Abstract
Background:
Agomelatine modulates brain-derived neurotrophic factor expression via its interaction with melatonergic and serotonergic receptors and has shown promising results in terms of brain-derived neurotrophic factor increase in animal models.
Methods:
Twenty-seven patients were started on agomelatine (25mg/d). Venous blood was collected and brain-derived neurotrophic factor serum levels were measured at baseline and after 2 and 8 weeks along with a clinical assessment, including Hamilton Depression Rating Scale and Snaith-Hamilton Pleasure Scale.
Results:
Brain-derived neurotrophic factor serum concentration increased after agomelatine treatment. Responders showed a significant increase in brain-derived neurotrophic factor levels after 2 weeks of agomelatine treatment; no difference was observed in nonresponders. Linear regression analysis showed that more prominent brain-derived neurotrophic factor level variation was associated with lower baseline BDNF levels and greater anhedonic features at baseline.
Conclusions:
Patients affected by depressive disorders showed an increase of brain-derived neurotrophic factor serum concentration after a 2-week treatment with agomelatine. The increase of brain-derived neurotrophic factor levels was found to be greater in patients with lower brain-derived neurotrophic factor levels and marked anhedonia at baseline.
Keywords: BDNF, agomelatine, major depressive disorder
Introduction
Agomelatine is a novel antidepressant possessing melatonergic receptor agonistic (MT1 and MT2) and 5-HT2C receptor antagonistic properties (Millan et al., 2005; Olie et al., 2007), which displays robust antidepressant and anxiolytic-like actions in preclinical models (Papp et al., 2003; Millan et al., 2005) and alleviates major depression in humans. There is growing evidence for its role in the treatment of depression, as shown in recent short-term, double-blind, randomized controlled trials in which agomelatine was found to be more effective than placebo and at least as effective as available antidepressants (Taylor et al., 2014).
This efficacy seems to be related to the novel pharmacological profile of agomelatine. In particular, agomelatine acts synergistically on MT1/MT2 and 5HT2C receptors, with increases in dopamine and noradrenaline in the prefrontal cortex and no increase in serotonin levels. Also, agomelatine leads to an early improvement of anhedonic symptoms (Martinotti et al., 2012). Agomelatine appears to be well tolerated, does not induce withdrawal symptoms, and preserves sexual function, as opposed to SSRIs.
Neurotrophins are a class of proteins that serve as survival factors for selected populations of CNS neurons. In particular, brain-derived neurotrophic factor (BDNF) plays a major role in the modulation of synaptic plasticity and the maintenance of plasticity and trophism of midbrain dopaminergic and cholinergic neurons (Kuczewski et al., 2010). Accordingly, impaired neurotrophin production in the brain can lead to a variety of CNS dysfunctions.
Several clinical studies have shown that patients with depressive disorders exhibit a significant reduction in plasma BDNF levels (Yoshida et al., 2012), an effect partially or completely reversed by antidepressant treatment (Teixeira et al., 2010). Besides their potentiating effects on circulating BDNF, several clinically beneficial therapies, including SSRIs, TCA, and electroconvulsive therapy, were reported to increase hippocampal BDNF expression (Castren et al., 2007). Moreover, antidepressant drugs have been shown to increase neurogenesis and survival of newborn neurons in the hippocampal dentate gyrus (Malberg et al., 2000), possibly thus contributing to the efficacy of antidepressants (Santarelli et al., 2003; Li et al., 2008).
Recent animal studies on the effects of agomelatine on cellular processes involved in antidepressant mechanisms have shown that the drug increases BDNF expression in the prefrontal cortex and hippocampus (Molteni et al., 2010; Gumuslu et al., 2014) as well as the expression of activity-regulated cytoskeleton associated protein in the prefrontal cortex (Racagni et al., 2011). Moreover, Molteni et al. (2010) demonstrated that acute agomelatine treatment modulates BDNF expression by interacting with melatonergic MT1/MT2 and serotonergic 5-HT2C receptors. In line with this, prolonged treatment with agomelatine increases neurogenesis within the hippocampus, particularly via enhancement of neuronal cell survival, reducing stress-induced glutamate release in the prefrontal/frontal cortex (Racagni et al., 2011).
Based on these observations, the first aim of this study was to investigate the effects of agomelatine treatment on BDNF serum levels in a sample of depressed patients. The secondary aim was to investigate if BDNF variations were correlated with clinical improvement.
Methods
Participants, Assessment, Study Procedures
This study was performed at the Department of Neuroscience, Imaging, and Clinical Sciences, Chair of Psychiatry, “G d’Annunzio” University of Chieti-Pescara, Italy. Between September and December 2013, 50 patients were screened and 27 were recruited at the outpatient unit of the Department. Inclusion criteria were: age between 18 and 65 years; presence of a DSM-IV diagnosis of: major depression; minor depression; dysthymia, corresponding to the current DSM-5 area of Depressive Disorders. Exclusion criteria were the following: current or lifetime diagnosis of organic mental disorder, schizophrenia, schizophrenia-spectrum, or other psychotic disorders, bipolar disorders, substance-related disorders, uncontrolled or severe medical conditions (eg, cirrhosis, renal impairment, unstable hypertension, hypotension, diabetes mellitus, convulsions). Female patients who were pregnant, breastfeeding, or not using effective contraception were also excluded. Any current psychopharmacological treatment was an exclusion criterion. Benzodiazepines were allowed.
Fifty patients were screened for the study and 27 enrolled. Screening failures included 10 patients who were not psychiatrically eligible, 8 with concomitant psychopharmacological treatments, 4 who withdrew consent, and 1 who was not medically eligible. After the screening period, patients were started on agomelatine at an initial dose of 25mg/d per os. The dosage regimen involved the administration of 25mg/d agomelatine in a single dose at 8:00 pm. In case of no clinical response and following approval from the examiner, the dosage could be increased to 50mg/d, administered in a single dose, after the first 2 weeks of therapy. The medical examination was performed by qualified physicians at baseline (T0) and after 2 weeks (T1) and 8 weeks (T2) from the beginning of the study, and it included checking vital signs, recording other concomitant medication, and assessing the severity of any adverse effects. Safety parameters were monitored through ECG and haematological and clinical chemical analyses of blood samples, with liver function monitoring carried out at T0, T1, and T2.
The clinical assessment, performed at T0, T1, and T2, included: the Structured Clinical Interview for DSM-IV Axis-I disorders Patient Version (First et al., 1997), the Snaith-Hamilton Pleasure Scale (SHAPS) for anhedonia, the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS) to assess mood and anxiety symptoms, and the Clinical Global Impression scale. The Structured Clinical Interview for DSM-IV Axis-I disorders Patient Version was administered to patients by psychiatrists trained and certified in the use of these instruments.
Twenty-nine healthy control subjects (HC), homogeneous with respect to socio-demographic characteristics, were also enrolled. Healthy volunteers were recruited from the hospital staff and their relatives. Before the inclusion in the study, they were tested to exclude current and past psychiatric conditions.
The study protocol complied fully with the guidelines of the Ethics Committee of the University “G.d’Annunzio” of Chieti-Pescara and was approved by the Institutional Review Boards in accordance with local requirements. It was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki (1964) and subsequent revisions. After receiving information about the drug, any possible side effects and the dosing rate as well as information on the possibility of dropping out of the study at any time, all patients (or their legal representatives) provided their written informed consent prior to randomization.
Determination of BDNF Content in Serum
At T0, T1, and T2 venous blood was collected between 7:30 and 9:00 am into sampling tubes that were centrifuged within 20 minutes after sampling at 2000 × g for 20 minutes. Serum was then aliquoted and stored at −80°C until analysis.
BDNF was detected in sandwich ELISAs according to the manufacturer’s instructions (R and D Systems, Minneapolis, MN). This sandwich ELISA is set to measure natural and recombinant human mature BDNF in cell culture medium and serum. All assays were performed on F-bottom 96-well plates (Nunc, Wiesbaden, Germany). Tertiary antibodies were conjugated to horseradish peroxidase. Wells were developed with tetramethylbenzidine and measured at 450/570nm. BDNF content was quantified against a standard curve calibrated with known amounts of protein. The detection limits were 4 pg/mL. Measurements were performed in duplicate and are expressed as pg/mL. Cross-reactivity to related neurotrophins (NGF, NT-3, NT-4) was <3%.
Statistical Analysis
All analyses were conducted using nonparametric testing. Mann-Whitney U Test for independent variables was used to compare BDNF levels of treated depressed patients with that of healthy matched controls. Friedman Test and Wilcoxon Test for paired variables were used to monitor changes in scores on psychometric scales and serum BDNF levels in the patient group. Correlations between scores on psychometric scales and BDNF levels were measured by means of Spearman’s rank correlation coefficient. Statistical analysis was performed using SPSS for Windows, Version 20.0 (SPSS Inc, Chicago, IL).
Results
Clinical Efficacy
Twenty-seven patients were enrolled in the study and compared with 29 matched HC. Mean age was 49.59±13.96 years (vs 44.31±13.65 in HC). Ten of 27 subjects were male (37% vs 44.8% in HC). Years of education were 13.9±3.7 (vs 14.5± 4 in HC). A total of 40.7% of subjects had familiarity for mood and other psychiatric disorders. Mean age of onset was 30±13.39 years, with a disease duration of 19.85±15.81 years.
Sixteen patients terminated early; 3 before T1 due to patient choice and 13 before T2 were either lost to follow-up (n=8) or dropped out due to lack of efficacy (n = 5). No patients terminated the study due to side effects. No patients had worsening of mood symptoms or were hospitalized.
Friedman nonparametric testing and posthoc Wilcoxon testing revealed that levels of depression (HDRS) (χ2 = 13.23 P<.01), anxiety (HARS) (χ2 = 14 P<.001), and anhedonia (SHAPS) (χ2 = 11.2 P<.01) decreased over time (Table 1). Eleven of 24 subjects (45.8 %) were responders at week 2; 9 of 11 (81.8%) were responders by the end of the trial.
Table 1.
BDNF Serum Levels and Clinical Assessment at Baseline and After Agomelatine Treatment
| Baseline | T1 | T2 | Statistical Analysis | |
|---|---|---|---|---|
| N | 27 | 24 | 11 | - |
| Time (weeks) | 0 | 2 | 8 | - |
| BDNF | 117.35 (34.02) | 150.79 (42.78) | 126.34 (37) | χ2 = 17.18 P<.001; T1>T0**; T2>T0** |
| CGI | 3.23 (1.02) | 2.27 (0.88) | 1.86 (0.69) | χ2 = 11.2 p=0.004; T1<T0**. T2<T0* |
| HDRS scores | 21.41 (6.15) | 13.86 (5.78) | 10.78 (5.54) | χ2 = 13.23 P<.01; T1<T0**. T2<T0* |
| SHAPS scores | 5.82 (1.87) | 3.5 (1.82) | 3.11 (1.05) | χ2 = 11.2 P<.01; T1<T0**. T2<T0* |
| HARS scores | 20.55 (4.95) | 14 (4.4) | 15.29 (5.09) | χ2 = 14 P<.001; T1<T0**. T2<T0* |
| YMRS scores | 4.23 (2.93) | 3.91 (3.39) | 2.86 (2.34) | NS |
Abbreviations: BDNF, brain-derived neurotrophic factor; CGI, Clinical Global Impression; HDRS, Hamilton Depression Rating Scale; SHAPS, Snaith-Hamilton Pleasure Scale; HARS, Hamilton Anxiety Rating Scale; T0, baseline; T1, after 2 weeks of treatment; T2, after 8 weeks of treatment; YMRS, Young Mania Rating Scale.
*P<.05; **P<.01.
There was no serious adverse events. The most common study-related adverse events were dizziness (n=2) and nausea (n=2). No aminotransferase elevations were noted. No subjects became manic.
The mean dosage at the end of the study was 29.5±10.1mg/d.
BDNF Levels
Mann-Whitney U-test was performed to compare baseline BDNF levels between patients and HC (Figure 1). Baseline BDNF levels were significantly lower in depressed individuals (117.34±34.02ng/mL vs 170.8±30.09ng/mL in HC; Z = -4.75, P<.0001). Spearman’s rank correlation coefficient revealed no relationship between BDNF levels at baseline (T0) and depressive symptoms severity (HDRS).
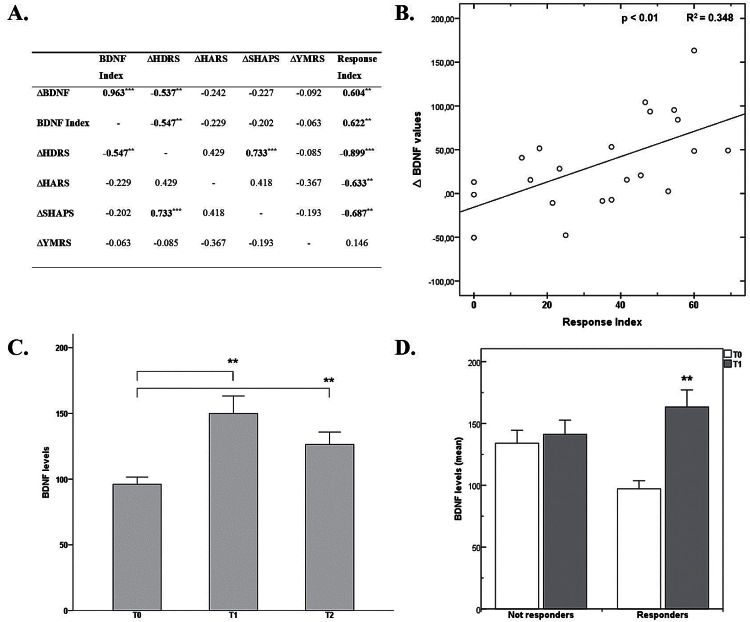
Figure 1.
(A) Correlation between brain-derived neurotrophic factor (BDNF) serum levels and psychometric variables after 2 weeks of agomelatine treatment. ∆BDNF values were obtained as the difference between after 2 weeks (T1) − baseline (T0) values. BDNF index was calculated as ∆BDNF on BDNF at baseline. ∆ Hamilton Depression Rating Scale (HDRS), ∆ Hamilton Anxiety Rating Scale (HARS), ∆ Snaith-Hamilton Pleasure Scale (SHAPS), and ∆ Young Mania Rating Scale (YMRS) values were respectively calculated as (T1-T0) values. *P<.05; **P<.01; ***P<.001. (B) Correlation between BDNF and HDRS variation after 14 days of agomelatine treatment. ∆BDNF indicates the change in BDNF after agomelatine treatment. The Response Index indicates the improvement (expressed as percentage) in depressive symptoms from baseline as measured by HDRS; response index = (∆HDRS*100)/(HDRS T0). (C) BDNF levels at baseline and follow-up. Data are expressed as mean±SEM. **P<.01. (D) BDNF levels variation in patients with and without a clinical response. **P<.01.
BDNF levels detected in the study population were as follows: T0 (117.35±34.02ng/mL), T1 (150.79± 42.78ng/mL), and T2 (126.34±37ng/mL). During the study period, Friedman’s test showed significant changes of BDNF serum levels over the 3 assessment time-points (χ2 = 17.18, P<.001). Wilcoxon’s posthoc analysis showed significant differences between T0 and T1 (P<.01), and T0 and T2 (P<.01), but not between T1 and T2 (Table 1; Figure 1).
The increase of serum BDNF concentration elicited by agomelatine was correlated with the improvement observed in symptoms of depression, as measured by reduction on HDRS scores (Rho Spearman = -0.537; P<.01), as well as response index (0.604; P<.01) (Figure 1). It is worth noting that responders showed a significant difference in BDNF levels after 2 weeks of agomelatine treatment, while no difference was observed in nonresponders (Figure 1).
Finally, results from the multivariate stepwise linear regression analysis showed that more prominent BDNF level variation was associated with lower baseline BDNF levels and greater anhedonic features at baseline, as measured with the SHAPS (R2 = 0.576; P<.01).
Discussion
In this study, we investigated whether agomelatine, a new and specific antidepressant drug able to increase BDNF in the rat brain (Soumier et al., 2009; Païzanis et al., 2010; Racagni et al., 2011; Ladurelle et al., 2012; Gumuslu et al., 2014), could induce a similar effect on BDNF serum levels in depressed patients.
First of all, consistent with other studies in patients with depressive and other major psychiatric disorders (Castren et al., 2007; Yoshida et al., 2012; Angelucci et al., 2014), we observed that depressed subjects had lower levels of baseline BDNF serum concentration compared with HC.
Moreover, our study, though with an open design, provides preliminary evidence that agomelatine’s antidepressant properties are associated to the enhancement of BDNF gene expression, as is the case for other antidepressant drugs (Calabrese et al., 2011; Gumuslu et al., 2014).
It is worth noting that this increase in serum BDNF has a strong correlation with improvement in depression, while the multivariate stepwise linear regression analysis showed that a more prominent BDNF level variation was associated with lower baseline BDNF levels and greater anhedonic features at baseline. This is in line with previous data demonstrating improvement in anhedonic symptoms following treatment with agomelatine (Martinotti et al., 2012; for review, see Di Giannantonio and Martinotti, 2012). This effect is possibly functionally correlated with the increase in BDNF levels. Moreover, this finding is consistent with the observation that absence of an early increase in BDNF levels may represent a peripheral marker of future treatment failure in MDD patients.
BDNF levels do not seem to increase between T1 (2 weeks) and T2 (8 weeks), though remain significantly higher than baseline values; this result needs to be evaluated in larger samples, given that in this study only 11 subjects fully completed the study. We hypothesize that agomelatine-induced increase in neurotrophic factors possibly triggers a chain of events ultimately related to its antidepressant action.
Agomelatine’s specific action on the circadian rhythm could be related to its effect on BDNF given that neurotrophin expression is activity dependent (Bramham and Messaoudi, 2005) and is regulated by the light and dark cycle in rats and humans (Faraguna et al., 2008).
Given the capability of agomelatine to stimulate the expression of BDNF mRNA and protein levels in different brain areas (prefrontal cortex, hippocampus) through the functional interaction with melatonergic MT1/MT2 and 5HT2C receptors (Molteni et al., 2010), Soumier et al. (2009) postulated that agomelatine produces major transcriptional changes in the hippocampus, where significant upregulation of BDNF was observed. Moreover, the levels of BDNF protein were elevated by agomelatine in both the hippocampus and the prefrontal cortex (Calabrese et al., 2011). These findings support the hypothesis that the increase of BDNF expression in different brain areas may be important for the antidepressant response (Tardito et al., 2006; Soumier et al., 2009; Ladurelle et al., 2012; Gumuslu et al., 2014) and provide new information regarding the molecular mechanisms that contribute to the chronic effects of the new antidepressant agomelatine on brain function. The ability of agomelatine to modulate the expression of these neuroplastic molecules, which are also involved in the physiological modulation of the circadian rhythm, may contribute to its antidepressant action.
This study has several limitations: (1) the sample size is small and the follow-up period was only 8 weeks; (2) in this study, we focused only on the mature form of BDNF, given that the sensitivity of the ELISA kit for the proBDNF did not guarantee a sufficient level of sensitivity (Yoshida et al., 2012); (3) the open design may limit the interpretation of results; (4) the absence of any comparator with regard to outcome; (5) the concomitant, though limited, intake of other drugs. With respect to the latter, it is important to note that the intake of any psychotropic drug (with the exception of benzodiazepines) was an exclusion criterion.
In conclusion, this study demonstrated that patients affected by depressive disorders showed an increase of BDNF serum concentration after a 2-week treatment with agomelatine, along with an improvement of depressive symptoms, significantly correlated with BDNF levels. Furthermore, the increase of BDNF levels following agomelatine treatment was found to be greater in patients with lower BDNF levels and marked anhedonia at baseline. Considering the important roles of both proBDNF and mature BDNF in physiological functions, future studies should measure individual levels of both forms in response to agomelatine treatment. Though the role of BDNF in eliciting an antidepressant response following treatment with agomelatine requires further investigation, the present study suggests that BDNF is a crucial factor, with important implications for both the clinical management of depressed patients and the future development of specific psychopharmacological treatments.
Statement of Interest
None.
Acknowledgments
We thank Dr. M. Lupi, Dr. F. Vellante, and Dr. F. Sarchione for clinical assistance in the recruitment of patients, and Dr. L. De Risio for language revision.
This work was partly supported by the National Institute of Health.
References
- Angelucci F, Ricci V, Gelfo F, Martinotti G, Brunetti M, Sepede G, Signorelli M, Aguglia E, Pettorruso M, Vellante F, Di Giannantonio M, Caltagirone C. (2014) BDNF serum levels in subjects developing or not post-traumatic stress disorder after trauma exposure. Brain Cogn 84:118–122. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76:99–125. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Gabriel C, Mocaer E, Racagni G, Riva MA. (2011) Modulation of neuroplastic molecules in selected brain regions after chronic administration of the novel antidepressant agomelatine. Psychopharmacology (Berl) 215:267–275. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. (2007) Role of neurotrophic factors in depression. Curr Opin Pharmacol 7:18–21. [DOI] [PubMed] [Google Scholar]
- Di Giannantonio M, Martinotti G. (2012) Anhedonia and major depression: The role of agomelatine. European Neuropsychopharmacology 22 (3): S505–S510 [DOI] [PubMed] [Google Scholar]
- Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. (2008) A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 28:4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumuslu E, Mutlu O, Sunnetci D, Ulak G, Celikyurt IK, Cine N, Akar F, Savli H, Erden F. (2014) The antidepressant agomelatine improves memory deterioration and upregulates CREB and BDNF gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights 8:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Gaiarsa JL. (2010) Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur J Neurosci 32:1239–1244. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Gabriel C, Viggiano A, Mocaer E, Baulieu EE, Bianchi M. (2012) Agomelatine (S20098) modulates the expression of cytoskeletal microtubular proteins, synaptic markers and BDNF in the rat hippocampus, amygdala and PFC. Psychopharmacology (Berl) 221:493–509. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. (2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Sepede G, Gambi F, Di Iorio G, De Berardis D, Di Nicola M, Onofrj M, Janiri L, Di Giannantonio M. (2012) Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: a pilot study. J Clin Psychopharmacol 32:487–491. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Dekeyne A. (2005) Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology (Berl) 177:448–458. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Pisoni S, Gabriel C, Mocaer E, Racagni G, Riva MA. (2010) Synergistic mechanisms in the modulation of the neurotrophin BDNF in the rat prefrontal cortex following acute agomelatine administration. World J Biol Psychiatry 11:148–153. [DOI] [PubMed] [Google Scholar]
- Olie JP, Kasper S. (2007) Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol 10:661–673. [DOI] [PubMed] [Google Scholar]
- Païzanis E, Renoir T, Lelievre V, Saurini F, Melfort M, Gabriel C, Barden N, Mocaër E, Hamon M, Lanfumey L. (2010) Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptor-impaired mice. Int J Neuropsychopharmacol 13:759–774. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Boyer P A, Mocaer E. (2003) Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology 28:694–703. [DOI] [PubMed] [Google Scholar]
- Racagni G, Riva MA, Molteni R, Musazzi L, Calabrese F, Popoli M, Tardito D. (2011) Mode of action of agomelatine: synergy between melatonergic and 5-HT2C receptors. World J Biol Psychiatry 12:574–587. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, Kerkerian-Le-Goff L, Gabriel C, Millan MJ, Mocaer E, Daszuta A. (2009) Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology 34:2390–2403. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. (2006) Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev 58:115–134. [DOI] [PubMed] [Google Scholar]
- Tardito D, Molteni R, Popoli M, Racagni G. (2012) Synergistic mechanisms involved in the antidepressant effects of agomelatine. Eur Neuropsychopharmacol 22:S482–486. [DOI] [PubMed] [Google Scholar]
- Taylor D, Sparshatt A, Varma S, Olofinjana O. (2014) Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ 348:g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AL, Barbosa IG, Diniz BS, Kummer A. (2010) Circulating levels of brain-derived neurotrophic factor: correlation with mood, cognition and motor function. Biomark Med 4:871–887. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K. (2012) Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One 7:e42676. [DOI] [PMC free article] [PubMed] [Google Scholar]