Abstract
Objective
To evaluate UBASH3A (rs11203203) as a predictor of persistent islet autoimmunity and type 1 diabetes.
Research Design and Methods
The Diabetes Autoimmunity Study in the Young (DAISY) followed prospectively for development of persistent islet autoimmunity (IA; autoantibodies to insulin, GAD65, IA-2 or ZnT8 on at least 2 consecutive exams) and diabetes 1715 non-Hispanic white children at increased genetic risk for type 1 diabetes. The DAISY participants were genotyped for rs11202203 (UBASH3A).
Results
UBASH3A allele A was associated with development of IA (HR=1.46, 95%CI=1.11–1.91, p=0.007) and diabetes (HR=1.84, 95%CI=1.28–2.64, p=0.001), controlling for presence of HLA-DR3/4,DQB1*0302 and having a first-degree relative with type 1 diabetes. The UBASH3A AA genotype conferred higher risk of persistent IA (12.7%) and diabetes (6.1%) by age 10 than for AG (7.7% and 3.1%, respectively) or GG (5.3% and 2.0%) genotype (p=0.009 for IA, p=0.0004 for diabetes). Among children with no family history of type 1 diabetes, but HLA-DR3/4,DQB1*0302 and UBASH3A AA genotype, 35.9% developed IA and 50.6% developed diabetes by age 15.
Conclusions
UBASH3A appears to be an independent predictor of IA and type 1 diabetes in children, including those free of family history of type 1 diabetes but carrying the HLA-DR3/4,DQB1*0302 genotype. If confirmed, UBASH3A may prove useful in type 1 diabetes risk prediction and pre-screening of the general population children for clinical trials.
Keywords: UBASH3A, Islet Autoimmunity, Type 1 Diabetes
INTRODUCTION
Type 1 diabetes (T1D) is a known autoimmune disease with strong genetic contribution, resulting in an autoimmune destruction of the insulin producing beta cells. The HLA region on chromosome 6p21 is considered the major susceptibility locus for type 1 diabetes 1. Several non-HLA genes have been shown to be associated with type 1 diabetes, including INS (11p15.5), PTPN22 (1p13.2), and CTLA4 (2p33.2). With the advent of genome wide association studies (GWAS), more than 40 non-HLA susceptibility gene markers for type 1 diabetes have recently been discovered 2,3,4,5. DAISY (Diabetes Autoimmunity Study in the Young) cohort participants who have been followed from birth for development of persistent islet autoimmunity and type 1 diabetes have been genotyped for 20 non-HLA genes. We have previously reported on association of INS and PTPN22 with persistent islet autoimmunity (IA) and T1D in the DAISY cohort 6. Among the other non-HLA genetic markers tested in DAISY, UBASH3A seems to be another important gene influencing development of IA and T1D.
UBASH3A (ubiquitin associated and SH3 domain containing A) has been associated with type 1 diabetes 7,8,9, celiac disease and rheumatoid arthritis 10. The gene is located on chromosome 21q22.3 11 and expressed in a limited number of tissues including spleen, peripheral blood leukocytes, and bone marrow.
In this study, we report the influence of UBASH3A on the development of IA and T1D in non-Hispanic white (NHW) children participating in DAISY and confirm the association in another prospective study BABYDIAB.
METHODS
Study population
The Diabetes Autoimmunity Study in the Young (DAISY) has followed two cohorts of young children at an increased risk for type 1 diabetes since 1993, the sibling and offspring cohort of relatives of type 1 diabetes patients, and the general population newborn cohort, identified through screening of over 31,000 newborns with susceptibility HLA-DR/DQ genotypes in Denver, Colorado. The details of screening and follow-up have been previously published 12. Children in this cohort have been followed from birth to an average age of 8.8 years (range: 4 months–23 years) and have been genotyped for rs11203203 (UBASH3A). Informed consent was obtained from the parents of each study subject. The Colorado Multiple Institutional Review Board approved all study protocols.
BABYDIAB is a similar German study that prospectively follows 1650 Caucasian offspring of mothers and/or fathers with type 1 diabetes from birth 13. Recruitment into the study began in 1989 and ended in 2000. Children are prospectively monitored for the development of IA and diabetes. The median follow-up for the antibody negative controls is 7.6 years. In this study, we genotyped rs11203203 (UBASH3A) in all BABYDIAB children with high risk HLA DR3/4-DQB1*0302 (n=28) and onset of IA before age 5. Matched autoantibody negative controls were selected on presence of HLA DR3/4-DQB1*0302 genotype and longest follow up time (n=29). All families gave written informed consent to participate in the BABYDIAB study. The study was approved by the ethical committee of Bavaria, Germany.
Islet Autoantibodies
Measurement of biochemical islet autoantibodies was performed in the laboratory of Dr. George Eisenbarth at the Barbara Davis Center for Childhood Diabetes in Denver, CO14. IA was defined as presence of ≥1 of the autoantibodies to insulin, GAD65, IA-2 or ZnT8 on at least 2 consecutive visits.
Genotyping
Genomic DNA isolation from whole blood was performed using the QuickGene 610L [Autogen, MA USA] and then amplified using Qiagen RepliG whole genome amplification kit [Qiagen, CA USA]. Taqman SNP genotyping assays [Applied Biosystems, CA USA] was then utilized to obtain genotype information on rs11203203. For each 12.5ul volume assay, 20ng of amplified gDNA template (1ul) was used with 6.35ul Taqman Genotyping master mix [Applied Biosystems, CA USA], 0.15ul SNP assay probe mix, and 5ul PCR grade water. PCR cycling conditions were as follows; 95C for 10 minutes, and 40 cycles of 92C for 15 seconds and 60C for 1 minute. Genotype results were obtained using an AB7000 Sequence Detection System and analysis software
Statistical Analysis
Univariate and multivariate analyses were performed in SAS version 9.1. (SAS Institute Inc, Cary, North Carolina) using the additive model. All analyses were limited to NHW only. The rs11203203 SNP was in Hardy Weinberg equilibrium. Multivariate analyses were run adjusting for HLA-DR3/4,DQB1*0302, gender and family history of type 1 diabetes. We performed survival analysis of progression to persistent IA and type 1 diabetes with PRISM software, using the log-rank test and an alpha level for significance set at 0.05. Follow-up time was defined as the age of the child at the 1st of the 2 consecutive positive visits for affected children and age of the child at the last visit for unaffected children.
RESULTS
Affected children with IA (N=107) compared with unaffected (N=1608) were more likely to carry the HLA-DR3/4,DQB1*0302 genotype (37.4% vs 17.2%) and have a first degree relative (FDR) with T1D (70.1% vs 47.8%). UBASH3A allele A was associated with development of IA (HR=1.52, 95%CI=1.16–2.00, p=0.002) and T1D (HR=2.02, 95%CI=1.40–2.91, p=0.0002). After controlling for presence of HLA-DR3/4,DQB1*0302 and having a first-degree relative with T1D, UBASH3A allele A remained associated with development of IA (HR=1.46, 95%CI=1.11–1.91, p=0.007) and T1D (HR=1.84, 95%CI=1.28–2.64, p=0.001) (data not shown).
Cumulative incidence of development of persistent IA and T1D by genotypes, estimated by survival analysis, showed a higher risk of persistent IA by age 10 years for the UBASH3A AA genotype (12.7%) compared to those having the AG (7.7%) or GG (5.3%) genotype (p=0.0087) (Figure 1A). Risk of development of T1D by age 10 years was also higher in subjects with the AA genotype (6.1%) compared to those with the AG (3.1%) or GG (2.0%) genotype (p=0.0004) (Figure 1B).
Figure 1.
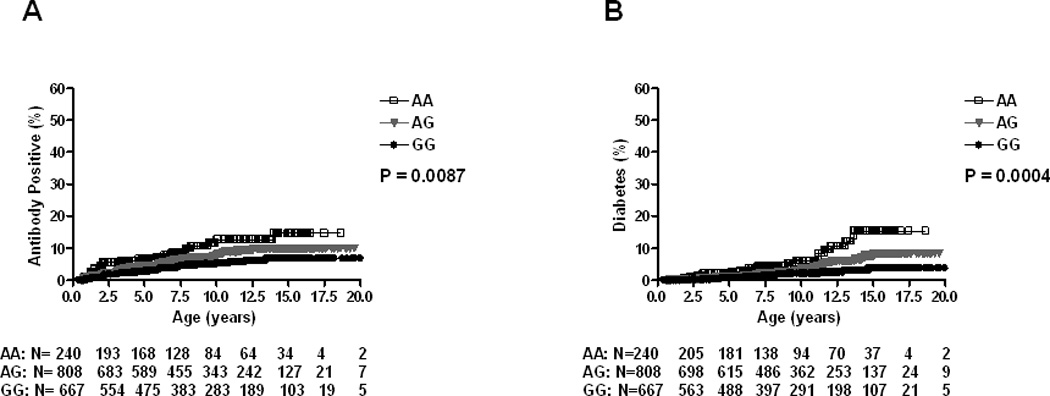
Progression to Islet Autoimmunity (A) and Diabetes (B) in DAISY NHW population (N=1715) by UBASH3A genotype
NHW= non-Hispanic White
When limiting the analyses to HLA-DR3/4,DQB1*0302 subjects (N=316), cumulative risk for persistent IA by age 10 years for the UBASH3A AA genotype was 34.6% compared to AG (17.6%) or GG (8.3%) genotype respectively (p=0.004) (Figure 2A). Cumulative incidence of T1D by age 10 years showed similar risk (p=0.01) (Figure 2B).
Figure 2.
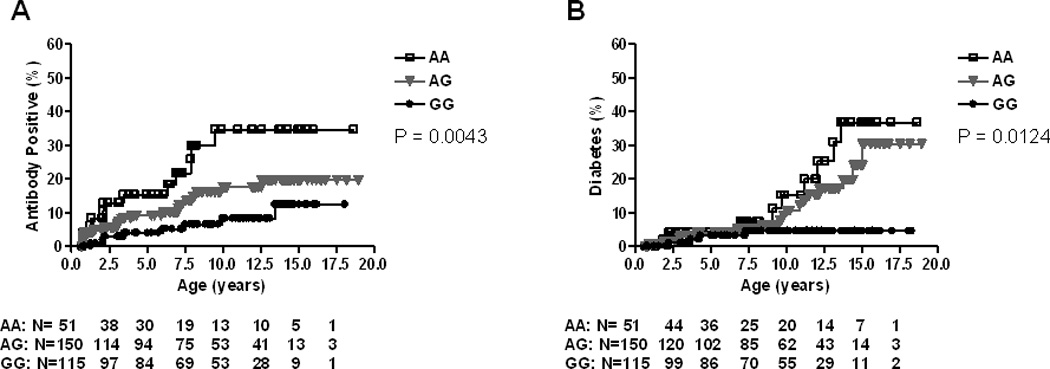
Progression to Islet Autoimmunity (A) and Diabetes (B) in DAISY DR3/4 NHW subjects (N=316) by UBASH3A genotype
NHW= non-Hispanic White
To determine if UBASH3A is helpful in predicting diabetes more accurately in the general population where most cases of T1D arise, we performed survival analysis in the DAISY general population children with HLA-DR3/4,DQB1*0302 (N=239). The cumulative risk of persistent IA among these general population children reached 35.9% in those with the AA genotype by age 10 (Figure 3A), while the cumulative risk for T1D was 22.2% (Figure 3B).
Figure 3.
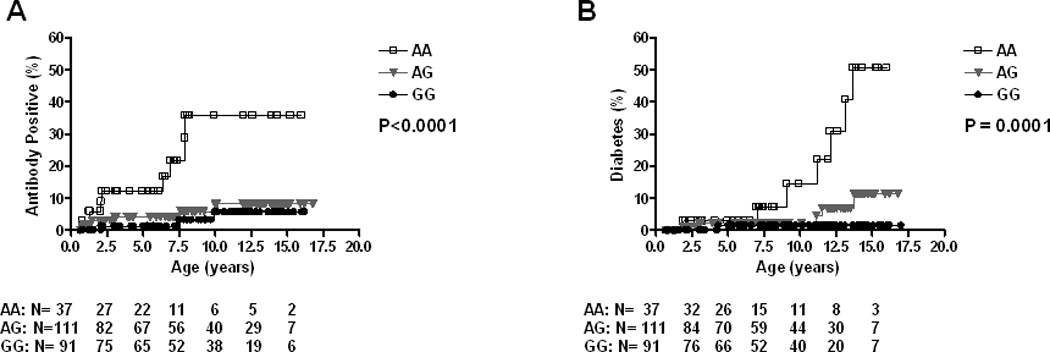
Progression to Islet Autoimmunity (A) and Diabetes (B) in DAISY DR3/4 general population children (N=239) by UBASH3A genotype
In the BABYDIAB cohort, the results were not statistically significant, likely due to small numbers. However, the trends were similar with UBASH3A AA genotype having a higher frequency in cases vs. controls for both IA (14.8% vs 7.1%) and type 1 diabetes (18.2% and 9.1% respectively). (Table 1).
Table 1.
Association of UBASH3A (rs11203203) in BABYDIAB population
| rs11203203 | Islet Autoimmunity | Type 1 Diabetes | ||||
|---|---|---|---|---|---|---|
| Case (Freq %) |
Control (Freq %) |
P Value |
Case (Freq %) |
Control (Freq %) |
P Value |
|
| AA | 4 (14.8%) | 2 (7.1%) | 0.11 | 2 (18.2%) | 4 (9.1%) | 0.61 |
| AG | 14 (51.9%) | 22 (78.6%) | 6 (54.5%) | 30 (68.2%) | ||
| GG | 9 (33.3%) | 4 (14.3%) | 3 (27.3%) | 10 (22.7%) | ||
DISCUSSION
The HLA region on chromosome 6p21 is the major susceptibility locus for type 1 diabetes with an estimated 30–50% of the genetic risk for type 1 diabetes attributed to the MHC region. Recently, a number of genome wide association studies have discovered more than 40 non-HLA genes that may be contributing to development of type 1 diabetes. To date, polymorphisms of the insulin gene and PTPN22 gene have been confirmed to contribute to type 1 diabetes independently of HLA Class II alleles 6. DAISY NHW participants have been genotyped for 20 additional SNPs that had previously been associated with type 1 diabetes. DAISY is a prospective cohort allowing definition of the age of conversion to islet autoimmunity and the cumulative risk of IA and type 1 diabetes. In addition to confirmation of the effect of the HLA Class II genotypes, we have previously confirmed in the DAISY population the independent effect of INS and PTPN22 on development of both IA and type 1 diabetes 6,15. In the current report, UBASH3A (rs11203203), is a novel gene marker significantly associated with IA and type 1 diabetes. However, there are potentially important differences in the age of type 1 diabetes diagnosis (younger in DAISY than in the previous GWAS studies). This is the first evaluation of this marker as predictor of persistent IA in a prospective cohort; the association was not statistically significant in BABYDIAB subjects with onset of IA before age 5, but showed a similar trend.
UBASH3A spans 40kb and contains 15 exons, with an alternate splicing resulting from skipping of exon 5. This protein is composed of two domains: the ubiquitin associated (UBA) domain is involved in the ubiquitin pathway and the SH3 domain is known for protein-protein interaction modules which bind to proline-rich proteins involved in cell polarization and signal transduction 11. UBASH3A is expressed predominantly in T-cells, acting by inhibiting the c-CBL-mediated downregulation of protein tyrosine kinases that are activated upon T-cell receptor stimulation 16,9. Similarly, the lymphoid-specific phosphatase (LYP), encoded by PTPN22, is involved in preventing spontaneous T-cell activation by directly downregulating some of the same protein tyrosine kinases. The C1858T PTPN22 SNP results in a missense mutation and thereby abrogates the ability of the molecule to bind to the signaling molecule Csk but the diabetes associated allele results in a “gain of function” inhibiting T cell receptor signaling 17,18. Both of these genes seem to play an important role in T-cell receptor stimulation and in several autoimmune diseases: PTPN22 has been confirmed to be associated with Graves’ disease 19, rheumatoid arthritis 20 and systemic lupus erythematosus 21, while UBASH3A has recently been associated with celiac disease and rheumatoid arthritis 10.
While HLA class II susceptibility alleles are being used to identify high-risk relatives for developing type 1 diabetes in combination with autoantibody positivity, no genetic risk factors are currently able to predict type 1 diabetes with enough accuracy in the general population. In the future, preventive therapies may be applied prior to diabetes onset or even prior to the appearance of IA in individuals who are genetically at risk for type 1 diabetes. Children who have a family history of type 1 diabetes and the HLA risk genotypes DR3/4-DQB1*0302 or DR4/DR4 have a 20% or higher risk for developing islet autoantibodies during childhood 22. Primary intervention studies such as Pre-POINT (Primary Oral INsulin Trial) and TrialNet Oral Insulin studies are currently recruiting siblings with high genetic risk for type 1 diabetes. However, only 10% of new onset type 1 diabetes subjects have a relative with type 1 diabetes. Determining extreme genetic risk in the general population is a prerequisite for the implementation of primary prevention trials in the general population, where most new cases of type 1 diabetes arise, approximately 90%.
In conclusion, the UBASH3A SNP rs11203203 predicts development of persistent IA and type 1 diabetes; however, confirmation studies in similar young populations are needed. With more accurate prediction, intervention trials may become possible for individuals at greatest risk.
Acknowledgments
This research was supported by NIH grants R37 DK32493 (DAISY), P30 DK57516 (DERC Clinical Investigation Core) and NIH Grant 5 K12 DK63722. A.K.S. was supported by the JDRF Grant 11-2010-206 Early Career Patient-oriented Diabetes Award. The authors have no conflict of interest.
Footnotes
Author Contributions: K.J researched data and wrote manuscript. R.W and K.J.B researched data. G.K, M.J.R and A.K.S contributed to discussion, reviewed/edited manuscript.
Reference List
- 1.Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: Molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59(5):1134–1148. [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009 doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todd JA, Walker NM, Cooper JD, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy MP, Wang H, Liu S, et al. Association between type 1 diabetes and GWAS SNPs in the southeast US Caucasian population. Genes Immun. 2011;12(3):208–212. doi: 10.1038/gene.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steck AK, Zhang W, Bugawan TL, et al. Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes? diab. 2009;58(4):1028–1033. doi: 10.2337/db08-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper JD, Walker NM, Smyth DJ, Downes K, Healy BC, Todd JA. Follow-up of 1715 SNPs from the Wellcome Trust Case Control Consortium genome-wide association study in type I diabetes families. Genes Immun. 2009;10(Suppl 1):S85–S94. doi: 10.1038/gene.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant SF, Qu HQ, Bradfield JP, et al. Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. diab. 2009;58(1):290–295. doi: 10.2337/db08-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Concannon P, Onengut-Gumuscu S, Todd JA, et al. A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes. 2008 doi: 10.2337/db08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhernakova A, Stahl EA, Trynka G, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7(2):e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wattenhofer M, Shibuya K, Kudoh J, et al. Isolation and characterization of the UBASH3A gene on 21q22.3 encoding a potential nuclear protein with a novel combination of domains. Hum Genet. 2001;108(2):140–147. doi: 10.1007/s004390000453. [DOI] [PubMed] [Google Scholar]
- 12.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY) diabetol. 1996;39(7):807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 13.Bonifacio E, Hummel M, Walter M, Schmid S, Ziegler AG. IDDM1 and multiple family history of type 1 diabetes combine to identify neonates at high risk for type 1 diabetes. Diabetes Care. 2004;27(11):2695–2700. doi: 10.2337/diacare.27.11.2695. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Rewers M, Gianani R, et al. Anti-islet autoantibodies develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81(12):4264–4267. doi: 10.1210/jcem.81.12.8954025. [DOI] [PubMed] [Google Scholar]
- 15.Emery LM, Babu S, Bugawan TL, et al. Newborn HLA-DR,DQ genotype screening: age- and ethnicity-specific type 1 diabetes risk estimates. Pediatr Diabetes. 2005;6(3):136–144. doi: 10.1111/j.1399-543X.2005.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpino N, Turner S, Mekala D, et al. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20(1):37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- 17.Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36(4):337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 18.Vang T, Congia M, Macis MD, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37(12):1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 19.Smyth D, Cooper JD, Collins JE, et al. Replication of an Association Between the Lymphoid Tyrosine Phosphatase Locus (LYP/PTPN22) With Type 1 Diabetes, and Evidence for Its Role as a General Autoimmunity Locus. diab. 2004;53(11):3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 20.Begovich AB, Carlton VE, Honigberg LA, et al. A Missense Single-Nucleotide Polymorphism in a Gene Encoding a Protein Tyrosine Phosphatase (PTPN22) Is Associated with Rheumatoid Arthritis. Am J Hum Genet. 2004;75(2):330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyogoku C, Langefeld CD, Ortmann WA, et al. Genetic Association of the R620W Polymorphism of Protein Tyrosine Phosphatase PTPN22 with Human SLE. Am J Hum Genet. 2004;75(3):504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker JM, Barriga K, Yu L, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J Clin Endocrinol Metab. 2004;89:3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]


