, and may undergo a subsequent “un-silencing” process through recruiting AMPARs to stabilize the synapses. Silent synapses can also be generated by removal or desensitization of AMPARs from existing excitatory synapses, and these destabilized synapses may be pruned away subsequently. Silent synapses are abundant during circuitry development and decline to very low levels after development (Durand et al., 1996). The generation of silent synapses and subsequent maturation or elimination of these synapses may not only strengthen or weaken the overall excitatory synaptic transmission but also redefine the anatomical architectures of affected circuits, leading to profound circuitry remodeling.
The overall levels of silent synapses are low in the NAc (i.e. <10%), and juvenile animals exhibit higher levels (e.g. 10%) than adult animals (e.g. ~3%; Huang et al., 2009; Lee et al., 2013). Regardless of the developmental regulation (Durand et al., 1996), 1 or 2 days after repeated intraperitoneal injections of cocaine (Huang et al., 2009) or morphine (Graziane and Dong, unpublished results), the levels of silent synapses in the NAc are increased substantially (to ~30%). Further results demonstrate that cocaine-induced generation of silent synapses involves synaptic insertion of new, GluN2B-containing NMDARs (Huang et al., 2009) and is accompanied by an increase in the density of dendritic spines (Robinson and Kolb, 2004; Brown et al., 2011), whereas morphine-induced generation of silent synapses involves internalization of AMPARs and is accompanied by a decrease in the density of dendritic spines (Robinson and Kolb, 2004). Thus, exposure to cocaine and morphine—two abused drugs with distinct pharmacological effects but both causing addiction—triggers opposing synaptic modifications and circuitry remodeling. These results also raise a number of important questions, which are discussed below.
Mechanisms Underlying Drug-Induced Generation of Silent Synapses
Accompanying cocaine-induced generation of silent synapses and increases in spine density, several neuronal substrates that are important for synapse or circuitry formation during brain development are up-regulated in the NAc, including synaptic GluN2B-containing NMDARs, transcription factor cAMP response element-binding protein (CREB), and neurotrophins (Dong and Nestler, 2014). Based on these correlative results, cocaine-induced generation of silent synapses has been hypothesized as a synaptogenesis process involving transient reactivation of certain developmental mechanisms in the adult brain (Dong and Nestler, 2014; Huang et al., 2015).
What developmental mechanisms may be reactivated after exposure to cocaine for the potential synaptogenesis process? The potential mechanisms may exhibit two important features. First, cocaine-induced generation and the subsequent maturation of silent synapses is projection-specific (Lee et al., 2013; Ma et al., 2014), involving both postsynaptic construction and directed presynaptic growth. Thus, there should be trans-synaptic signaling that directs and coordinates pre- and postsynaptic approaching and connecting. Second, given the prominent effects of cocaine on dopamine release, the mechanisms are likely activated or regulated by dopamine signaling and are likely active preferentially in the mesolimbic dopamine systems. One such candidate is the interaction between netrin1 and its presynaptic receptor deleted in colorectal cancer (DCC). During circuitry development, DCC, located at the axon growth cone, directs axon growth toward a specific postsynaptic area where netrin1 is released; disruption of DCC-netrin1 interactions disrupts synapse and circuitry formation (Barallobre et al., 2005; Round and Stein, 2007). Both netrin1 and DCC are highly enriched during early embryonic stages, when synapse and circuitry development is highly active. After development, netrin1 and DCC are reduced to very low levels in most brain regions (Barallobre et al., 2005; Round and Stein, 2007), except in some midbrain sites (Osborne et al., 2005). The netrin1-DCC-based synapse and circuitry development can be resumed following exposure to drugs of abuse. It has been shown in a cell-based analysis that persistent activation of dopamine signaling, a major pharmacological consequence of cocaine exposure, quickly increases the level of DCC (Jassen et al., 2006). Furthermore, in a mouse line in which the expression of DCC is compromised (dcc-/+), reduced amphetamine-induced locomotor sensitization is observed (Flores et al., 2005). These observations lead to a hypothesis that cocaine-induced generation of silent synapses may be initiated by DCC-netrin1-based pre- and postsynaptic approaching.
Similar to cocaine, exposure to morphine also generates silent synapses in the NAc (Graziane and Dong, unpublished results). However, in contrast to cocaine, morphine-induced generation of silent synapses in the NAc is accompanied by a decrease in the density of dendritic spines, suggesting opposing underlying mechanisms (Graziane and Dong, unpublished results). Furthermore, a molecular manipulation that prevents AMPAR internalization prevents morphine-induced, but not cocaine-induced, generation of silent synapses in the NAc (Graziane and Dong, unpublished results). These results lead to a hypothesis that morphine-induced generation of silent synapses is mediated by AMPAR internalization from preexisting synapses (Graziane and Dong, unpublished results). This initial synaptic weakening process, if followed by a subsequent synapse elimination process, may result in a complete loss of affected synapses during morphine withdrawal. Similar to synaptogenesis, synapse elimination is also highly active in the developing brain. These two opposing processes coordinate and cooperate with each other to establish and refine new neurocircuits during development. As such, the potential morphine-induced synapse elimination process can also be triggered by a reactivation of certain developmental mechanisms.
The above analyses raise the possibility that exposure to cocaine and morphine triggers two opposing synaptic effects in the NAc, namely synapse formation and elimination. While this notion is intriguing, a legitimate question is whether these opposing synaptic effects produce similar circuitry consequences, given many similar behavioral consequences are induced after exposure to cocaine and morphine. The following section makes an attempt to address this critical question.
Circuitry Impact
There are at least two levels at which drug-induced generation of silent synapses can remodel NAc circuits. At the afferent level, as elaborated above, there are several excitatory projections to the NAc, each presumably carrying out different aspects of addiction-related emotional and motivational information. A critical question is whether drug-induced generation of silent synapses is a cross-board event, occurring in all projections, or a projection-specific occurrence. Thus far, we have examined the projections from the basolateral amygdala (BLA) and medial prefrontal cortex (mPFC) after cocaine self-administration and observed similar generation of silent synapses in both projections (Lee et al., 2013; Ma et al., 2014). However, the evolution of cocaine-generated silent synapses in these projections varies substantially. For example, a portion of cocaine-generated silent synapses in the projections from the BLA and infralimbic mPFC to the NAc mature by recruiting GluA2 subunit-lacking, calcium-permeable AMPARs (CP-AMPARs; Lee et al., 2013; Ma et al., 2014). Compared to regular AMPARs, CP-AMPARs have a higher single channel conductance and, more importantly, can activate intracellular calcium-coupled signaling; these properties may profoundly alter the synaptic properties within the affected circuits (Liu and Zukin, 2007). On the other hand, cocaine-generated silent synapses in the prelimbic mPFC to NAc projection mature by recruiting regular, calcium-impermeable AMPARs (Ma et al., 2014). These different maturation processes indicate that silent synapse-based circuitry remodeling not only quantitatively but also qualitatively changes the circuitry properties in the NAc (Figure 1). Thus far, little is known about whether morphine-induced generation of silent synapses and potential subsequent maturation of these synapses exhibit such afferent specificity.
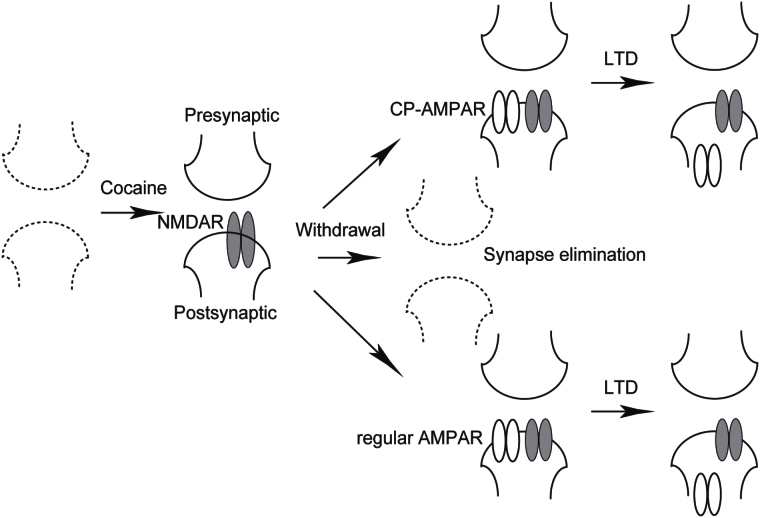
Figure 1.
Schematic diagram showing a hypothesized process of generation and evolution of silent synapses in cocaine-exposed animals. It is hypothesized that exposure to cocaine triggers a synaptogenesis process by forming nascent, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-silent excitatory synapses in the nucleus accumbens (NAc; left; Dong and Nestler, 2014). After withdrawal from cocaine self-administration, these silent synapses may: (1) mature by recruiting calcium-permeable AMPARs (CP-AMPARs), (2) mature by recruiting nonCP-AMPARs, or (3) be eliminated through metabolic turnover (middle). Generation and maturation of cocaine-generated silent synapses have been detected in a number of NAc afferents. Matured silent synapses contain both AMPARs and N-methyl-D-aspartate receptors (NMDARs) and are indistinguishable via most electrophysiological measurements. However, some of these matured silent synapses contain CP-AMPARs, and thus are susceptible to CP-AMPAR-oriented manipulations. Application of an in vivo long-term depression (LTD) protocol that preferentially targets synaptic CP-AMPARs to a specific NAc afferent may selectively internalize CP-AMPARs from matured silent synapses within this afferent, pushing these synapses from the matured state (containing both AMPARs and NMDARs) back to the immature state (containing only stable NMDARs; right).
At the efferent level, the principal NAc neurons can be roughly divided into two subpopulations, dopamine D1 receptor-expressing neurons and D2-expressing neurons, which preferentially project to the ventral tegmental area (direct pathway) and ventral pallidum (indirect pathway), respectively (Smith et al., 2013). Thus far, all prior studies that examine drug-induced generation of silent synapses randomly sample NAc neurons without distinguishing their subtypes. It is tempting to speculate that exposure to cocaine and morphine preferentially generates silent synapses in D1-expressing and D2-expressing neurons, respectively. As discussed above, cocaine- and morphine-induced generation of silent synapses likely involves two opposing cellular processes, one leading to synaptogenesis and the other leading to synapse elimination. However, exposure to cocaine and morphine induces many similar behavioral alterations. Given the potentially opposing roles of NAc D1- and D2-expressing neurons in drug-induced behaviors (Smith et al., 2013), the two potentially opposing processes of silent synapse generation after exposure to cocaine and morphine may lead to the same shift in the balance of excitatory inputs between D1- and D2-expressing NAc neurons, contributing to the same or similar behavioral outputs. This speculation remains to be tested.
Behavioral Impact
The behavioral consequences of drug-induced generation and the subsequent maturation of silent synapses start to be explored in a rodent relapse model called incubation of cue-induced cocaine craving. After drug self-administration, cue-induced drug seeking becomes increasingly intensified over the withdrawal period, a phenomenon termed incubation of cue-induced drug craving (Grimm et al., 2001). The incubated drug craving increases the likelihood of drug relapse during drug abstinence, and manipulating the incubation process may serve as an effective approach to reduce drug relapse.
Using in vivo optogenetic approaches after long-term withdrawal from cocaine self-administration, matured silent synapses within specific NAc afferents can be selectively reversed through in vivo long-term depression (LTD) procedures. To selectively target matured silent synapses in cocaine-exposed animals, the in vivo LTD procedure should meet at least three key standards. First, to minimize nonspecific effects, the LTD procedure should not affect pre-existing synapses. As such, the basal synaptic transmission (e.g. transmission in saline-exposed animals) should not be altered. Second, the LTD procedure should preferentially target CP-AMPARs, which are enriched in matured silent synapses. Third, the LTD procedure is likely projection-specific, as different projections often exhibit different synaptic properties. Bearing with these considerations, different LTD procedures are developed and verified, with which matured silent synapses within the BLA-to-NAc or PFC-to-NAc projection can be “re-silenced” to their immature state as observed right after cocaine exposure (Lee et al., 2013; Ma et al., 2014). Reversing silent synapse maturation in the BLA to NAc shell projection reverses incubation of cue-induced cocaine craving, indicating the critical role of silent synapse-based remodeling of BLA-to-NAc projection in cocaine relapse (Lee et al., 2013). Similarly, reversing silent synapse maturation in the prelimbic PFC to NAc core projection also reverses the incubated cocaine craving that has developed during drug withdrawal (Ma et al., 2014). An interesting future direction would be to determine what mechanisms connect silent synapse-based circuitry remodeling in these two separate NAc projections such that alterations in either projection is required for incubation of cocaine craving.
In contrast to the pro-relapse effects of the above two projections, reversing maturation of cocaine-generated silent synapses within the infralimbic mPFC to NAc shell projection after long-term withdrawal from cocaine self-administration results in enhanced incubation of cocaine craving (Ma et al., 2014). Thus, silent synapse-based remodeling of infralimbic mPFC to NAc shell projection functions to counteract the development of cocaine incubation. These results raise two important points: (1) for NAc-based behavioral outputs, the function of glutamatergic synapses is highly pathway specific, and should be considered separately; and (2) endogenous anti-relapse mechanisms exist and can be activated after exposure to drugs of abuse. The efficacies of these anti-relapse mechanisms are masked by stronger pro-relapse mechanisms, but, if boosted up through appropriate manipulations, these anti-relapse mechanisms can be unleashed to reduce drug craving after drug abstinence.
Concluding Remarks
This short review summarizes several critical questions provoked by recent findings of silent synapse-based circuitry remodeling. Addressing these questions will deepen our understanding about the cellular and molecular basis underlying drug-induced circuitry remodeling and may present circuitry-oriented therapeutic opportunities for treating drug addiction.
Statement of Interest
The author declares no conflict of interest related to the data presented in this manuscript. The paper meets the ethical guidelines provided by the University of Pittsburgh and NIH.
Acknowledgments
Dr Dong is supported by NIH funds DA023206, DA030379, and DA034856.
References
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E. (2005) The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev 49:22–47. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas PW, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schluter OM. (2011) A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci 31:8163–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. (2014) The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 35:374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. (1996) Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381:71–75. [DOI] [PubMed] [Google Scholar]
- Flores C, Manitt C, Rodaros D, Thompson KM, Rajabi H, Luk KC, Tritsch NX, Sadikot AF, Stewart J, Kennedy TE. (2005) Netrin receptor deficient mice exhibit functional reorganization of dopaminergic systems and do not sensitize to amphetamine. Mol Psychiatry 10:606–612. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Seth H, Riebe I. (2013) AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci 14:839–850. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schluter OM, Zukin RS, Dong Y. (2009) In vivo cocaine experience generates silent synapses. Neuron 63:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schluter OM, Dong Y. (2015) Silent synapses speak up: updates of the neural rejuvenation hypothesis of drug addiction. Neuroscientist 21:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. (1995) Evidence for silent synapses: implications for the expression of LTP. Neuron 15:427–434. [DOI] [PubMed] [Google Scholar]
- Jassen AK, Yang H, Miller GM, Calder E, Madras BK. (2006) Receptor regulation of gene expression of axon guidance molecules: implications for adaptation. Mol Pharmacol 70:71–77. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schluter OM, Dong Y. (2013) Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 16:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. (1995) Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375:400–404. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134. [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y. (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne PB, Halliday GM, Cooper HM, Keast JR. (2005) Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience 131:671–681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Supp 1):33–46. [DOI] [PubMed] [Google Scholar]
- Round J, Stein E. (2007) Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol 17:15–21. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. (2013) Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol 23:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. (1987) The role of reward pathways in the development of drug dependence. Pharmacol Ther 35:227–263. [DOI] [PubMed] [Google Scholar]
- Wolf ME. (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720. [DOI] [PubMed] [Google Scholar]
- Wolf ME. (2010) The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]