Abstract
The study objective was to construct and validate a subject-specific knee model that can simulate full six degree of freedom tibiofemoral and patellofemoral joint behavior in the context of full body movement. Segmented MR images were used to reconstruct the geometry of 14 ligament bundles and articular cartilage surfaces. The knee was incorporated into a lower extremity musculoskeletal model, which was then used to simulate laxity tests, passive knee flexion, active knee flexion, and human walking. Simulated passive and active knee kinematics were shown to be consistent with subject-specific measures obtained via dynamic MRI. Anterior tibial translation and internal tibial rotation exhibited the greatest variability when uncertainties in ligament properties were considered. When used to simulate walking, the model predicted knee kinematic patterns that differed substantially from passive joint behavior. Predictions of mean knee cartilage contact pressures during normal gait reached 6.2 and 2.8 MPa on the medial tibial plateau and patellar facets, respectively. Thus, the dynamic modeling framework can be used to simulate the interaction of soft tissue loads and cartilage contact during locomotion activities, and therefore provides a basis to simulate the effects of soft tissue injury and surgical treatment on functional knee mechanics.
Keywords: Knee mechanics, Gait, Computational biomechanics, Elastic foundation model
INTRODUCTION
Dynamic musculoskeletal models provide a consistent framework to simulate soft tissue loads and joint kinematics in locomotor activities. For example, prior studies have used dynamic simulations to investigate muscular contributions to tibiofemoral loads in walking,47 and the influence of quadriceps coordination on patellofemoral joint force in running.38 However, most current gait models use a highly simplified representation of the knee. Specifically, the knee is often represented as a one degree of freedom joint, in which patellofemoral kinematics and secondary tibiofemoral kinematics are constrained functions of knee flexion.4,16 There are two fundamental limitations with this approach. First, a one degree of freedom knee joint does not provide estimates of the ligament and cartilage loading patterns that act to constrain secondary joint motion. Second, joint motion constraints are often assumed based on the passive behavior of cadaveric joints,4,16,49,50,52 which intrinsically ignores load-dependent variations in joint kinematics.9,18,26,30,33,51 Loading effects are likely very relevant during locomotion activities, where secondary tibiofemoral kinematics vary substantially over the gait cycle.18,30
Anatomically detailed knee models have been introduced that allow for full six degree of freedom (DOF) kinematics at the tibiofemoral and patellofemoral joints. Finite element (FE) formulations are attractive since they provide for estimates of internal tissue stresses and strains.17 However, FE models remain computationally challenging to solve within the context of a multibody simulation of gait.24 Dynamic multi-body knee models provide a viable alternative for simulating gait, where elastic ligament bundles can capture overall joint laxity11,44,45,48 and elastic foundation models can provide estimates of cartilage contact pressure that are comparable to FE predictions.25 However, computing coordinated muscle actions needed to control six DOF joints remains challenging. We recently introduced a modified computed muscle control (CMC) algorithm that can modulate muscle excitations to track knee flexion, while secondary knee motions evolve naturally due to muscle, ligament, cartilage contact, and external loading.48 While we have shown that the modified CMC framework can accurately simulate tibiofemoral contact loads in a total knee replacement,48 the capacity to simulate natural knee behavior has not yet been demonstrated.
The purpose of this study was to develop and validate a subject-specific, multi-body knee model that allows for six DOF motion at both the tibiofemoral and patellofemoral joints. We first used high resolution magnetic resonance (MR) images to create a knee model that included ligamentous constraints and articular cartilage contact. We then compared model predictions of tibiofemoral and patellofemoral kinematics with dynamic MRI measures obtained under passive and active loading conditions.3,28 Probabilistic simulations were performed to assess the propagation of uncertain ligament properties onto knee kinematics. Finally, we used the model to simulate secondary knee kinematics in walking, and compared the results to those obtained via conventional gait models.
MATERIALS AND METHODS
Participant Information
MR imaging and gait analysis were performed on a 23 year old female subject (height = 1.65 m, mass = 61 kg) with no history of chronic knee pain, injury, or surgery. The study was approved by the University of Wisconsin-Madison’s Health Sciences Institutional Review Board, and appropriate written informed consent was obtained prior to testing.
MR Imaging
The subject underwent a MR examination in a clinical 3.0-T MR scanner (MR750, General Electric Healthcare, Waukesha, WI). An eight-channel phased-array extremity coil (Precision Eight TX/pulse repetition time (TR) High Resolution Knee Array; Invivo, Orlando, FL) was positioned about her dominant (right) knee in an extended posture. Two high-resolution static scans were performed, which included a 3D IDEAL19 spoiled gradient-echo (SPGR) sequence (256 × 256 × 108 cubic voxels with 0.78 mm spacing) and a fast-spin echo (FSE) cube sequence (512 × 512 × 392 cubic voxels with 0.30 mm spacing).
We manually segmented (MIMICS, Materialise Group, Leuven, Belgium) the bone geometries from the static IDEAL-SPGR images, and the articular cartilage surfaces and knee ligaments from the FSE Cube images. Bone geometries were smoothed (<0.1 mm average deviation from original, unsmoothed model) and decimated to 7000 triangles for the femur and tibia and to 2000 triangles for the patella (Geomagic, Research Triangle Park, NC). Cartilage surface geometries were likewise smoothed (<0.02 mm average deviation from original, unsmoothed model) and decimated to 4000, 2000, and 2000 triangles for the femoral, tibial, and patellar cartilages, respectively.
Anatomical reference frame orientations were established for each bone using a automatic algorithm based on geometric and inertial properties of the 3D segments.37,41 The medial–lateral axis of femur was aligned with the centerline of a best-fit cylinder to the condyles and the superior-inferior axis is based off the smallest inertial axis of the diaphysis. The tibial axes were defined as the first, second, and third inertial axes of the plateaus. The anterior–posterior axis of the patella was defined as the principal axis corresponding with the smallest mass moment of inertia. The superior–inferior axis was aligned with the median ridge. The origins of the femoral and tibial reference frames were both placed at the center of the best-fit cylinder of the femoral condyles. The origin of the patellar reference frame was placed at the center of mass.
The subject was next positioned supine on a MR-compatible loading device46 with her dominant knee centered in a 16-channel flex coil (GEM Flex, NeoCoil, Pewaukee, WI) coil. The knee was cyclically flexed and extended under three conditions: voluntary motion against an inertial load which induced quadriceps loading with the knee flexed,28,51 voluntary motion against an elastic load which induced quadriceps loading with the knee extended,51 and a passive case in which a researcher cyclically flexed the subject’s relaxed leg. Inertial loading was applied via rotating disks and elastic loading was applied via a torsion spring as described in Westphal et al.51 Knee range of motion was limited by the MRI bore size (60 cm diameter), ranging from 36° in the passive task to 45° in the active tasks. Cyclic task rates (0.5 Hz) were maintained via an audible metronome, with repeatability enhanced by having the subjects practice the tasks prior to the MR examination. A dynamic SPGR sequence in conjunction with vastly-undersampled isotropic projections (SPGR-VIPR, 160 × 160 × 160 cubic voxels with 1.5 mm spacing, 75,000 unique lines, 5 min scan time) was used to continuously acquire volumetric image data over 150 motion cycles of each motion task.28
The dynamic images were used to track tibiofemoral and patellofemoral kinematics using methods that have been described previously.28 Briefly, an encoder measurement of knee angle was used to retrospectively sort SPGR-VIPR projections into 60 bins over the motion cycle. Sorted projections were used to reconstruct 60 volumetric image sets. Femur, tibia, and patella bone model geometries were then registered to each image set using numerical optimization.40 This resulted in 3D trajectories of the translations and rotation angles for the femur, tibia, and patella over the motion cycle, which were subsequently low-pass filtered (5 Hz cutoff frequency). Tibiofemoral kinematics were described by three translations and three successive body-fixed rotations (flexion, adduction, and internal rotation) of the tibia relative to the femur.22 Similarly, patellofemoral orientation was defined by successive body-fixed rotations consisting of flexion, lateral rotation, and medial tilt of the patella relative to the femur.
Knee Model
The following ligament volumes were constructed from the FSE Cube images: superficial and deep medial collateral ligament (sMCL, dMCL), lateral collateral ligament (LCL), anteriomedial and posteriolateral anterior cruciate ligament (aACL, pACL), anteriolateral and posteriomedial posterior cruciate ligament (aPCL, pPCL), patellar tendon (PT), medial and lateral patellofemoral ligaments (MPFL, LPFL), popliteofibular ligament (PFL), posteriomedial capsule (pmCAP), the posterior capsule (CAP), and the iliotibial band (ITB). Ligaments were smoothed and centerlines were calculated using the MIMICS Medcad module. Each ligament was represented by a bundle of non-linear springs spanning from the ligament origin footprint to its insertion footprint. Wrap objects were defined for the medial collateral ligament, posterior capsule, and patellar tendon so that they wrapped appropriately around the tibia, femur, and patella respectively.
Each ligament bundle was represented by a discrete number of strands. Tensile forces were computed assuming each strand behaved as a non-linear stiffening spring at low strains (ɛ < 0.06), and having a linear stiffness at higher strains.10 The linear stiffness of each ligament were estimated as the product of the average ligament cross-sectional area and an assumed ligament elastic modulus of 125 MPa.13 Ligament cross-sectional areas for the MPFL, LPFL, PFL, ITB, pmCAP, and CAP could not be well estimated from the images, such that the values for cross-sectional area or stiffness were adapted from other modeling and cadaveric studies.1,6,27,36,39,43,48 Ligament stiffness was equally portioned to all strands included in the bundle. The reference strains of each ligament strand in an extended knee posture were adapted from the literature.43,45,48 See the Supplemental Material for ligament properties used in this model.
Tibiofemoral and patellofemoral cartilage contact pressures were computed using an elastic foundation model in which pressure is assumed to be a function of the depth of penetration between contacting cartilage surfaces. Regions of cartilage contact were determined at each time step using ray-casting techniques together with hierarchical bounding boxes.48 At each triangle of the tibia plateau, the contact pressure was computed using a non-linear elastic foundation formulation,8 with cartilage assumed to have an elastic modulus of 5 MPa,10,12 a Poisson’s ratio of 0.45,5,10,12,45 and a uniform cartilage thickness of 3 mm over each surface (i.e., 6 mm total thickness).42
The knee model was incorporated into a generic lower extremity musculoskeletal model with a six DOF pelvis, a three DOF ball-in-socket representation of the hip, and a one DOF ankle allowing for dorsi/plantarflexion (Fig. 1).4 The model included 44 musculotendon units acting about the right hip, knee and ankle. To incorporate the knee model, the femoral skeletal and cartilage geometries were positioned to closely align with the femoral geometry in the generic lower extremity model. Tibial and patellar geometries were then positioned to just contact the femoral surfaces in an upright posture. The full model was implemented in SIMM,15 with the Dynamics Pipeline (Musculographics Inc., Santa Rosa, CA) and SD/Fast (Parametric Technology Corp., Needham, MA) used to generate code describing muscle–tendon dynamics and the multibody equations of motion.
FIGURE 1.
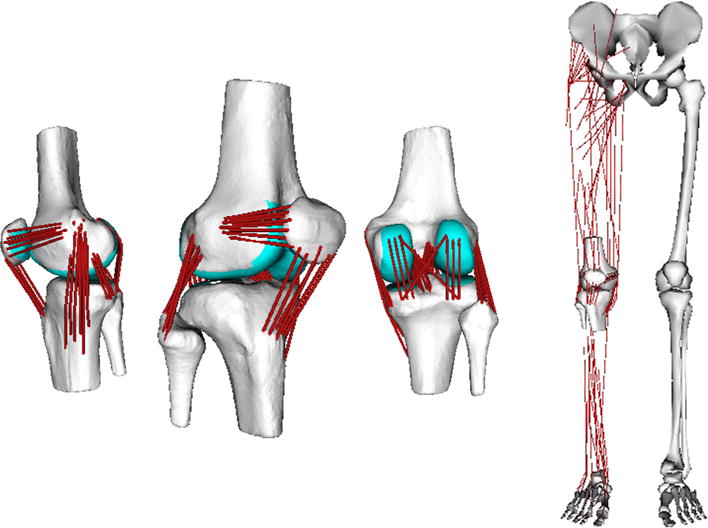
(Left) Ligament, skeletal and cartilage geometries were reconstructed from segmented MR images. The 12 degree of freedom knee model included 14 ligament bundles (76 elements) acting across the tibiofemoral and patellofemoral joints. (Right) The knee model was included into a generic lower extremity musculoskeletal model4 for simulating functional movement.
Dynamic Simulations
As a first assessment of veracity of the model, we simulated laxity tests and compared the resulting knee kinematics to those measured in cadaveric studies.2,20,21,35 In these tests, the femur was fixed and muscle activations were set to 0. Anterior–posterior (A–P) laxity was assessed by applying a ±100 N force on the tibia at a position 10 cm inferior to the tibial reference frame origin. Forward dynamics was used to simulate the resulting tibial and patellar motion. Rotational laxity was assessed by applying a ±5 Nm torque about the long axis of the tibia and using dynamic simulation to predict the resulting tibiofemoral rotations. Both A–P and rotational laxity was assessed at 10° increments of knee flexion between 0° and 90°.
Simulations were then performed to compare model predictions against the in vivo tibiofemoral and patellofemoral kinematic measured from the dynamic MRI experiments. For these simulations, we emulated the dynamic MRI setup by prescribing posterior pelvic tilt to 90° and hip flexion to 20°. The passive knee flexion task was then simulated by setting muscle activations to a low level (activation a = 0.01) and prescribing knee flexion to cycle between 0° and 50° of knee flexion at 0.5 Hz. The active loading cases were simulated by using a modified computed muscle control algorithm48 to compute muscle excitations needed to drive cyclic knee flexion against simulated inertial and elastic loads applied to the tibia. Applied tibia load magnitudes were set such that peak knee extensor moments of 30 Nm were generated, as was measured previously in the comparison dynamic MRI experiments.51 Ankle plantarflexion was also actively controlled to track a fixed value of 20°. The resulting tibiofemoral and patellofemoral kinematics were compared to both subject-specific and population51 measures obtained with dynamic MRI.
We performed Monte Carlo simulations (n = 2000) to evaluate the effects of uncertainty in ligament stiffness and reference strains on passive knee kinematics. For each simulation, ligament stiffness and reference strains were selected from a normal distribution with mean value set to the nominal model value. The standard deviation chosen for reference strain was 0.02 and for stiffness was 30% of the nominal ligament stiffness.7 Perturbations to reference strain and stiffness were independently assigned for each ligament bundle, but all strands within a bundle were prescribed the same parametric variations.
The subject underwent gait analysis during overground walking at a preferred speed. Whole body kinematics were recorded using an eight-camera, passive motion capture system (Motion Analysis, Santa Rosa, CA) to track 44 retroreflective markers, which included 25 markers on anatomical landmarks and 14 markers on rigid plates attached to the thigh and shank segments. The lower extremity model was scaled to the subject based on segment lengths determined in a standing upright posture. Pelvis, thigh, and shank reference frames were set using anatomical markers placed over the anterior superior iliac spine, posterior superior iliac spine, and the medial and lateral epicondyles and malleoli. A functional calibration routine was used to determine hip joint centers in the pelvis reference frame.31 During walking, marker kinematics were collected at 100 Hz and then low-pass filtered at 12 Hz. Ground reaction forces were simultaneously collected at 2000 Hz (forceplate model BP400600, AMTI, Watertown, MA) and then low-pass filtered at 50 Hz.
A global optimization inverse kinematics routine determined pelvis translations, pelvis rotations, hip angles, knee flexion and ankle dorsiflexion that best agreed with marker positions measured during gait.34 At this stage, secondary tibiofemoral and all patellofemoral DOF were assumed to be a constrained function of knee flexion, with these functions based on our simulated passive knee behavior.4 We then used a modified CMC algorithm to compute muscle excitations that drove the dynamic multi-body model to track measured hip kinematics, knee flexion and ankle dorsiflexion trajectories over a gait cycle.48 The pelvis coordinates were prescribed to reproduce measured values, and measured ground reaction forces were applied directly to the feet. It should be explicitly noted that only knee flexion was tracked in the gait simulation, with all other tibiofemoral and patellofemoral DOFs being allowed to evolve as a function of muscle and ligament forces and cartilage contact. We then compared simulated tibiofemoral and patellofemoral kinematics during gait to our passive knee joint behavior to better understand the importance of load-dependent effects.
RESULTS
Knee Laxity
The nominal model-predicted patterns of translational and rotational laxity were consistent with measures obtained on cadaveric specimens (Fig. 2). A 100 N anterior force induced tibial translations of 1–7 mm with a peak at 30° knee flexion, while a 100 N posterior force induced translations of 1–7 mm with a peak at 10° knee flexion. A 5 Nm internal rotation moment induced internal tibial rotation angles ranging from 10° to 22° across the 90° of knee flexion considered, while external rotation moments induced rotations of 9°–18°.
FIGURE 2.
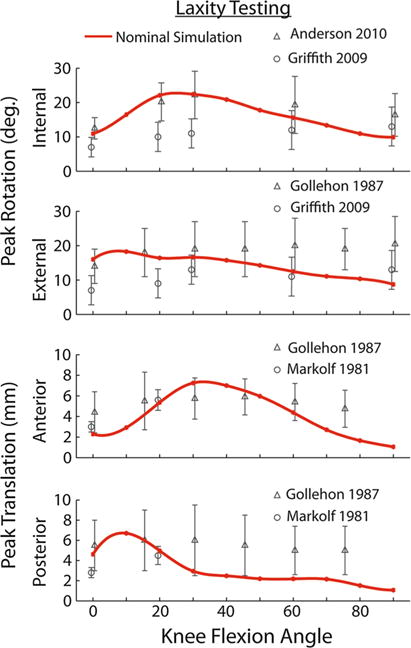
The passive knee model exhibited anterior-posterior translational laxity and internal-external rotation laxity that was consistent with prior cadaveric studies (symbols 5 mean, error bars 5 one standard deviation).2,20,21,35
Passive Knee Flexion–Extension
Dynamic MRI measurements of tibiofemoral and patellofemoral kinematics in the passive flexion–extension tasks were consistent with model predictions, generally falling within the simulated uncertainty range (Fig. 3). One exception was patellofemoral tilt, with the model predicting a small amount of medial tilt (1.8° on average) with tibiofemoral flexion, while the MRI results showed approximately 3.0° of lateral tilt. In addition, anterior patellar translation was slightly underpredicted relative to MRI measures. The nominal model did exhibit some kinematic hysteresis, with the kinematics being predicted during flexion and extension being slightly disparate for anterior tibial translation, internal tibial rotation and patellar flexion. Tibiofemoral translations, internal tibial rotation, superior patellar translation, and patellar flexion exhibited the greatest sensitivity to ligament properties. For example, one standard deviation in internal tibiofemoral rotation ranged from 1.5° to 5.8° over the knee flexion range of motion, while adduction varied only 0.6° to 1.1°.
FIGURE 3.
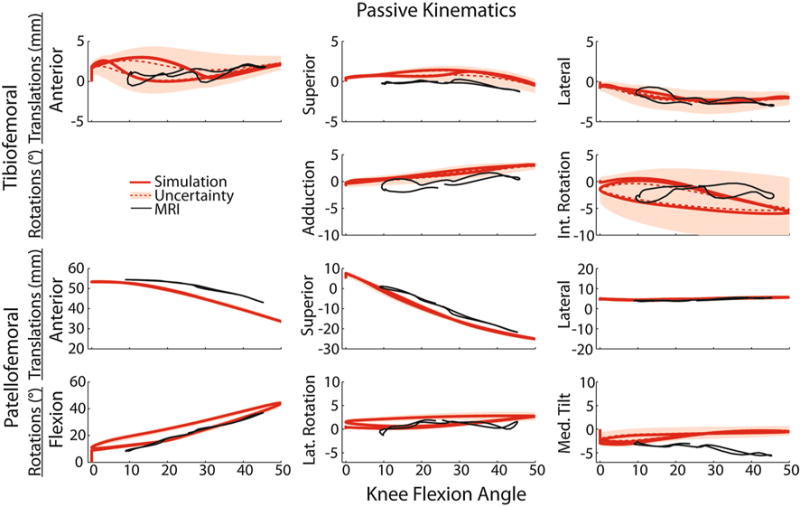
Forward dynamic simulations of passive knee flexion–extension (solid red line) compared with subject-specific dynamic MRI measurements (solid black line). Shaded regions represent Monte Carlo simulation results (red dotted line = mean; shaded region = one standard deviation) obtained by accounting for uncertainty in ligament stiffnesses and reference strains. The effect of ligament uncertainty was directionally dependent, with the greatest variability seen in tibiofemoral translations, internal tibial rotation and medial patellar tilt.
Inertial and Elastic Loading Conditions
Simulations of the elastic and inertial loading conditions produced distinct tibiofemoral and patellofemoral kinematic patterns (Fig. 4). For example, when quadriceps activity occurred at extended knee postures (i.e., in the elastic case), greater anterior tibial translation (2.2 mm), internal tibial rotation (5.1°) and superior patellar translation (5.2 mm) were predicted in the extended position. Inertial loading (i.e., quadriceps active when knee was flexed) induced greater anterior (6.2 mm) and inferior (3.3 mm) tibial translation in a flexed knee posture. At the patellofemoral joint, there was increased superior (5.2 mm) and lateral (1.0 mm) translation of the patella in the elastic case at maximum extension. In addition, there was patella flexion (7.2°), and increased anterior (4.0 mm) and superior (4.2 mm) translation at maximum knee flexion during the inertial case. These load-dependent kinematic differences generally agreed with both subject-specific and population-average51 dynamic MRI measurements. For example, average difference between our subject-specific MRI and simulated results over a similar range of motion were 0.6 mm of superior translation, 0.7 mm of lateral translation, and 1.5° of adduction.
FIGURE 4.
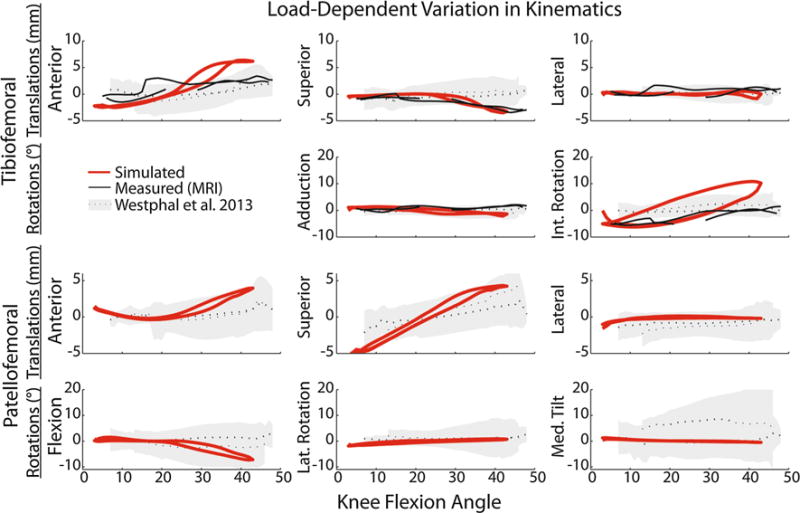
Tibiofemoral and patellofemoral kinematics were simulated and measured under inertial (peak quadriceps activity with flexion) and elastic (peak quadriceps activity with extension) loading conditions. Shown are the differences in kinematics observed between the inertial and elastic loading conditions, as predicted by the nominal simulation (red line) and measured with dynamic MRI (black line = subject-specific, shaded region represents ±1 SD reported in Westhphal et al.51). Non-zero values represent phases where load-dependent behavior is evident, with the load-dependent effects most prominent in anterior tibial translation, tibial rotation, superior patellar translation, and patellar flexion.
Gait Simulations
The gait simulation predicted secondary tibiofemoral and patellofemoral kinematics that differed considerably from what would be assumed based a constrained one DOF knee model created from the passive motion results (Figs. 5 and 6). In particular, the largest load-dependent effects occurred in tibial rotation and anterior tibial translation (Fig. 5). The gait simulation predicted external rotation through load acceptance, internal rotation through toe-off, followed by external tibia rotation during much of swing. In stance, the tibia was also substantially more anteriorly and laterally translated than a knee model based on passive behavior would predict (Fig. 6).
FIGURE 5.
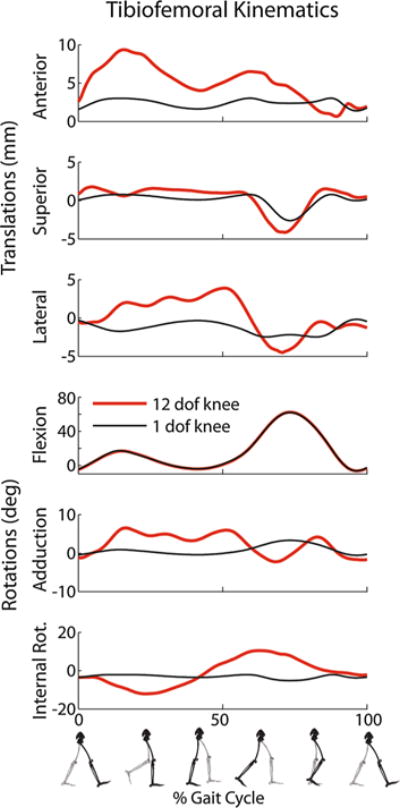
Simulation of tibiofemoral kinematics during a normal gait cycle. The simulation results (red line) differ substantially from what would be assumed via passive joint behavior.
FIGURE 6.
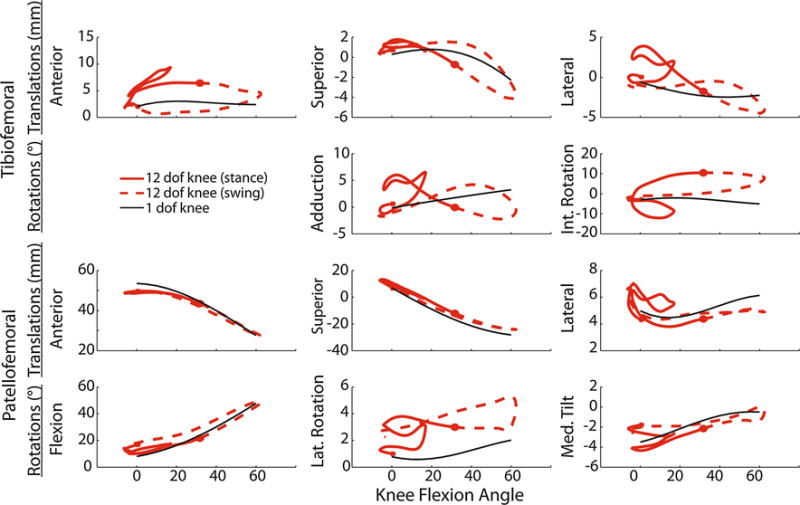
Plots of the predicted tibiofemoral and patellofemoral kinematics (solid red = stance, dashed red = swing) vs. knee flexion over a walking gait cycle. For many of the degrees of freedom (e.g., anterior tibial translation, internal tibial rotation), there are substantial variations in the predicted kinematics and that assumed when describing secondary tibiofemoral kinematics and patellofemoral kinematics as a constrained function of knee flexion (solid black line).
Model predictions of tibiofemoral contact pressures on the medial plateau were higher than those on the lateral plateau through the majority of stance. Medial tibial plateau contact pressures averaged 6.2 and 5.7 MPa at the first (15% gait cycle) and second (50% gait cycle) peaks of tibiofemoral loading, respectively (Fig. 7). Contact pressure on the patellar facets reached an average of 2.8 MPa at the first peak of tibiofemoral loading.
FIGURE 7.
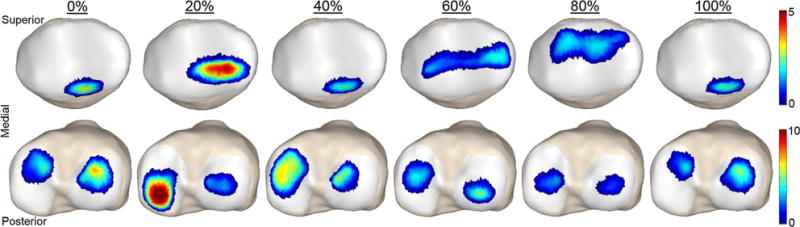
Predicted patellar and tibial plateau contact pressures (MPa) at different percentages of the gait cycle during normal walking.
DISCUSSION
We have introduced and validated a 12 DOF multi-body, dynamic knee model suitable for simulating load-dependent knee behavior during locomotor activities. A key feature of the model is the capacity to predict the tibiofemoral and patellofemoral mechanics that arise from the interaction of muscle, ligament, and contact forces, instead of relying on pre-assumed behavior based on cadaveric studies.4,16 We showed that the knee model produced kinematics that were consistent with in vivo measures obtained with dynamic MRI. When extended to gait, the framework predicted secondary knee kinematics that differ substantially from that is assumed based on traditional knee models used in gait analysis.4,16 Thus, multi-scale interactions that occur between internal joint mechanics and movement dynamics seem important to consider in simulations of human locomotion. Further, as demonstrated by our stochastic simulations, the new knee model may be extremely relevant in simulating movement in cases of altered ligament properties due to injury or disease.
Our knee model validation efforts were perhaps the most extensive ever performed, with model predictions directly compared to in vivo measures obtained under both passive and active conditions. We found that passive kinematic behavior of the model compared very well to that observed experimentally, but that specific degrees of freedom (notably tibiofemoral translations and internal tibial rotation) are highly dependent on ligament properties (Fig. 3). In contrast, tibiofemoral adduction and patellofemoral behavior were much less dependent on ligament properties, suggesting that cartilage geometries assume a more major role in guiding those movements. Inherent laxity in the knee model allowed for the prediction of load-dependent knee behavior that has been measured experimentally (Fig. 4).9,18,30,51 The model predicted an increase in anterior tibial translation and internal rotation with quadriceps loading in a flexed knee posture (i.e., in the inertial loading case), a result that was seen in the dynamic MRI (Fig. 4) and has been recorded previously in cadaveric studies.26,33 Such predictive capabilities are important when using knee models to investigate the influence of soft tissue injury and treatment on internal joint behavior.
When used to simulate gait, the co-simulation framework predicted variations in secondary knee kinematics that differed substantially from what is assumed based on passive behavior of cadaveric knees.4,50,53 In particular, phase plots clearly show that secondary kinematics are not simple functions of knee flexion, but also depend on the current internal and external loading state (Fig. 6). Notably, internal tibial rotation occurred with knee extension during the second half of stance, motion that is opposite of the classically defined screw home mechanism but is consistent with empirical studies of gait.9,18,30 Anterior tibial translation arises during the early stance phases of gait, an effect likely attributable to the quadriceps loading. An important feature of the knee model is the capacity to simultaneously estimate muscle forces and cartilage contact pressure patterns that arise during locomotion. In the nominal gait simulation, medial tibial plateau pressure was larger than the lateral tibial plateau pressure through much of stance and approximately two times greater than the patellar pressures through mid-stance (Fig. 7).
What is the clinical relevance of being able to simulate load-dependent knee behavior? There are two potentially important considerations. First is the recognition that slight variations in secondary knee kinematics can have a substantial impact on both the magnitude and location of cartilage contact pressure. It has been suggested that sudden shifts in the location of cartilage contact, due to ligament injury or surgical repair, may be a precipitator to tissue degeneration and osteoarthritis.14 Hence, the proposed knee model provides a systematic way of predicting how variations in treatment factors may influence cartilage tissue loading. Second, variations in knee kinematics can affect the capacity of soft tissues to induce joint motion. We began to assess this effect by computing the tibiofemoral finite helical axis during stance. This analysis shows that the tibiofemoral adduction and rotation during gait resulted in a skewing of the finite helical axis (FHA) and a distal migration of location of the FHA in the mid-sagittal plane (Fig. 8). Load-dependent shifts in the FHA alter the effective moment arms of the muscles and ligaments crossing the knee, such as has been previously observed for the patellar tendon.51 Variations in muscle moment arms are not currently considered in standard gait knee models, but would appear relevant in an intact knee and likely become an even more important consideration when joint laxity exists.
FIGURE 8.
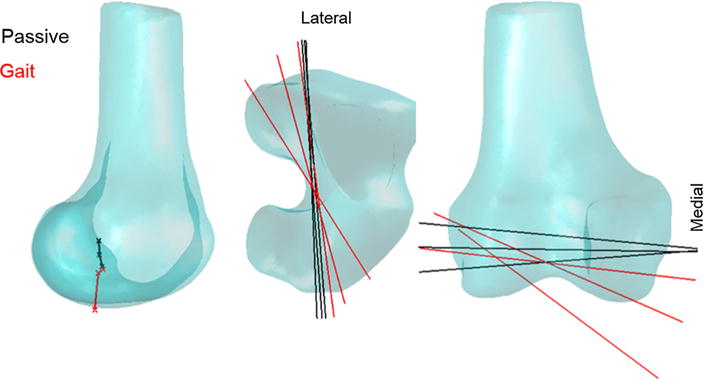
Shown is the position of the finite helical axis in the mid-sagittal plane (left), and its orientation in the transverse (middle) and frontal (right) planes at 5°, 10°, and 15° during passive flexion–extension and during the early stance phase of gait. Internal and external loading present during gait substantially altered the position of the finite helical axis, to a more inferior position and skewed the axis in both the transverse and frontal planes.
While our knee model has improved prediction of load-dependent kinematics, we must acknowledge some of its inherent limitations. We represented the ligaments as spring elements, rather than deformable 3D representations that account for spatial variations in strain. We were able to include some wrap objects to improve wrapping around the bony structures. However, these wrapping surfaces were simple geometric representations (e.g., cylinder, ellipse) and ligament–ligament interactions were not incorporated. Ligament reference strains are not measurable in vivo, so these parameters were estimated from the literature. We followed the approach of Baldwin et al. of using stochastic simulations to assess the influence of this uncertainty has on our model predictions.7 Our cartilage surface was represented as a constant thickness, an approximation which has been used by other groups.10,42 We believe that inclusion of variable cartilage thickness, as can be determined from MR images,29 will be straightforward to implement in the future. Further, the knee model does not currently have a meniscus. While the meniscus is not considered a primary constraint in low load conditions,32 incorporating a meniscus model23 will become important as the model is used for large-load tasks or in cases of ligamentous deficiency. Finally, our gait simulations applied measured ground reactions directly to the feet. Future improvements include adding a ground contact model that can simulate reaction forces arising from foot-floor interactions.
In conclusion, this study introduced a new, multi-body dynamic knee model that includes six DOF tibiofemoral and patellofemoral joints. The model provides simultaneous prediction of the ligament, muscle, and articular contact loads underlying human motion. We showed that model predictions compare well with load-dependent knee kinematic behavior measured in vivo. When extended to walking, the model predicted knee kinematic patterns that differ markedly from that traditionally assumed in gait analysis models. Thus, the modeling framework provides a powerful new approach to simulate alterations in knee mechanics that may arise due to injury or disease-related changes in soft tissue properties.
Acknowledgments
This project was supported in part by the Clinical and Translational Science Award program, through the NIH National Center for Advancing Translational Sciences, Grant UL1TR000427. Additional funding was provided by NIH F30AR065838, NIH EB015410, NIH AR062733, the National Science Foundation (0966535), and the UW Medical Scientist Training Program (T32GM008692). The authors also thank Anne Schmitz, PhD, and Kwang Won Choi, PhD, for their contributions to the modeling work.
Footnotes
Associate Editor Amit Gefen oversaw the review of this article.
CONFLICT OF INTEREST
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Amiri S, Wilson DR. A computational modeling approach for investigating soft tissue balancing in bicruciate retaining knee arthroplasty. Comput Math Methods Med. 2012;2012:652865. doi: 10.1155/2012/652865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CJ, Westerhaus BD, Pietrini SD, Ziegler CG, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Kinematic impact of anteromedial and posterolateral bundle graft fixation angles on double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2010;38:1575–1583. doi: 10.1177/0363546510364841. [DOI] [PubMed] [Google Scholar]
- 3.Anderst W, Zauel R, Bishop J, Demps E, Tashman S. Validation of three-dimensional model-based tibio-femoral tracking during running. Med Eng Phys. 2009;31:10–16. doi: 10.1016/j.medengphy.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Ann Biomed Eng. 2010;38:269–279. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askew M, Mow V. The biomechanical function of the collagen fibril ultrastructure of articular cartilage. J Biomech Eng. 1978;100:105–115. [Google Scholar]
- 6.Atkinson P, Atkinson T, Huang C, Doane R. A comparison of the mechanical and dimensional properties of the human medial and lateral patellofemoral ligaments. Proceedings of the 46th Annual Meeting of the Orthopaedic Research Society; Orlando, FL. 2000. [Google Scholar]
- 7.Baldwin MA, Laz PJ, Stowe JQ, Rullkoetter PJ. Efficient probabilistic representation of tibiofemoral soft tissue constraint. Comput Methods Biomech Biomed Eng. 2009;12:651–659. doi: 10.1080/10255840902822550. [DOI] [PubMed] [Google Scholar]
- 8.Bei Y, Fregly BJ. Multibody dynamic simulation of knee contact mechanics. Med Eng Phys. 2004;26:777–789. doi: 10.1016/j.medengphy.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benoit DL, Ramsey DK, Lamontagne M, Xu L, Wretenberg P, Renstrom P. In vivo knee kinematics during gait reveals new rotation profiles and smaller translations. Clin Orthop Relat Res. 2007;454:81–88. doi: 10.1097/BLO.0b013e31802dc4d0. [DOI] [PubMed] [Google Scholar]
- 10.Blankevoort L, Huiskes R. Ligament-bone interaction in a three-dimensional model of the knee. J Biomech Eng. 1991;113:263–269. doi: 10.1115/1.2894883. [DOI] [PubMed] [Google Scholar]
- 11.Bloemker KH, Guess TM, Maletsky L, Dodd K. Computational knee ligament modeling using experimentally determined zero-load lengths. Open Biomed Eng J. 2012;6:33. doi: 10.2174/1874230001206010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruntu DI, Hefzy MS. 3-D anatomically based dynamic modeling of the human knee to include tibio-femoral and patello-femoral joints. J Biomech Eng. 2004;126:44–53. doi: 10.1115/1.1644565. [DOI] [PubMed] [Google Scholar]
- 13.Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006;39:2943–2950. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 15.Delp SL, Loan JP. A computational framework for simulating and analyzing human and animal movement. Comput Sci Eng. 2000;2:46–55. [Google Scholar]
- 16.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 17.Dhaher YY, Kwon TH, Barry M. The effect of connective tissue material uncertainties on knee joint mechanics under isolated loading conditions. J Biomech. 2010;43:3118–3125. doi: 10.1016/j.jbiomech.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyrby CO, Andriacchi TP. Secondary motions of the knee during weight bearing and non-weight bearing activities. J Orthop Res. 2004;22:794–800. doi: 10.1016/j.orthres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Gerdes CM, Kijowski R, Reeder SB. IDEAL imaging of the musculoskeletal system: robust water–fat separation for uniform fat suppression, marrow evaluation, and cartilage imaging. AJR Am J Roentgenol. 2007;189:W284–W291. doi: 10.2214/AJR.07.2593. [DOI] [PubMed] [Google Scholar]
- 20.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69:233–242. [PubMed] [Google Scholar]
- 21.Griffith CJ, LaPrade RF, Johansen S, Armitage B, Wijdicks C, Engebretsen L. Medial knee injury part 1, static function of the individual components of the main medial knee structures. Am J Sports Med. 2009;37:1762–1770. doi: 10.1177/0363546509333852. [DOI] [PubMed] [Google Scholar]
- 22.Grood E, Suntay W. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:9. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 23.Guess TM, Thiagarajan G, Kia M, Mishra M. A subject specific multibody model of the knee with menisci. Med Eng Phys. 2010;32:505–515. doi: 10.1016/j.medengphy.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Halloran JP, Ackermann M, Erdemir A, Van den Bogert AJ. Concurrent musculoskeletal dynamics and finite element analysis predicts altered gait patterns to reduce foot tissue loading. J Biomech. 2010;43:2810–2815. doi: 10.1016/j.jbiomech.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halloran JP, Easley SK, Petrella AJ, Rullkoetter PJ. Comparison of deformable and elastic foundation finite element simulations for predicting knee replacement mechanics. J Biomech Eng. 2005;127:813–818. doi: 10.1115/1.1992522. [DOI] [PubMed] [Google Scholar]
- 26.Hirokawa S, Solomonow M, Lu Y, Lou ZP, D’Ambrosia R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am J Sports Med. 1992;20:299–306. doi: 10.1177/036354659202000311. [DOI] [PubMed] [Google Scholar]
- 27.Ishigooka H, Sugihara T, Shimizu K, Aoki H, Hirata K. Anatomical study of the popliteofibular ligament and surrounding structures. J Orthop Sci. 2004;9:51–58. doi: 10.1007/s00776-003-0733-8. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser J, Bradford R, Johnson K, Wieben O, Thelen DG. Measurement of tibiofemoral kinematics using highly accelerated 3D radial sampling. Magn Reson Med. 2013;69:1310–1316. doi: 10.1002/mrm.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthr Cartil. 2005;13:782–789. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Lafortune M, Cavanagh P, Sommer H, Kalenak A. Three-dimensional kinematics of the human knee during walking. J Biomech. 1992;25:11. doi: 10.1016/0021-9290(92)90254-x. [DOI] [PubMed] [Google Scholar]
- 31.Leardini A, Cappozzo A, Catani F, Toksvig-Larsen S, Petitto A, Sforza V, Cassanelli G, Giannini S. Validation of a functional method for the estimation of hip joint centre location. J Biomech. 1999;32:99–103. doi: 10.1016/s0021-9290(98)00148-1. [DOI] [PubMed] [Google Scholar]
- 32.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg. 1982;64A:883–888. [PubMed] [Google Scholar]
- 33.Li G, Rudy T, Sakane M, Kanamori A, Ma C, Woo SLY. The importance of quadriceps and hamstring muscle loading on knee kinematics and in situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 34.Lu TW, O’connor JJ. Bone position estimation from skin marker co-ordinates using global optimisation with joint constraints. J Biomech. 1999;32:129–134. doi: 10.1016/s0021-9290(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 35.Markolf KL, Bargar WL, Shoemaker SC, Amstutz HC. The role of joint load in knee stability. J Bone Joint Surg Am. 1981;63:570–585. [PubMed] [Google Scholar]
- 36.Maynard MJ, Deng X, Wickiewicz TL, Warren RF. The popliteofibular ligament. Rediscovery of a key element in posterolateral stability. Am J Sports Med. 1996;24:311–316. doi: 10.1177/036354659602400311. [DOI] [PubMed] [Google Scholar]
- 37.Miranda DL, Rainbow MJ, Leventhal EL, Crisco JJ, Fleming BC. Automatic determination of anatomical coordinate systems for three-dimensional bone models of the isolated human knee. J Biomech. 2010;43:1623–1626. doi: 10.1016/j.jbiomech.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neptune RR, Wright IC, van den Bogert AJ. The influence of orthotic devices and vastus medialis strength and timing on patellofemoral loads during running. Clin Biomech. 2000;15:611–618. doi: 10.1016/s0268-0033(00)00028-0. [DOI] [PubMed] [Google Scholar]
- 39.Nomura E, Inoue M, Osada N. Anatomical analysis of the medial patellofemoral ligament of the knee, especially the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2005;13:510–515. doi: 10.1007/s00167-004-0607-4. [DOI] [PubMed] [Google Scholar]
- 40.Powell MJ. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput J. 1964;7:155–162. [Google Scholar]
- 41.Rainbow MJ, Miranda DL, Cheung RT, Schwartz JB, Crisco JJ, Davis IS, Fleming BC. Automatic determination of an anatomical coordinate system for a three-dimensional model of the human patella. J Biomech. 2013;46:2093–2096. doi: 10.1016/j.jbiomech.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segal NA, Anderson DD, Iyer KS, Baker J, Torner JC, Lynch JA, Felson DT, Lewis CE, Brown TD. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J Orthop Res. 2009;27:1562–1568. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shelburne KB, Pandy MG, Anderson FC, Torry MR. Pattern of anterior cruciate ligament force in normal walking. J Biomech. 2004;37:797–805. doi: 10.1016/j.jbiomech.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J Orthop Res. 2006;24:1983–1990. doi: 10.1002/jor.20255. [DOI] [PubMed] [Google Scholar]
- 45.Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech. 2007;40:1145–1152. doi: 10.1016/j.jbiomech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Silder A, Westphal CJ, Thelen DG. A magnetic resonance-compatible loading device for dynamically imaging shortening and lengthening muscle contraction mechanics. J Med Device. 2009;3:034504. doi: 10.1115/1.3212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sritharan P, Lin YC, Pandy MG. Muscles that do not cross the knee contribute to the knee adduction moment and tibiofemoral compartment loading during gait. J Orthop Res. 2012;30:1586–1595. doi: 10.1002/jor.22082. [DOI] [PubMed] [Google Scholar]
- 48.Thelen DG, Choi KW, Schmitz AM. Co-simulation of neuromuscular dynamics and knee mechanics during human walking. J Biomech Eng. 2014;136:021033. doi: 10.1115/1.4026358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Eijden TM, de Boer W, Weijs WA. The orientation of the distal part of the quadriceps femoris muscle as a function of the knee flexion-extension angle. J Biomech. 1985;18:803–809. doi: 10.1016/0021-9290(85)90055-7. [DOI] [PubMed] [Google Scholar]
- 50.Walker PS, Rovick JS, Robertson DD. The effects of knee brace hinge design and placement on joint mechanics. J Biomech. 1988;21:965–974. doi: 10.1016/0021-9290(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 51.Westphal CJ, Schmitz A, Reeder SB, Thelen DG. Load-dependent variations in knee kinematics measured with dynamic MRI. J Biomech. 2013;46:2045–2052. doi: 10.1016/j.jbiomech.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson DR, Feikes JD, O’connor JJ. Ligaments and articular contact guide passive knee flexion. J Biomech. 1998;31:1127–1136. doi: 10.1016/s0021-9290(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 53.Wilson DR, Feikes JD, Zavatsky AB, Oconnor JJ. The components of passive knee movement are coupled to flexion angle. J Biomech. 2000;33:465–473. doi: 10.1016/s0021-9290(99)00206-7. [DOI] [PubMed] [Google Scholar]


