Abstract
Background:
We have previously reported associations between frontal D2/3 receptor binding potential positive symptoms and cognitive deficits in antipsychotic-naïve schizophrenia patients. Here, we examined the effect of dopamine D2/3 receptor blockade on cognition. Additionally, we explored the relation between frontal D2/3 receptor availability and treatment effect on positive symptoms.
Methods:
Twenty-five antipsychotic-naïve first-episode schizophrenia patients were examined with the Positive and Negative Syndrome Scale, tested with the cognitive test battery Cambridge Neuropsychological Test Automated Battery, scanned with single-photon emission computerized tomography using the dopamine D2/3 receptor ligand [123I]epidepride, and scanned with MRI. After 3 months of treatment with either risperidone (n=13) or zuclopenthixol (n=9), 22 patients were reexamined.
Results:
Blockade of extrastriatal dopamine D2/3 receptors was correlated with decreased attentional focus (r = -0.615, P=.003) and planning time (r = -0.436, P=.048). Moreover, baseline frontal dopamine D2/3 binding potential and positive symptom reduction correlated positively (D2/3 receptor binding potential left frontal cortex rho = 0.56, P=.003; D2/3 receptor binding potential right frontal cortex rho = 0.48, P=.016).
Conclusions:
Our data support the hypothesis of a negative influence of D2/3 receptor blockade on specific cognitive functions in schizophrenia. This is highly clinically relevant given the well-established association between severity of cognitive disturbances and a poor functional outcome in schizophrenia. Additionally, the findings support associations between frontal D2/3 receptor binding potential at baseline and the effect of antipsychotic treatment on positive symptoms.
Keywords: Schizophrenia, antipsychotic-naïve, epidepride, SPECT, frontal dopamine D2/3 receptor, psychopathology, cognition
Introduction
The literature generally supports an association between psychotic symptoms and both increased synthesis and release of dopamine in the associative striatum (Howes et al., 2012). Clinical and preclinical data additionally point to a key role of prefrontal dopamine activity for schizophrenic symptomatology, including cognitive deficits as well as psychopathology (Knable and Weinberger, 1997; Glenthoj et al., 2006; Floresco, 2013; Fagerlund et al., 2013; Arnsten, 2013). We have previously shown associations between frontal D2/3 receptor binding potential (BPND) values and cognitive measures of planning, attention, and set shifting in antipsychotic-naïve first-episode schizophrenia patients (Fagerlund et al., 2013). The data indicated that in schizophrenia patients, frontal dopamine D2/3 receptors are involved in cognition to a higher degree than in healthy subjects and that patients depend on frontal D2/3 availability for normal cognitive processing. Moreover, in the same cohort of antipsychotic-naïve patients, we additionally found a significant, positive correlation between BPND and psychopathology (Glenthoj et al., 2006).
Our clinical in vivo data on extrastriatal D2/3 receptors (Glenthoj et al., 2006; Fagerlund et al., 2013) as well as corresponding clinical in vivo data on extrastriatal D1 receptors (Abi-Dargham et al., 2002, 2012) are generally in agreement with the so-called two-state dynamic model of dopamine function in PFC proposed by Seamans and Yang (2004). The model suggests that predominant D1 receptor activation will increase the inhibition of intruding stimuli (closure of the gate), and initiation and stabilization of goal-related representations in working memory. In this way, D1 activation will enhance the robustness of working memory by facilitating the encoding of specific stimuli at the expense of other internally or externally derived representations. The authors call this network state 2. In contrast, the functional state with an open gate is denoted network state 1. In state 1, predominant D2 activity will reduce inhibition, which will allow multiple inputs to be represented at the same time. State 1 allows the organism to react upon important stimuli, but a switch to state 2 is necessary to avoid “contamination” of information processing by externally or internally derived distracters. Hence, Seamans and Yang (2004) propose that increased prefrontal D2 receptor activity result in random, tangential, or intrusive thoughts and development of positive psychotic symptoms. Our previous data support the clinical relevance of D2/3 receptor activity for state 1 in the model (Glenthoj et al., 2006; Fagerlund et al., 2013).
In the present study, we related blockade of frontal D2/3 receptors to cognitive functions. Based on our previous data (Fagerlund et al., 2013), we expected high occupancy to further compromise selected cognitive functions. Additionally, we explored if higher BPND at baseline (Glenthoj et al., 2006) was associated with more pronounced reductions in positive psychotic symptoms.
Methods and Materials
The study was approved by the ethical committee of Copenhagen and Frederiksberg (KF 01-078/97 and 01-012/98). After complete description of the study, written informed consent was obtained.
Participants
Patients were included if fulfilling criteria for the international classification of diseases 10th version (ICD-10) for schizophrenia. The diagnosis was confirmed by a trained psychiatrist using Schedules for Clinical Assessment in Neuropsychiatry 2.0 (Wing et al., 1990). This was the first admission for treatment of psychotic symptoms for all patients, and none had been exposed to antipsychotic medication at the baseline examinations. Only antipsychotic-naïve patients were included. Patients with known retardation or who were compulsorily hospitalized were excluded (Glenthoj et al., 2006; Fagerlund et al., 2013). Patients received antipsychotic medication within normal clinical range during the treatment period (Table 1).
Table 1.
Demographics, Clinical Ratings, and Treatment
| All Patients (n = 25) | Zuclopenthixol (n = 9) | Risperidone (n = 16) | |
|---|---|---|---|
| Age | 26.5 (5.0) | 26.3 (5.2) | 27.2 |
| Sex M/F | 18/7 | 6/3 | 12/4 |
| DUP (months) | 19.4 (18.2) | 15.3 (8.0) | 21.7 (21.9) |
| PANSS Baseline | |||
| Positive | 20.2 (3.9) | 18.4 (2.3) | 21.2 (4.3) |
| Negative | 19.6 (5.3) | 17.8 (5.1) | 20.6 (5.3) |
| Total | 70.0 (12.6) | 64.4 (12.3) | 73.1 (12.1) |
| PANSS follow-up | |||
| Positive | 10.4 (2.2) | 9.7 (1.9) | 10.9 (2.3) |
| Negative | 16.5 (3.4) | 15.3 (2.9) | 17.1 (3.6) |
| Total | 47.7 (6.1) | 19.8 (2.3) | 21.0 (2.9) |
| PANSS change | |||
| Positive | 9.76 (3.2)** | 8.78 (2.2)** | 10.31 (3.6)** |
| Negative | 3.12 (4.4)** | 2.44 (3.5) | 3.50 (4.9)** |
| Total | 22.24 (10.6)** | 19.67 (10.7)** | 23.69 (10.6)** |
| Treatment period, wk | 12.6 (3.4) | 12.8 (4.2) | 12.5 (3.0) |
| Medication dose, mg | 9.6 (6.3) [4–26] | 3.8 (1.7) [1–7] | |
| Extrastriatal occupancy | 65% | 66% | 65% |
Abbreviations: DUP, duration of psychosis; PANSS, Positive and Negative Syndrome Scale.
Variables are provided in mean values. Standard deviations are provided in () and range in []. Significant differences between groups are marked with*, P < .05. Significant changes in PANSS score over time are marked with **, P < .05. There were no other significant differences between groups.
Baseline data relating BPND to cognitive functions or psychopathology in the same cohort of antipsychotic-naïve patients and matched healthy controls have previously been reported (Glenthoj et al., 2006; Fagerlund et al., 2013) as have longitudinal single-photon emission computerized tomography (SPECT) data on [123I]epidepride binding to cerebellar receptors (Pinborg et al., 2007). In addition, we have reported longitudinal and/or baseline data on disturbances in sensorimotor gating, structural correlates of sensorimotor gating, brain structure, and cognitive data on patients compared with matched controls in the same cohort of patients and controls (Mackeprang et al., 2002; Fagerlund et al., 2004, 2013; Glenthoj et al., 2007; Hammer et al., 2011, 2013).
Medication
Patients were randomly allocated to either zuclopenthixol or risperidone treatment. Medication dose was individually determined based on the severity of symptoms and reported adverse effects. The use of benzodiazepines was allowed but restricted on days of examinations. The study is part of a longitudinal cohort study. When the study was originally planned, we expected, but did not find, differential effects of first- and second-generation antipsychotic compounds on cognitive functions and sensory motor gating (Mackeprang et al., 2002; Fagerlund et al., 2004). Risperidone and zuclopenthixol were chosen because at that time they were the most commonly used first- and second-generation antipsychotic compounds in Denmark, respectively. Based on our previous observations, we did not expect differential effects of the two compounds with respect to neither cognition nor psychopathology nor frontal D2/3 receptor occupancy.
Psychopathology and Cognition
Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Cognitive measures were obtained with Cambridge Neuropsychological Test Automated Battery (CANTAB) (Sahakian and Owen, 1992; Lowe and Rabbitt, 1998). For the present analyses, we a priori selected a total of 7 cognitive measures addressing domains of attention, executive function, and processing speed (Fagerlund et al., 2004, 2013). Selected cognitive measures were CANTAB Rapid visual information processing; CANTAB Stockings of Cambridge minimal number of moves; CANTAB Stockings of Cambridge initial thinking time; CANTAB Stockings of Cambridge subsequent thinking time; CANTAB intra extra dimensional set shifting task; Verbal semantic fluency; and Trail making B (minus A).
SPECT
SPECT scans were performed using a Tomomatic 232 scanner (Medimatic, Copenhagen), a fast rotating (6rpm), brain-dedicated SPECT scanner that simultaneously recorded radiation from 2 parallel, trans-axial slices of the brain with a slice thickness of 17mm and distance between mid-slice levels of 10mm. Four recording sessions of 15 minutes began approximately 6 hours after the bolus injection. Data were then achieved from 8 orbitomeatal (OM) planes covering the brain from OM +20 to OM +90, generating 8 slices. The spatial resolution in the trans-axial plane was 12mm full width half maximum (FWHM). The energy window was set to 140 to 180 KeV. For quantification of D2/3 receptors in extrastriatal regions, we used [123I]epidepride (Kessler et al., 1991) and a bolus/infusion approach (Pinborg et al., 2000). Tracer steady-state conditions were obtained in extrastriatal regions within 3 to 4 hours, but the infusion continued for 7 hours to minimize individual differences in plasma clearance and binding parameters. A 64x64 filtered back-projection reconstruction matrix was used to reconstruct data. The trans-axial slices were corrected with a uniform attenuation coefficient of 0.05cm-1. Subjects received approximately 150 MBq [123I]epidepride (MAP, Medical Technologies, Inc., Finland) per examination. The radiochemical purity was >99% and the specific activity >1.8x1014 Bq/nmol.
MRI
At baseline, high-resolution 3D T1-weighted sagittal MPRAGE scans of the whole head were acquired for structural analyses. Twenty-two patients were examined on a 1.5 Tesla Siemens Vision scanner (TE=4ms; TR=9.7ms; flip angle=12°; matrix= 256x256; FOV=250mm; 0.98x0.98x1mm voxels; 170 slices). Three patients were scanned on a 1.0 Tesla Siemens Impact scanner (TE=4.4ms; TR=11.4ms; flip angle=15°; matrix= 256x256; FOV=250mm; 0.98x0.98x1mm voxels; 170 slices). Patients had no clinically significant brain pathology as determined by neuroradiological examination.
Coregistration
Coregistration between SPECT and MRI images was performed using a Matlab (Mathworks Inc., Natick, MA) based semiautomatic program, Interactive Point Selection (Willendrup et al., 2004). For each image modality, at least 6 corresponding anatomical points were manually identified. A rigid transformation of the images was estimated automatically by minimizing the sum of squared errors between the defined points. Using the identified rigid transformation matrix, the MRI images were resliced to the planes defined by the SPECT images (10mm between slices and an in-plane resolution of 1x1mm as in the MRI images).
Region of Interest
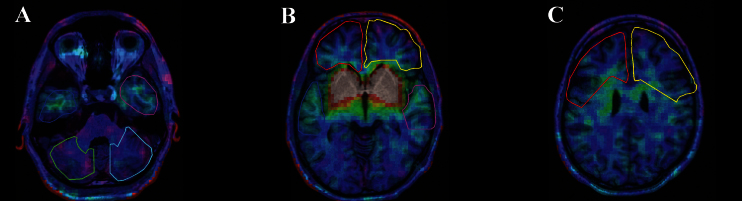
A set of 6 anatomical regions of interest (ROIs) (left and right frontal cortex, left and right temporal cortex, and left and right cerebellum) were manually delineated at 2D transverse MRI planes using a locally developed Matlab (Mathworks Inc., Natick, MA) based program (editroi) (Figure 1). ROIs were identified by means of a neuroanatomical atlas (Talairach and Tournaux, 1988, MNI). Subsequently, ROIs were applied to the SPECT images, and mean counts of the voxels included in each of the ROIs were extracted. BPND was calculated for left and right frontal cortex, left and right temporal cortex, and left and right cerebellum.
Figure 1.
Example of axial image coregistration of single-photon emission computerized tomography (SPECT) and magnetic resonance (MR) images in a patient. Regions delineated are: (A) cerebellum (left in green and right in turquoise); (B) temporal cortex (left in blue and right in purple); (C) frontal cortex (left in red and right in yellow). SPECT spatial resolution in the trans-axial plain was 12mm full width half maximum (FWHM) with a 17-mm slice thickness. MR images were resliced to the planes defined by the SPECT images giving an in-plane resolution of 1x1mm in the MRI images and 10mm between slices.
Quantification of Dopamine D2 Receptors
The BPND was used as a measure of regional D2/3 receptor availability before and after treatment (Innis et al., 2007). , where VT is the total volume of distribution of [123I]epidepride in a ROI and VND represents the volume of distribution of nondisplacable [123I]epidepride in tissue. Cerebellum is used as a representation of nondisplaceable [123I]epidepride binding (Glenthoj et al., 2006). For the calculation of extrastriatal D2/3 receptor occupancy, we used the paired distribution volumes before and after treatment. This was done using the paired distribution volumes before (the unblocked situation) and after treatment (the partially blocked situation) and the Lassen Plot (Lassen et al., 1995). For each patient, a Lassen Plot was generated, including the regions left and right frontal cortex, left and right temporal cortex, and left and right cerebellum. Occupancy was then calculated from the slope of the Lassen plot using linear regression analysis and provides a measure of extrastriatal D2/3 occupancy.
Statistical Analyses
All data analyses were performed using the statistical analysis software, SPSS 20 (SPSS, Statistics 20, IBM Corporation, Armonk, NY). Both in the complete dataset and in the 2 treatment groups, separately, the BPND and occupancy data object variables could be fitted approximately by normal distribution for all ROIs (Glenthoj et al., 2006). Differences in occupancy between treatment groups were consequently tested using ANOVA techniques. Change scores in psychopathology (follow-up minus baseline) were calculated separately for PANSS positive, negative, and total scores. PANSS scores were not normally distributed and consequently, group differences were tested using nonparametric techniques, for example, the Mann-Whitney U test, and changes over time were tested with Wilcoxon signed rank test. The Spearman’s rank-correlation coefficient was used to test potential associations between PANSS score, BPND, and occupancy.
Most of the cognitive measures could be fitted immediately by normal distribution and if not, a log transformation was applied to improve fit. Changes in cognitive function (follow-up minus baseline) were calculated for all 7 variables. Our measurement of change in cognitive variables was also analyzed using the residuals from a regression analysis of endpoint on baseline values, predicting endpoint from baseline. Possible associations between occupancy and cognitive function were tested using correlation analysis in accordance with the statistical properties of the variables. Since previous data have shown the need for extending linear analysis through possible quadratic associations, both occupancy and squared occupancy measures were used in the analyses. Potential (gender x occupancy) and (medication group x occupancy) interaction effects for both occupancy and occupancy squared measures were tested.
Results
Demographic and Clinical Data
Twenty-five patients (18 males, 7 females) entered the study. After baseline examinations, 9 patients were randomized to treatment with zuclopenthixol and 16 were allocated to risperidone. Twenty-two patients (16 males, 6 females) completed follow-up examinations; however, 1 of the patients (male) did not go through the cognitive examination but completed all other tests. Two patients (1 male, 1 female) were excluded from follow-up examinations because of compulsorily hospitalization. One patient (male) was excluded from follow-up analyses due to incomplete SPECT data. All 3 excluded patients were treated with risperidone. No significant differences in demographic, clinical, and neurochemical variables between dropouts and completers were found. The 2 patient groups were comparable regarding age, gender, treatment period, and mean duration of psychosis (Table 1).
Patients were moderately ill, and psychopathology improved significantly in the whole group and in the risperidone group during treatment. Improvement in negative symptoms was only trend level in the zuclopenthixol group (Table 1).
Extrastriatal Dopamine D2/3 Receptor Occupancy
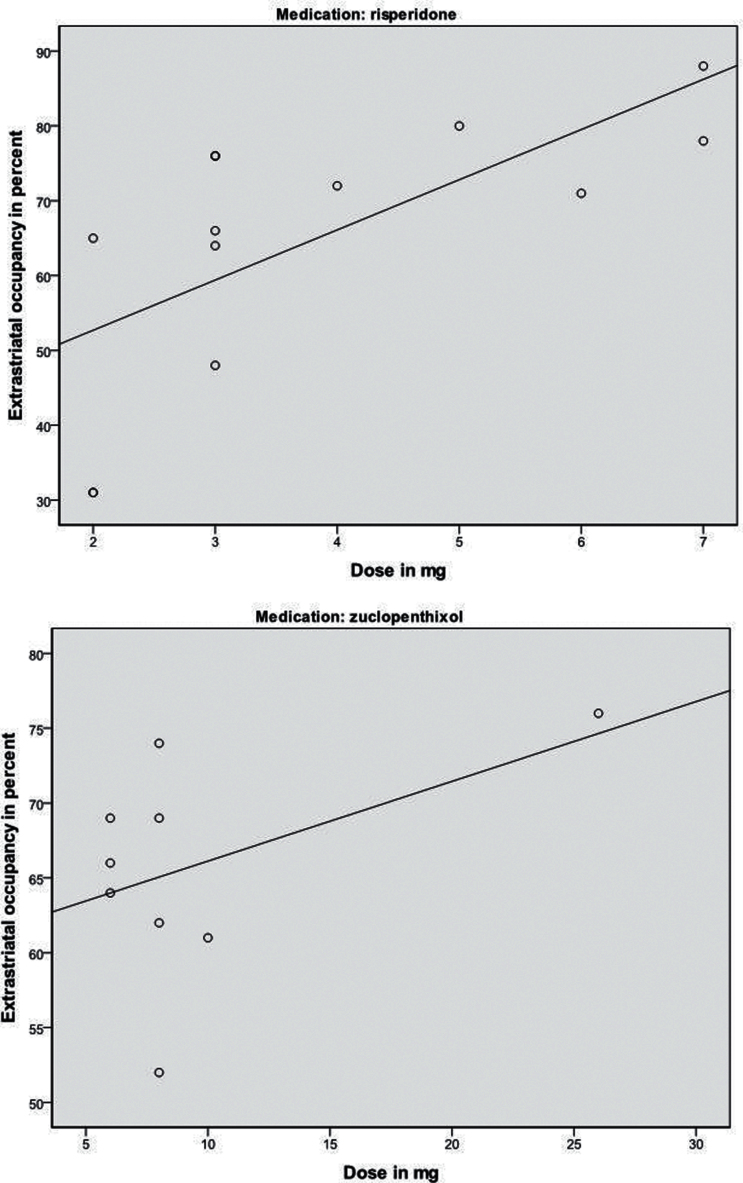
Mean extrastriatal D2/3 receptor occupancy in the whole group was 65% (n = 22, range 31–88%, spearman correlation r = 0.94); 66% (n = 9, range 52–76%, r = 0.95) in the zuclopenthixol group; and 65% (n = 13, range 31–88%, spearman correlation r = 0.93) in the risperidone group. There was no significant difference in occupancy between groups (Table 1). In the risperidone group, dose and extrastriatal occupancy were significantly correlated (n = 13, r = 0.68, P = .01) (Figure 3A) but not in the zuclopenthixol group (P>.2) (Figure 3B).
Figure 3.
(A-B) Scatter plots showing correlations between dose and extrastriatal dopamine D2/3 receptor occupancy in the 2 treatment groups. Patients treated with risperidone had significant correlations between dose and occupancy (n = 13, r = 0.68, P=.01). For patients treated with zuclopenthixol, no significant correlations were found (n = 9, r = 0.46, P=.2).
Extrastriatal Dopamine D2/3 Receptor Occupancy and Cognition
For all patients, extrastriatal D2/3 receptor occupancy showed a significant negative correlation with planning time at follow-up (Stockings of Cambridge initial thinking time, n = 21, r = -0.436, P=.048) (Figure 4a). Moreover, occupancy and improvement in attention from baseline to follow-up was negatively correlated in the whole group (signal detection measure A’ from the Rapid Visual Information Processing test, n = 21, r = -0.615, P=.003) (Figure 4b). In the zuclopenthixol group, we found a significant negative correlation between occupancy and attention at follow-up (signal detection A’ from the Rapid Visual Information Processing test, n = 9, rho = -0.795, P= .018) and at a trend level with planning latency also at follow-up (Stockings of Cambridge initial thinking time, n = 9, r = -0.70, P=.050). In the risperidone group, a significant negative correlation between occupancy and improvement in attention was found (signal detection A’ from the Rapid Visual Information Processing test, n = 13, r = -0770, P=.003). Even when using 2 different statistical methods, the overall results remained the same regardless of method.
Figure 4.
Scatter plots showing correlations between extrastriatal dopamine D2/3 receptor occupancy and cognitive measures. (A) Planning time at follow-up measured with CANTAB (Stockings of Cambridge initial thinking time) (n = 21, r = -0.436, P=.048). Extrastriatal occupancy is in percent, planning time in msek. (B) Improvement in attention measured with CANTAB (signal detection measure A’ from the Rapid Visual Information Processing test) (n = 21, r = -0.615, P=.003). Occupancy is in percent, and attention scores range from 0 to 1, with 1 indicating optimal signal detection.
Extrastriatal Dopamine D2/3 Receptor Occupancy and Psychopathology
No significant associations were found between extrastriatal dopamine D2/3 receptor occupancy and improvement in PANSS positive symptoms for the whole group. Separate analyses of the 2 treatment groups did not alter the results.
Frontal Dopamine D2/3 Receptor Availability and Treatment Response
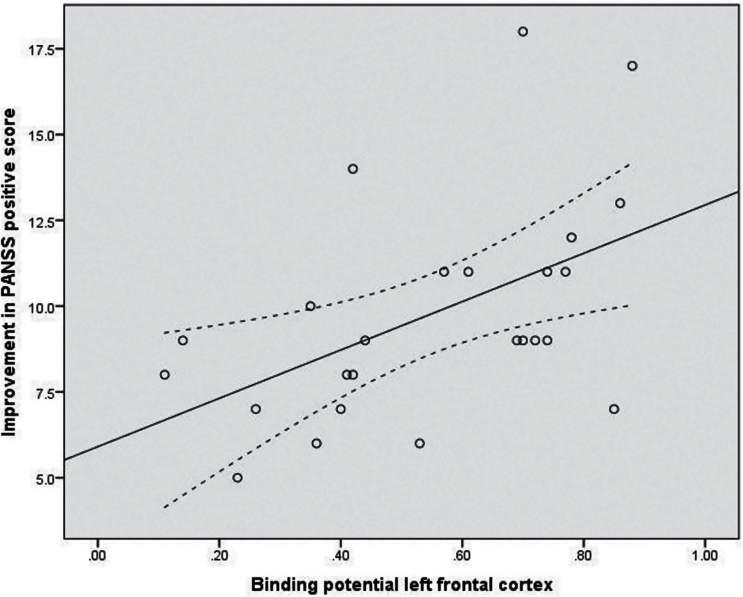
In the total patient group, we found significant positive correlations between BPND in frontal cortex bilaterally and improvement in positive symptoms (Figure 2) (BPND in left frontal cortex P=.003, rho = 0.56; BPND right frontal cortex P=.016, rho = 0.48). In the risperidone group, similar significant positive correlations between BPND in frontal cortex and improvement in positive symptoms were found (BPND left frontal cortex P=.007, rho = 0.64; BPND right frontal cortex P=.002, rho = 0.71) though not in the zuclopenthixol group (P>.4).
Figure 2.
Correlation between left frontal dopamine D2/3 receptor binding potential (BPND) in the antipsychotic-naïve state and treatment outcome. Improvement in Positive and Negative Syndrome Scale (PANSS) positive score (follow-up minus baseline) after 3 months of treatment: n=25, rho = 0.56, P<0.01. Linear regression shown as fully drawn line and confidence intervals as dashed lines.
Discussion
In the present study, we related blockade of frontal D2/3 receptors to cognitive functions and examined whether higher BPND at baseline was associated with reductions in positive psychotic symptoms. Our results showed that extrastriatal dopamine D2/3 receptor blockade was correlated with decreased attentional focus and planning time. Moreover, baseline frontal dopamine D2/3 BPND was positively correlated with positive symptom reduction. In line with our hypothesis, the data support that blockade of extrastriatal dopamine D2/3 receptors may further compromise specific cognitive functions in initially antipsychotic-naïve first-episode schizophrenia patients. The negative associations between occupancy and both attention at follow-up and improvement of attention scores further support a possible detrimental effect of high dopamine blockade on attention. The significant negative associations to follow-up scores on planning latency could in itself suggest a beneficial effect of high occupancy levels on planning, but since patients perform worse than healthy controls on planning efficiency both at baseline and follow-up, faster planning latencies do not indicate improved processing in lieu of improved planning. This suggests that faster planning latencies at follow-up were not advantageous, but rather indicate a lack of sufficient planning (Fagerlund et al., 2013). Even so, most of the cognitive tests were not related to D2/3 receptor blockade. Our results are in accordance with other findings suggesting that antipsychotics may worsen aspects of attention (Tost et al., 2006) and decision-making (Eisenegger et al., 2014) in healthy volunteers.
As expected, the variables significantly affected by blockade of frontal D2/3 receptors were the variables that in the baseline data showed quadratic associations with D2/3 BPND in line with an inverted U-curve (Fagerlund et al., 2013). This indicates that blockade of frontal D2/3 receptors by antipsychotics may worsen some cognitive domains following an inverted U-curve, possibly by overshooting the optimal window for cognitive processing, perhaps similar to the inverted U-curve involvement of frontal dopamine D1 receptors’ function in working memory (Brozoski et al., 1979; Williams and Goldman-Rakic, 1995; Arnsten, 2013). Although these cognitive domains did not worsen significantly from baseline to follow-up, the strength of the negative correlations between follow-up scores, especially on attention with occupancy, suggests that the impact of occupancy is not negligible. In the zuclopenthixol group, occupancy explained as much as 62% of the variance in attention at follow-up, indicating that attentional focus and selection may be more detrimentally affected by dopamine D2/3 receptor blockade than any of the other functions (Fagerlund et al., 2013). This is in line with other previous studies (Harris et al., 2009; Sakurai et al., 2013; Keedy et al., 2014), which together indicate that frontal D2/3 receptors appear to be involved in the selection of relevant information for processing (Seamans and Yang, 2004), while D1 receptors are crucially involved in the maintenance of information, for example, in working memory (Goldman-Rakic, 1995). This is clinically relevant, because some of the cognitive deficits that are present at illness onset may be further compromised by high D2/3 receptor blockade (Fervaha et al., 2014; Lepage et al., 2014), which is also indicated by our present data.
Our data on baseline frontal D2/3 receptor BPND and improvement of positive symptoms support that high frontal D2/3 receptor availability is associated with treatment response in antipsychotic-naïve first-episode schizophrenia patients. This was the case for the total group of patients as well as the group treated with risperidone. Since patients and controls did not differ significantly with regard to BPND at baseline (Glenthoj et al., 2006), it suggests a receptor-mediated effect which is in accordance with the model of Seamans and Yang (2004) and in line with the hypothesized dopamine D2 receptor high-affinity state model (Seeman, 2013). Thus, frontal BPND may predict treatment response in antipsychotic-naïve patients before the patient’s first treatment with a dopamine antagonist. We did not find significant correlations between D2/3 receptor BPND and change in PANSS positive scores in the smaller group of patients treated with zuclopenthixol. This is likely due to the uniform dosing with most patients receiving 8 or 10mg.
In apparent opposition to both the demonstrated connection between baseline D2/3 receptor BPND values, positive symptoms (Glenthoj et al., 2006), treatment outcome, as well as the previously described model by Seamans and Yang (2004), our data could not directly confirm an association between blockade of frontal D2/3 receptors and treatment effect on positive symptoms. A likely contributory factor to the seemingly conflicting results could be interactions between frontal and subcortical dopamine activity (Wilkinson, 1997; Clarke et al., 2014). Blockade of frontal D2/3 receptors is believed to cause an increase in dopamine release in the nucleus accumbens (Del and Mora, 2005), hereby opposing the effect of frontal blockade on positive psychotic symptoms. This is indirectly supported by a recent study on a different cohort from our group. In this study, low D2/3 receptor BPP in the caudate at baseline was significantly associated with effect of treatment on positive symptoms with the relatively selective dopamine D2/3 receptor antagonist, amisulpride, in initially antipsychotic-naïve first-episode schizophrenia patients (Wulff et al., 2015). Moreover, data also pointed to a negative effect of D2/3 receptor blockade on level of function, which is in agreement with the present findings of a negative association between occupancy and certain cognitive functions.
Strengths and Limitations
The inclusion of antipsychotic-naïve first-episode schizophrenia patients enables assessment of biochemical markers before confounding factors like medication and chronicity, and the longitudinal design is optimal for relating these biomarkers to changes in symptom domains over time. Before completion of all follow-up examinations, 3 patients dropped out, corresponding to a very low attrition rate of 12%. Apart from all being in the risperidone group, the dropouts did not differ with regard to clinical and neurochemical values. Treatment period and doses were also comparable with the other patients (Table 1) with a mean dose of 3.3mg. Hence, we judge attrition bias unlikely to have affected our results.
Inherently, correlation analyses show only associations and not a causal relationship between BPND and improvement in PANSS scores and our analysis on PANSS scores are not corrected for multiple comparisons, which increase the risk for type one errors, though we did have strong a priori hypotheses supporting the findings. As in the previous papers based on the same cohort of patients, we used the change measures in the PANSS positive score (calculated by subtraction of the 2 scores) to assess the effect of blockade on positive symptoms. This allows us to compare the present with previous data on the same patients. We have not included remission as an outcome measure, since our hypothesis was related to the change in the PANSS positive score. Even so, other factors like endogenous dopamine and other transmitter systems, for example, serotonin 2A receptors (Rasmussen et al., 2010, 2011; Ebdrup et al., 2011), may be involved in psychosis and affect our results.
We found that extrastriatal occupancy affected aspects of cognition. It is, however, important to keep in mind that extrastriatal occupancy is a global measure of cortical dopamine D2/3 receptor blockade, and we cannot separate frontal from temporal blockade in this study. Thus, we cannot exclude that direct blockade of temporal D2/3 receptors or interactions with temporal D2/3 receptors may also have affected cognition (Tregellas et al., 2014). Regarding cognition, our results would not have survived Bonferroni correction, but because we had a strong a priori hypothesis regarding the relation between dopamine blockade and change in cognition (Fagerlund et al., 2004, 2013), we did not correct for multiple comparisons.
Furthermore, the size of the ROIs hampers a more detailed analysis of the BPND of specific subregions, for example, dorsolateral prefrontal cortex (Figure 1). Finally, the affinity of epidepride makes it impossible to distinguish between D2- and D3 receptors, but we note that D3 receptors are sparsely represented in cortex (Beaulieu and Gainetdinov, 2011).
Conclusion
Taken together with our previous baseline data (Glenthøj et al. 2006; Fagerlund et al., 2013), the present results support that frontal dopamine D2/3 receptors are involved in psychopathological and some cognitive processes in antipsychotic-naïve first-episode schizophrenia patients. The data did not point to an association between high frontal occupancy and treatment effect. On the contrary, they support that blockade of frontal D2/3 receptors by antipsychotics may worsen some cognitive domains possibly by compromising the optimal prefrontal network functioning, which is required for cognitive performance, motivation, and learning. This is highly clinically relevant given the association between cognitive performance and functional outcome. In addition, the data showed an association between higher extrastriatal baseline BPND in antipsychotic-naïve patients and the effect of their first antipsychotic treatment on positive symptoms. This further supports the hypothesis of a differentiated treatment outcome of antipsychotic treatment based on the patient’s dopamine activity at baseline.
Statement of Interest
Dr. Glenthøj is the leader of a Lundbeck Foundation Center of Excellence for CINS, which is partially financed by an independent grant from the Lundbeck Foundation based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administrated by them. She has nothing else to declare. Dr. Ebdrup has received lecture fees from Bristol-Myers Squibb, Otsuka Pharma Scandinavia AB, and Eli Lilly and Company and is part of the Advisory Board of Eli Lilly Danmark A/S and Takeda Pharmaceutical Company Ltd. All other authors declare no conflict of interest.
Acknowledgments
We thank Bente Dall and Eva Broedsgaard for their superior technical assistance and would also like to thank Anne Bjerring Jensen for linguistic proofreading.
The Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS) is partially funded by an independent grant from the Lundbeck Foundation based in international review (R25-A2701). Additionally, the study has obtained financial support from the Danish Medical Research Council, Mental Health Services in the Capital Region of Denmark, H:S (Copenhagen Hospital Cooperation) Research Council, and a nonrestricted grant from Janssen-Cilag A/S and the Novo Nordic Foundation. None of the parties sponsoring the study had any role in the design or conduct of the study, nor in collection, management, analysis, or interpretation of data, nor in preparation, review, or approval of the manuscript.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van HR, Gorman JM, Laruelle M. (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, Narendran R, Hwang DR, Laruelle M, Slifstein M. (2012) Increased prefrontal cortical D(1) receptors in drug naive patients with schizophrenia: a PET study with [(1)(1)C]NNC112. J Psychopharmacol 26:794–805. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. (2013) The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937–2003. Cereb Cortex 23:2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–932. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Cardinal RN, Rygula R, Hong YT, Fryer TD, Sawiak SJ, Ferrari V, Cockcroft G, Aigbirhio FI, Robbins TW, Roberts AC. (2014) Orbitofrontal dopamine depletion upregulates caudate dopamine and alters behavior via changes in reinforcement sensitivity. J Neurosci 34:7663–7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del AA, Mora F. (2005) Glutamate-dopamine in vivo interaction in the prefrontal cortex modulates the release of dopamine and acetylcholine in the nucleus accumbens of the awake rat. J Neural Transm 112:97–109. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Rasmussen H, Arnt J, Glenthoj B. (2011) Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin Investig Drugs 20:1211–1223. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Mackeprang T, Gade A, Glenthoj BY. (2004) Effects of low-dose risperidone and low-dose zuclopenthixol on cognitive functions in first-episode drug-naive schizophrenic patients. CNS Spectr 9:364–374. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Pinborg LH, Mortensen EL, Friberg L, Baare WF, Gade A, Svarer C, Glenthoj BY. (2013) Relationship of frontal D(2/3) binding potentials to cognition: a study of antipsychotic-naive schizophrenia patients. Int J Neuropsychopharmacol 16:23–36. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G. (2014) Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand 130:290–299. [DOI] [PubMed] [Google Scholar]
- Floresco SB. (2013) Prefrontal dopamine and behavioral flexibility: shifting from an “inverted–U” toward a family of functions. Front Neurosci 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenthoj A, Glenthoj BY, Mackeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL, Baare WF. (2007) Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res 154:199–208. [DOI] [PubMed] [Google Scholar]
- Glenthoj BY, Mackeprang T, Svarer C, Rasmussen H, Pinborg LH, Friberg L, Baare W, Hemmingsen R, Videbaek C. (2006) Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry 60:621–629. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. (1995) Cellular basis of working memory. Neuron 14:477–485. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Fagerlund B, Bro H, Glenthoj BY. (2011) Stability of prepulse inhibition and habituation of the startle reflex in schizophrenia: a 6-year follow-up study of initially antipsychotic-naive, first-episode schizophrenia patients. Int J Neuropsychopharmacol 14:913–925. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Skimminge A, Aggernaes B, Ebdrup BH, Glenthoj B, Baare W. (2013) Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. J Psychiatry Neurosci 38:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MS, Wiseman CL, Reilly JL, Keshavan MS, Sweeney JA. (2009) Effects of risperidone on procedural learning in antipsychotic-naive first-episode schizophrenia. Neuropsychopharmacology 34:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry 69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, et al. (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Reilly JL, Bishop JR, Weiden PJ, Sweeney JA. (2014) Impact of antipsychotic treatment on attention and motor learning systems in first-episode schizophrenia. Schizophr Bull 41:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RM, Ansari MS, Schmidt DE, de PT, Clanton JA, Innis R, al-Tikriti M, Manning RG, Gillespie D. (1991) High affinity dopamine D2 receptor radioligands. 2. [125I]epidepride, a potent and specific radioligand for the characterization of striatal and extrastriatal dopamine D2 receptors. Life Sci 49:617–628. [DOI] [PubMed] [Google Scholar]
- Knable MB, Weinberger DR. (1997) Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 11:123–131. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, Osman S, Bloomfield PM, Jones T, Patsalos PN. (1995) Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab 15:152–165. [DOI] [PubMed] [Google Scholar]
- Lepage M, Bodnar M, Bowie CR. (2014) Neurocognition: clinical and functional outcomes in schizophrenia. Can J Psychiatry 59:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C, Rabbitt P. (1998) Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Cambridge Neuropsychological Test Automated Battery. International Study of Post-Operative Cognitive Dysfunction. Neuropsychologia 36:915–923. [DOI] [PubMed] [Google Scholar]
- Mackeprang T, Kristiansen KT, Glenthoj BY. (2002) Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biol Psychiatry 52:863–873. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Videbaek C, Ziebell M, Mackeprang T, Friberg L, Rasmussen H, Knudsen GM, Glenthoj BY. (2007) [123I]epidepride binding to cerebellar dopamine D2/D3 receptors is displaceable: implications for the use of cerebellum as a reference region. Neuroimage 34:1450–1453. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Ebdrup BH, Erritzoe D, Aggernaes B, Oranje B, Kalbitzer J, Pinborg LH, Baare WF, Svarer C, Lublin H, Knudsen GM, Glenthoj B. (2011) Serotonin2A receptor blockade and clinical effect in first-episode schizophrenia patients treated with quetiapine. Psychopharmacology (Berl) 213:583–592. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B, Kalbitzer J, Madsen J, Pinborg LH, Baare W, Svarer C, Lublin H, Knudsen GM, Glenthoj B. (2010) Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry 67:9–16. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. (1992) Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Bies RR, Stroup ST, Keefe RS, Rajji TK, Suzuki T, Mamo DC, Pollock BG, Watanabe K, Mimura M, Uchida H. (2013) Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull 39:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74:1–58. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2013) Are dopamine D2 receptors out of control in psychosis? Prog Neuropsychopharmacol Biol Psychiatry 46:146–152. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R. (2014) Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 171:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LS. (1997) The nature of interactions involving prefrontal and striatal dopamine systems. J Psychopharmacol 11:143–150. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575. [DOI] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N. (1990) SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 47:589–593. [DOI] [PubMed] [Google Scholar]
- Wulff S, Pinborg LH, Svarer C, Jensen LT, Nielsen MO, Allerup P, Bak N, Rasmussen H, Frandsen E, Rostrup E, Glenthoj BY. (2015) Striatal D2/3 binding potential values in drug-naive first-episode schizophrenia patients correlate with treatment outcome. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]