Abstract
Biogenesis of the primary cilium, a cellular organelle mediating various signaling pathways, is generally coordinated with cell cycle exit/re-entry. Although the dynamic cell cycle-associated profile of the primary cilium has been largely accepted, the mechanism governing the link between ciliogenesis and cell cycle progression has been poorly understood. Using a human genome-wide RNAi screen, we identify genes encoding subunits of the spliceosome and proteasome as novel regulators of ciliogenesis. We demonstrate that 1) the mRNA processing-related hits are essential for RNA expression of molecules acting in cilia disassembly, such as AURKA and PLK1, and 2) the ubiquitin-proteasome systems (UPS)-involved hits are necessary for proteolysis of molecules acting in cilia assembly, such as IFT88 and CPAP. In particular, we show that these screen hit-associated mechanisms are crucial for both cilia assembly and cell cycle arrest in response to serum withdrawal. Finally, our data suggest that the mRNA processing mechanism may modulate the UPS-dependent decay of cilia assembly regulators to control ciliary resorption-coupled cell cycle re-entry.
Keywords: High-content screen, Ciliogenesis, Cell cycle, mRNA processing, Ubiquitin-proteasome system
Introduction
For many years, the primary cilium had been considered an obscure structure of no importance. However, recent studies suggest that this cellular organelle acts as a major hub for multiple signal transduction pathways that are necessary for diverse cellular phenomena, including differentiation, polarity, and homeostasis [1–3]. The primary cilium is an immotile and microtubule-based sensory organelle that extends from a mother centriole in most types of cells. The mother centriole generally contributes to formation of the mitotic spindle in a dividing cell, but in a quiescent cell it becomes associated with a Golgi-derived vesicle, migrates to the cell surface, and attaches to the plasma membrane [4]. Once it attaches, the mother centriole is referred to as the basal body, which serves as a nucleation region for the growth of ciliary axonemal microtubules [5]. Because of the ambilateral roles of the mother centriole in ciliogenesis and cell division, the events of cilia biogenesis and cell cycle progression are mutually exclusive.
In general, assembly of the primary cilium is coupled with cell cycle exit and entry into quiescence, whereas its disassembly is coupled with resumption of proliferation [6–8]; these facts present long-standing evidence for a link between ciliogenesis and cell cycle progression. Although the idea that cilia are resorbed as the cells enter mitosis was once prevalent, recent studies have indicated that cilia disassembly proceeds in two waves as quiescent cells re-enter the cell cycle: the first wave occurs at the G1→S transition, and the second wave occurs at the G2→M transition [9]. It has also been suggested that the inhibition of ciliary resorption halts cell cycle progression. For example, depletion of Nde1, Tctex-1, and Aurora kinase A (AURKA) results in the delay of cell cycle re-entry with abnormal retention of cilia [10, 11]. Thus, it appears that not only is a suppressive mechanism of ciliogenesis active during cell proliferation, but a stimulatory mechanism of ciliary resorption may also be active. Together, these observations imply that the disassembly of the primary cilium is a prerequisite for cells to cease being quiescent and re-enter the cell cycle.
Recent studies have shown that autophagy- and ubiquitin-proteasome system (UPS)-mediated proteolysis of cilia-related molecules are involved in cell cycle exit and subsequent cilia assembly [12, 13]. For instance, UPS-mediated degradation of Trichoplein, which is composed of Cul3-RING ubiquitin ligases (CRL3s) and KCTD17 as a substrate-adaptor, inactivates AURKA and initiates ciliogenesis [13]. Therefore, it is conceivable that the formation of the primary cilium is regulated by degradation of proteins involved in cilia disassembly prior to exit from the cell cycle. Although there are several clues as to the basis of coordinated regulation of ciliogenesis and cell cycle progression, some questions remain about how resorption of the primary cilium modulates progression of the cell cycle. A mechanistic explanation does not exist for how the presence or absence of the primary cilium can serve as a checkpoint at the G1→S transition.
In the present study, we perform a genome-wide high-content screen and uncover roles of mRNA processing and UPS mechanisms in cell cycle arrest and ciliogenesis. We find that mRNA processing-related hits are required to control RNA expression of cilia disassembly regulators (such as AURKA, PLK1, and NEK2), and UPS-related hits are necessary for the proteolysis of cilia assembly regulators (such as CPAP, KATANIN, and IFT88). Furthermore, our data suggest that mRNA processing is a stimulatory mechanism of cilia disassembly, whereas UPS is a suppressive mechanism of cilia assembly. Finally, we demonstrate that the mRNA processing and UPS-mediated ciliary resorption provide a link to the G1→S transition during cell cycle re-entry.
Results
Identification of novel modulators of ciliogenesis and cell cycle progression
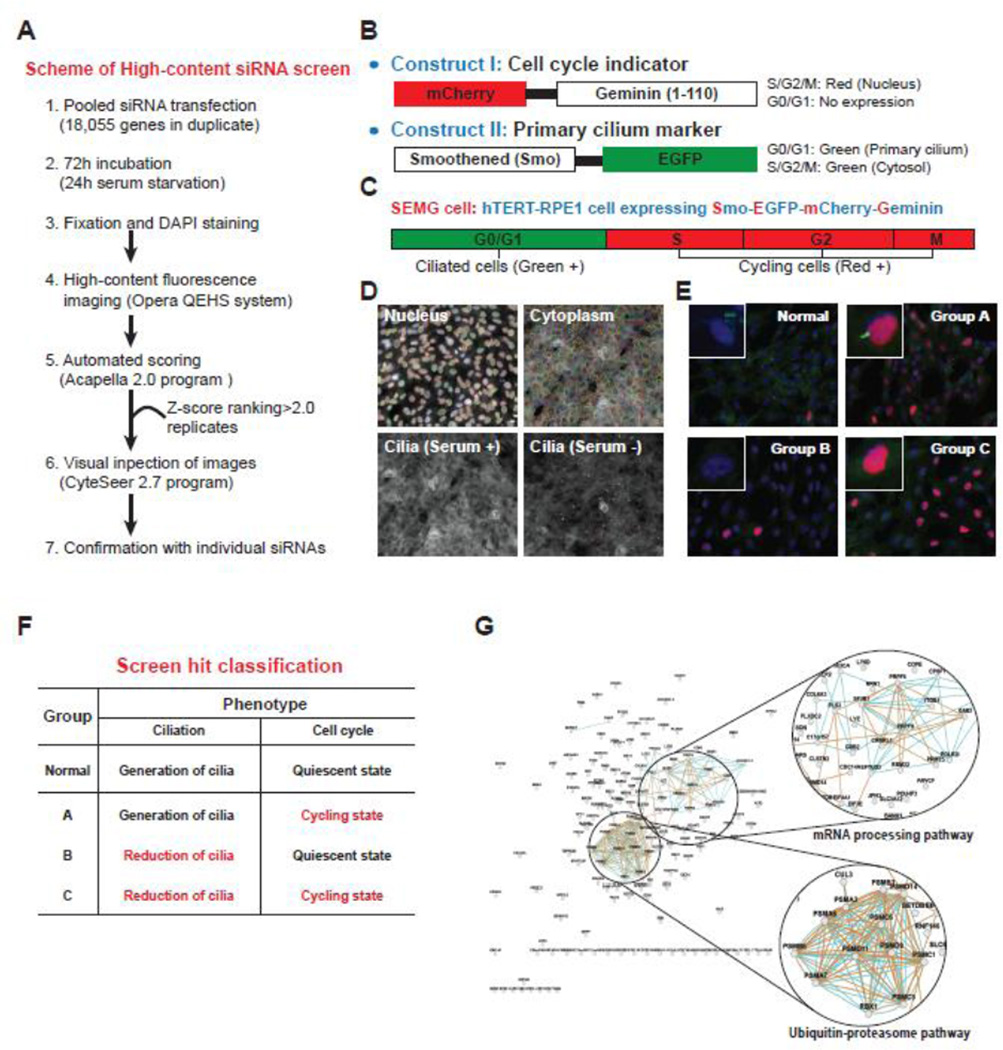
To obtain a list of candidates and to gain a comprehensive understanding of the molecular mechanism connecting ciliogenesis to cell cycle progression, we utilized a functional genomics approach. We performed a high-content screening (HCS) procedure using small interfering RNA (siRNA) [14] to analyze the genetic intersection of ciliogenesis and cell cycle state (Fig. 1A). This screen utilized a subclone of the hTERT-immortalized ciliary retinal pigment epithelial (RPE) cell line expressing both EGFP-tagged Smoothened, a transmembrane protein that accumulates in the cilium [15], and the N-terminal 110 amino acid residues of Geminin, a nuclear protein stabilized during S, G2, and M phases, fused to mCherry [16] (Fig. 1B). We predicted that this cell line, abbreviated SEMG (Smo-EGFP and mCherry-Geminin), would display a green fluorescent primary cilium during the G0/G1 phases and a red fluorescent nucleus during S, G2, and M phases (Fig. 1C).
Figure 1. High-content RNAi screen to identify novel regulators of ciliogenesis and cell cycle.
A. The workflow started with reverse transfection of the pooled siRNA library in a medium for 72 h, with 24 h of these involving serum starvation. We then performed fixation, DAPI staining, fluorescent imaging, identification of hits based on Z-score ranking, and visual inspection of the hits. B. The mCherry-Geminin (1–110) construct was transfected as a cell cycle indicator and was expressed in the nucleus during the S, G2, and M phases of the cell cycle. The Smo-EGFP construct was transfected as a primary cilium marker and was expressed in the cytosol of cycling cells and primary cilia of quiescent cells. C. The hTERT-RPE1 cell line that was stably transfected with mCherry-Geminin (1–110) and Smo-EGFP was named the SEMG cell line. The cells showed prominent expression of EGFP in the primary cilium in the G0/G1 phases and mCherry in the nucleus at S, G2, and M phases. D. The algorithm properly segmented the nucleus, cytoplasm, and primary cilium in SEMG cells under the conditions with and without serum. E. The screen hits were analyzed by manual image inspection using Cytseer 2.7 and classified into three groups. F. The classified hits were profiled in each group on the basis of the knockdown phenotype (red letters) in terms of ciliogenesis and cell cycle progression. G. The hit genes were clustered into two main functional groups, mRNA processing and UPS, by network analysis using GeneMANIA. Scale bars, 10 µm (E).
Because serum starvation induces cell cycle arrest in the G0/G1 phases and ciliary assembly in RPE cells, we hypothesized that serum withdrawal would yield a majority of cells with EGFP-positive cilia and mCherry-negative nuclei, whereas addition of serum would cause the majority of the cells to display mCherry-positive nuclei with EGFP-negative cilia. The cells were subjected to automated fluorescent imaging and scoring, and we found that ~70% of SEMG cells that were cultured in the presence of fetal bovine serum (FBS) showed mCherry-positive nuclei, with few if any EGFP-positive cilia. In contrast, 24 h after serum deprivation, ~60% of the cells had EGFP-positive cilia with few mCherry-positive nuclei (Fig. 1D). Additionally, we found that the depletion of positive control genes including KIF3A, ACTR3, and CRNKL1, which are involved in ciliogenesis or cell cycle regulation, led to abnormal numbers of EGFP-cilia and mCherry-nuclei in the serum-starved SEMG cells [14]. The rationale for performing our genome-wide RNAi screen with SEMG cells was to identify genes that, when downregulated, interfered with the mutually exclusive expression of mCherry and EGFP.
A total of 18,055 target genes representing the known human ENCODE transcriptome were individually knocked down in duplicate with the siRNA library for 72 h, including 24 h of serum starvation (Fig. 1A). The siRNA library was pooled such that four individual siRNAs targeted each gene (Supplementary Table S1). The cells were then subjected to automated fluorescent imaging and scoring followed by visual inspection (Fig. 1E) and secondary screening in triplicate. Through manual visual inspection, we confirmed that the non-specific scrambled siRNA-transfected cells under serum starvation had mostly EGFP-labeled cilia and few cells with mCherry-labeled nuclei. Finally, we obtained the screen hits, which displayed abnormal numbers of EGFP-cilia and mCherry-nuclei with Z-scores > 2; the hits were clustered into three outcome groups (Fig. 1F). Among the classified hits that surpassed the threshold, we identified 201 in Group A, 460 in Group B, and 164 in Group C (Supplementary Table S2). In addition, we found that several genes such as NEK2, PLK1, APCs, and cyclins, which are known to be associated with ciliogenesis-linked cell cycle control, were included in our screen hits.
Because the hits from Groups A, B, and C were likely to be largely non-overlapping, we profiled the results separately. Group A had a normal number of EGFP-cilia but a higher than normal number of mCherry-nuclei. This result indicated that silencing of Group A genes might cause inhibition of ciliary resorption and failed entry into mitosis. Group B had a lower than normal number of EGFP-cilia but a normal number of mCherry-nuclei, suggesting that the Group B genes might have no direct effect on the cell cycle but might block ciliogenesis. Furthermore, we found that the Group B list included many known ciliopathy genes and, accordingly, identified a novel Joubert Syndrome causative gene, KIAA0586, from the list [14]. Group C had a lower than normal number of EGFP-cilia and a higher than normal number of mCherry-nuclei. This finding suggested that depletion of Group C genes triggered a blockade of entry into or maintenance of a quiescent state and a concurrent (or resulting) failure of ciliation. Therefore, we focused on Group C because the results suggested dual regulation of ciliogenesis and cell cycle progression. To extract information regarding the mechanisms by which ciliogenesis is coupled to cell cycle regulation, we analyzed the list of hits using public databases such as DAVID [17] and GeneMANIA [18]. The results of combined analyses showed enrichment within two functional clusters: mRNA processing and the Ubiquitin-proteasome system (UPS) (Fig. 1G). The mRNA processing module (GO: 0006397) is involved in the conversion of a primary mRNA transcript into one or more mature mRNAs prior to translation into a polypeptide. The UPS module (proteasome-mediated ubiquitin-dependent protein catabolic process; GO: 0043161) is involved in the breakdown of a protein (this process is initiated by attachment of ubiquitin, which is mediated by the proteasome).
The machineries of mRNA processing and UPS are necessary for ciliogenesis and cell cycle control
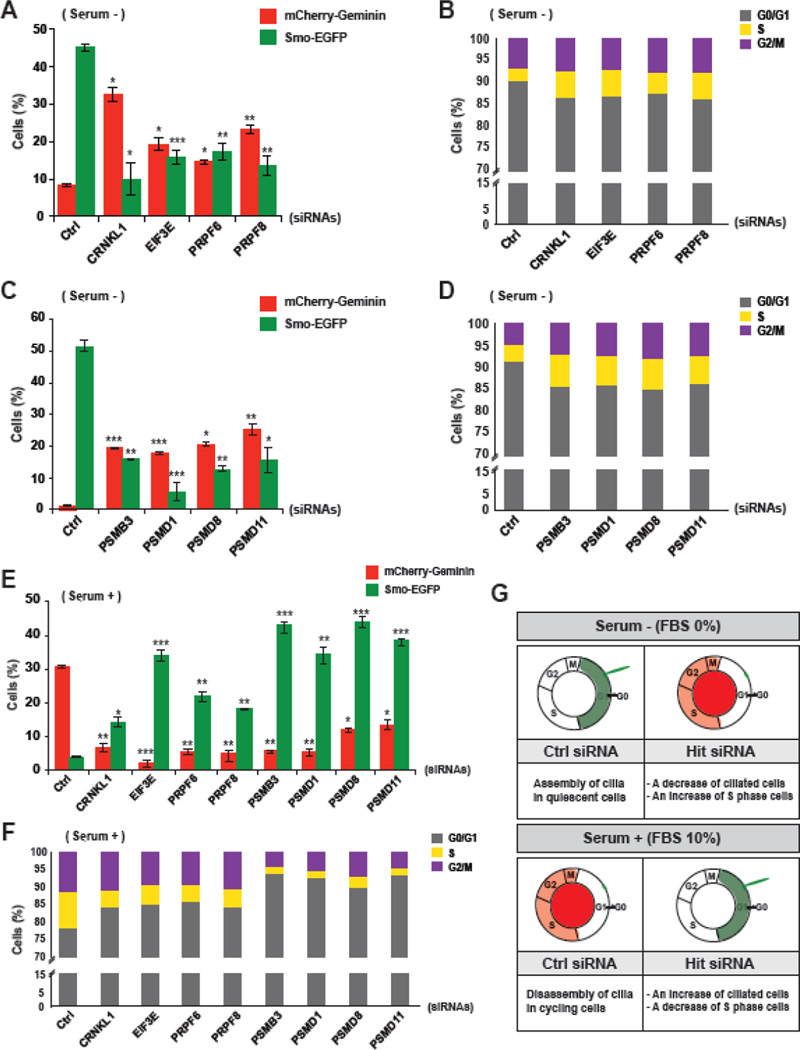
To validate the role of hit genes in the coupling of ciliogenesis with cell cycle progression, we performed fluorescent imaging and fluorescence-activated cell sorting (FACS) analyses on knockdown cells transfected with individual siRNAs against representative hits. For the validation tests, we prioritized groups of genes that were predicted to have roles in similar pathways within each cluster shown in Fig. 1G. The individual siRNAs used to target each hit were selected based on a qRT-PCR knockdown efficiency test (Supplementary Fig. S1). We counted Smo-EGFP-positive ciliated cells and mCherry-Geminin-positive cycling cells from the imaging data and compared the results to the FACS data. In the mRNA processing pathway cluster, crooked neck pre-mRNA splicing factor 1 (CRNKL1); eukaryotic translation initiation factor 3, subunit E (EIF3E); pre-mRNA-processing factor 6 (PRPF6); and pre-mRNA-processing factor 8 (PRPF8) were selected. As expected, the depletion of each hit resulted in a decrease of Smo-EGFP-positive cells and an increase of mCherry-Geminin-positive cells (Fig. 2A). Further analysis using FACS implied that the increased number of cycling cells might be due to an increase of S phase cells (Fig. 2B). In the UPS pathway cluster, proteasome subunit type-3 (PSMB3); proteasome 26S subunit, non-ATPase 8 (PSMD8); and proteasome 26S subunit, non-ATPase 11 (PSMD11) were chosen; proteasome 26S subunit, non-ATPase 1 (PSMD1), which is not a hit from the screen, was also included. Because 11 over 30 human proteasome subunits were hits in our screen (Fig. 1G and Supplementary Fig. S2), we wanted to determine if any specific subunit of the proteasome complex plays a role in the link between ciliogenesis and cell cycle progression. The depletion of the chosen proteasome subunits showed decreases of Smo-EGFP-positive cells and increases of mCherry-Geminin-positive cells that were consistent with the effects of knocking down mRNA processing-related hits (Fig. 2C). The FACS analysis also suggested that the increased number of cycling cells was caused by an increase of S phase cells after gene silencing (Fig. 2D). We therefore focused on the common knockdown phenotypes of mRNA processing and UPS-related hits, which were a decrease of ciliated cells and an increase of S phase cells.
Figure 2. Validation of hit genes involved in the coupling of ciliogenesis with cell cycle control.
A. The SEMG cells were transfected with individual siRNAs targeting mRNA processing-related hits under serum starvation and subjected to fluorescent imaging to analyze ciliation (Smo-EGFP) and cell cycling (mCherry-Geminin). B. The cell cycle state of the hit-silenced SEMG cells was analyzed by FACS under serum starvation. C. The SEMG cells were transfected with individual siRNAs targeting proteasome subunits under serum starvation and subjected to fluorescent imaging to analyze ciliation and cell cycling. D. The cell cycle state of the proteasome subunit-silenced SEMG cells was analyzed by FACS under serum starvation. E. The screen hit-depleted SEMG cells were subjected to fluorescent imaging to analyze ciliation and cell cycling in the presence of serum. F. The cell cycle state of the hit gene-depleted SEMG cells was analyzed by FACS in the presence of serum. The data are shown as the mean ± SEM, One-way ANOVA: *P < 0.05, **P < 0.005, and ***P < 0.001 (n = 3, [A, C, and E]). G. Based on the results of fluorescent imaging and FACS analyses, the roles of screening hit genes were predicted.
It seemed that the increased number of S phase cells was correlated with the decreased number of G0/G1 phase cells (Fig. 2B, D). From the data, the cell cycle-related roles of the screen hits were inferred, and we predicted that the silencing of the hits might lead to the bypass of G0 arrest from G1 phase or a failure to maintain G0/G1 arrest under serum starvation. We therefore hypothesized that the hit genes played roles in G1 phase and that the dysregulation of hit-mediated mechanisms resulted in the abnormal transition of cells to S phase from G1 phase. To determine if function of the screen hits during G1 phase affect the G1→S transition, we simply examined the down-regulation effect of the hits on cell cycle progression in the presence of serum (Fig. 2E, F, and Supplementary Fig. S3A). The knockdown led to more Smo-EGFP-positive cells and fewer mCherry-Geminin-positive cells (Fig. 2E). Remarkably, FACS data showed that the silencing of hits caused an increase in the number of G0/G1 arrested cells (Fig. 2F). These data implied possible roles for the screen hits in the regulation of the G1→S transition as well as ciliogenesis (Fig. 2G). Taken together, our validation analysis with the screen hits showed not only that our screening was robust but also that mRNA processing- and UPS-associated mechanisms might be important for controlling coordination of the G1→S transition of the cell cycle with cilia biogenesis.
The mRNA processing and UPS mechanisms are essential for ciliary formation and function in vivo
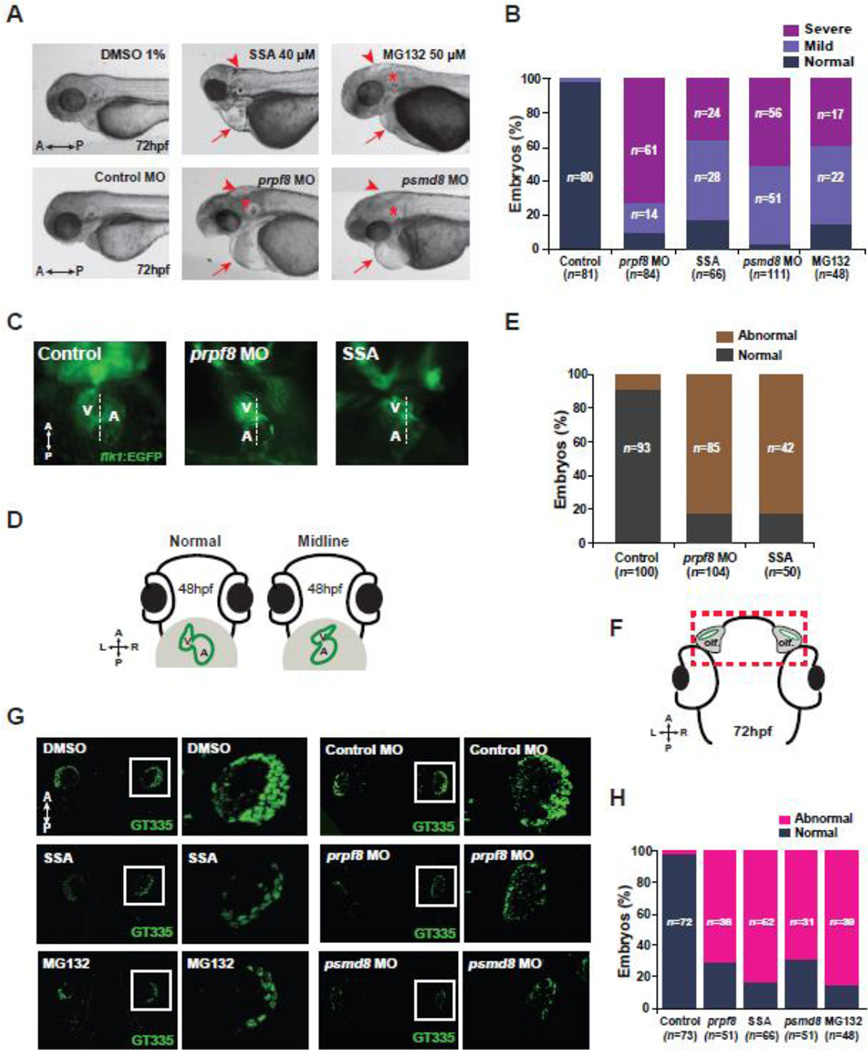
Because most of the screen hits have not been previously reported as ciliary genes, we wondered whether the machineries for mRNA processing and UPS are necessary for ciliogenesis/ciliary function in vivo. We therefore examined the inhibitory effect of mRNA splicing by utilizing spliceostatin A (SSA) [19] and morpholinos (MOs) targeting prpf8, and that of UPS by utilizing MG132 and MOs targeting psmd8 in zebrafish (Supplementary Fig. S3B). We found that the zebrafish larvae treated with SSA or MG132 and injected with prpf8 MOs or psmd8 MOs showed typical ciliary defects, such as peripheral heart edema, small brain/hydrocephalus, abnormal otoliths (abnormal angle between two otholiths) and curved tails [20] (Fig. 3A, B, and Supplementary Fig. S4A). According to previous findings showing that malformed or dysfunctional cilia result in disruption of heart asymmetry zebrafish [20, 21], we analyzed the heart laterality the embryos of Tg(flk1:egfp) zebrafish (an endocardium-specific transgenic reporter line) treated with SSA or MG132 and injected with prpf8 or psmd8 MOs. We found that the inhibition of the spliceosome by SSA and prpf8 MOs caused failed laterality of the ventricle and atrium in the zebrafish heart (Fig. 3C–E). Moreover, the drug-treated or MOs-injected larvae showed attenuated ciliary formation in the cells of the olfactory organ at 72 h post fertilization (hpf) (Fig. 3F–H). We tested the rescue for psmd8 MO, but not for prpf8 MO, because the coding sequence of zebrafish prpf8 was very large to obtain an expression construct. We found that the morphological defects were not due to off-target effects of MOs (Supplementary Fig. S4B–C) and also confirmed that the reduced cilia were not due to less olfactory cells by drug-treatment or MOs injection (Supplementary Fig. S5). Taken together, these data suggest essential roles of mRNA processing, such as RNA splicing, and the UPS in the regulation of ciliogenesis and ciliary function in vivo.
Figure 3. The function of spliceosome and proteasome is essential for ciliogenesis in vivo.
A. Overall morphological defects in the MOs-injected, SSA- and MG132-treated zebrafish embryos at 72 hpf. Arrows indicate heart edema; arrowheads point hydrocephalus; asterisks indicate otolith defects in the ear. B. The embryos showing ciliary defects are quantified according to severity, and the data were presented in the graph. C. The asymmetry of the heart, controlled by cilia in the Kupffer’s vesicle, was observed in the prpf8 MO-injected and SSA-treated Tg(flk1:EGFP) zebrafish larvae. V: ventricle, A: atrium. D. The diagrams show normal and abnormal (midline) heart asymmetry of the zebrafish at 48 hpf. E. The Tg(flk1:EGFP) zebrafish larvae showing defects in heart asymmetry were counted and the quantified data were presented in the graph. F. The diagram shows head area of the zebrafish larva containing olfactory organs at 72 hpf. The rectangle indicates the photographed region after immunostaining with anti-GT335 antibodies. Olf.: olfactory organs, hpf: hours postfertilization. G. Zebrafish larvae were treated with 40 µM SSA and 50 µM MG132 to inhibit function of the spliceosome and proteasome, respectively. The larvae were immunostained with anti-GT335 antibodies and the olfactory regions were compared to those of the DMSO-treated control larvae at 72 hpf. The olfactory cilia of zebrafish larvae, injected with control MO, prpf8 MO, or psdm8 MO were also observed by GT335 immunostaining. The magnified images are indicated by insets and presented in the right panels. H. The individual zebrafish larvae showing ciliogenesis defects within the olfactory organs were counted. The quantified data were presented in the graph. A: anterior, P: posterior, L: left, R: right (A, C, D, F, and G).
The control of cilia disassembly regulator expression is crucial for the link between ciliary resorption and cell cycle re-entry
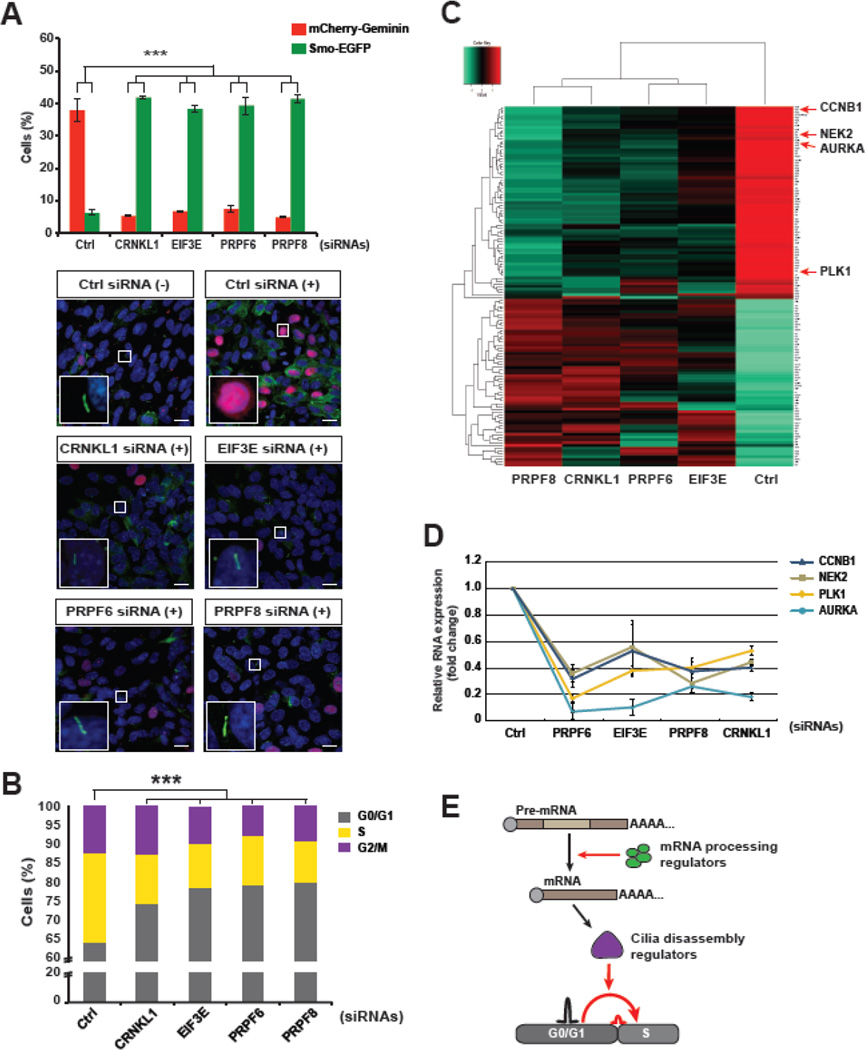
Several studies have shown that most cells with cilia in the G0/G1 arrested state lose them when the quiescent cells re-enter the cell cycle [8, 22]. According to our validation data, suggestive of a ciliary disassembly-associated role by the molecular mechanism acting in the G1→S transition, we assessed the knockdown effects of the screen hits on cilia disassembly. We first explored the mRNA processing regulators including CRNKL1, EIF3E, PRPF6, and PRPF8 (Fig. 3). In general, the human telomerase reverse transcriptase-transformed retinal pigment epithelium (hTERT-RPE1) cells that are forced to generate cilia by serum deprivation and resorb the cilia 18 h after serum is added [22]. The cell cycle states of SEMG cells were also changed by incubation with serum (Supplementary Fig. S3C, S6). In line with other studies, control siRNA-transfected SEMG cells were forced to disassemble cilia and re-enter the cell cycle 18 h after cessation of serum starvation. In contrast, knockdown of the mRNA processing-related hits blocked cilia disassembly and cell cycle re-entry in SEMG cells (Fig. 4A). According to FACS analysis, hit gene-silenced SEMG cells maintained G0/G1 arrest instead of re-entering the cell cycle after addition of serum (Fig. 4B). These results indicated that the mRNA processing mechanism is important for ciliary resorption and the G1→S transition in cell cycle re-entry.
Figure 4. The mRNA processing hits control RNA expression of cilia disassembly regulators and promote the G1-S transition.
A. The ciliation and cell cycle of the mRNA processing hit-silenced SEMG cells were analyzed by fluorescent imaging analysis 18 h after cessation of serum starvation. The cells showed inhibition of cilia disassembly and cell cycle re-entry. (−), serum starvation; (+), serum re-addition for 18 h. B. The cell cycle state of the knockdown cells was analyzed by FACS and compared to that of controls after addition of serum for 18 h. The quantified data were presented in the graph. C. Whole-transcriptome sequencing data obtained from the cells with a knockdown of mRNA processing-related hits was compared with those of control siRNA-transfected cells, and the resulting heatmap showed a decrease of the expression levels of primary cilia disassembly-related genes including AURKA, CCNB1, NEK2, and PLK1 in the knockdown cells. D. The reduced RNA expression levels of the cilia disassembly regulators that were putatively targeted by the mRNA processing hits were confirmed by qRT-PCR. E. A proposed model for the essential roles of mRNA processing regulators in coupling of ciliary resorption with cell cycle re-entry. One-way ANOVA: ***P < 0.001 (A, B). The data are presented as the mean ± SEM (n = 3, [A]). Insets: representative images of each knockdown at higher magnification. Scale bars, 10 µM (A).
To determine the possible molecular mechanism, we performed whole transcriptome sequencing (RNA-Seq), which can analyze overall mRNA expression and pre-mRNA splicing events. We compared the RNA-Seq results of CRNKL1-, EIF3E-, PRPF6-, and PRPF8- depleted cells with those of control siRNA knockdown cells. The results showed that few genes caused alternative splicing, and the genes were not common among the knockdown cells. However, expression levels of several genes were changed and commonly targeted (Fig. 4C and Supplementary Table S3). This result was not surprising because the mRNA processing hits included not only splicing factors but also translation regulators such as EIF3E, which controls mRNA expression of target genes by regulating nonsense-mediated mRNA decay [23–25]. Furthermore, it has been reported that splicing factors also regulate expression of mRNA via direct interaction with mature RNA [26]. We hypothesized that the knockdown phenotype would involve altered expression of certain genes crucial for either ciliogenesis or cell cycle control. Accordingly, the heatmap generated by analysis of the genes commonly targeted by the screen hits showed an obvious contrast between the clusters of genes with decreased and increased expression levels (Fig. 4C). Within the cluster of genes with decreased expression were AURKA, PLK1, NEK2, and CCNB1. These genes have been reported to play a role in cilia disassembly, which is independent of their well-known roles in mitotic control of the cell cycle [22, 27–29]. Moreover, our data were consistent with those of another report showing that knockdown of AURKA gave rise to G0/G1 phase arrest and ablated cilia disassembly in hTERT-RPE1 cells [30]. Upon validation with quantitative real-time PCR (qRT-PCR) analysis, we confirmed that the mRNA expression levels of all four candidates in the knockdown cells were lower than those of control cells (Fig. 4D). Taken together, these data suggest an influence of the mRNA processing mechanism on the cilia resorption and G1→S transition mediated by ciliary genes, such as AURKA, PLK1, NEK2, and CCNB1 (Fig. 4E).
Proteolysis of cilia assembly regulators promotes ciliary resorption and cell cycle re-entry
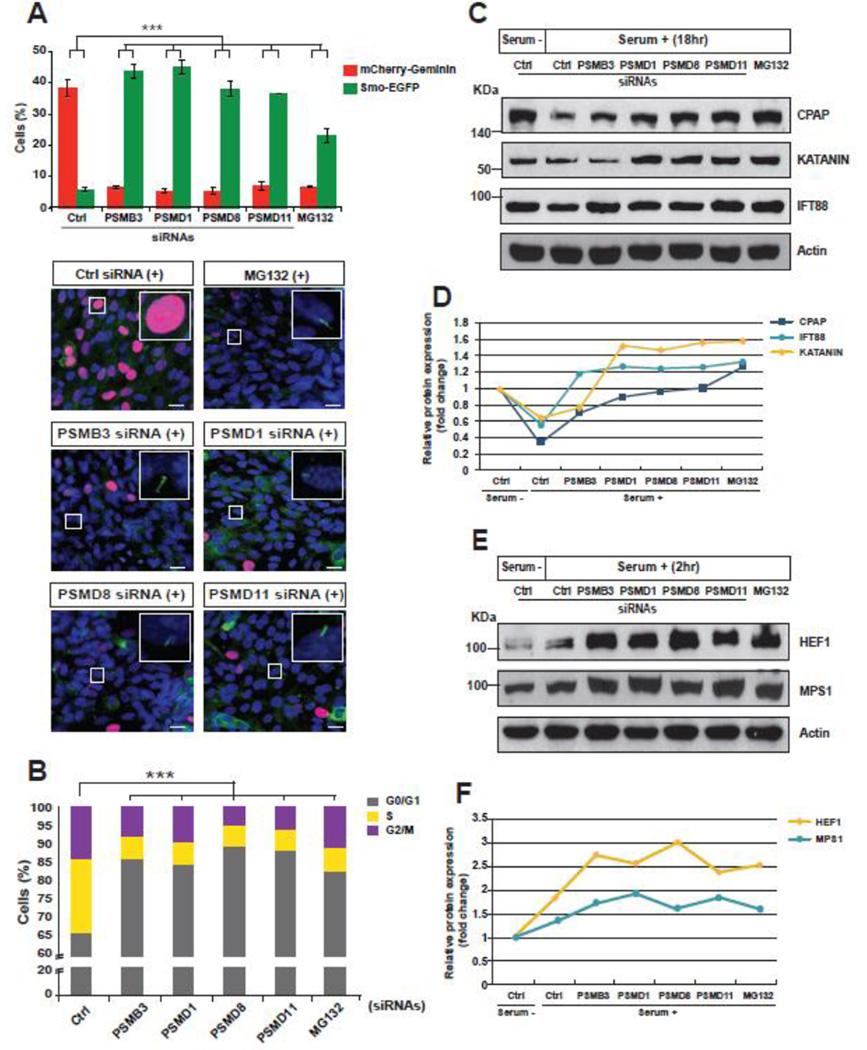
Next, to elucidate a required role of the UPS mechanism in the link between cilia disassembly and cell cycle re-entry, we additionally compared the phenotypes of SEMG cells treated with MG132 to those of proteasome subunit-depleted cells. Both the knockdown and MG132-treated cells displayed an inhibition of ciliary resorption and cell cycle re-entry 18 h after serum re-addition (Fig. 5A and Supplementary Fig. S3C). Moreover, the disruption of the UPS mechanism led to an increased number of cells in the G0/G1 phases, indicating an important role of UPS in cilia disassembly and control the G1→S transition (Fig. 5B).
Figure 5. Proteasome-dependent decay of cilia assembly regulators is necessary for cilia disassembly and cell cycle re-entry.
A. The SEMG cells were silenced with siRNAs targeting proteasome subunits or treated with MG132 and were analyzed by fluorescent imaging analysis 18 h after cessation of serum starvation. The cells showed inhibition of cilia disassembly and cell cycle re-entry. B. The cell cycle state of the knockdown or MG132-treated cells was analyzed by FACS and compared to that of controls 18 h after addition of serum. The quantified data were presented in the graph. C. The protein levels of cilia assembly regulators, including CPAP, KATANIN, and IFT88, in the proteasome subunit-depleted or MG132-treated cells were compared to those of control cells by Western blot assay. The protein levels of control cells were decreased, while those of knockdown and MG132-treated cells were high 18 h after cessation of serum starvation. D. The fold change of protein expression levels of cilia assembly regulators were quantified. E. The expression levels of cilia disassembly-related proteins including HEF1 and MPS1 in the proteasome subunit-depleted or MG132-treated cells were compared to those of control cells by western blot assay. The protein levels of control cells were slightly increased 2 h after serum re-addition, while those of knockdown and MG132-treated cells were much higher than controls. F. The fold change in protein expression levels of cilia disassembly regulators was quantified. The data are presented as the mean ± SEM (n = 4, [A, B]). One-way ANOVA: ***P < 0.001 (A, B). Insets: representative images of each knockdown at higher magnification. Scale bars, 10 µM (A).
Because the function of the proteasome complex has not been previously implicated in ciliary resorption, we assumed that the cilia-associated molecules undergoing UPS-dependent proteolysis might be key regulators that connect cell cycle re-entry to cilia disassembly. Based on previous studies, we selected several ciliary regulators as candidates [28, 30–34]. We then examined the protein expression levels of the candidate molecules by Western blot analysis. We found that the protein levels of cilia assembly regulators, such as CPAP, KATANIN, and IFT88, were reduced in control cells 18 h after serum addition, whereas the protein levels in the knockdown and MG132-treated cells were as high as or higher than those of control cells under serum deprivation (Fig. 5C, D).
It has been reported that the hTERT-RPE1 cells undergo cilia disassembly at two different time points, in early G1 and S phases, 2 and 18 h after serum re-addition, respectively [22, 35]. Therefore, in G1 phase we analyzed the protein levels of HEF1 and MPS1, which were believed to be important for the G0→G1 transition and cilia disassembly [36–38] (Supplementary Fig. S3D). Consistent with other studies, serum addition induced higher protein expression of HEF1 and MPS1 than controls under serum deprivation. However, the serum-induced increase of HEF1 and MPS1 protein levels in the UPS-dysregulated cells was greater than that of controls (Fig. 5E, F). In S phase, we analyzed the protein levels of AURKA and PLK1, which were known to play a role in cilia disassembly and be highly expressed 18 h after serum addition in the RPE1 cells [39]. We found that their protein levels in the UPS-deregulated cells were not appreciably different from those of controls, which were increased by serum re-addition (Supplementary Fig. S7A, B). Taken together, our data suggest that the UPS-dependent decay of the ciliary proteins is necessary for cilia resorption and cell cycle re-entry in G1 phase. Finally, these results imply a dual role of UPS in G1 phase: 1) Proteolysis of any suppressor of cilia disassembly regulators such as HEF1 and MPS1 to control G0→G1 transition; and 2) Proteolysis of cilia assembly regulators to control G1→S transitions.
The mechanisms to control expression of ciliary molecules regulate the cell cycle progression
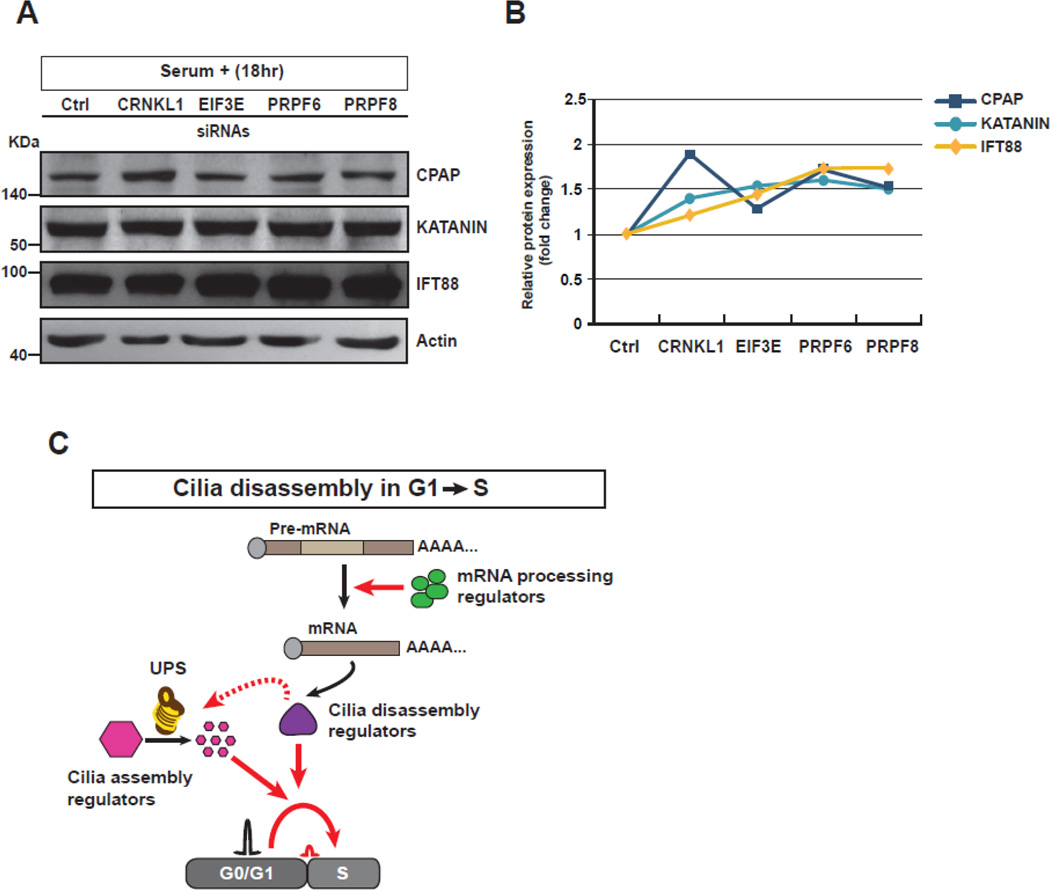
The similar effects of mRNA processing- and UPS-dysregulation on ciliogenesis and cell cycle progression prompted us to explore a functional relationship between these two mechanisms. Although the functional importance of the mRNA processing mechanism was in the regulation of expression of AURKA and PLK1, the protein levels were not affected by UPS deficiency 18 h after serum re-addition (Fig. 4C and Supplementary Fig. S7A, B). Furthermore, 2 h after cessation of serum starvation, when AURKA and PLK1 were normally activated for cilia disassembly [37], no obvious change of their protein levels was observed (Supplementary Fig. S7C, D). The results suggested that the activity of cilia disassembly regulators such as AURKA and PLK1 than their expression is required to link ciliary resorption to the G1→S transition. Alternatively, we hypothesized that cilia disassembly regulators, activated by the mRNA processing mechanism, might be required to promote UPS-dependent proteolysis of cilia assembly regulators. To address this, we examined the expression levels of cilia assembly proteins such as CPAP, KATANIN, and IFT88 in the mRNA processing hit-depleted SEMG cells, and found that the protein levels (especially IFT88) in the knockdown cells were higher than those of controls 18 h after serum re-addition (Fig. 6A, B). In conclusion, these data suggest that the mRNA processing mechanism may be required for the function of UPS to break down cilia assembly regulators in the G1→S transition of cell cycle re-entry, thereby implying a possible role of cilia disassembly regulators in suppressing the activity of cilia assembly regulators (Fig. 6C).
Figure 6. A proposed model for the role of screen hits in a link between ciliogenesis and cell cycle progression.
A. The protein levels of cilia assembly regulators including CPAP, KATANIN, and IFT88 in the mRNA processing-deficient cells were compared to those of control cells by western blot assay. The protein levels of knockdown cells were higher than those of control cells 18 h after cessation of serum starvation. B. The fold change of protein expression levels of cilia assembly regulators were quantified. C. The schematic diagram shows possible roles of screen hits including mRNA processing and UPS regulators in cilia disassembly and G1-S transition.
Discussion
The ciliary dynamics undergoing assembly and disassembly during cell cycle progression is the subject of a long-standing debate regarding whether the primary cilium functions as a cell cycle checkpoint. The mechanism governing the link between ciliogenesis and cell cycle progression has been poorly understood. In the present study, we used a whole-genome RNAi screening to uncover the underlying mechanism that connects ciliogenesis to cell cycle progression. The machineries associated with mRNA processing and the UPS were identified as the screen hits, and follow-up functional assays suggested that the screen hits are required for ciliary resorption and the G1→S transition in cell cycle re-entry. In particular, we demonstrated that the mRNA processing-mediated expression/activity of cilia disassembly regulators and the UPS-dependent proteolysis of cilia assembly regulators are essential for a link between ciliary biogenesis and cell cycle control.
It has recently been suggested that UPS is one of the core mechanisms in regulating ciliogenesis as well as cell cycle progression. For example, cilia-related roles of Anaphase-Promoting Complex (APC), which is a well-known ubiquitin E3 ligase in cell cycle control, have been suggested [40]. To induce cilia disassembly, APC/CCDC20 is activated when cells exit from the quiescent state [41]. In contrast, APC/CCDH1 is necessary for cilia assembly by ensuring the optimal level of ciliary proteins, such as Dishevelled, are present when cells enter the quiescent state [42]. Along with APCs, Cul3-RING ubiquitin ligases are involved in governing ciliogenesis by targeting ciliary proteins, such as Trichoplein [13]. Although these data have indicated the importance of UPS in the dynamic profile of cilia, the role of the proteasome complex in ciliogenesis has been poorly studied. Nevertheless, MG132-treated SEMG cells showed both defects in cilia assembly and disassembly, implying a definite function of the proteasome in ciliary dynamics (Supplementary Fig. S8). Furthermore, it has been recently reported that several subunits of the proteasome are localized at the base of cilia, and their activity is essential for cilia-mediate signal transduction [43]. Therefore, our findings not only support the previous results showing that UPS is necessary for cilia biogenesis but also contribute to a comprehensive understanding of the role of the proteasome in governing a connection between ciliogenesis and cell cycle progression.
Although it has been widely accepted that the cilium is disassembled in two waves, in the G1 and S phases, in RPE1 cells, the regulatory mechanism has been unknown [22, 35]. In our study, abnormally increased levels of HEF1, MPS1, and cilia assembly regulators, such as CPAP, KATATIN, and IFT88 in the G1 phase inhibited cilia disassembly and cell cycle re-entry in the UPS deficient cells (Fig. 5). Therefore, these results implied that cilia assembly regulators might suppress HEF1 and MPS1; thus, the failed decay of cilia assembly proteins might interfere with the role of HEF1 and MPS1 that control cilia disassembly and cell cycle re-entry. Therefore, our findings suggest that the UPS-mediated proteolysis of cilia assembly regulators is a stimulatory mechanism of ciliary resorption in the G1 phase.
Because mechanisms to regulate ciliogenesis and cell cycle have been associated with several diseases, including cancer, our results have prompted us to explore an involvement of the screen hits in cancer. We therefore analyzed whole-exome sequencing data including public databases and our own samples from several types of cancers and found considerable mutation frequencies in various cancers for most of the hit genes (Supplementary Fig. S9). In conclusion, our findings suggest that a better understanding of mRNA processing- and UPS-dependent mechanisms to connect ciliogenesis to the cell cycle progression may help to identify new therapeutic targets in cancers.
Methods
Whole-genome siRNA library screening
The primary screening
An arrayed library containing pooled siRNAs targeting 18,055 human genes (GE Healthcare Dharmacon Inc.) was screened in duplicate. Assay plates (384-well plates with an optical bottoms; Greiner) were spotted with 1 µl of 0.5 µM siRNA using the Velocity 11-Bravo Pipettor with a 384 ST head. Reverse transfection was performed using Lipofectamine RNAiMAX with a final siRNA concentration of 10 nM. SEMG cells were resuspended in DMEM/F12 supplemented with 10% FBS and seeded in assay plates using the Matrix-Well Mate (2,000 cells in 40 µl medium in each well). The culture medium was replaced with DMEM 24 h after the transfection using the TiterTek-MAP-C, and the cells were incubated for another 48 h before fixation.
Imaging and image analysis
Imaging related to the siRNA screening was performed on the Opera QEHS system (Perkin Elmer). Six fields were photographed in each well at wavelengths of three dyes: DAPI, Cy3, and Cy5. Acapella 2.0 software (Perkin Elmer) was used to perform image segmentation and cytometry with algorithms similar to those previously described by Kim et al. [44]. Plate-to-plate variability was normalized using a control-based method; the associated control samples were aggregated, and the mean and variance across the wells were determined. The Z-score for all wells with a siRNA knockdown was calculated using the mean and variance from the control data, and the final results were obtained using a threshold of more than two standard deviations (>2 SD). Additional invalid data that were ascribed to cell toxicity or out-of-focus images were excluded through manual inspection using the CyteSeer 2.7 software.
Confirmation screening
During this procedure, re-arrayed siRNAs targeting a total of 1,481 genes (Group A hits from the primary screening) were tested in triplicate. SEMG cells (1,300 cells for the serum-containing medium and 1,850 cells for the serum-free medium in 40 µl of the medium for each well) were transfected and incubated with or without serum for 24 h before fixation. The confirmation screening was based on measurement of the percentage of ciliated cycling cells under serum-free conditions. For plate normalization, the quantification data were converted to a Z-score using unique non-targeting siRNAs included in each plate as reference data points. Genes were defined as confirmed screen hits if the genes selected with a cut-off of >2 SD were subsequently validated by manual image analysis in the CyteSeer 2.7 software.
Hit classification
To classify the primary screen hits, the apparent knockdown phenotypes were compared to those of control cells with cilia by maintaining the quiescent state via serum starvation. Three groups were distinguished according to changed ciliogenesis and/or cell cycle features on the basis of measurement of the percentage of ciliated or cycling cells: Group A, formation of cilia in cycling cells; Group B, reduction of cilia in quiescent cells; and Group C, decreased number of ciliated cells and increased number of proliferating cells in the condition of quiescence.
Cell culture
hTERT-RPE1 cells were cultured in the DMEM/F12 medium supplemented with 10% FBS under standard conditions (37°C, 5% CO2). Plasmid DNAs carrying mouse Smo-EGFP and mCherry-geminin (1–110) fusion genes were transfected into hTERTRPE1 cells, and the stable cell line Smo-EGFP-mCherry-Geminin/hTERT-RPE1 (SEMG), was established via G418 selection. To induce ciliogenesis, the cells were serum-starved in the serum-free DEMEM/F12 medium for 24–48 h before fixation.
siRNA reagents
A Dharmacon human ON-TARGET-PLUS pooled siRNA library (GE Healthcare Dharmacon Inc.) was used, in a standard 384-well assay plate with an optical bottom (Greiner) using the Velocity 11-Bravo Pipettor with a 384 ST head. Each gene was targeted using a pool of four duplexes per well, and 18,055 genes of the entire library targeted a total of 18,301 human genes in the initial screening procedure. The pooled siRNAs targeting KIF3A were used as a positive control for the transfection, and AURKA, NEK1, BUB1B, and OFD1 siRNAs were included in the confirmation screening plates as positive controls for effects on ciliogenesis-coupled cell cycle. For functional studies, individual oligos of Dharmacon ON-TARGET-PLUS siRNAs were used at the final concentration of 20 nM.
siRNA transfections
Reverse transfection procedures were set up using Lipofectamine® RNAiMAX (Invitrogen). The final siRNA concentration was 20 nM, and SEMG cells were resuspended in DMEM/F12 supplemented with 10% FBS and then seeded on Lab-Tek™ 8-well chamber slides (Nunc). The siRNAs were diluted in Transfectagro™ (Corning) and mixed with Lipofectamine® RNAiMAX in Transfectagro™ at the 1:1 ratio by volume. The medium was refreshed for the transfected cells after 6 h of incubation, and the cells were assayed after 48–72 h, according to the experimental conditions.
Spliceostatin A (SSA) treatment
SSA (AdooQ Bioscience, cat. # A12700), which as dissolved in 0.1% dimethyl sulfoxide (DMSO) was diluted in a cell culture media (DMEM/F12 supplemented with 10% FBS, 1% penicillin streptomycin). The cells, plated on Lab-Tek™ 8-well chamber slides (Nunc) were treated with 50 ng/ml of SSA for 24 h.
Image analysis
After the siRNA transfection, the SEMG cells at 90–100% confluence were fixed with 4% paraformaldehyde (PFA) and stained with DAPI. To monitor the primary cilia and cell cycle state, we used an LSM700 confocal microscope (Carl Zeiss). The total numbers of ciliated cells (green) and cycling cells expressing Geminin (red) were counted using the Image J software (NIH) and were analyzed using one-way analysis of variance (ANOVA) followed by independent Tukey’s post hoc test using SigmaPlot 12 for at least three independent experiments.
Cell cycle analysis
siRNA transfection was performed on SEMG cells seeded in 100 mm dishes and grown to approximately 70% confluence. The cells were trypsinized and fixed in 70% ethanol, followed by incubation with 100 g/ml RNase A (Mentos) for 15 min at room temperature. To determine cell cycle state of each cell, the cells were incubated with 50 g/ml propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at room temperature, and the DNA contents were analyzed by FACS analysis using a BD FACSVerse™ flow cytometer and BD FACSuite software (Becton Dickinson). The statistical analysis involved one-way ANOVA followed by an independent Tukey’s post hoc test using SigmaPlot 12 for at least three independent experiments.
Whole-transcriptome sequencing
SEMG cells were cultured in 6-well plates (Corning) in a serum-containing (FBS 10%) or serum-free (FBS 0%) medium after siRNA transfection and were harvested 72 h later. The cells were washed with PBS, and total RNA was extracted using the RNA extraction kit (Qiagen). RNA was tested for quality and yield using a NanoDrop 1000 spectrophotometer and an Agilent 2100 Bioanalyzer. cDNA was synthesized from the total RNA according to the standard protocol of Illumina Inc. for highthroughput sequencing, and the transcriptome was sequenced using Illumina Hiseq2000. The reads from the FASTQ files were mapped against the hg19 human reference genome using TopHat version 2.0.6 (http://tophat.cbcb.umd/edu/). The output files in BAM format were analyzed in Cufflinks version 2.1.1 (http://cufflinks.cbcb.umd.edu/) to estimate the transcript abundance and assemble transcripts. The transcript assembly was compared with reference annotation Ensemble GTF in the Cuffcompare software, and the differentially expressed genes were extracted using Cuffdiff with the following criteria: q-value <0.05 and a 1.5-fold change. The heatmap of genes with expression levels significantly different between control and siRNA knockdown groups was generated using the heatmap.2 feature of the gplots R package (http://CRAN.R-project.org/package=gplots), with the mRNA abundance expressed in FPKM (fragments per kilobase of exon per million reads mapped).
Quantitative real-time PCR
The reaction of reverse transcription (RT) with oligo[dT]20 primers (Invitrogen) and 1 µg of total RNA as a template was performed using SuperScript III reverse transcriptase (Invitrogen). The RT-generated cDNAs were used as the templates, and the Power SYBR Green PCR Master Mix (Applied Biosystems) and primers designed for each gene (listed below) were added to each reaction mixture for qRT-PCR. To prevent formation of primer dimers, PCR conditions were optimized, and the results were subsequently confirmed by analyzing amplimer dissociation curves after quantitative PCR. The qRT-PCR reactions were conducted using a 7300 Real-Time PCR System (Applied Biosystems); a constitutively expressed housekeeping gene, GAPDH, served as the biomass reference. The following primers were used for the qRT-PCR:
CCNB1_F, 5’- ACATGGTGCACTTTCCTCCT- 3’
CCNB1_R, 5’-AGGTAATGTTGTAGAGTTGGTGTCC- 3’
NEK2_F, 5’- CATTGGCACAGGCTCCTAC- 3’
NEK2_R, 5’-TGGAGCCATAGTCAAGTTCTTTC- 3’
AURKA_F, 5’- GCAGATTTTGGGTGGTCAGT- 3’
AURKA_R, 5’-TAGTCCAGGGTGCCACAGA- 3’
PLK1_F, 5’-CACAGTGTCAATGCCTCCA- 3’
PLK1_R, 5’-TTGCTGACCCAGAAGATGG- 3’
Western blotting
The proteins of SEMG cells were separated on 10% SDS-PAGE gels (we loaded 30 µg of protein per lane), followed by transfer onto nitrocellulose membranes (GE Healthcare). After incubation in blocking buffer (5% skim milk in TBST [Tris-buffered saline pH7.5 with 0.5% Tween-20]) for 1 h at room temperature, membranes were blotted with a mouse anti-Aurora A (cat. #12100, Cell Signaling), rabbit anti-CPAP (cat. #11517-I-AP, Proteintech), rabbit anti-IFT88 (cat. #13967-I-AP, Proteintech), mouse anti-MPS1 (cat. #ab11108, Abcam), mouse anti-PLK1 (cat. #ab17057, Abcam), mouse anti-HEF1 (cat. #ab18056, Abcam), mouse anti-beta-Actin (C4) (cat. #sc-47778, Santa Cruz Biotechnology), and mouse anti-Katanin p60 (cat. #sc-373814, Santa Cruz Biogechnology) antibodies overnight at 4 °C. The membranes were then washed two times with TBST and incubated with secondary antibodies: a goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated antibody (cat. #sc-2031, Santa Cruz Biotechnology) and a goat anti-rabbit IgG HRP-conjugated antibody (cat. #sc-2030, Santa Cruz) for 1 h at room temperature. After washing the membranes two times with TBST, we visualized the bands using the ECL detection system (Bio-Rad).
In vivo experiments
Zebrafish housing and manipulations
Adult zebrafish were maintained at 28.5 °C and pH 7.0–7.9 with a cycle of light (13 h) and dark (11 h) per day in the automatic system (Constructed by Genomic-Design, Korea). The zebrafish embryos were collected by natural breeding and incubated in the E3 medium (297.7 mM NaCl, 10.7 mM KCl, 26.1 mM CaCl2, and 24.1 mM MgCl2) containing 1% methylene blue (Sigma-Aldrich) at 28.5 °C. To inhibit formation of melanin, we raised the zebrafish larvae (after 24 hpf) in the E3 medium containing 0.2 mM N-phenylthiourea (PTU; Sigma- Aldrich Chemistry, cat. # P7629).
Microinjection with morpholino oligos (MOs)
Translation-blocking MOs were designed and synthesized by Gene Tools (Philomath, OR 97370, USA). Each MOs were diluted in distilled water at a concentration of 4–5 µg/µl and then injected into the yolk of zebrafish embryos at one to four cell stages using a gas-used microinjection system (PV83 Pneumatic PicoPump, SYS-PV830, World Precision Instruments, USA). The following MO sequences were used for the microinjection:
prpf8 ATG-MO, 5’-AAAGCAGTTTCTCGCCAGTGACTGT-3’
psmd8 ATG-MO, 5’-CAGTCTCTTTCAACACAGACGCCAT-3’
control MO, 5’-CCTCTTACCTCAGTTACAATTTATA-3’.
Microinjection with mRNA
The full-length zebrafish psmd8 was amplified by RT-PCR using zebrafish cDNA, which was synthesized with AccuPower® Rocket Script RT Premix (K-2101, Bioneer, Korea) from total RNA, extracted from zebrafish embryos at 24hpf using the Accuzol™ Total RNA Extraction Reagent (K-3090, Bioneer, Korea). The zebrafish psmd8 cDNA was subcloned into pCS2+ (Clontech) and the Capped mRNA was synthesized using the mMESSAGE mMACHINE® SP6 Kit (AM1340, Thermo Fisher Scientific). The zebrafish psmd8 mRNA (200 pg/nl) was injected into the psmd8 MO-injected embryos. The following primer sequences were used for the RT-PCR:
Forward primer, 5’-CCGGAATTCATGGGCGTCTTGTTGAAAGAG-3’,
Reverse primer, 5’-GCGCGTCTAGACTACACAATCATTTCCAGCTGTCTTGC-3’.
Whole-mount immunostaining of zebrafish larvae
To induce ciliary defects, we treated zebrafish embryos with either 40 µM SSA (AdooQ Bioscience, cat. # A12700) or 50 µM MG132 (Calbiochem, cat. # 474790) at 24 hpf (Supplementary Fig. S3B), and the control embryos were treated with 1% DMSO. The drug-treated embryos were raised at 28.5 °C until 3 days postfertilization (dpf) and fixed in Dent’s fixative (80% MeOH, 20% DMSO) at room temperature for 3 h. The fixed zebrafish larvae were immunostained with an anti- GT335 antibody (AdipoGen, cat. # A20631002; 1:400 dilution) as a primary antibody at 4 °C overnight and with a goat anti-mouse Alexa Fluor® 488-conjugated antibody (Invitrogen, cat. # A11001, 1:1000) as a secondary antibody and 1X DAPI (Molecular probes, cat. #D3571) at room temperature for 2 h in a half-diluted blocking solution (10% normal goat serum, 0.5% Tween 20 in PBS). The fluorescent images were obtained using a LSM700 confocal microscope (Carl Zeiss).
Supplementary Material
Highlights.
Genome-wide siRNA screen identifies the mechanism to connect ciliogenesis with cell cycle
mRNA processing and UPS are key mechanisms to correlate cilia disassembly and cell cycle re-entry
Expression of cilia disassembly regulators by mRNA processing mechanism is pivotal for G1-S transition
UPS-dependent proteolysis of cilia assembly regulators is required for G1-S transition
Acknowledgments
We thank J.-S. Lee (Korea Research Institute of Bioscience and Biotechnology) for Tg(flk1:EGFP) zebrafish; and E.-J. Choi (Korea University) and H.-Y. Yoo (Sungkyunkwan University) for supplying several antibodies and scientific comments. This work was supported by the Samsung Medical Center (CRP1500082 to J.E.L.) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2013R1A1A1059056 to J.E.L.), the US National Institutes of Health (NS052455 to J.G.G.). J.G.G. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
J.H.K. and S.M.K. performed molecular biological and cell experiments and the in vivo experiments on zebrafish. J.K. designed and performed the primary siRNA screening, with assistance from P.A.-B. on screening and from S. H.-G. on scoring. E.S. performed statistical analysis of the screening results. J.E.L. and J.G.G. wrote the manuscript and designed the experiments with substantial contributions from J.-G.J. and C.H.K.
Competing Financial Interests
The authors declare that they have no competing financial interests.
References
- 1.Kim S, Dynlacht BD. Assembling a primary cilium. Current opinion in cell biology. 2013;25:506–511. doi: 10.1016/j.ceb.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Current opinion in genetics & development. 2013;23:429–437. doi: 10.1016/j.gde.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nature reviews. Molecular cell biology. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 4.Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nature cell biology. 2013;15:1387–1397. doi: 10.1038/ncb2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke YN, Yang WX. Primary cilium: an elaborate structure that blocks cell division? Gene. 2014;547:175–185. doi: 10.1016/j.gene.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle. 2011;10:2683–2690. doi: 10.4161/cc.10.16.17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nature cell biology. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto H, Inoko A, Inagaki M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cellular and molecular life sciences : CMLS. 2013;70:3893–3905. doi: 10.1007/s00018-013-1302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J, Seeger-Nukpezah T, Golemis EA. The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cellular and molecular life sciences : CMLS. 2013;70:1849–1874. doi: 10.1007/s00018-012-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nature cell biology. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nature cell biology. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, Zhong Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502:254–257. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasahara K, Kawakami Y, Kiyono T, Yonemura S, Kawamura Y, Era S, Matsuzaki F, Goshima N, Inagaki M. Ubiquitin-proteasome system controls ciliogenesis at the initial step of axoneme extension. Nature communications. 2014;5:5081. doi: 10.1038/ncomms6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roosing S, Hofree M, Kim S, Scott E, Copeland B, Romani M, Silhavy JL, Rosti RO, Schroth J, Mazza T, Miccinilli E, Zaki MS, Swoboda KJ, Milisa-Drautz J, Dobyns WB, Mikati MA, Incecik F, Azam M, Borgatti R, Romaniello R, Boustany RM, Clericuzio CL, D'Arrigo S, Stromme P, Boltshauser E, Stanzial F, Mirabelli-Badenier M, Moroni I, Bertini E, Emma F, Steinlin M, Hildebrandt F, Johnson CA, Freilinger M, Vaux KK, Gabriel SB, Aza-Blanc P, Heynen-Genel S, Ideker T, Dynlacht BD, Lee JE, Valente EM, Kim J, Gleeson JG. Functional genome-wide siRNA screen identifies KIAA0586 as mutated in Joubert syndrome. eLife. 2015;4:e06602. doi: 10.7554/eLife.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 16.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 17.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 18.Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q. GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome biology. 2008;9(Suppl 1):S4. doi: 10.1186/gb-2008-9-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrionero A, Minana B, Valcarcel J. Reduced fidelity of branch point recognition and alternative splicing induced by the anti-tumor drug spliceostatin A. Genes & development. 2011;25:445–459. doi: 10.1101/gad.2014311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, Schlossman AM, Merriman B, Attie-Bitach T, Logan CV, Glass IA, Cluckey A, Louie CM, Lee JH, Raynes HR, Rapin I, Castroviejo IP, Setou M, Barbot C, Boltshauser E, Nelson SF, Hildebrandt F, Johnson CA, Doherty DA, Valente EM, Gleeson JG. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nature genetics. 2012;44:193–199. doi: 10.1038/ng.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Current biology : CB. 2004;14:685–690. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Spalluto C, Wilson DI, Hearn T. Evidence for reciliation of RPE1 cells in late G1 phase, and ciliary localisation of cyclin B1. FEBS open bio. 2013;3:334–340. doi: 10.1016/j.fob.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchsbaum S, Morris C, Bochard V, Jalinot P. Human INT6 interacts with MCM7 and regulates its stability during S phase of the cell cycle. Oncogene. 2007;26:5132–5144. doi: 10.1038/sj.onc.1210314. [DOI] [PubMed] [Google Scholar]
- 24.Morris C, Jalinot P. Silencing of human Int-6 impairs mitosis progression and inhibits cyclin B-Cdk1 activation. Oncogene. 2005;24:1203–1211. doi: 10.1038/sj.onc.1208268. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Endler A, Uchida K, Horiguchi S, Morizane Y, Iijima O, Toi M, Shibasaki F. Int6/eIF3e silencing promotes functional blood vessel outgrowth and enhances wound healing by upregulating hypoxia-induced factor 2alpha expression. Circulation. 2010;122:910–919. doi: 10.1161/CIRCULATIONAHA.109.931931. [DOI] [PubMed] [Google Scholar]
- 26.Gupta SK, Carmi S, Waldman Ben-Asher H, Tkacz ID, Naboishchikov I, Michaeli S. Basal splicing factors regulate the stability of mature mRNAs in trypanosomes. The Journal of biological chemistry. 2013;288:4991–5006. doi: 10.1074/jbc.M112.416578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoko A, Matsuyama M, Goto H, Ohmuro-Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura S, Kiyono T, Izawa I, Inagaki M. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. The Journal of cell biology. 2012;197:391–405. doi: 10.1083/jcb.201106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KH, Johmura Y, Yu LR, Park JE, Gao Y, Bang JK, Zhou M, Veenstra TD, Yeon Kim B, Lee KS. Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. The EMBO journal. 2012;31:3104–3117. doi: 10.1038/emboj.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spalluto C, Wilson DI, Hearn T. Nek2 localises to the distal portion of the mother centriole/basal body and is required for timely cilium disassembly at the G2/M transition. European journal of cell biology. 2012;91:675–686. doi: 10.1016/j.ejcb.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, van Dyk D, Choe V, Yan J, Majumder S, Costanzo M, Bao X, Boone C, Huo K, Winey M, Fisk H, Andrews B, Rao H. Ubiquitin ligase Ufd2 is required for efficient degradation of Mps1 kinase. The Journal of biological chemistry. 2011;286:43660–43667. doi: 10.1074/jbc.M111.286229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck J, Maerki S, Posch M, Metzger T, Persaud A, Scheel H, Hofmann K, Rotin D, Pedrioli P, Swedlow JR, Peter M, Sumara I. Ubiquitylation-dependent localization of PLK1 in mitosis. Nature cell biology. 2013;15:430–439. doi: 10.1038/ncb2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang CJ, Fu RH, Wu KS, Hsu WB, Tang TK. CPAP is a cell-cycle regulated protein that controls centriole length. Nature cell biology. 2009;11:825–831. doi: 10.1038/ncb1889. [DOI] [PubMed] [Google Scholar]
- 33.Pletz N, Medack A, Riess EM, Yang K, Kazerouni ZB, Huber D, Hoyer-Fender S. Transcriptional activation of Odf2/Cenexin by cell cycle arrest and the stress activated signaling pathway (JNK pathway) Biochimica et biophysica acta. 2013;1833:1338–1346. doi: 10.1016/j.bbamcr.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Maddika S, Chen J. Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nature cell biology. 2009;11:409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. Journal of cell science. 2010;123:511–518. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugacheva EN, Golemis EA. HEF1-aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5:384–391. doi: 10.4161/cc.5.4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumder S, Fisk HA. VDAC3 and Mps1 negatively regulate ciliogenesis. Cell Cycle. 2013;12:849–858. doi: 10.4161/cc.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G, Chen Q, Zhang X, Zhang B, Zhuo X, Liu J, Jiang Q, Zhang C. PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry. Journal of cell science. 2013;126:1355–1365. doi: 10.1242/jcs.114918. [DOI] [PubMed] [Google Scholar]
- 40.Fujita T, Liu W, Doihara H, Wan Y. An in vivo study of Cdh1/APC in breast cancer formation. International journal of cancer. Journal international du cancer. 2009;125:826–836. doi: 10.1002/ijc.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Wu T, Kirschner MW. The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium. eLife. 2014;3:e03083. doi: 10.7554/eLife.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganner A, Lienkamp S, Schafer T, Romaker D, Wegierski T, Park TJ, Spreitzer S, Simons M, Gloy J, Kim E, Wallingford JB, Walz G. Regulation of ciliary polarity by the APC/C. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17799–17804. doi: 10.1073/pnas.0909465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerhardt C, Lier JM, Burmuhl S, Struchtrup A, Deutschmann K, Vetter M, Leu T, Reeg S, Grune T, Ruther U. The transition zone protein Rpgrip1l regulates proteasomal activity at the primary cilium. The Journal of cell biology. 2015;210:115–133. doi: 10.1083/jcb.201408060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.