Abstract
Human hepatocellular carcinoma (HCC) has a high rate of tumor recurrence and metastasis, resulting in shortened survival times. The efficacy of current systemic therapies for HCC is limited. In this study, we used xenograft tumor models to investigate the use of antibodies that block CD47 and inhibit HCC tumor growth. Immunostaining of tumor tissue and HCC cell lines demonstrated CD47 over-expression in HCC as compared to normal hepatocytes. Macrophage phagocytosis of HCC cells was increased after treatment with CD47 antibodies (CD47mAbs) that block CD47 binding to SIRPα. Further, CD47 blockade inhibited tumor growth in both heterotopic and orthotopic models of HCC, and promoted the migration of macrophages into the tumor mass. Our results demonstrate that targeting CD47 by specific antibodies has potential immunotherapeutic efficacy in human HCC.
Keywords: CD47, HCC, Antibody therapy, Xenograft models
Introduction
Hepatocellular carcinoma (HCC) is a malignancy with poor clinical prognosis due to presentation at advanced stages and high post-surgical recurrence rates [1]. Efficacious modalities for treatment of HCC with conventional chemotherapeutic agents remain limited. However, advances in the use of immunomodulation to promote anti-tumor effects hold promise for more effective therapy to treat HCC. The activation of macrophage anti-tumor activity is an area of active investigation, in particular regarding the blockade of the interaction between tumor CD47 and macrophage signal regulatory protein-α (SIRPα). CD47 is a transmembrane protein that is widely-expressed in normal tissues and acts as a “self” signal on normal cells by inhibiting macrophage phagocytosis when it interacts with macrophage SIRPα [2]. This “self” signal is mediated through tyrosine phosphorylation of SIRPα at the cytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs) and the recruitment of the phosphatase, SHP-1 [3,4]. In a co-optation of this “self” signal, the overexpression of CD47 found in many malignancies is thought to be a mechanism by which cancer cells increase their “selfness” thus evading macrophage tumoricidal activity. Clinically, higher CD47 expression was also found in tumor tissues obtained from patients with HCC [5]. These associations suggest that the targeting of CD47 may have a therapeutic benefit in the treatment of HCC. We sought to evaluate the therapeutic effects of CD47 blockade using specific antibodies in mouse models of HCC. We showed that CD47 blockade resulted in a significant increase in HCC cell phagocytosis by macrophages in vitro as well as increased migration of macrophages into the HCC mass in vivo. Furthermore, CD47 blockade resulted in diminished tumor growth both in heterotopic and orthotopic xenograft models of HCC.
Materials and methods
Immunohistochemistry
CD47 protein expression was evaluated by immunostaining on paraffin-embedded tissues. Specimens from ten HCC patients who had undergone surgical resection were obtained under study protocol approved by the Institutional Review Board at Washington University School of Medicine. Normal liver samples free of malignancy or chronic liver disease were obtained from organ donors. Sections (4 μm) were made from the paraffin blocks, blocked with 1% BSA and incubated with sheep anti-CD47 antibody at 0.2 μg/mL (RD Systems, AF4670) overnight at 4 °C. The sections were then incubated with donkey anti-sheep IgG conjugated with FITC (RD Systems, NL012) for 1 hour, followed by nuclear counterstaining with Hoechst (Invitrogen). The stained sections were imaged and immunofluorescence intensity was measured using NIS-Elements Microscope Imaging Software (Nikon).
Flow cytometry
HepG2-luc2 (Caliper Life Sciences) and H3B (Hep-3B, ATCC) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS). Normal human hepatocytes were cultured in Hepatocyte Basal Medium (HBM) with ultraglutamine-1 (Clonetics). These cell cultures were maintained at 37 °C in 5% CO2 with exchanges of media every 2–3 days.
To evaluate the expression of CD47 on hepatocytes, HepG2 and H3B cells, cell suspensions were incubated with polyclonal anti-CD47 antibody (RD Systems, AF4670) or IgG control antibody at 0.2 μg/mL for 12 hours at 4 °C. FITC-conjugated secondary antibody (RD Systems, NL012) was used for fluorescent visualization. Flow cytometry analysis was performed on a BD FACSAria cell sorter (Becton Dickinson). To determine the purity of isolated macrophages, cells were incubated with PE-conjugated anti-CD11b (Biolegend, 101207) and FITC-conjugated anti-CDF4/80 (Biolegend, 123107).
Antibody preparation and MTT assay
The anti-CD47 monoclonal antibodies (mAbs) 2D3, B6H12 and CD47 monoclonal antibody (Ab400) (CD47mAb400) were purified from hybridomas. MAbs 2D3 and B6H12 bind only to human/non-human primate CD47 while CD47mAb400 exhibits cross species binding to both mouse and human/non-human primate CD47. Cells were cultured using standard protocols, and low endotoxin antibodies were purified with MEP HyperCel™ chromatography (Pall Corporation).
To assess for potential cytotoxic effects after treatment with anti-CD47 antibodies, the cell viability of normal hepatocytes, HepG2 and H3B cells was measured. IgG control, 2D3, B6H12 or CD47mAb400 antibodies were added to the wells to final concentrations of 10 μg/mL, 3.3 μg/mL or 1.1 μg/mL. After 24 hours, 48 hours and 72 hours of incubation at 37 °C, 20 μg/mL MTT (Promega, G4000) was added to a 96-well plate. After four additional hours of incubation at 37 °C with MTT, the media from the wells were removed and MTT solubilization solution was added and incubated for 15 min, after which absorbance was measured at 590 nm with a reference filter of 620 nm.
In vitro phagocytosis assay
Peritoneal macrophages were isolated following injection of 1 mL 3% thioglycollate medium into the peritoneal cavity of six-week old NOD/SCID/IL2γnull (NSG) mice. These mice harbor a mutation in SIRPa that allows it to bind human CD47 on the HCC cells [6]. After 3 days, a peritoneal wash was performed using 10 mL DMEM/F12 medium (10% fetal bovine serum (FBS; Hyclone) in DMEM/F12 medium (Invitrogen, 10565). The macrophage-containing medium was withdrawn, and the macrophages were cultured in DMEM/F12 medium. To perform the in vitro phagocytosis assay, 5 × 104 macrophages were plated per well in a 24-well tissue-culture plate. Tumor cells were labeled with 2.5 μM carboxy fluorescein diacetate succinimidyl ester (CFSE) according to the manufacturer’s protocol (Invitrogen). Macrophages were incubated in serum-free medium for 2 hours prior to adding 2 × 105 CFSE-labeled, live tumor cells. The antibodies 2D3, B6H12 and CD47mAb400 or IgG control (BioXcell, #BE0085) were added at a concentration of 10 μg/mL and incubated for 2 hours at 37 °C. The macrophages were then washed and subsequently imaged using confocal microscopy. The phagocytic index was calculated as the number of phagocytosed CFSE+ cells per 100 macrophages.
Xenograft models
All animals and experiments were maintained and performed under protocols approved by the Washington University Animal Studies Committee. Male NSG mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in cages in temperature and light-controlled environments with access to water and food ad libitum. For the heterotopic xenograft model, HepG2-luc2 cells were suspended in DMEM containing 25% (v/v), and 1 × 106 cells were implanted subcutaneously into the axillary subcutaneous space of 4- to 8-wk-old NSG mice. After 2 weeks of growth, antibody treatment commenced with twice-weekly 400 μg intraperitoneal injections of anti-CD47 antibodies CD47mAb400 or B6H12, or human isotype-matched IgG control for 6 weeks. Luciferin bioluminescence and direct tumor dimension measurements were performed weekly. Tumor volumes were calculated using (height × weight)/0.6. After 6 weeks of treatment, animals were euthanized and tumors were resected, weighed and fixed in 10% formalin for histological and IHC analysis. Concomitantly, the lungs and liver of each mouse were fixed in formalin for histological analysis and stained with W6-32 human HLA antibody to assess for the presence of human cells representing distant metastases. For the orthotopic xenograft model, we first grew HepG2-luc2 cells subcutaneously in NSG mice for 3 weeks. The tumors were then excised, and 1 mm3 fragments were implanted into the livers of a second set of NSG mice. After two weeks of tumor growth within the liver, the mice were imaged for luciferin bioluminescence to confirm the presence of tumor. Treatment then commenced with bi-weekly intraperitoneal injections of 400 μg anti-CD47 CD47mAb400, B6H12 or human IgG control for 4 weeks. Bioluminescence imaging was performed weekly. Six weeks after tumor implantation, the animals were euthanized, tumors excised, measured, and then fixed for histological and immunohistochemical examination.
Bioluminescent imaging
In vivo bioluminescence was imaged using the IVIS Spectrum system (Caliper Life Science) with Living Image 4.0 software. A 1.7% solution of D-luciferin potassium salt (Biosynth) in PBS was prepared, and 150 mg/kg body weight of luciferin was injected into the peritoneum of mice. Bioluminescent imaging was performed until peak radiance was achieved, and total flux (photons/second) was measured from a delineated region of interest.
Statistical analysis
Comparisons between groups were performed using one-way analysis of variance; differences with p-values <0.05 based on Tukey’s multiple comparisons test were considered significant. Statistical analyses were performed using the R statistical package. To model the longitudinal bioluminescence data, a mixed-effects model was used with the R statistical package library nlme [7].
Results
CD47 is over-expressed in HCC compared with normal liver
Our initial insight into the role of CD47 in HCC came from a comparison of CD47 expression levels between HCC and normal liver. Immunostaining with antibodies specific to CD47 showed higher CD47 expression in HCC tissue compared to adjacent non-tumor liver from human resection specimens (Fig. 1A and B, Supplementary Table S1). Furthermore, there were significantly lower levels of CD47 expression in normal liver tissues compared to HCC (Fig. 1B). We then examined the expression of CD47 on two human HCC cell lines, HepG2 and H3B. There were higher levels of CD47 in HepG2 and H3B relative to that of normal human liver hepatocytes (Fig. 1C). These data suggested that CD47 was overexpressed in human HCC tissue and HCC cell lines as compared to normal liver tissue and hepatocytes.
Fig. 1.
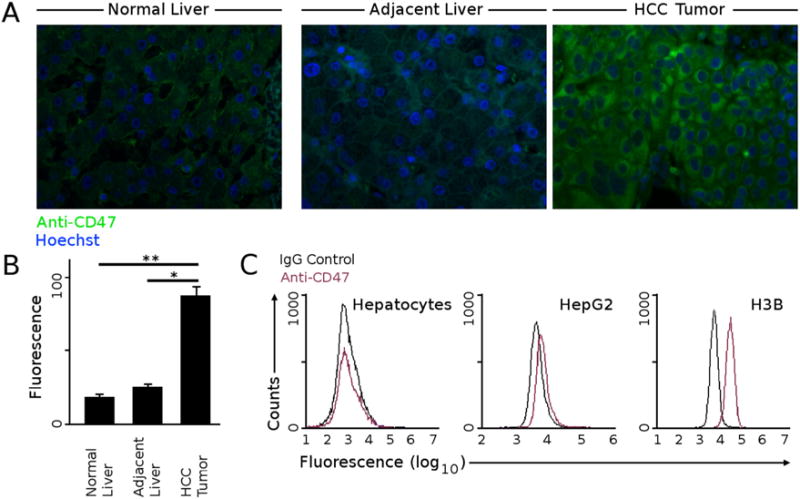
CD47 is expressed at higher levels in HCC. (A) Immunofluorescence staining with anti-CD47 antibody showed low but detectable CD47 staining in normal liver without chronic disease or tumors. This is similar to the liver adjacent to HCC tumors obtained from resection specimens, whereas HCC tissue stained highly for CD47. Representative images are shown here. (B) The relative fluorescence values from immunofluorescence staining were quantified from six normal livers and ten HCC and matched adjacent non-tumor livers (* p < 2e-5 (paired t-test), ** p < 8e-6 (t-test)). (C) Surface CD47 protein expression of normal hepatocytes, HepG2 and H3B were measured with flow cytometry. HepG2 and H3B tumor cells expressed increased levels of CD47 compared to normal hepatocytes.
CD47 blockade using specific antibodies increases macrophage phagocytosis of HCC tumor cells
We next investigated the effect of CD47 overexpression on HCC cells and macrophage phagocytosis. HepG2 and H3B tumor cells were labeled with CFSE and individually co-cultured with peritoneal-derived macrophages from NOD/SCID/IL2γnull (NSG) mice. To identify the macrophages, flow cytometry for macrophage markers using F4/80 and CD11b were performed (Fig. 2D). Macrophages and tumor cells incubated with control antibodies IgG or 2D3 (a control antibody that binds to CD47 but does not block CD47 interactions with SIRPα) resulted in low levels of tumor phagocytosis by macrophages (Fig. 2A–C). In contrast, CD47 blockade using B6H12 or CD47mAb400 antibodies resulted in significantly higher rates of macrophage phagocytosis of HepG2 and H3B. Furthermore, the phagocytosis index was higher with the CD47mAb400 antibody compared to B6H12 (Fig. 2B). Both monoclonal B6H12 and CD47mAb400 antibodies block CD47 interactions with SIRPα; B6H12 has specificity to only human CD47, whereas CD47mAb400 binds to CD47 of multiple species (including mouse and human) and has higher affinity for binding to CD47 than B6H12 (Supplementary Fig. S1). These results demonstrated that CD47 blockade enhances phagocytosis of HCC tumor cells by macrophages.
Fig. 2.

CD47 blockade increases macrophage phagocytosis of tumor cells in vitro. (A) HepG2 tumor cells were labeled with CFSE and cultured with peritoneal-derived macrophages from NSG mice. The cells were incubated with control IgG or with the non-blocking 2D3 or blocking B6H12 or CD47mAb400 anti-CD47 antibodies. Representative 200× magnification images are shown. Incubation with blocking anti-CD47 antibodies resulted in increased phagocytosis of CFSE labeled HepG2 cells (arrows). (B) Increased macrophage phagocytosis was also seen with CFSE labeled H3B cells when incubated with blocking anti-CD47 antibodies B6H12 and CD47mAb400. (C) The extent of macrophage phagocytosis with each antibody was quantified for HepG2 (ANOVA p < 3e-16; * p < 3e-9, ** p < 1e-13, *** p < 1e-20 (Tukey post-hoc)) and H3B cells (ANOVA p < 3e-16; * p < 8e-9, ** p < 5e-13, *** p < 1e-20 (Tukey post-hoc)). (D) Flow cytometry confirmed that the majority of cells obtained from the peritoneal fluid in NSG mice were CD11b+ ad F4/80+ macrophages.
The CD47mAb400 is not cytotoxic to HCC cells and normal hepatocytes
To test whether the CD47mAb400 antibodies have direct cytotoxic effects on tumor cells and normal hepatocytes, we used the MTT cell proliferation assay to measure cell viability after incubation with IgG control, non-blocking 2D3, or blocking B6H12 and CD47mAb400 antibodies. Normal hepatocytes and HepG2 and H3B cells were exposed to these antibodies over a range of antibody concentrations (1.1 μg/mL, 3.3 μg/mL, and 10 μg/mL) over 24–72 hours, followed by determination of viability. Within each antibody concentration and duration of exposure, there was no significant difference in viability of normal hepatocytes and HCC tumor cells, whether exposed to CD47 blocking antibodies (B6H12 and CD47mAb400) or control antibodies (IgG and 2D3) compared to no antibody conditions (Fig. 3). This result suggested that the therapeutic effect of CD47 blocking antibodies (CD47mAb400 and B6H12) is unlikely due to direct toxicity to the tumor cells. Also, these CD47 blocking antibodies did not result in detectable reduction in viability of normal hepatocytes, suggesting that direct in vivo injury to the liver from these antibodies would not be expected.
Fig. 3.
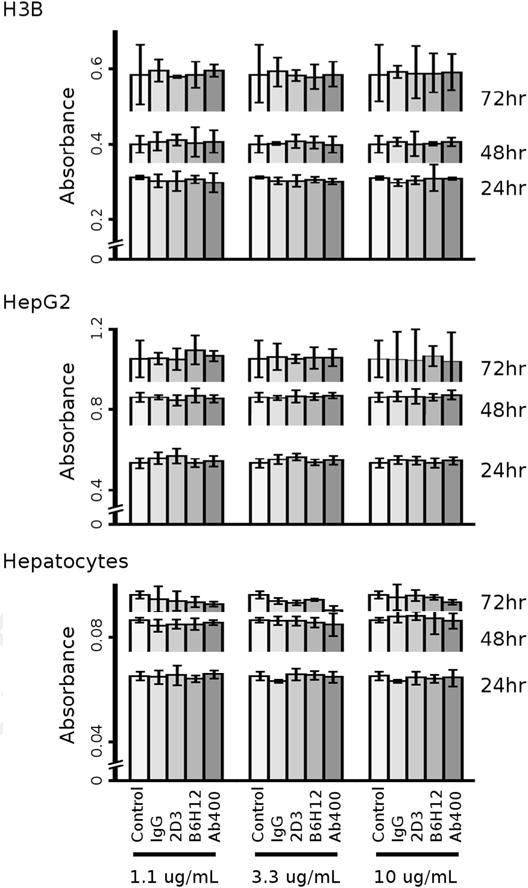
CD47 specific antibodies B6H12 and CD47mAb400 do not have direct cytotoxic properties in vitro. Normal hepatocytes and HCC tumor cells H3B and HepG2 were cultured and incubated with no antibody control, IgG control antibody, 2D3 (non-blocking anti-CD47 antibody), B6H12 or CD47mAb400 antibodies at escalating concentrations. Antibody exposures ranged from 24 to 72 hours. The MTT assay was assessed for cell viability and did not show a statistical difference between antibody type or concentration within an exposure duration.
CD47 blockade reduces tumor size in mice bearing heterotopic tumors
To test the effect of CD47 blockade on tumor growth in vivo, a heterotopic xenograft model was established by implanting HepG2-luc2 labeled tumor cells into the axillary subcutaneous space of NSG mice. HepG2-luc2 cells were injected subcutaneously and allowed to grow for 2 weeks, after which 400 μg of IgG control or CD47 blocking B6H12 or CD47mAb400 antibodies was injected intraperitoneally twice weekly (Fig. 4A). The progression of tumor burden was evaluated by bioluminescence as well as direct measurements of tumor size. A mixed-effects model was fitted with bioluminescence as the response variable. The antibodies (IgG, B6H12 and CD47mAb400) were treated as fixed intercepts, and the random effects were grouped by mice. Compared to mice treated with control IgG, the tumor bioluminescence was less in the B6H12 (bioluminescence difference = −4.65e9 photons/sec [95% CI: −9.16e9 to −1.33e8], p < 0.04) and CD47mAb400 (bioluminescence difference = −8.10e9 photons/sec [95% CI: −12.6e9 to −3.59e9], p < 0.003) treated mice (Fig. 4B and C). In addition, the rate of tumor growth was significantly lower for mice treated with B6H12 (growth difference = −0.16 cm3/wk [95% CI: −0.20 to −0.13], p < 1e-5) and CD47mAb400 (growth difference = −0.29 cm3/wk [95% CI: −0.33 to −0.26], p < 1e-5) as compared to those treated with control IgG (Fig. 4D). At the end of 8 weeks, the tumors were excised from the subcutaneous space and weighed. Similar to the results obtained using bioluminescence imaging, direct measurements of the subcutaneous tumors showed that CD47mAb400 treated mice had a reduction in tumor size at a more rapid rate and to a greater degree over the 6-week antibody course compared to B6H12 treated mice (p < 1e-5, Fig. 4D–F). Furthermore, mice treated with CD47mAb400 and B6H12 antibodies maintained overall higher body masses compared to IgG control (Supplementary Fig. S2). These data indicated that CD47 blockade inhibited tumor growth and progression of HCC in vivo in a heterotopic tumor model.
Fig. 4.

CD47 specific antibodies inhibit tumor growth in a heterotopic HCC model. (A) HepG2-luc2 cells were injected subcutaneously into the axilla of NSG mice and allowed to grow for 2 weeks. Intraperitoneal injections of IgG, B6H12 or CD47mAb400 were administered twice-weekly thereafter. (B) Representative bioluminescent images of mice at week 2 and week 8 are shown. (C) Mice treated with anti-CD47 antibodies had less tumor bioluminescence than those treated with IgG antibody, with CD47mAb400 having a greater effect than B6H12 (mixed-effects model; p < 0.04 IgG vs B6H12; p < 0.003 IgG vs CD47mAb400). (D) Direct tumor size measurements showed that tumor growth initially continued with all antibodies. After the third week tumor growth was inhibited with B6H12 antibody, and tumor size regressed with CD47mAb400 antibody (mixed-effects model; p < 1e-5 for both IgG vs B6H12 and IgG vs CD47mAb400). (E) After 8 weeks from initial tumor implantation, the tumors excised from IgG-treated mice were significantly larger than that of anti-CD47 antibody treated mice (ANOVA p < 8e-6, * p < 0.003, ** p < 3e-4). (F) Images of excised subcutaneous tumors are shown.
CD47 blockade reduces tumor size in mice bearing orthotopic tumors
We next examined the effect of CD47 blockade on HepG2 tumors in an orthotopic xenograft model, which simulates a more native microenvironment to assess tumor response to therapeutic agents. HepG2 tumors were first grown in the subcutaneous space of NSG mice, and then tumor fragments were implanted into the livers of a second set of NSG mice (Fig. 5A). These tumors were allowed to grow for two weeks and then imaged for tumor bioluminescence to establish baseline levels prior to antibody treatments. Weekly imaging for four subsequent weeks showed that animals treated with antibodies specific to CD47 had slower increase in tumor bioluminescence than animals treated with IgG control (Fig. 5B and C). The tumor bioluminescence of mice treated with control IgG was found to increase at an average of 2.8e9 photons/sec/week (95% CI: 1.8e9–3.8e9). For animals treated with B6H12, the increase in tumor bioluminescence was lower than control IgG but was not statistically significant (growth difference = −7.7e8 photons/sec/week [95% CI: −2.1e9 to 5.6e8], p = 0.25). However, animals treated with CD47mAb400 did show a slower increase in tumor bioluminescence as compared to control IgG (growth difference = −1.3e9 photons/sec/week [95% CI: −2.7e9 to −1.9e6], p < 0.004; Fig. 5C).
Fig. 5.
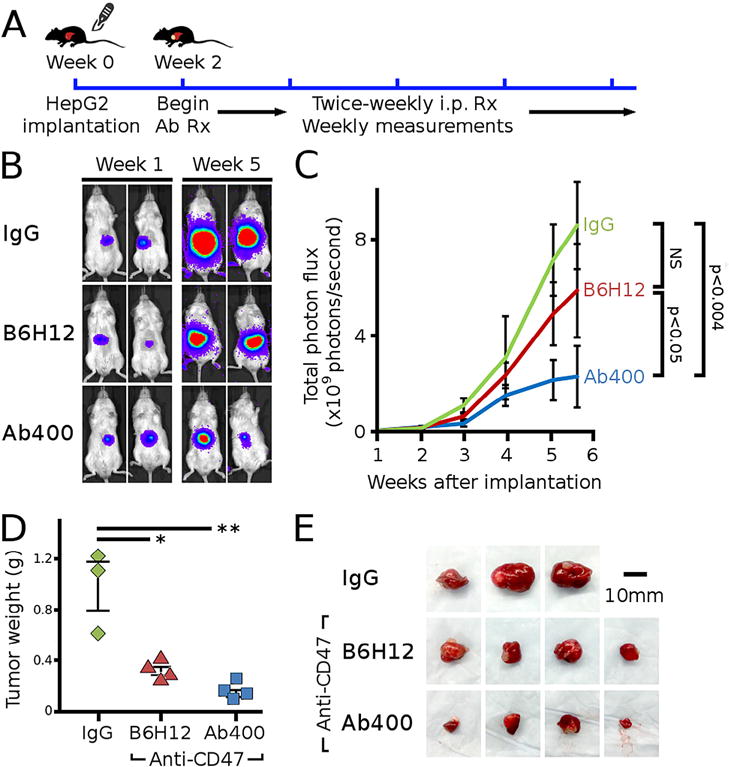
CD47 specific antibodies inhibit tumor growth in an orthotopic HCC model. (A) HepG2-luc2 tumors were implanted into the liver of NSG mice and allowed to grow for 2 weeks, followed by twice-weekly intraperitoneal injections of antibodies. (B) Representative bioluminescent images of mice at week 1 and week 5 are shown. (C) Mice treated with CD47mAb400 antibodies had less tumor bioluminescence than those treated with IgG antibody (mixed-effects model; p < 0.004 IgG vs CD47mAb400). The B6H12 data trended lower than IgG but did not reach statistical significance. (D) After 6 weeks from initial tumor implantation, the tumors excised from IgG-treated mice were significantly larger than those from anti-CD47 antibody treated mice (ANOVA p < 6e-4, * p < 0.003, ** p < 6e-4). (E) Images of excised subcutaneous tumors are shown.
The changes in tumor bioluminescence correlated well with final measurements of tumor dimensions. The tumors extracted from the liver of mice treated with CD47mAb400 were significantly smaller than those treated with IgG control (p < 6e-4, Fig. 5D and E). The final tumor sizes in animals treated with B6H12 were also lower than those treated with IgG control, and in contrast to the bioluminescence data, these differences were significant (p < 0.003). These data confirmed that CD47 blockade could suppress tumor growth of HCC in vivo.
To assess for the presence of metastatic lesions in all treatment groups, the lungs of each mice were fixed and stained with W6-32 human HLA antibody to identify the presence of metastatic human tumor cells. There was no evidence of lung metastasis found in the IgG, B6H12 or CD47mAb400 treatment groups (data not shown).
CD47 blockade increases intra-tumor migration of macrophages
In human HCC, macrophages have been found to reside at a higher density in the peri-tumoral space than within the tumor [8]. We found a similar pattern of macrophage distribution when we examined macrophage distribution around and within the orthotopic HepG2 tumors. Using F4/80 staining to identify macrophages, we observed a high density of macrophages in the peri-stromal region and a low density of macrophages within the tumor (Fig. 6A). With CD47 blockade using specific antibodies (B6H12 or CD47mAb400), there was an increased proportion of macrophages which migrated into the tumor compared to the IgG control treated animals (Fig. 6B). Furthermore, the density of macrophages which appeared within the tumor increased approximately two-fold more with CD47mAb400 when compared to that of B6H12 (Fig. 6C). These data suggest that CD47 blockade leads to a significant increased migration of macrophases into the HCC mass in vivo.
Fig. 6.

CD47 blockade increases macrophage infiltration of HCC tumors. (A) F4/80 staining (brown) of orthotopic xenografted HepG2 tumors from control IgG-treated mice shows that tumor associated macrophages are concentrated in the peritumoral stroma. (B) Representative images of F4/80 staining of tumors of mice treated with control IgG, B6H12 and CD47mAb400. (C) Macrophage counts in tumors from mice treated with anti-CD47 antibodies were greater than tumors from IgG-treated mice, with CD47mAb400 having the greatest effect (ANOVA p < 1e-4; * p < 0.02; ** p < 0.003). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussion
With these results, we for the first time demonstrate that CD47 blockade using monoclonal antibodies has anti-tumor effects against HCC in vitro and in vivo. Consistent with the hypothesis that the overexpression of CD47 confers a survival advantage to tumor cells, we found CD47 to be expressed at higher levels in human HCC. This was true both in comparisons of HCC cell lines (HepG2 and H3B) compared to isolated, normal hepatocytes, and in patient-derived HCC tumors compared to matched adjacent non-tumor liver or normal liver tissue. The increased CD47 expression in HCC is correlated with the low rate of macrophage phagocytosis of HepG2 and H3B tumor cells in vitro. However, the rate of tumor cell phagocytosis increased significantly with CD47 blockade using the CD47mAb400 or B6H12 monoclonal antibodies, which can specifically interrupt the interaction of CD47 and SIRPα. This effect was not seen with the 2D3 antibody, which is specific to CD47 but does not block SIRPα binding to CD47.
These anti-tumor effects of CD47 blockade in cell culture were also demonstrated using HCC xenograft models in mice. The heterotopic model with subcutaneous implantation allowed direct measurements of tumor size throughout the course of antibody administration. These data correlated with the bioluminescence of HepG2-luc tumors and showed that CD47 blockade with B6H12 or CD47mAb400 antibodies resulted in significant inhibition of tumor growth as compared to control IgG. We also assessed the effects of CD47 blockade in an orthotopic model in which the surrounding liver provided a more native microenvironment in which to evaluate tumor progression and response [9]. Serial imaging of bioluminescence from liver-implanted HepG2-luc tumors showed the inhibition of tumor growth with CD47 blockade as did the final tumor weights 6 weeks after implantation of tumors.
Based on our in vitro observations as well as those of others that tumor cell phagocytosis by macrophages is increased with CD47 blockade [10,11], this anti-tumor effect likely involves increased macrophage activity. We further observed that CD47 blockade resulted in the increased migration of macrophages into the tumor tissue. In HCC, macrophages reside predominantly in the peri-tumor stroma, with fewer macrophages present within the HCC tumor itself [8]. We found that CD47 blockade alters this characteristic distribution of macrophages, and that macrophages had increased infiltration into the HCC tumors following treatment with the CD47 blocking antibodies.
The results of the heterotopic model showed that the engrafted tumors continued to increase in size for several weeks after commencement of CD47 blockade with antibodies. This delayed pattern of response has been recognized to occur with immunomodulatory agents and underscores the importance of serial measurements to accurately assess tumor response [12]. These measurements also showed that the CD47mAb400 antibody resulted in a gradual decrease in tumor size, whereas the B6H12 antibody halted tumor growth but did not decrease tumor size. This tumoristatic effect of B6H12 was also seen with a bladder tumor xenograft study in which B6H12 administration resulted in static tumor growth without a significant decrease in tumor size [11]. These in vivo data were consistent with our finding that in vitro blockade of CD47 with CD47mAb400 enhanced macrophage tumor phagocytosis to a greater extent than CD47mAb B6H12. One possible factor to account for the difference between the two antibodies is that the binding affinity of CD47mAb400 to CD47 is greater than two-fold that of B6H12. Another important observation is that CD47mAb400, which can bind to CD47 on the mouse (host) macrophages, elicited a greater infiltration of macrophages than mAb B6H12 which can only block CD47 on the human HCC cells but cannot bind to CD47 on the mouse macrophages. Our use of the multi-species CD47 binding antibody CD47mAb400 allows for simultaneous assessment of potential toxicities as well as anti-tumor effects of CD47 blockade. In vitro, we did not detect any direct cytotoxicity of B6H12 or CD47mAb400 on normal hepatocytes or HCC tumor cells. This result suggested that the therapeutic effect of antibodies specific to CD47 is not due to direct toxicity to the tumor cells. In vivo, we did not observe any evidence of systemic toxicity with B6H12 or CD47mAb400 antibody administration to NSG mice. Furthermore, NSG mice with heterotopic or orthotopic xenografted tumors maintained a higher body mass with CD47mAb400 treatments, potentially due to an anti-cachectic effect, suggesting a net beneficial effect from the anti-tumor activity.
Although in recent publications [10,11] the main focus of anti-CD47 cancer therapy has been on blockade of the “don’t eat me” signal sent by tumor cell CD47 to host macrophages, additional potential mechanisms may also contribute to the anti-tumor effects of CD47 antibodies. In human tumor xenograft models in immunodeficient NSG mice, the macrophage involvement is likely to be a predominant mechanism since NSG mice completely lack T and B cells and NK cells. However, in mouse tumor models in syngeneic, immunocompetent hosts, additional mechanisms may also augment the anti-tumor effect of CD47mAbs such as effects of CD47 blockade on host T cells and other cells of the immune system. A recent study by Soto-Pantoja et al. [13] suggests that blockade of CD47 on host immune cells relieves an inhibitory effect of the endogenous thrombospondin-1/CD47 interaction thereby augmenting an immune response. In addition, Tseng et al. [14] reported that increased phagocytosis of tumor cells upon treatment with a blocking CD47mAb can lead to an increased adaptive anti-tumor response. Another possible mechanism suggested by Lee et al. [5] that may contribute to an anti-tumor activity following blockade/down regulation of CD47 is the interruption of an autocrine signaling loop involving cathepsin S activation of PAR2. However, in that study, they did not test the role of increased phagocytosis which could have explained their observations.
In summary, we found that CD47 blockade using specific antibodies resulted in increased macrophage phagocytosis of HCC tumor cells. This was correlated with the reduction of xenografted HCC tumors in animals given the CD47mAb400 antibody, and a dramatically increased macrophage infiltration into the tumor tissues. Our results provide a rationale for further evaluation of the clinical efficacy of anti-CD47 antibody therapy for HCC.
Supplementary Material
Acknowledgments
The authors thank Julie L. Prior of the BRIGHT Institute and Molecular Imaging Center for her expertise with the bioluminescent imaging experiments. S.L. received support from the National Institutes of Health (NIH) Fellowship Grants F32HL110473 and K99HL119617. This work was supported in part by a grant from the Elsa U. Pardee Foundation.
Abbreviations
- CD47mAb400
CD47 monoclonal antibody (Clone 400)
- CFSE
carboxy fluorescein diacetate succinimidyl ester
- H3B
Hep3B
- HCC
hepatocelluar carcinoma
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NSG
NOD/SCID/IL2γnull
- SIPRα
signal regulatory protein alpha
Appendix: Supplementary material
Supplementary data to this article can be found online doi:10.1016/j.canlet.2015.02.036.
Footnotes
Conflict of interest
P.T. Manning, R.R. Hiebsch and B.J. Capoccia are employees and shareholders of Vasculox. W.A. Frazier is a shareholder of Vasculox. All other authors declare no conflicts of interest.
References
- 1.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, Brown EJ, et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–2554. doi: 10.4049/jimmunol.167.5.2547. < http://www.ncbi.nlm.nih.gov/pubmed/11509594> (accessed 08.23.14) [DOI] [PubMed] [Google Scholar]
- 4.Braun D, Galibert L, Nakajima T, Saito H, Quang VV, Rubio M, et al. Semimature stage: a checkpoint in a dendritic cell maturation program that allows for functional reversion after signal-regulatory protein-alpha ligation and maturation signals. J Immunol. 2006;177:8550–8559. doi: 10.4049/jimmunol.177.12.8550. < http://www.ncbi.nlm.nih.gov/pubmed/17142753> (accessed 08.23.2014) [DOI] [PubMed] [Google Scholar]
- 5.Lee TKW, Cheung VCH, Lu P, Lau EYT, Ma S, Tang KH, et al. Blockade of CD47 mediated CTSS-PAR2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60(1):179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- 6.Kwong LS, Brown MH, Barclay AN, Hatherley D. Signal-regulatory protein α from the NOD mouse binds human CD47 with an exceptionally high affinity – implications for engraftment of human cells. Immunology. 2014;143:61–67. doi: 10.1111/imm.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. Springer Verlag; New York: 2009. [Google Scholar]
- 8.Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–595. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 9.Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–879. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edris B, Weiskopf K, Volkmer AK, Volkmer JPJP, Willingham SB, Contreras-Trujillo H, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA. 2012;109:6656–6661. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 13.Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, et al. CD47 in the tumor microenvironment limits cooperation between antitumor t-cell immunity and radiotherapy. Cancer Res. 2014;74:6771–6783. doi: 10.1158/0008-5472.CAN-14-0037-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


