Abstract
Sternal wound infections represent one of the most frequent complications after cardiac surgery and are associated with high postoperative mortality. Several preventive methods have been introduced, and recently, gentamicin-impregnated collagen sponges (GICSs) have shown a promising effect in reducing the incidence of this type of complications. Gentamicin is an aminoglycoside antibiotic that has been widely used to treat infections caused by multiresistant bacteria; despite its effectiveness, its systemic use carries a risk of toxicity. GICSs appear to overcome this side effect, topically delivering high antibiotic concentrations to the wound and thus reducing the toxic-related events. Although several retrospective analyses and randomized controlled trials have studied the use of GICSs in cardiac surgery, conclusions regarding their efficacy in preventing sternal wound infection are inconsistent. We have reviewed the current literature focusing on high-risk patients.
Keywords: gentamicin, wound infection, topical drug administration
Introduction
Sternal wound infections (SWIs) represent one of the most challenging postoperative complications following cardiac surgery, associated with increased hospital stay, mortality, and costs.1 It is widely acknowledged that several factors (eg, smoking, obesity, insulin-dependent diabetes, emergent surgery, prolonged operative time, reoperation, bilateral internal mammary artery (BIMA) harvesting, transfusions, and prolonged ventilation/intensive care unit [ICU] stay) predispose to a higher risk of both superficial and deep SWIs; many of these conditions are observed with increasing frequency in the modern cardiac surgery scenario.2–4 Historically, the cornerstones of prevention of SWIs have been preoperative skin asepsis and administration of prophylactic antimicrobial drugs and several surgical maneuvers intended to maintain sterility and achieve a stable sternal fixation.5–7 During the past few years, local administration of gentamicin through a surgically inserted collagen sponge has been gaining popularity in different surgical fields, and it has been increasingly used in cardiac surgery.8 The reported incidence of SWIs varies between 0.5% and 6% throughout the literature, although it is considerably higher among high-risk individuals, ranging between 12% and 20%.6 SWIs can be classified as superficial SWIs (SSWIs) or deep SWIs (DSWIs), according to the extension of the infective process from the skin and subcutaneous layer to the bone and mediastinum. The Centers for Disease Control and Prevention and the National Institute of Clinical Excellence have developed guidelines for the diagnosis and management of surgical site infections.9,10 However, currently, the assessment and treatment of SWIs seem to be quite heterogeneous among different centers and even among different surgeons from the same center. It is clear that DSWIs strongly impact on postoperative morbidity and mortality and that SSWIs are a common cause of prolonged hospital stay. In 2008, Mauermann et al reviewed data regarding 11 series of postoperative SWIs, reporting an overall mortality of 13% (ranging between 6% and 30%). They also concluded that it is difficult to precisely assess the impact of SWIs on hospital costs due to the variability in protocols among different centers.1 Since SWIs significantly prolong ICU stay and hospitalization, they are widely known to be a major cause of increased health-care costs in cardiac surgery.1 Both DSWIs and SSWIs are usually caused by gram-positive skin bacteria such as Coagulase-negative staphylococci (CoNS) and Staphylococcus aureus; in a minority of cases, they can be caused by gram-negative rods such as Escherichia coli and Pseudomonas aeruginosa and fungi such as Candida albicans.11–13 As shown in Table 1, gram-positive bacteria are isolated in 60%–80% of postoperative SWIs, gram-negative rods in 20%–40% of cases, and fungi in about 5% (polymicrobial isolations are frequent and occur in 10%–40% of cases).1,14,15
Table 1.
Microbiology of SWIs.
| MICROORGANISM | FREQUENCY |
|---|---|
| Gram-positive Cocci | 60–80% |
| Staphylococcus aureus | 40–45% |
| Coagulase-negative staphylococcus | 20–35% |
| Gram-negative rods | 20–40% |
| Enterobacter spp | 10% |
| Pseudomonas spp | 2–10% |
| Klebsiella spp | 3–8% |
| Escherichia coli | 5% |
| Proteus spp | 2–3% |
| Fungi | 5% |
| Polymicrobial | 10–40% |
The emergence of multiresistant bacterial strains has led to a challenging situation: beta-lactam antibiotics have become ineffective against most CoNS and S. aureus clusters, and the routine use of vancomycin as a prophylactic agent is not advisable in order to avoid further antibiotic resistances.13,16 Furthermore, CoNS have the intrinsic ability to adhere to foreign bodies (eg, sternal wires) and produce a biofilm, thus increasing their resistance to antibiotics and the probability of a chronic infection.17,18
Advantages of Gentamicin Local Delivery
Local administration of antibiotics such as gentamicin, tobramycin, tetracycline, minocycline, teicoplanin, and sulbactam–cefoperazone has been performed in several surgical fields.8 Gentamicin has gradually become the most used molecule for this purpose due to a combination of characteristics such as broad spectrum, low cost, and favorable pharmacokinetics and pharmacodynamics when administered topically.8,19
Gentamicin is an aminoglycoside antibiotic that has been widely used to treat infections caused by multiresistant bacteria (Fig. 1). Although its spectrum is mainly directed toward gram-negative species, gentamicin is also effective against several gram-positive strains.19 Furthermore, gentamicin also shows a synergy with beta-lactam antibiotics, especially against gram-positive species such as S. aureus and CoNS.16 Nevertheless, the principal factor limiting its systemic use is represented by its intrinsic toxicity; when administered intravenously or intramuscularly, gentamicin accumulates into renal cortex and into endolymph and perilymph of the inner ear, causing kidney injury and hearing loss.20 These drawbacks can be partially eluded by administering gentamicin locally, reducing the systemic toxicity. Kidney and inner ear accumulations seem to appear when gentamicin serum concentration exceeds 10–12 mg/L, although a precise cutoff has not been established.8,21 Interestingly, it has been observed that, after local administration in the sternal region, the drug serum concentration does not exceed 1 mg/L, while mediastinal fluid concentration remains above 300 mg/L for 36 hours.21 Moreover, gentamicin exhibits a concentration-dependent effect, especially against gram-negative rods: this means that a high concentration of the drug circumscribed to the surgical site can lead to a bactericidal effect not only toward sensitive bacteria but also toward poorly sensitive or even resistant ones;20 an acute peak concentration in the surgical site, combined with a low serum level of the drug, is protective against the selection of resistant bacteria; in fact, prolonged high serum concentrations promote the so-called adaptive resistance to gentamicin.20
Figure 1.

Chemical structure of gentamicin.
Notes: Atoms are represented as spheres with conventional color coding: white represents hydrogen, gray represents carbon, blue represents nitrogen, and red represents oxygen.
Pharmacokinetics of GICS
Surgical implants impregnated with gentamicin started to be used in the 1970s, primarily in orthopedic surgery, aiming to treat or prevent prosthetic infections. The first devices had the disadvantage that they were not made of reabsorbable materials; hence, they had to be surgically removed once the infection had been treated.22
As a consequence, biodegradable polymers such as polylactic acid, polyglycolic-polylactic acid, poly(ortho esters), and polyhydroxybutyrate-co-hydroxyvalerate were developed to carry antibiotic drugs to the surgical site.23,24
Finally, since the 1980s, collagen implants began to be used in several surgical specialties for local antibiotic delivery, mainly due to collagen biocompatibility and pharmacokinetic versatility.23,24
Regarding pharmacokinetics, collagen is a unique polymer because it has a complex, well-known three-dimensional structure with different hierarchical levels: primary, secondary, tertiary, and quaternary.25 The physiochemical characteristics of the final polymer can be modified by intervening on the molecular structure (eg, intra- and intermolecular cross-links) as well as linking collagen with other polymers to obtain different drug-releasing temporal curves. Hence, when collagen is used as a carrier for a drug, any structural modification can lead to different pharmacokinetic profiles.8,26,27
Collagen biocompatibility and absorbability represent essential characteristics with respect to infection prevention or treatment, because they allow to avoid a further surgical procedure (which could be as well complicated by infection) to remove the drug-carrying device.
Gentamicin-impregnated collagen sponges (GICSs) can be inserted in three different regions during sternal closure, depending on the desired primary site of action:
– behind the posterior surface of the sternum, between the bone and the sternal wires;
– between sternal halves; and
– on the anterior surface of the sternum, under the muscular fascia.
Every different position of the GICS corresponds to a slightly different spatial distribution of gentamicin, according to the closest sternal region.28,29 For instance, when a GICS was placed posteriorly to the sternum, its effect was more evident in preventing DSWI rather than SSWI.30
One or two GICSs per patient, corresponding to 130 and 260 mg of gentamicin, respectively, were used in most of the reviewed studies, with various combinations of the above-mentioned positions. There is some evidence that collagen sponges should not be soaked in saline solution prior to use.31,32 Bennett-Guerrero et al conducted a multicenter randomized double-blind trial comparing high-risk cardiac surgical patients receiving a GICS with patients receiving a standard sternal closure. The authors found no advantages for the study group over the control group regarding the incidence of SWI up to 90 days after surgery. The GICS were soaked in saline as per the study protocol, and the authors have been criticized due to this maneuver.31 Gentamicin is a highly water-soluble molecule, and in vitro studies have showed that exposing a GICS to saline causes the loss of 6.7%, 40.5%, and 100% of the gentamicin after 2 seconds, 1 minute, and 6 hours, respectively.32 Manufacturers recommend not to soak the GICS prior to use.
GICS use in High-risk Cardiac Surgery Patients
Obesity represents one of the most important risk factors for the development of SWI in cardiac surgery,15 but there are contrasting data on the effect of the GICS on preventing this complication in this subgroup of patients. An important multicenter randomized controlled trial has involved 1502 high-risk patients in 48 centers (Table 2).31 In this study, 1006 patients (67% of the population) were diabetic and 1137 patients (76%) were obese with a median body mass index (BMI) of 32.9 kg/m2. With a 90-day postoperative follow-up, the authors reported a comparable incidence of SWIs in the intention-to-treat analysis, with an incidence of DSWI of 8.4% in the GICS group vs 8.7% in the control group (P = 0.83). Similar results have been reported in the per-protocol analysis (8.4% vs 8.6%, P = 0.89). A further subanalysis of this study, targeting the very high-risk group and including patients who were both obese and diabetic, showed no differences between the two groups (11.1% vs 13.8%, P = 0.30). The authors concluded that the use of a GICS did not reduce the 90-day SWI rate.31 One of the most controversial points regarding this study was the fact that the GICS was wetted in saline before application: the consequence of this may have influenced the gentamicin release,32,33 and therefore the outcome of the study would have been affected.
Table 2.
Comparison of various studies that have investigated GICS use in high-risk cardiac surgery patients.
| AUTHOR | TYPE OF STUDY | SUBGROUP | OUTCOME | GICS | NO GICS | P-VALUE |
|---|---|---|---|---|---|---|
| Bennett-Guerrero et al31 | Multicenter RCT, 1502 pts | All pts | Any SWI (ITT) | 8.4% | 8.7% | 0.83 |
| Any SWI (PP) | 8.4% | 8.6% | 0.89 | |||
| BMI > 30 | Any SWI | 8.1% | 4.2% | 0.07 | ||
| Diabetes | Any SWI | 4% | 5.4% | 0.52 | ||
| BMI > 30 + diabetes | Any SWI | 11.1% | 13.8% | 0.30 | ||
| Friberg et al16 | Two-centers RCT, 2000 pts | All pts | Any SWI | 4.3% | 9% | <0.001 |
| SSWI | 1.9% | 5.7% | <0.001 | |||
| DSWI | 2.3% | 3.3% | 0.20 | |||
| No reoperation or early death | SSWI | 2.7% | 6.7% | <0.001 | ||
| DSWI | 2.1% | 3.3% | 0.088 | |||
| Diabetes | SSWI | 1.67% | 7.47% | 0.0086 | ||
| DSWI | 3.89% | 9.77% | 0.028 | |||
| BMI > 25 | SSWI | 2.16% | 6.45% | <0.001 | ||
| DSWI | 2.47% | 4.45% | 0.050 | |||
| Birgand et al35 | Single center CS, 552 pts | BIMA + BMI > 30 or ID diabetes | DSWI | 12.6% | 13.8% | NS |
Abbreviations: BIMA, bilateral internal mammary; CS, cohort study; DSWI, deep sternal wound infection; BMI, body mass index; GICS, gentamicin-impregnated collagen sponge; ID, insulin-dependent; ITT, intention-to-treat; NS, not significant; PP, per-protocol; pts, patients; RCT, randomized controlled trial; SSWI, superficial sternal wound infection; SWI, sternal wound infection.
Different results were reported by a previously published randomized controlled trial,16 where the impact of obesity was specifically subanalyzed. With a cutoff of BMI > 25 kg/m2 (1299 patients), the authors reported a significantly reduced incidence of SSWIs (2.16% vs 6.45%, P < 0.001) and DSWIs (2.47% vs 4.45%, P = 0.05) in the GICS group. The analysis of the whole population confirmed these results only for SSWIs, whereas there were no differences in terms of DSWIs (P = 0.2); these outcomes can be interpreted as a stronger effect of GICS in preventing DSWI in the obese group of patients.
Another subgroup at high risk of SWI includes patients who undergo BIMA harvesting during coronary artery bypass grafting (CABG), as this technique can impair sternal vascularization.34 A single-center study, published in 2012 by Birgand et al (Table 2), compared the incidence of DSWI requiring surgery in high-risk patients defined as BIMA harvesting plus overweight (BMI > 30 kg/m2) and/or insulin-dependent diabetes.35 The authors did not find any difference regarding the incidence of DSWI between patients receiving a GICS and those who did not receive it (12.6% vs 13.8%, respectively); interestingly, in that series, the probability of DSWI caused by a gentamicin-resistant bacterium was higher in the GICS group (21/27, 77.8%) compared with the other patients (23/56, 41.1%; P < 0.01). Again, as in the above-mentioned study, GICS pre-soaking in saline solution represented a weakness.
Once we consider the patients who undergo an early reoperation for bleeding (or other reasons) in the immediate postoperative course, the risk of a SWI is increased. In their prospective randomized trial, Friberg et al16 investigated the beneficial effect of GICS in 2000 patients undergoing cardiac surgery (Table 2); their results showed a reduced incidence of SWI at two months in the treatment group (4.3% vs 9%, RR 0.47; 95% confidence interval [CI] 0.33–0.68, P < 0.001). This result appears to be even more important if we consider that the treatment group had a higher number of early reoperations for bleeding (4% vs 2.3%, P = 0.03), thus suggesting that the use of a GICS is effective in reducing SWI even in the presence of early resternotomy. The authors repeated their analysis, excluding the patients reoperated for bleeding (or other reasons) and those who died within two months; in this subgroup, there was still a significantly lower incidence of SSWIs (2.5% vs 6.7%, P = 0.001) and DSWIs (2.1 vs 3.3%, P = 0.088) in the GICS group.
In 2012, Creanor et al published a meta-analysis including randomized controlled trials, which had previously investigated the use of GICS in cardiac surgery.36 One of the subanalyses of this study was conducted considering high-risk patients; a statistically significant difference between treatment and control group was found with regard to DSWIs (odds ratio [OR] 0.62, 95% CI 0.39–0.98), while no difference was found with regard to any SWI (OR 0.60, 95% CI 0.24–1.52).36
In a recent study published in 2014, Benedetto and Raja33 identified the most important risk factors for DSWI after cardiac surgery. In their large analysis involving 8750 cardiac surgical patients, the authors identified several variables that can have an impact on the development of this complication: female gender, obesity, insulin-dependent diabetes, need for re-exploration, isolated or combined CABG, and the use of bilateral mammary artery.33 Using these variables, the authors were able to create a risk score to guide the use of GICS, demonstrating that an individual assessment of DSWI risk is realizable. Patients were classified as low, moderate, or high risk for DSWI, depending on their baseline score (Table 3 and Fig. 2). According to the authors’ findings, the use of GICS allowed to reclassify patients with moderate predicted risk of DSWI in the low-risk class. On the other hand, in high-risk patients, the observed DSWI incidence was lower than expected when a GICS was implanted, but the authors concluded that these patients should still be considered at higher risk.33 Till date, this score appears to be the only available guide in the decision-making process for the use of GICS in cardiac surgery.
Table 3.
Score to calculate DSWI predicted risk.
| VARIABLE | POINTS |
|---|---|
| Female gender | 26 |
| Insulin-dependent diabetes mellitus | 20 |
| CABG (isolated or combined) | 19 |
| BIMA harvesting | 15 |
| Need for re-exploration | 51 |
| BMI | See Figure 2 |
Notes: Data from Ref. 33. Overall score < 136: low risk of DSWI. Overall score between 136 and 199: moderate risk of DSWI. Overall score > 199: high risk of DSWI.
Abbreviations: CABG, coronary artery bypass grafting; BMI, body mass index.
Figure 2.
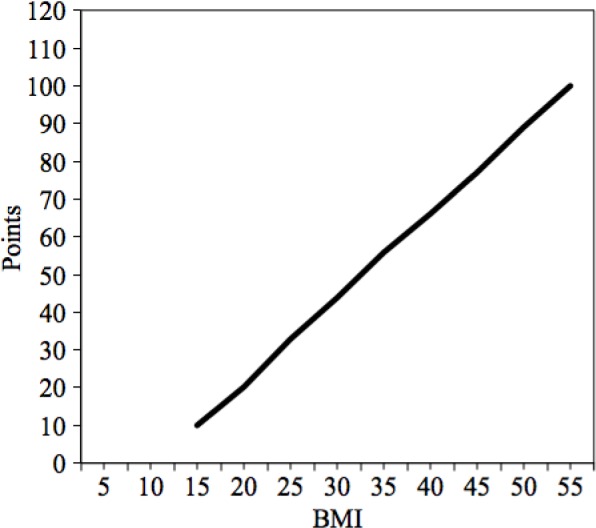
Baseline score points according to BMI (Data from Ref. 33).
Abbreviation: BMI, body mass index.
Conclusion
GICSs represent a promising option to prevent the occurrence of SWIs after heart surgery. Their main advantage is related to a high local concentration of gentamicin to the surgical site, combined with low serum levels of the drug, thus avoiding systemic side effects.
Most of the current knowledge on their use in cardiac surgery derives from underpowered studies, with different techniques of application. Hence, the real clinical beneficial effects in high-risk patients undergoing cardiac surgery have not been completely established, although there seems to be a tendency toward a reduced incidence of SWIs with their use. This is particularly evident when the device is not soaked in saline solution, as the results appear to be negatively affected by this maneuver.
Currently, there are no guidelines on the use of GICS in cardiac surgery; the scoring system, proposed by Benedetto and Raja,33 seems to be the most reliable tool presently available for the indication of their use in high-risk patients.
However, further prospective randomized controlled trials, particularly in high-risk patients, are needed to better clarify the impact of GICS in preventing SWIs.
Footnotes
ACADEMIC EDITOR: Anuj Chauhan, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 333 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: FR and VDB. Wrote the first draft of the manuscript: FR, VDB, and CZ. Contributed to the writing of the manuscript: PC, GG, and RM. Agreed with manuscript results and conclusions: FR, CZ, GG, RM, PC, and VDB. Jointly developed the structure and arguments for the paper: FR, CZ, GG, RM, PC, and VDB. Made critical revisions and approved the final version: FR, CZ, GG, RM, PC, and VDB. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Mauermann WJ, Sampathkumar P, Thompson RL. Sternal wound infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):423–436. doi: 10.1016/j.bpa.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Tang GHL, Maganti M, Weisel RD, Borger MA. Prevention and management of deep sternal wound infection. Semin Thorac Cardiovasc Surg. 2004;16(1):62–69. doi: 10.1053/j.semtcvs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Mavros MN, Mitsikostas PK, Alexiou VG, Peppas G, Falagas ME. Gentamicin collagen sponges for the prevention of sternal wound infection: a meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg. 2012;144(5):1235–1240. doi: 10.1016/j.jtcvs.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg. 2004;77(2):676–683. doi: 10.1016/S0003-4975(03)01523-6. [DOI] [PubMed] [Google Scholar]
- 5.Lador A, Nasir H, Mansur N, et al. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(3):541–550. doi: 10.1093/jac/dkr470. [DOI] [PubMed] [Google Scholar]
- 6.Mishra P, Ashoub A, Salhiyyah K, et al. Role of topical application of gentamicin containing collagen implants in cardiac surgery. J Cardiothorac Surg. 2014;9(1):122. doi: 10.1186/1749-8090-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckmann A, Doebler K, Schaefer E, Koetting J, Gastmeier P, Graf K. Sternal surgical site infection prevention—is there any room for improvement? Eur J Cardiothorac Surg. 2011;40(2):347–351. doi: 10.1016/j.ejcts.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Ruszczak Z, Friess W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Deliv Rev. 2003;55(12):1679–1698. doi: 10.1016/j.addr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence . NICE Clinical Guideline 74: Surgical Site Infection. National Institute for Health and Clinical Excellence; Manchester, United Kingdom: 2008. pp. 1–31. [Google Scholar]
- 11.Gårdlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery—microbiology and pathogenesis. Eur J Cardiothorac Surg. 2002;21(5):825–830. doi: 10.1016/s1010-7940(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 12.Charbonneau H, Maillet JM, Faron M, et al. Mediastinitis due to gram-negative bacteria is associated with increased mortality. Clin Microbiol Infect. 2013;20(3):O197–O202. doi: 10.1111/1469-0691.12369. [DOI] [PubMed] [Google Scholar]
- 13.Friberg Ö, Dahlin L-G, Källman J, Kihlström E, Söderquist B, Svedjeholm R. Collagen-gentamicin implant for prevention of sternal wound infection; long-term follow-up of effectiveness. Interact Cardiovasc Thorac Surg. 2009;9(3):454–458. doi: 10.1510/icvts.2009.207514. [DOI] [PubMed] [Google Scholar]
- 14.Lepelletier D, Bourigault C, Roussel JC, et al. Epidemiology and prevention of surgical site infections after cardiac surgery. Méd Mal Infect. 2013;43(10):403–409. doi: 10.1016/j.medmal.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Filsoufi F, Castillo JG, Rahmanian PB, et al. Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23(4):488–494. doi: 10.1053/j.jvca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Friberg Ö, Svedjeholm R, Söderquist B, Granfeldt H, Vikerfors T, Källman J. Local gentamicin reduces sternal wound infections after cardiac surgery: a randomized controlled trial. Ann Thorac Surg. 2005;79(1):153–161. doi: 10.1016/j.athoracsur.2004.06.043. discussion 161–162. [DOI] [PubMed] [Google Scholar]
- 17.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 18.Olsson E, Friberg Ö, Venizelos N, Koskela A, Källman J, Söderquist B. Coagulase-negative staphylococci isolated from sternal wound infections after cardiac surgery: attachment to and accumulation on sternal fixation stainless steel wires. APMIS. 2007;115(2):142–151. doi: 10.1111/j.1600-0463.2007.apm_559.x. [DOI] [PubMed] [Google Scholar]
- 19.Brunton L, Chabner B, Knollman B. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. p. 2011. [Google Scholar]
- 20.Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME. Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs. 2011;71(17):2277–2294. doi: 10.2165/11597020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Leyh RG, Bartels C, Sievers HH. Adjuvant treatment of deep sternal wound infection with collagenous gentamycin. Ann Thorac Surg. 1999;68(5):1648–1651. doi: 10.1016/s0003-4975(99)00836-x. [DOI] [PubMed] [Google Scholar]
- 22.Wahlig H. Gentamicin-PMMA beads: a drug delivery system in the treatment of chronic bone and soft tissue infections. J Antimicrob Chemother. 1982;10(5):463–465. doi: 10.1093/jac/10.5.463. [DOI] [PubMed] [Google Scholar]
- 23.Sendil D, Gürsel I, Wise DL, Hasirci V. Antibiotic release from biodegradable PHBV microparticles. J Control Release. 1999;59(2):207–217. doi: 10.1016/s0168-3659(98)00195-3. [DOI] [PubMed] [Google Scholar]
- 24.Türesin F, Gürsel I, Hasirci V. Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: in vitro antibiotic release. J Biomater Sci Polym Ed. 2001;12(2):195–207. doi: 10.1163/156856201750180924. [DOI] [PubMed] [Google Scholar]
- 25.Brown JC, Timpl R. The collagen superfamily. Int Arch Allergy Immunol. 1995;107(4):484–490. doi: 10.1159/000237090. [DOI] [PubMed] [Google Scholar]
- 26.Ramshaw JA, Werkmeister JA, Glattauer V. Collagen-based biomaterials. Biotechnol Genet Eng Rev. 1996;13:335–382. doi: 10.1080/02648725.1996.10647934. [DOI] [PubMed] [Google Scholar]
- 27.Rao KP. Recent developments of collagen-based materials for medical applications and drug delivery systems. J Biomater Sci Polym Ed. 1995;7(7):623–645. doi: 10.1163/156856295x00526. [DOI] [PubMed] [Google Scholar]
- 28.Schersten H. Modified prophylaxis for preventing deep sternal wound infection after cardiac surgery. APMIS. 2007;115(9):1025–1028. doi: 10.1111/j.1600-0463.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- 29.Kozioł M, Targońska S, Stążka J, Kozioł-Montewka M. Gentamicin-impregnated collagen sponge for preventing sternal wound infection after cardiac surgery. Kardiochir Torakochirurgia Pol. 2014;11:21–25. doi: 10.5114/kitp.2014.41925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schimmer C, Özkur M, Sinha B, et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: a controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg. 2012;143(1):194–200. doi: 10.1016/j.jtcvs.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Bennett-Guerrero E, Ferguson TB, Lin M, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA. 2010;304(7):755–762. doi: 10.1001/jama.2010.1152. [DOI] [PubMed] [Google Scholar]
- 32.Lovering AM, Sunderland J. Impact of soaking gentamicin-containing collagen implants on potential antimicrobial efficacy. Int J Surg. 2012;10(suppl 1):S2–S4. doi: 10.1016/j.ijsu.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Benedetto U, Raja SG. Scoring system to guide decision making for the use of gentamicin-impregnated collagen sponge to prevent deep sternal wound infection. J Thorac Cardiovasc Surg. 2014;148(5):2390.e1–2396.e1. doi: 10.1016/j.jtcvs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Toumpoulis IK, Theakos N, Dunning J. Does bilateral internal thoracic artery harvest increase the risk of mediastinitis? Interact Cardiovasc Thorac Surg. 2007;6(6):787–791. doi: 10.1510/icvts.2007.164343. [DOI] [PubMed] [Google Scholar]
- 35.Birgand G, Radu C, Alkhoder S, et al. Does a gentamicin-impregnated collagen sponge reduce sternal wound infections in high-risk cardiac surgery patients? Interact Cardiovasc Thorac Surg. 2013;16(2):134–141. doi: 10.1093/icvts/ivs449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creanor S, Barton A, Marchbank A. Effectiveness of a gentamicin impregnated collagen sponge on reducing sternal wound infections following cardiac surgery: a meta-analysis of randomised controlled trials. Ann R Coll Surg Engl. 2012;94(4):227–231. doi: 10.1308/003588412X13171221590179. [DOI] [PMC free article] [PubMed] [Google Scholar]


