Abstract
Aims/hypothesis
The discordance status of (autoimmune) type 1 diabetes within monozygotic twin pairs points to the importance of environmental factors. The aim of this study was to investigate whether the environmental events causing type 1 diabetes influence thyroid autoimmunity.
Methods
Monozygotic and dizygotic twins discordant for type 1 diabetes from the UK and USA were tested for thyroid peroxidase autoantibodies (TPOA) by radioimmunoassay. Using quantitative genetic model fitting of a liability-threshold model we estimated the contribution of genetic (heritability) and environmental factors to TPOA.
Results
TPOA positivity was higher in females than in males in both cohorts and was associated with later age at diagnosis in the UK and combined cohorts (p<0.01). TPOA did not specifically segregate with type 1 diabetes in the twin pairs (p>0.2 in all groups). The best-fitting models showed heritability (95% CI) estimates for TPOA of 63% (37%, 80%) for the UK and 80% (51%, 92%) for US twins, while the best-fitting meta-analysis model of the two twin cohorts combined included additive genetic and unique environmental factors with a heritability estimate of 69% (50%, 82%).
Conclusions/interpretation
Risk of thyroid autoimmunity, defined by TPOA, in the context of autoimmune diabetes is, substantially, genetically determined in discordant twin pairs. Environmental factors leading to type 1 diabetes were not the same as those involved with thyroid autoimmunity. It follows that it is as important to investigate for thyroid autoimmunity in relatives of type 1 diabetes patients as it is in the patients themselves.
Keywords: Autoantibodies, Autoimmunity, Thyroid, TPO, Type 1 diabetes
Introduction
Autoimmune diseases result from the interaction of genetic and non-genetic (probably environmental) factors. These diseases affect about 10% of the population and are characterised immunogenetically by the presence of disease-associated autoantibodies and an association with Class I and Class II HLA genotypes [1]. Type 1 diabetes is one such disease associated with the presence of diabetes-associated autoantibodies (DAA) including insulin autoantibodies (IAA), glutamic acid decarboxylase autoantibodies (GADA), insulinoma-associated antigen-2 autoantibodies (IA-2A) and zinc transporter 8 autoantibodies (ZnT8A) [2]. Type 1 diabetes is associated with other autoimmune diseases, including Hashimoto’s thyroiditis, characterised by thyroid peroxidase autoantibodies (TPOA) [3]. Both type 1 diabetes and Hashimoto’s thyroiditis are, in part, determined by non-genetic factors as exemplified by a notable discordance for each disease between identical twin pairs [4].
We previously showed that the DAA are determined by unique environmental events in twins discordant for type 1 diabetes [5]. Data from birth cohorts from families with type 1 diabetes suggest the appearance of TPOA has a later peak incidence, distinct from that associated with DAA (peak age 12–16 vs 0.75–2 years, respectively) [6]. Nevertheless, Bonifacio et al observed that these children at risk for type 1 diabetes frequently show TPOA (cumulative risk 20.3% by age of 14) [7]. Thyroid autoimmunity may be present at diagnosis of type 1 diabetes, reaching a peak at puberty, or it can be detected several years after diagnosis of type 1 diabetes (15%– 30% in adults) [8]. These differences imply distinct environmental events inducing DAA and TPOA. However, it is unknown whether the early environmental triggers leading to DAA might also increase the risk for TPOA development, for example through environmentally induced epigenetic changes.
The objective of the current study was to determine if such stochastic (environmental) events are associated with a heightened susceptibility to develop thyroid autoimmunity. We therefore assessed in two twin cohorts discordant for type 1 diabetes from the UK and the USA whether TPOA status is largely determined by unique environmental factors and whether it segregates with type 1 diabetes.
Methods
Participants
Two cohorts of monozygotic (MZ) and dizygotic (DZ) twins discordant for type 1 diabetes were tested for TPOA to determine the relative influence of genetic and environmental factors. Initially, type 1 diabetes-discordant twin pairs were selected from the British Diabetic Twin Study [5] and a US twin cohort [4]. The basic characteristics of the twins are shown in Table 1. These individuals fulfilled the following criteria: (1) twin pairs initially disease discordant; (2) both twins available for study; (3) neither twin receiving drugs other than human insulin; (4) all had normal plasma creatinine; and (5) diabetes initially excluded in the co-twin by OGTT and random whole-blood glucose <7.0 mmol/l. Monozygosity was established using both clinical data and DNA fingerprinting (data not shown) and type 1 diabetes was defined by standard criteria [9].
Table 1.
Characteristics of the UK and US twin pairs
| Characteristic | Type 1 diabetic twins | Non-diabetic co-twins | p value |
|---|---|---|---|
| UK | |||
| MZ (n) | 55 | 55 | – |
| Male, n (%) | 35 (63.6) | 35 (63.6) | – |
| Duration (years) | 2.82 (0.56, 14.46) | – | – |
| Age at diagnosis (years) | 12.53 (8.56, 19.05) | – | – |
| Age at test (years) | 18.94 (11.61, 36.10) | 18.94 (11.59, 35.24) | 0.53 |
| TPOAa (IU/ml) | 0.07 (0.05, 0.13) | 0.10 (0.06, 0.15) | 0.38b |
| TPOA+, n (%) | 10 (18.52) | 9 (16.36) | 0.34b |
| DZ (n) | 32 | 32 | – |
| Male, n (%) | 12 (37.5) | 13 (40.6) | – |
| Duration (years) | 8.41 (3.28, 17.30) | – | – |
| Age at diagnosis (years) | 13.43 (7.12, 31.35) | – | – |
| Age at test (years) | 22.25 (13.17, 39.60) | 22.25 (13.17, 39.58) | 0.49 |
| TPOA (IU/ml) | 0.90 (0.10, 18.75) | 0.40 (0.15, 1.00) | 0.56b |
| TPOA+, n (%) | 15 (46.9) | 6 (18.8) | 0.03* |
| US | |||
| MZ (n) | 25 | 25 | – |
| Male, n (%) | 14 (56.0) | 14 (56.0) | – |
| Duration (years) | 7.50 (2.25, 12.67) | – | – |
| Age at diagnosis (years) | 8.59 (4.59, 11.58) | – | – |
| Age at test (years) | 15.50 (9.42, 22.00) | 15.50 (9.42, 22.00) | 0.55 |
| TPOA (IU/ml) | 0.50 (0.00,1.50) | 0.50 (0.00, 99.50) | 0.24b |
| TPOA+, n (%) | 5 (20.0) | 7 (28.0) | 0.34b |
| DZ (n) | 25 | 25 | – |
| Male, n (%) | 15 (60.0) | 12 (48.0) | – |
| Duration (years) | 6.91 (1.75, 11.58) | – | – |
| Age at diagnosis (years) | 7.66 (3.66, 12.25) | – | – |
| Age at test (years) | 14.58 (9.50, 22.58) | 14.58 (9.50, 22.17) | 0.54 |
| TPOA (IU/ml) | 0.50 (0.00, 1.50) | 0.50 (0.00, 0.50) | 0.36b |
| TPOA+, n (%) | 5 (20.0) | 5 (20.0) | 0.81b |
Median (IQR) is shown unless indicated otherwise
For one of the MZ twin pairs the TPO value of the diabetic twin was missing TPOA+, defined as >1.5 IU/ml
p values were based on a conditional logistic regression model with disease status as outcome and TPOA level or TPOA+ as predictor, with sex included as covariate
p<0.05
For the UK and US DZ pairs, 17 (53.13%) and 13 (52.0%) pairs were opposite sex, respectively
UK twins
Twin pairs were selected from the British Diabetic Twin Study and ascertained by referral through their physicians from 1971 to present. From this collection, we identified all twin pairs discordant for type 1 diabetes of similar age at diagnosis and disease duration at sampling. All participants gave informed consent, and the East London Health Authority Research Ethics Committee approved the study (reference 07/Q0604/10).
US twins
Twin pairs were selected from the US twin cohort of diabetes-discordant identical and non-identical twins. Twins were initially ascertained through the Joslin Diabetes Center (1962–1992), and subsequently through the Barbara Davis Center patient clinic, the Diabetes Prevention Trial, TrialNet and physician referrals (1992–2012). Affected twins and their unaffected co-twins were matched for age at TPOA testing. Samples were selected as drawn at or nearly at the same time for twins and co-twins and were restricted to those drawn before diabetes onset for the initially unaffected twin. Furthermore, affected MZ and DZ probands were matched by age, diabetes duration and sex. Informed consent was obtained from each study participant. The Colorado Multiple Institutional Review Board approved all study protocols.
TPOA assay
TPOA were determined in both cohorts by radioimmunoassay, using the same assay system (RSR, Cardiff, UK; Kronus, Star, ID, USA), according to the manufacturer’s instructions. All UK serum samples were stored at −20°C prior to analysis and assayed in a batched assay at Ulm University, Ulm, Germany, to avoid assay batch variations. The lower detection limit for TPOA was 0.03 U/ml and values >0.3 U/ml (=1.5 IU/ml [WHO international units]) were scored as positive, in accordance with the manufacturer’s instructions. All TPOA data are expressed in WHO IU in this paper.
Analytical approach
As the distribution of TPOA could not be transformed to normal, it was categorised into four groups (ordinal values of 0, 1, 2 or 3) for analysis in both twin cohorts, with the highest category representing a level above the clinical cut-off of TPOA positivity (>1.5 IU/ml) (Table 2).
Table 2.
Cumulative proportions of participants in the four TPOA categories
| Category | Value (IU/ml) | Cumulative proportion (%) |
|---|---|---|
| UK | ||
| 1 | TPO=0.00 | 7.32 |
| 2 | 0.00<TPO≤0.50 | 57.72 |
| 3 | 0.50<TPO≤1.50 | 81.30 |
| 4 | >1.50 | 100 |
| US | ||
| 1 | TPO=0.00 | 33 |
| 2 | 0.00<TPO≤0.50 | 66 |
| 3 | 0.50<TPO≤1.50 | 78 |
| 4 | >1.50 | 100 |
As twin pairs were discordant for diabetes, the design is that of a co-twin case–control study in which twins in a pair are matched for age, genes (for MZs completely, for DZs in part) and shared childhood environmental exposures. As such, we used conditional logistic regression to examine differences within MZ and DZ twin pairs in TPOA (i.e. disease effects) and age at testing after adjustment for sex in the DZ pairs only. Significantly higher TPOA in either the diabetic or the nondiabetic twins would imply a non-genetic origin, as we have previously observed for DAA [5]. Spearman’s rank correlations (and logistic regression with sex as covariate) were used to examine associations of TPOA (and TPOA positivity) with age at diagnosis and disease duration in affected twins (MZ and DZ combined).
A liability-threshold model was used in all twin modelling of TPOA [10]. A normal liability distribution was assumed to underlie the ordered categories, with the three estimated thresholds (z values) explaining the proportions (counts) of each class. The thresholds were adjusted for age and sex. First, a so-called saturated model was fitted to: (1) estimate polychoric correlations within zygosity groups; (2) test whether thresholds could be set equal between twin pairs (twin 1 vs twin 2) and across zygosity groups (MZ vs DZ) by comparing a model in which these thresholds are freely estimated with models in which they are constrained to be equal across twin pairs (within zygosity groups) and across zygosity groups; and (3) estimate the age and sex effects on the thresholds. Second, we conducted quantitative genetic-model-fitting analysis to estimate the influence of genetic and environmental factors on TPOA [11]. In brief, we compared polychoric correlations calculated by the saturated model in MZ and DZ twin pairs and quantified sources of individual differences by separation of observed phenotypic variance into additive genetic (A), common (shared) environmental (C), dominant genetic (D) and unique (or non-shared) environmental (E) components. The full starting model was based on the pattern of correlations within zygosity groups: ACE if the DZ correlation was larger than half the MZ correlation and ADE if the DZ correlation was smaller than half the MZ correlation [12]. The significance of components A and C was assessed by testing deterioration in model fit after each component was dropped from the full model (ACE or ADE). Standard hierarchic χ2 tests were used to select the best-fitting model in combination with Akaike’s information criterion ([AIC]=χ2 − 2df). Generally, the lowest AIC indicates the best balance of goodness-of-fit and parsimony.
First, model fitting was conducted separately within the UK and US cohorts. Subsequently, meta-analysis was conducted to test whether estimates of genetic and environmental factors could be set equal between the UK and US samples, by comparing the fit of the heterogeneity model (variance components estimated separately) with that of the homogeneity model (variance components set equal across the UK and US cohorts). That there was no significant difference between the fits of these two models indicated there were no cohort differences and the UK and US samples could be combined to estimate overall variance components.
Data handling and descriptive analyses were performed with SAS 9.3 software (SAS Institute, Cary, NC, USA). Twin model fitting was carried out with OpenMx 1.3.2 software (http://openmx.psyc.virginia.edu/).
Results
From the UK and US collections, we analysed TPOA in all initially type 1 diabetes-discordant twin pairs of similar age at sampling, testing both MZ pairs (UK 55 pairs, US 25 pairs) and DZ pairs (UK 32 pairs, US 25 pairs). Neither the UK nor the US MZ twins showed significant intra-pair differences in TPOA between diabetic and healthy twins, i.e. TPOA did not associate with type 1 diabetes (Table 1). However, in the UK DZ group the difference for TPOA positivity did reach significance (p=0.03, without adjustment for multiple testing) (Table 1).
In the affected twins, Spearman’s rank correlations of TPOA with age at type 1 diabetes diagnosis (AD) and disease duration (DD) were 0.12 and 0.01 for the UK sample, and 0.26 and 0.20 for the US sample, respectively (for all p >0.05). In the logistic regression model with TPOA positivity as outcome variable and AD, DD and sex as independent variables, AD (but not DD) showed a significant effect (p= 0.01) in the UK cohort. This significant effect of AD (p<0.01) was confirmed in a model that combined the UK and US samples and added cohort as covariate.
Results from the saturated model showed that thresholds between twins and co-twins (p=0.74 within MZ and p=0.54 within DZ pairs) as well as zygosity groups (p=0.73) could be set equal for the US twins. The same was true for thresholds between twins and co-twins in the UK cohort (p=0.18 within MZ and p=0.06 within DZ pairs). However, thresholds between the UK zygosity groups could not be set equal (p<0.01) and were estimated separately in all further models. Sex effects on thresholds were significant in both the UK and US twins (p<0.01 for both). That is, females had a higher prevalence of TPOA positivity than males (UK: female 30%, male 16%; US: female 42%, male 5%). The effect of age was not significant in either the UK or the US twins.
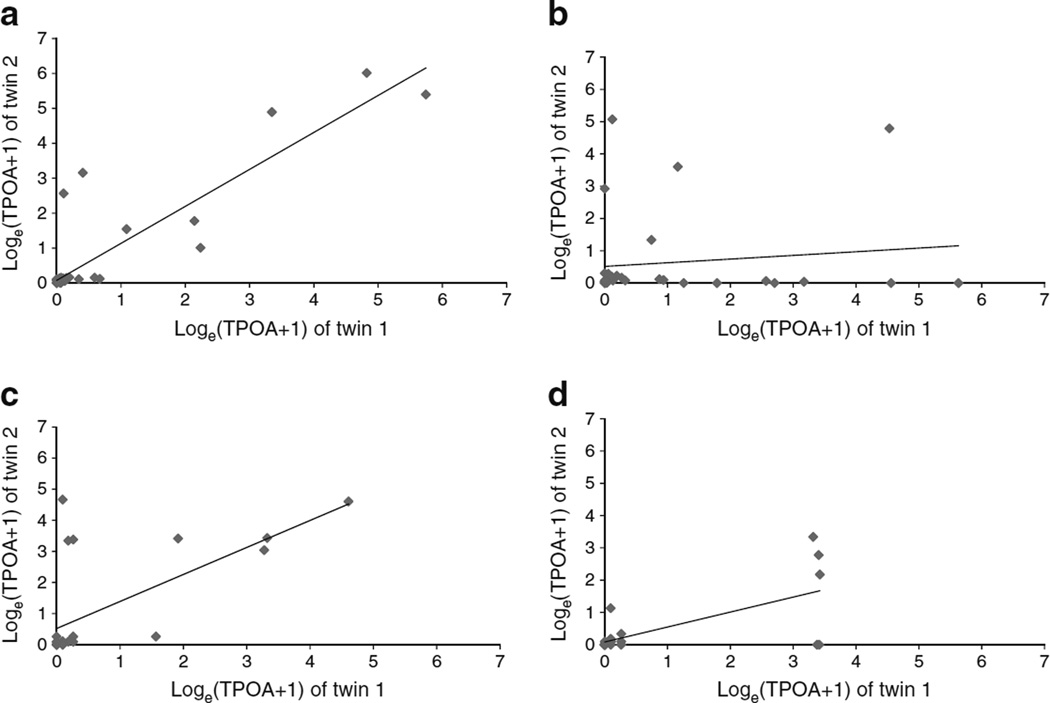
The UK polychoric DZ correlation was not significantly different from zero and was less than half of the MZ correlation (rMZ [95% CI] 0.72 (0.50, 0.85); rDZ [95% CI] −0.24 [−0.62, 0.23]). As such, an important contribution of genetics including an effect of the D component would be expected. For the US twins, MZ and DZ correlations (rMZ [95% CI] 0.79 [0.45, 0.92]; rDZ [95% CI] 0.52 [0.08, 0.79]) suggested a contribution of the C component. These conclusions were confirmed by Fig. 1, which shows a scatterplot of Loge (TPOA+1) values of twin 1 (diabetic) vs twin 2 (non-diabetic) for MZ and DZ twin pairs in UK and US samples.
Fig. 1.
Scatterplot of TPOA values (calculated as the natural logarithm of TPOA+1) of twin 1 (diabetic) vs twin 2 (non-diabetic) for MZ (a) and DZ (b) twin pairs in UK samples and for MZ (c) and DZ (d) twin pairs in US samples. In (a) r2=0.89 and p<0.01; in (b) r2=0.13 and p<0.49; in (c) r2=0.64 and p<0.01; and in (d) r2=0.68 and p<0.01
Separate model-fitting analysis in the UK and US twins was carried out first with adjustment of age and sex on thresholds to estimate the specific genetic and environmental variance components. Model fit characteristics, parameter estimates and 95% CIs of these models are presented in Table 3. UK twins model fitting showed the C component could be dropped from the full ACE model without deterioration in fit (ACE vs AE Δχ2[df=1]=0.00, p=1.00). The A component could not be dropped from the full model because the fit deteriorated (ACE vs CE Δχ2[df=1]=8.00, p<0.005). However, D was not significant (ADE vs AE: Δχ2[df=1]= 3.42, p=0.06) and could be dropped from the full ADE model. Thus, the AE model showed the best fit, confirmed by the lowest ΔAIC. In the US twins neither A nor C was significant in the model. However, they could not be dropped from the model simultaneously (ACE vs E Δχ2[df=2]=12.89, p<0.01), confirming a contribution of familial factors. Due to the small sample size, we could not discriminate between A and C components although the AE model showed the best fit based on the lowest ΔAIC. These best-fitting AE models showed heritability (95% CI) estimates of 0.63 (0.37, 0.80) for the UK twins and 0.80 (0.51, 0.92) for the US twins.
Table 3.
Model fitting characteristics, parameter estimates and 95% CIs in the UK and US samples
| Model | −2LL | AIC | Δχ2 | Δdf | p | ΔAIC | Variance component (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|
| A | C/D | E | |||||||
| UK | |||||||||
| ACE | 392.49 | 66.49 | – | – | – | – | 0.63 (0.27, 0.80) | 0.00 (0.00, 0.27) | 0.37 (0.20, 0.63) |
| AE | 392.49 | 64.49 | 0.00 | 1 | 1.00 | −2.00 | 0.63 (0.37, 0.80) | – | 0.37 (0.20, 0.63) |
| CE | 400.49 | 72.49 | 8.00 | 1 | <0.005 | 6.00 | – | 0.42 (0.18, 0.62) | 0.58 (0.38, 0.82) |
| E | 411.00 | 81.00 | 18.51 | 2 | <0.001 | 14.51 | – | – | 1.00 |
| ADE | 389.07 | 63.07 | – | – | – | – | 0.00 (0.00, 0.69) | 0.67 (0.00, 0.82) | 0.33 (0.18, 0.56) |
| AE | 392.49 | 64.49 | 3.42 | 1 | 0.06 | 1.42 | 0.63 (0.37, 0.80) | – | 0.37 (0.20, 0.63) |
| E | 411.00 | 81.00 | 21.93 | 2 | <0.001 | 17.93 | – | – | 1.00 |
| US | |||||||||
| ACE | 229.31 | 43.31 | – | – | – | – | 0.54 (0.00, 0.92) | 0.25 (0.00, 0.78) | 0.21 (0.08, 0.55) |
| AE | 229.71 | 41.71 | 0.40 | 1 | 0.53 | −2.40 | 0.80 (0.51, 0.92) | – | 0.21 (0.08, 0.49) |
| CE | 231.26 | 43.26 | 1.94 | 1 | 0.16 | −0.05 | – | 0.65 (0.36, 0.82) | 0.36 (0.18, 0.64) |
| E | 246.20 | 56.20 | 16.89 | 2 | <0.001 | 12.89 | – | – | 1.00 |
In all models the thresholds were adjusted for effects of age and sex
(−2LL, minus twice the log likelihood)
To increase power and provide more stable variance components estimates we combined the UK and US samples in a meta-analysis. The homogeneity ACE model, in which all variance components were set equal between the UK and US data, was not significantly different from the heterogeneity model (p=0.66). This was also true for the AE model (p= 0.55). Thus, we could set the variance components of A, C, and E equal across the UK and US samples. The best-fitting model was the AE model with a heritability (95% CI) of 0.69 (0.50, 0.82) and E component (95% CI) of 0.31 (0.18, 0.50). No significant C component was found in the meta-analysis (Table 4). Next, we tested whether the upper threshold representing the clinical cut-off point for TPOA positivity (>1.50 IU/ml) was different between the UK and US samples by comparing a model in which these thresholds were freely estimated with a model in which they were constrained to be equal across US and UK samples. The upper threshold in the US twins could be set equal to the UK DZ twins (p=0.98), but not to the UK MZ twins (p=0.03) who showed a somewhat lower prevalence of TPOA positivity also reflected in slightly lower TPOA levels (Table 1).
Table 4.
Model fitting characteristics, parameter estimates and 95% CIs of the meta-analysis of the UK and US twin cohorts
| Model | −2LL | AIC | Δχ2 | Δdf | p | ΔAIC | Variance component (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|
| A | C | E | |||||||
| ACE | 623.41 | 109.41 | – | – | – | – | 0.69 (0.34, 0.82) | 0.00 (0.00, 0.29) | 0.31 (0.18, 0.50) |
| AE | 623.41 | 107.41 | 0.00 | 1 | 1.00 | −2.00 | 0.69 (0.50, 0.82) | – | 0.31 (0.18, 0.50) |
| CE | 633.43 | 117.43 | 10.02 | 1 | <0.005 | 8.02 | – | 0.51 (0.32, 0.66) | 0.49 (0.34, 0.68) |
| E | 657.21 | 139.21 | 33.80 | 2 | <0.001 | 29.80 | – | – | 1.00 |
In all models the thresholds were adjusted for effects of age and sex
(−2LL, minus twice the log likelihood)
After a median (interquartile range [IQR]) follow-up time of 5.5 (0.6–19.5) and 0.75 (0–1.5) years for UK and US twins, respectively, 15 initially healthy MZ co-twins from the UK and two initially healthy MZ co-twins from the US developed type 1 diabetes. None of the healthy DZ co-twins developed type 1 diabetes. Of ten initially non-diabetic twins with at least two diabetes-associated autoantibody types, nine developed type 1 diabetes on follow-up. Sensitivity analyses excluding the 15 UK MZ pairs and two US MZ pairs who became concordant on follow-up yielded virtually identical results. With the exception of the UK DZ group, TPOA still did not associate with type 1 diabetes. AE models remained best-fitting for both UK and US samples. Variance components could be set equal between the UK and US data in the meta-analysis, with an overall heritability (95% CI) of 0.66 (0.44, 0.81).
Discussion
The current study tested the hypothesis that a stochastic (environmental) trigger is associated with a heightened susceptibility to develop thyroid autoimmunity. However, our hypothesis was rejected as, especially in the MZ pairs that have the most optimal matching, levels or frequency of TPOA were not higher in the type 1 diabetes twin. On the contrary, even in these disease-discordant pairs, TPOA levels showed twin correlations in the combined twin cohorts, translating into a heritability estimate of 69%. The results show that TPOA levels are substantially genetically determined, consistent with an inherited susceptibility to thyroid autoimmunity in type 1 diabetes.
Previous studies have estimated the heritability of TPOA, but not in the context of type 1 diabetes risk. One such study put TPOA heritability at 0.41 in Old Order Amish families in the US [13]. An early twin study carried out about 50 years ago in Scotland in a sample of 145 healthy pairs (68 MZ and 77 DZ pairs) reported concordance in thyroid ‘microsomal’ autoantibody positivity [14]. Using their published data we were able to calculate case-wise concordance values of 0.45 for MZ and 0.35 for DZ twins. Quantitative genetic liability-threshold modelling in OpenMx on the same data yielded tetrachoric twin correlations of 0.59 (95% CI 0.15, 0.86) for MZ and 0.52 (95% CI 0.02, 0.84) for DZ twins and a significant familial contribution (A+C) to the variance in the best-fitting model of 58%, which was very similar to our result. However, due to power limitations we could not determine from that study whether the familial contribution of 58% was most likely explained by additive genetic or common environmental factors. A more recent study in healthy twins from the Danish Twin Registry measured TPOA in 283 MZ and 403 DZ pairs and showed that genetic components accounted for 73% (95% CI 46%, 89%) of the liability of being thyroid-antibody positive. The covariate adjusted estimate for genetic influence on serum TPOA concentrations was 61% (49%, 70%) in males and 72% (64%, 79%) in females [15].
Our present study in type 1 diabetes disease-discordant twin pairs showed the AE model fitted best with an A component (i.e. additive genetic) proportion of 69%. Similar to the Danish twin study, the genetic effect on TPOA was most important, with no C component (i.e. shared environmental effect) found. The unexpected negative DZ correlation in our UK sample was likely due to small sample size fluctuation. This made it difficult to distinguish between A and D components in the UK cohort alone, prompting us to combine the US and UK studies in a meta-analysis of the 137 twin pairs to boost both sample size and power.
Our results in type 1 diabetes-discordant twin pairs showed a significant effect of sex on the distribution of TPOA, but no age effect. Both the Danish twin study [15] and a British twin study [16] found sex and age had highly significant influences on TPOA in healthy non-diabetic individuals. Similarly, the incidence of TPOA was higher in girls than in boys in a German cohort of offspring of type 1 diabetes patients [6] and a Belgian cohort of patients with type 1 diabetes [17]. The Belgian study found TPOA was not associated with age of type 1 diabetes onset, but was associated with type 1 diabetes disease duration [17]; we found the converse in our affected twins. We anticipate that some of the children we studied here will develop TPOA at a later age. As autoimmune autoantibodies appear at different ages, our observations support the proposal that TPOA and type 1 diabetes have a distinct pathogeneses [7].
This present study is the first twin study of thyroid autoimmunity in the context of type 1 diabetes. The study benefits from the comparative matching of the twin pairs and the use of type 1 diabetes-discordant twins to test a clear hypothesis, which was rejected. Importantly, the results were strengthened in sensitivity analyses that excluded those pairs that became concordant for type 1 diabetes on follow-up. Further, we used the same TPOA assay system in both twin cohorts. Limitations were the modest size of the cohorts and the initial analysis on the separate cohorts due to distinct methods of ascertainment, which limited the analytical power, and the lack of prolonged prospective analysis. However, we have previously shown how these UK and US cohorts behave similarly in terms of progression to type 1 diabetes [4], so the differences are likely limited, as indeed were the estimates of heritability, while the meta-analysis demonstrated comparable heritability of TPOA to that in each cohort considered separately. Further, we did not estimate thyroglobulin autoantibodies or thyroid function as neither was relevant to the question we posed for TPOA [8].
Given these caveats, we can conclude with some confidence that thyroid autoimmunity in the context of risk of autoimmune diabetes is substantially genetically determined, consistent with an inherited susceptibility to thyroid autoimmunity in families at risk for type 1 diabetes. By implication, it will be as important to screen relatives for TPOA as patients with type 1 diabetes. Moreover, autoimmune thyroid disease is genetically distinct from type 1 diabetes, though they share large regions of clustered or contiguous enhancer genes, e.g. a candidate causal gene in the interleukin-2 receptor super-enhancer region is associated with type 1 diabetes but has no effect on autoimmune thyroiditis disease risk [18]. Importantly, from this present study the environmental component contributing to thyroid autoimmunity also appears to be distinct from that leading to type 1 diabetes [5].
Acknowledgments
Funding PRF was supported by the Children’s Diabetes Foundation in Denver, the University of Colorado Denver Diabetes and Endocrinology Research Center (National Institutes of Health [NIH] Grant P30-DK-57516), and the JDRF International Autoimmunity Center Consortium; BOB was supported by Deutsche Forschungsgemeinschaft (GRK 1041), the State Baden Wuerttemberg Centre of Excellence ‘Metabolic Disorders’, and the Ministry of Education, Singapore; RDGL was supported by grants from the British Diabetic Twin Research Trust and the JDRF International.
Abbreviations
- A
Additive genetic variance component
- AIC
Akaike’s information criterion
- C
Common (shared) environmental variance component
- D
Dominant genetic variance component
- DAA
Diabetes-associated autoantibodies
- DZ
Dyzygotic
- E
Unique (non-shared) variance component
- MZ
Monozygotic
- TPOA
Thyroid peroxidase autoantibodies
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement BW, MIH, FVR, PRF and SA contributed to the acquisition, analysis and interpretation of the data and drafting the work. BOB, AKS, HS and RDGL contributed to the conception and design of the work, and the analysis and interpretation of the data, and revised the paper critically for important intellectual content. All authors approved the final version of the paper to be published. RDGL is the guarantor of the work as a whole and is responsible for its integrity.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 4.Redondo MJ, Yu L, Hawa M, et al. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354–362. doi: 10.1007/s001250051626. [DOI] [PubMed] [Google Scholar]
- 5.Beyan H, Riese H, Hawa MI, et al. Glycotoxin and autoantibodies are additive environmentally determined predictors of type 1 diabetes: a twin and population study. Diabetes. 2012;61:1192–1198. doi: 10.2337/db11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler AG, Bonifacio E BABYDIAB-BABYDIET Study Group. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55:1937–1943. doi: 10.1007/s00125-012-2472-x. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacio E, Mayr A, Knopff A, Ziegler AG. Endocrine autoimmunity in families with type 1 diabetes: frequent appearance of thyroid autoimmunity during late childhood and adolescence. Diabetologia. 2009;52:185–192. doi: 10.1007/s00125-008-1206-6. [DOI] [PubMed] [Google Scholar]
- 8.Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31:126–135. doi: 10.1111/dme.12318. [DOI] [PubMed] [Google Scholar]
- 9.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis, Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 10.MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43:30–37. doi: 10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Boker S, Neale M, Maes H, et al. An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snieder H, Boomsma DI, van Doornen LJ, de Geus EJ. Heritability of respiratory sinus arrhythmia: dependency on task and respiration rate. Psychophysiology. 1997;34:317–328. doi: 10.1111/j.1469-8986.1997.tb02402.x. [DOI] [PubMed] [Google Scholar]
- 13.Pauls DL, Zakarija M, McKenzie JM, Egeland JA. Complex segregation analysis of antibodies to thyroid peroxidase in Old Order Amish families. Am J Med Genet. 1993;47:375–379. doi: 10.1002/ajmg.1320470315. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan WW, Boyle JA, Greig WR, et al. Distribution of certain autoantibodies in monozygotic and dizygotic twins. Ann Rheum Dis. 1996;25:463–468. doi: 10.1136/ard.25.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen PS, Brix TH, Iachine I, Kyvik KO, Hegedüs L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity. A study of healthy Danish twins. Eur J Endocrinol. 2006;154:29–38. doi: 10.1530/eje.1.02060. [DOI] [PubMed] [Google Scholar]
- 16.Phillips DI, Osmond C, Baird J, Huckle A, Rees-Smith B. Is birthweight associated with thyroid autoimmunity? A study in twins. Thyroid. 2002;12:377–380. doi: 10.1089/105072502760043440. [DOI] [PubMed] [Google Scholar]
- 17.de Block CE, de Leeuw IH, Vertommen JJ, et al. Belgian Diabetes Registry Beta-cell, thyroid, gastric, adrenal and coeliac autoimmunity and HLA-DQ types in type 1 diabetes. Clin Exp Immunol. 2001;126:236–241. doi: 10.1046/j.1365-2249.2001.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farh KK, Marson A, Zhu J, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]