Abstract
Background
Retaining HIV patients in medical care promotes access to antiretroviral therapy, viral load suppression, and reduced HIV transmission to partners. We estimate the programmatic costs of a US multisite randomized controlled trial of an intervention to retain HIV patients in care.
Methods
Six academically affiliated HIV clinics randomized patients to intervention (enhanced personal contact with patients across time coupled with basic HIV education) and control [standard of care (SOC)] arms. Retention in care was defined as 4-month visit constancy, that is, at least 1 primary care visit in each 4-month interval over a 12-month period. We used microcosting methods to collect unit costs and measure the quantity of resources used to implement the intervention in each clinic. All fixed and variable labor and nonlabor costs of the intervention were included.
Results
Visit constancy was achieved by 45.7% (280/613) of patients in the SOC arm and by 55.8% (343/615) of patients in the intervention arm, representing an increase of 63 patients (relative improvement 22.1%; 95% confidence interval: 9% to 36%; P <0.01). The total annual cost of the intervention at the 6 clinics was $241,565, the average cost per patient was $393, and the estimated cost per additional patient retained in care beyond SOC was $3834.
Conclusions
Our analyses showed that a retention in care intervention consisting of enhanced personal contact coupled with basic HIV education may be delivered at fairly low cost. These results provide useful information for guiding decisions about planning or scaling-up retention in care interventions for HIV-infected patients.
Keywords: HIV, retention in care, microcosting, cost study
INTRODUCTION
When taken as prescribed, antiretroviral therapy helps HIV-infected patients achieve and maintain viral suppression, which improves their health, and lowers their probability of transmitting HIV to others.1,2 To receive the full benefits of antiretroviral therapy, HIV-infected patients must engage and remain in continuous care.3–6 Some HIV-infected patients, however, delay entry into care, or fail to remain or re-engage in care.7–9 In 2010, the National HIV/AIDS Strategy (NHAS) set a goal to improve retention in HIV care by retaining 80% of patients who are in the Ryan White HIV/AIDS Program.10
An estimated 1.1 million people are living with HIV in the United States, and approximately 964,000 of them have been diagnosed and are aware of their infection.11 Recent studies have found that approximately 75% of HIV-infected patients were linked to HIV care within 3–4 months of diagnosis, but only 50%–60% of them were retained in care.12,13 Mugavero et al14 measured retention in 6 different ways, including a 4-month visit constancy measure, that is, at least 1 kept visit with an HIV primary care provider in each 4-month interval, and found that all 6 measures were significantly associated with viral load suppression.
In addition to observational studies of retention, several studies have reported results of interventions to improve retention in care.15 However, there have been no cost or cost-effectiveness analyses of clinical trials on retention in HIV care. This study is a cost analysis of the programmatic aspects of delivering a clinic-based retention intervention that was part of a multisite randomized controlled trial in the United States.
METHODS
The multisite randomized controlled trial was conducted in 6 academically affiliated HIV clinics at the University of Alabama at Birmingham, AL; Jackson Memorial Hospital, University of Miami, FL; Johns Hopkins University Medical Center, Baltimore, MD; Boston Medical Center, Boston, MA; Downstate Medical Center, State University of New York, Brooklyn, NY; and Thomas Street Health Center, Baylor College of Medicine, Houston, TX. The intervention targeted patients with a recent history of missed visits and those newly enrolled in HIV care. At each clinic, patients were randomly assigned to one of the 2 intervention arms or a standard of care (SOC) comparison arm. The patients in the enhanced contact only (EC-only) intervention arm received basic HIV education and personal contacts across time from dedicated project staff to improve retention in care. The intervention included brief face-to-face meetings with patients at primary care visits to discuss progress and provide positive reinforcement for attending clinic, brief interim phone contacts approximately halfway between primary care appointments, appointment reminder phone calls 7 days and 2 days before scheduled appointments, and missed visit calls. The interventionist did not perform traditional case management activities but referred patients to case managers for unmet needs beyond the scope of the intervention. The details of the intervention and its content are reported elsewhere.16
Patients in the enhanced contact plus skill-building (EC-plus) intervention arm received EC-only elements plus training in behavioral skills relevant to retention in care (eg, organizational skills, problem solving, and communication with providers). Patients in the SOC arm and the intervention arms received usual clinical care that was available to all patients, including social worker or case manager encounters, and preexisting appointment reminders, such as computerized phone calls or letters mailed to patients. We conducted a cost analysis of the programmatic aspects of the EC-only intervention delivered by 2 trained interventionists at each clinic; each interventionist had an average caseload of 50 EC-only patients. The EC-plus arm was excluded in our analyses because they did not produce results that were statistically significantly different from the EC-only arm.16
Patients were eligible to enroll in the trial if they were new patients to the clinic, established patients who had missed 1 or more scheduled visits in past 12 months, or patients who failed to attend clinic in 2 consecutive 6-month periods before enrollment. Eligible patients were those who understood and spoke English or Spanish, were able to give informed consent, were 18 years of age or older (19 in Alabama), were not planning to move out of the area for 12 months, and had not been hospitalized or incarcerated as the reason for a previous missed visit. Recruitment occurred between June 2010 and February 2011. The intervention lasted 12 months from the time each patient enrolled in the study. For all arms, retention in care was defined as at least 1 visit in each of the 3 consecutive 4-month periods (12-month visit constancy).16 Written informed consent was obtained from each participant, and the study was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board (IRB) and the IRBs at each participating clinic.
Cost Measures
We used microcosting direct measurement methods to account for all fixed and variable labor and nonlabor costs attributable to the programmatic implementation of the EC-only intervention.17–21 Labor in the cost calculations was based on hours spent by the 2 interventionists and programmatic and clinical supervisors, per clinic. Fixed costs remained constant regardless of the number of patients in the intervention, and the fixed cost per patient decreased as the number of patients increased.17 The fixed costs in our analyses included project meetings, supervision, general administration, travel for training, and utilities such as telephone costs. They also included durable items, such as computers, printers, and office space. We based office space costs on average rental rates in the local market. We annuitized the cost of durable items (ie, determined a constant annual value of a capital item) over the useful life of each item using straight-line depreciation.22 Variable costs, which increase in direct proportion to the number of patients, included staff time spent on management of trial patients and personal contacts with them. Variable costs also included office supplies.
We collected monthly cost data during October–November 2010 and multiplied the costs by 12 to express them as annualized costs. Staff time data were collected for hours spent on programmatic activities over a typical work week during the intervention period. These included management of trial participants, program supervision, administrative duties, quality assurance, training, and travel. However, patient encounter activity time, that is, the time spent with patients during meetings, on the phone, or discussing appointment-related issues, was based on actual hours spent on activities with patients during the entire intervention period. Staff time spent on intervention activities was multiplied by the wage and fringe rates paid at each clinic to estimate labor costs.
Outcome Measures
Analyses focused on 3 cost measures: annual intervention cost, cost per patient, and cost per additional patient retained in care beyond the number retained in the SOC arm. We also provided microcosting estimates of fixed and variable costs that form the basis of the aggregated costs. Cost per additional patient retained in care was calculated by dividing the additional costs of the intervention arm by the number of intervention patients retained in care beyond that observed in the SOC arm. We used a health care provider’s perspective in that we did not include costs associated with the patients’ time and productivity cost. All costs were expressed in 2010 US dollars.17
RESULTS
Table 1 presents a summary of the results, including the number of patients enrolled in the SOC arm and the intervention arm. Among the patients enrolled, visit constancy was achieved by 280 (45.7%) patients in the SOC arm and by 343 (55.8%) patients in the intervention arm, a difference of 63 patients during the 12-month intervention period. Estimated annual total cost of the intervention in the 6 clinics was $241,565. The median cost over the 6 clinics was $43,523 per year (range: $20,917–$53,587; Table 2). The average cost per patient was $393 ($241,565 divided by 615 intervention patients). The estimated cost per additional patient retained in care was $3834, calculated by dividing the total cost of $241,565 by the 63 patients retained in care beyond that observed in the SOC arm.
TABLE 1.
Summary Results of the Cost of a Multisite Retention in HIV Care Intervention in the United States, Retention in Care Study, 2010–2012
| Intervention | SOC | |
|---|---|---|
| Number of patients | 615 | 613 |
| Number and percentage of patients with 4-month visit constancy* | 343 (55.8%) | 280 (45.7%) |
| Number of additional patients retained in care beyond expected in SOC† | 63 | — |
| Total program cost per year, US$ | 241,565‡ | — |
| Cost per patient in the intervention§ | 393 | — |
| Cost per additional patient retained in care beyond expected in SOC‖ | 3834 | — |
Patient visit constancy defined as at least 1 kept primary care visit in each 4-month interval over 12 months.
Calculated by subtracting the number of SOC arm patients with 4-month visit constancy from intervention arm patients with 4-month constancy. The trial showed a relative improvement for the retention arm compared with the SOC arm to be 22.1%; 95% confidence interval: 9% to 36%; P < 0.01 (Gardner et al16).
Total of itemized costs listed in Table 3. We collected additional costs associated with the EC-only intervention, and we did not collect the cost of SOC.
Calculated by dividing $241,565 by 615 patients in the intervention arm.
Calculated by dividing $241,565 by 63 patients retained in care beyond expected in SOC arm.
TABLE 2.
Variation in Intervention Cost by Primary Care Clinic Site, Retention in Care Study, 2010–2012
| Total of All 6 Clinics | Median | Range | |
|---|---|---|---|
| Overall cost, US$ | 241,565 | 43,523 | 20,917–53,587 |
| Cost per patient | 393 | 415 | 207–531 |
| Cost per patient with 12-month visit constancy | 704 | 703 | 505–864 |
| Labor costs, % of the total | |||
| Labor | 89 | 89 | 81–92 |
| Nonlabor | 11 | 11 | 08–19 |
| Variable costs, % of the total | |||
| Variable | 54 | 52 | 38–62 |
| Fixed | 46 | 48 | 38–62 |
The cost per patient and cost per patient with 12-month visit constancy varied across clinics, ranging from $207 to $531 and from $505 to $864, respectively (Table 2). We found that approximately 89% of total intervention costs were attributable to labor costs, which ranged from 81% to 92% between clinics. Overall, 54% of the total costs were variable costs, primarily attributable to the management of trial patients and patient encounters (Table 3). The cost for patient encounter activities varied by the type of activity, ranging from $1944 for missed visit calls (ie, those occurring shortly after missed primary care visits) to $14,151 for face-to-face meetings with patients at primary care visits (Table 3). The variation in cost across patient encounter activities was associated with staff time spent per patient on each activity (Fig. 1), where the time spent per patient was a function of both time per contact and number of contacts with the patient under the activity (Fig. 2). Staff persons spent approximately 1 hour per patient in face-to-face meetings at primary care visits (ie, on average, four 15-minute contacts during the intervention period). Staff persons spent only about 30 minutes per patient in appointment reminder contacts, and they were spread over approximately 9 reminder contacts during the intervention.
TABLE 3.
Variable and Fixed Costs of a Multisite Retention in HIV Primary Care Intervention in the United States, Retention in Care Study, 2010–2012
| Total Cost of All 6 Clinics (US$) | Median Cost (US$) | |
|---|---|---|
| Variable cost: labor | ||
| Patient management | ||
| Preparing for face-to-face meeting | 14,105 | 2433 |
| Checking and verifying patients’ next scheduled visit | 22,316 | 3985 |
| Waiting to meet with patients | 12,122 | 1581 |
| Writing case notes and other documentation of patient contacts | 13,616 | 1572 |
| Entering data from patient encounter form | 22,784 | 4281 |
| Patient encounter | ||
| Face-to-face meeting with patient at primary care visit (FFT) | 14,151 | 2395 |
| Brief interim phone contact between primary care appointments (BIT) | 9562 | 1614 |
| Appointment reminder phone call before scheduled appointment (ARCT) | 8319 | 1224 |
| Missed visit call shortly after no-show primary care visit (MVT) | 1944 | 169 |
| Contact with case manager to discuss patient unmet needs (CMT) | 428 | 30 |
| Contact with medical team to discuss patient concerns (MTT) | 153 | 27 |
| Variable cost: nonlabor | ||
| Office supplies | 10,459 | 1687 |
| Fixed cost: labor | ||
| Project supervisor’s time on project administration, monitoring, and quality assurance | 18,590 | 3332 |
| Clinical supervisor’s time on reviewing case load and patients visit and meeting with interventionists | 13,360 | 2215 |
| Project interventionists’ time | ||
| Administrative duties | 16,769 | 2333 |
| Patients identification and enrollment | 11,761 | 1788 |
| Project-related meetings | 10,374 | 1488 |
| Quality assurance checks of encounter form | 15,094 | 2099 |
| Travel for off-site training | 9531 | 1435 |
| Fixed cost: nonlabor | ||
| Computer | 3288 | 438 |
| Other equipment | 512 | 102 |
| Facility space | 10,622 | 1299 |
| Utilities | 1709 | 193 |
FIGURE 1.
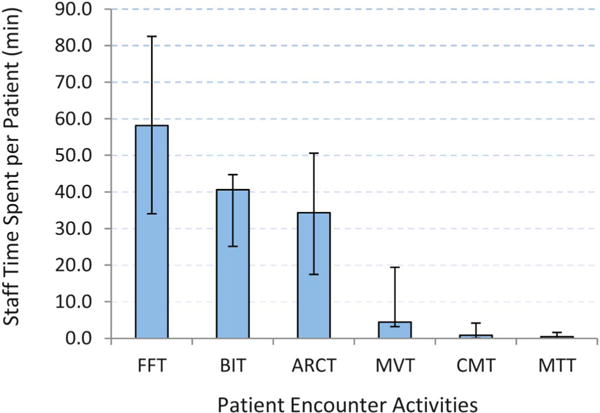
Staff time spent (minutes per person) on patient encounter activities, Retention in Care Study, 2010–2012. Time expressed as the median per-patient contact time over the 6 clinics by activity under EC-only arm. Error bars show ranges on per-patient contact time (minutes). FFT, face-to-face contact time; BIT, brief interim phone contact time; ARCT, appointment reminder phone contact time; MVT, missed visit contact time; CMT, case manager contact time; MTT, medical team contact time.
FIGURE 2.
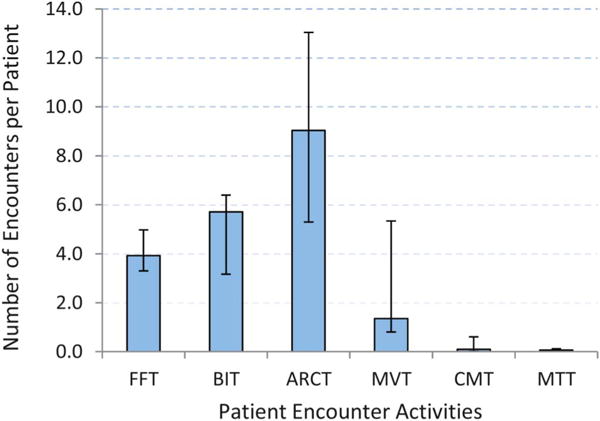
Number and type of patient encounter activities, Retention in Care Study, 2010–2012. Number of encounters expressed as the median number of staff encounters per patient over the 6 clinics by activity under EC-only arm. Error bars show ranges on the number of encounters per patient. FFT, face-to-face contact time; BIT, brief interim phone contact time; ARCT, appointment reminder phone contact time; MVT, missed visit contact time; CMT, case manager contact time; MTT, medical team contact time.
Fixed costs were mainly attributable to supervisory and administrative activities. Supervisory costs related to programmatic supervision, which included project administration, monitoring, and quality assurance ($18,590 total), were calculated separately from clinical supervision, for example, reviewing cases with interventionists ($13,360). Similarly, costs because of project interventionists’ time spent on administrative duties ($16,769), patient identification and enrollment ($11,761), and their meetings ($10,374) were reported separately. The intervention incurred a substantial cost ($15,094) for interventionists to review the patients’ encounter forms for data entry errors and quality control. Office space cost was estimated to be $10,622 per year.
DISCUSSION
We estimated the cost of a retention in care intervention consisting of enhanced personal contacts with patients across time coupled with basic HIV education. We estimated the cost per intervention patient to be $393, and the cost per additional patient retained in HIV primary care beyond that observed in the SOC arm to be $3834. These results provide the first estimates of the programmatic cost of implementing a clinic-based randomized controlled trial to improve retention in care for HIV-infected patients. Our cost estimates are comparable to those for an intervention that improved linkage to care.23 In the Antiretroviral Treatment Access Study (ARTAS), linkage-to-care intervention cost was estimated to be $599 per patient and $3993 (2005 US dollars) per additional client linked to care beyond that observed under the SOC. Thus, our retention in care costs are in line with other published results of HIV interventions that are not easily translated into cost-effectiveness units (eg, mortality and morbidity) but do directly translate to improvement in the care continuum.12,24
We found that labor cost was the largest part (81%– 92%) of total cost across all intervention clinics, consistent with microcosting data reported in the literature on other HIV prevention interventions.20 Clinics may improve the cost efficiency of retention in care interventions through better utilization of labor resources. The intervention cost varied by clinic site in part because of the differences in cost of living and clinic’s performance in delivering the intervention. For example, the median compensation (wage and fringe benefit) per hour of the project staff over the 6 clinics ranged from $24.34 in Birmingham, AL, to $33.63 in Brooklyn, NY (data not reported), thus contributing to the variation in labor cost. The cost per patient over the 6 clinics varied widely, ranging from $207 to $531, with the upper bound at 256% of the lower bound cost, but this variation was less in the cost per patient who achieved 12-month visit constancy, ranging from $505 to $864, with an upper bound only 171% of the lower bound cost.
Despite receiving an interim phone call between primary care appointments, and an appointment reminder phone call before the scheduled appointment, some patients failed to show up to their appointment or canceled the appointment at the last minute, resulting in a cost to clinics if there was insufficient time to schedule another patient in that time slot. However, the trial results showed that the EC-only intervention significantly increased kept visits and decreased no-show visits—without a significant difference in canceled visits—compared with SOC,16 suggesting our cost estimate reflects the true cost of the intervention.
The retention in care trial enrolled, in part, patients at risk for inadequate retention based on their recent history of missed visits, that is, 1 or more missed scheduled visits, or failure to be seen twice in the past 12 months. Other investigators or practitioners might identify other parameters on which to target patients with poor attendance or identify patients who are at risk of falling out of care. Such targeted approaches may be the most efficient use of scarce resources.
Interventions aimed at improving clinic attendance can benefit patients’ health, lower HIV transmission risk, and prevent new infections. Additionally, interventions that reduce missed visit rates have economic implications for the clinic in terms of reimbursement of services and generation of revenue. The microcosting approach showed details of where clinics incurred costs and allowed clinic administrators to better integrate intervention activities within the existing clinic structure.
Our analysis has several limitations. Although we may have misspecified some of the itemized costs, that misspecification may have been minimized by averaging costs over 6 clinics. Our sample was drawn from academic medical centers; thus, caution should be applied when generalizing our findings to other HIV care settings. In our analysis, we used a health care provider’s perspective in that the providers are particularly concerned about the cost of delivering the retention in care intervention, and an accurate estimate of the programmatic cost of delivering the intervention is an essential first step for scaling up the intervention beyond the clinical trial. A successful retention in HIV care intervention might incur a substantial amount of patient’s time and effort, thus our estimate may provide a minimum cost for conducting a cost-effectiveness analysis from a societal perspective.
Given that our outcome was retention in care, we did not collect information on patients’ viral loads. The intervention period was 12-month long, which may not be optimal for observing the full range of retention in care benefits, particularly those that can only be observed after a long period of sustained retention in care. Nevertheless, there is reason to believe from other studies that observed improvements in retention can translate into improved clinical outcomes.3,25
A recent modeling study showed that if a retention in HIV care intervention could generate a 17% or better improvement in retention at the cost of $869 per patient, the intervention would be cost saving (CDC, unpublished data, 2014). Because our retention in care trial had a higher intervention efficacy (22.1%) and a lower estimated cost per patient ($393), the intervention is likely to be cost saving.
CONCLUSIONS
Our analyses provide an estimated cost of a clinic-based retention in care intervention that included enhanced personal contact with patients coupled with basic HIV education. The results demonstrate that the intervention may be delivered to patients at a fairly low cost and can significantly boost visit constancy over a 12-month intervention period. Importantly, the cost analyses we provide could be replicated in other settings to inform decisions about initiating or expanding retention in care interventions for HIV-infected patients.
Acknowledgments
Supported by the Centers for Disease Control and Prevention (CDC) and the Health Resources and Services Administration (HRSA) (contracts 200-2007-23685, 200-2007-23690, 200-2007-23689, 200-2007-23687, 200-2007-23684, and 200-2007-23692).
M.S. has received institutional grant support from BMS, Gilead, Merck, ViiV, Jannsen, and GSK.
Footnotes
Presented in part as an oral presentation at the American Society of Health Economists, Fifth Biennial Conference, June 22–25, 2014, Los Angeles, CA.
The remaining authors have no conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV surveillance–United States, 1981–2008. MMWR Morb Mortal Wkly Rep. 2011;60:689–693. [PubMed] [Google Scholar]
- 3.Mugavero MJ, Amico KR, Westfall AO, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59:86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehia BR, Fleishman JA, Metlay JP, et al. Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. JAMA. 2012;308:339–342. doi: 10.1001/jama.2012.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18:81–88. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care and STDs. 2009;23:41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleishman JA, Yehia BR, Moore RD, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249–259. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dombrowski J, Fleming M, Simoni J, et al. Surveillance-based outreach to promote HIV care engagement and ARV use: results from the pilot phase of a health department intervention. Paper presented at: CROI 20132012; March 3–6, 2013; Atlanta, GA. (abstract number 1031b) [Google Scholar]
- 10.The White House Office of National AIDS Policy. National HIV/AIDS Strategy. Washington, DC: Jul, 2010. Available at: http://www.whitehouse.gov/administration/eop/onap/nhas. Accessed August 15, 2013. [Google Scholar]
- 11.Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data–United States and 6 US Dependent Areas–2011. 5. Vol. 18. Atlanta, GA: Centers for Disease Control and Prevention; 2013. (HIV Surveillance Supplemental Report 2013). [Google Scholar]
- 12.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment–United States. MMWR Morb Mortal Wkly Rep. 2011;60:1618–1623. [PubMed] [Google Scholar]
- 13.Marks G, Gardner LI, Craw J, et al. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24:2665–2678. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 14.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61:574–580. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higa DH, Marks G, Crepaz N, et al. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9:313–325. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner LI, Giordano TP, Marks G, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59:725–734. doi: 10.1093/cid/ciu357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold MR, Siegel JE, Russell LB, et al. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 18.Haddix AC, Teutsch SM, Corso PS. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 19.Frick KD. Microcosting quantity data collection methods. Med Care. 2009;47(7 Suppl 1):S76–S81. doi: 10.1097/MLR.0b013e31819bc064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrestha RK, Sansom SL, Farnham PG. Comparison of methods for estimating the cost of human immunodeficiency virus-testing interventions. J Public Health Manag Pract. 2012;18:259–267. doi: 10.1097/PHH.0b013e31822b2077. [DOI] [PubMed] [Google Scholar]
- 21.Smith MW, Barnett PG. Direct measurement of health care costs. Med Care Res Rev. 2003;60:74S–91S. doi: 10.1177/1077558703257001. [DOI] [PubMed] [Google Scholar]
- 22.Drummond MF, McGuire A. Economic Evaluation in Health Care: Merging Theory With Practice. Oxford, NY: Oxford University Press; 2001. [Google Scholar]
- 23.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 24.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]


