Abstract
Cartilage damaged by trauma has a limited capacity to regenerate. Current methods for treating small chondral defects include palliative treatment with arthroscopic debridement and lavage, reparative treatment with marrow stimulation techniques (e.g. microfracture), and restorative treatment, including osteochondral grafting and autologous chondrocyte implantation. Larger defects are treated by osteochondral allografting or total joint replacements. However, the future of treating cartilage defects lies in providing biologic solutions through cartilage regeneration. Laboratory and clinical studies have examined the treatment of larger lesions using tissue engineered cartilage. Regenerated cartilage can be derived from various cell types, including chondrocytes, mesenchymal stem cells, and pluripotent stem cells. Common scaffolding materials include proteins, carbohydrates, synthetic materials, and composite polymers. Scaffolds may be woven, spun into nanofibers, or configured as hydrogels. Chondrogenesis may be enhanced with the application of chondroinductive growth factors. Finally, bioreactors are being developed to enhance nutrient delivery and provide mechanical stimulation to tissue-engineered cartilage ex vivo. The multi-disciplinary approaches currently being developed to produce cartilage promise to bring the dream of cartilage regeneration in clinical use to reality.
Introduction
Osteoarthritis is a condition characterized by cartilage destruction that affects over 27 million adults in the United States1 and has limited treatment options. Cartilage has a decreased ability to self-repair because of its inherent limited vascularity that results in poor replicative capacity of chondrocytes, the main cell type in cartilage. Current methods for treating well-defined osteochondral defects include drilling, autologous chondrocyte implantation, and osteochondral allografting. These treatment options result in the formation of fibrocartilage that contains both collagen type I and type II, which has decreased strength and resilience compared to cartilage. The coefficient of friction of the fibrocartilage scar tissue is also higher than cartilage, which can hinder motion compared to the smooth surface of cartilage and lead to earlier degeneration. Degenerated joints with larger cartilage defects or lesions, as seen in osteoarthritis, are often treated ultimately by total joint replacement with metal implants. While these current treatments reduce pain and increase mobility, there is an increasing need for treatment options that restore the native biological properties of cartilage.
Due to the limited capacity of damaged cartilage to regenerate, and the potential morbidity associated with implanting or transferring bone and cartilage, cartilage regeneration is an attractive alternative. The field of cartilage tissue engineering is being advanced to create biologically compatible, synthetic cartilage constructs. These constructs are composed of appropriate cell types seeded within biomaterial scaffolds to produce a durable tissue repair system that can potentially be implanted in a single step. The sections below describe different components that are necessary to construct tissue engineered cartilage, including the various constituent cell types, biomimetic scaffolds, inductive bioactive factors, gene therapy, and the use of bioreactors for ex vivo cartilage tissue engineering.
Cell types
Chondrocytes
The initial cell based therapy for repairing cartilage lesions logically began with chondrocytes, the principal cell type found within cartilage. The technique of autologous chondrocyte implantation (ACI) was first described by Brittberg et.al.,2 where chondrocytes are harvested from a non-weight bearing portion of the joint and are expanded ex vivo. During a separate surgical procedure, an autologous periosteal flap is harvested, is sewn over the osteochondral defect, and chondrocytes are injected in a collagen-containing suspension into the defect and sealed with fibrin glue. While this procedure decreases pain and swelling for limited-size lesions, there is associated donor site morbidity from harvesting the periosteum and chondrocytes.3 The second-generation ACI technique is an improvement, since a collagen membrane is used instead of periosteum to cover the lesion and prevents compromise to other regions of bone or cartilage. This technique is also known as collagen-covered ACI (CACI). In the third generation, further improvement was made by placing chondrocytes onto biomaterial scaffolds that were subsequently placed into lesions. This technique is also known as matrix-assisted ACI (MACI), and has the advantage of maintaining chondrocytes within the matrix, instead of injecting them within the lesion. While these techniques have been shown to be effective for improving patient function, these harvested chondrocytes are grown in vitro, which can lead to dedifferentiation or loss of phenotype, which renders them useless for the regeneration of hyaline cartilage.4 A more viable chondrocyte option are neonatal or fetal chondrocytes, which grow significantly faster than adult chondrocytes and more closely resemble cells from native cartilage, with higher proteoglycan, collagen type II, and collagen type IX contents.5 However, as with adult chondrocytes, juvenile chondrocytes also have limited availability.
Pluripotent stem cells
Given these limitations, other cell types have been used that can differentiate into chondrocytes. Pluripotent stem cells, such as embryonic stem cells (ESCs), are attractive options for tissue regeneration given their potential for indefinite self-renewal and their ability to differentiate into multiple tissue types. However, ESCs are derived from the inner cell mass of blastocyst stage embryos,6 and their derivation raises ethical concerns. An alternative is another pluripotent cell type called induced pluripotent stem cells (iPSCs). These are ESC-like stem cells that are developed from a patient’s own skin or blood cells by means of gene transduction using ESC-specific transcription factors, and in principle can be used in multiple tissue applications.7 Although these pluripotent cells have multiple capabilities, their undifferentiated nature and tendency to grow without restraint may lead to the development of tumor, as teratoma formation in vivo is well recognized.8 Clinical studies utilizing ESCs are currently underway for implantation in spinal cord regeneration and for treating Stargardt’s Macular Degeneration. While ESCs and iPSCs may be viable options for future treatment of these conditions, their direct differentiation into chondrocytes is at an early stage of investigation (Ref), and there are no current clinical studies examining the use of pluripotent stem cells to treat cartilage damage.
Mesenchymal stem cells
Another cell alternative for regenerating cartilage that has minimal tumorigenic capacity is mesenchymal stem cells (MSCs), or adult tissue derived cells that have a high proliferative capacity and are multipotent in terms of differentiation capability. MSCs have the ability to differentiate along various cell lineages including chondrocytes, adipocytes, osteoblasts, and myocytes.9 MSCs are an ideal option for cartilage regeneration because they represent a readily available and accessible supply of cells, and have high expansion and differentiation capacity. Growth factors, such as transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP), are used to induce chondrogenesis in MSCs. MSCs can also migrate and incorporate into musculoskeletal tissue, exert effects on tissue microenvironment, and have anti-inflammatory and immunosuppressive properties that may be useful when treating osteoarthritis or rheumatoid arthritis.10 A study in a porcine model demonstrated that an intra-articular injection of MSCs with hyaluronic acid could facilitate cartilage regeneration after induced injury.11
Of the multiple sources of MSCs reported, bone marrow stem cells (BMSCs) are the most commonly used. An in vitro model demonstrated that osteochondral defects in rabbits could be marginally repaired with injected BMSCs and fully repaired with BMSCs embedded in a synthetic extracellular matrix.12 Full thickness osteochondral defects created in the trochlear ridge of femurs in horses demonstrated improved macroscopic filling of the lesion and higher collagen type II content when microfracture was combined with concentrated bone marrow aspirate containing MSCs versus microfracture alone.13 While multiple animal studies have been performed examining the capacity of BMSCs to repair cartilage, few clinical studies have been conducted. Initial case reports utilizing BMSCs embedded in a collagen gel implanted within an autologous periosteum cover to treat patellar articular cartilage defects demonstrated pain-free walking for over 4 years after the index procedure.14 Another study compared patients who did and did not undergo BMSC transplantation for medial femoral condyle osteochondral defects, and results demonstrated that patients had similar clinical outcomes, but those with BMSC transplantation improved arthroscopic and histologic articular cartilage growth 42 weeks post-operatively.15
Besides BMSCs, adipose-derived stem cells (ADSCs) are another MSC alternative that are less chondrogenic than BMSCs, but are more plentiful and easily accessible.16 These cells can produce cartilage with a high total collagen content but lower levels of collagen type II. Previous studies comparing chondrogenic capacity between MSCs derived from different sites demonstrated that synovial-derived stem cells (SDSCs) were superior to bone marrow, periosteum, skeletal muscle, and adipose.17 However, SDSCs may retain some fibroblastic capacity after implantation, making this cell type less effective as a cartilage substitute. A summary of MSC types potentially applicable for cartilage regeneration is provided in Table 1.
Table 1.
Cell types used for cartilage tissue engineering
| Cells | Advantages | Disadvantages | |
|---|---|---|---|
| Differentiated | Adult chondrocytes |
|
|
| Neonatal/Fetal chondrocytes |
|
|
|
|
Pluripotent stem cells |
Embryonic stem cells (ESCs) |
|
|
| Induced pluripotent stem cells (iPSCs) |
|
|
|
|
Mesenchymal stem cells (MSCs) |
Bone marrow stem cells (BMSCs) |
|
|
| Adipose-derived stem cells (ADSCs) |
|
|
|
| Synovial-derived stem cells (SDSCs) |
|
|
Scaffolds
For tissue engineering, cells must be seeded on a temporary structure to establish a three-dimensional structure that retains the seeded cells and provides mechanical support to aid in the development of cartilage over time. Thus, scaffold biomaterials must be biodegradable, noncytotoxic, mechanically competent (similar to surrounding tissue), able to regulate cell activity, have appropriate surface chemistry, and may be shaped into different sizes and forms. There are four main groups of scaffolding that may be applied for cartilage tissue engineering: (1) protein-based polymers, (2) carbohydrate-based polymers, (3) synthetic polymers, and (4) composite polymers, which contain combinations of biomaterials from the first three groups (Table 2).18
Table 2.
Commonly used scaffold biomaterials in cartilage tissue engineering
| Scaffold Class | Scaffolding Material | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Protein-based | Fibrin |
|
|
|
| Collagen |
|
|
NeoCart | |
|
Carbohydrate- based |
Hyaluronic acid |
|
|
Hyalograft C autograft |
| Alginate |
|
|
||
|
Synthetic polymers |
Polylactic acid (PLA) |
|
|
Kensey Nash Cartilage Repair Device |
| Polyglycolic acid (PGA) |
|
|
||
| Polycaprolactone (PCL) |
|
|
||
| Polylactic-co-glycolic acid (PLGA) |
|
|
TruFit | |
| Bioceramics | Hydroxyapatite (Ca10(PO4)6(OH)2) |
|
|
MaioRegen |
Protein-based polymers
Fibrin, gelatin, and collagen are examples of protein-based polymers used in bioengineered scaffolds. Fibrin, a protein matrix derived from fibrinogen, is a key component of blood clots, and gelatin is formed from denatured collagen and can bind growth factors, proteins, and peptides, as well as allow for cell adhesions. Since collagen is the major structural component of the extracellular matrix, its role as a scaffolding material allows cells to retain their phenotypes and cellular events are regulated by integrin binding.19
In terms of clinical studies, NeoCart (Histogenics, Waltham, MA), a collagen type I scaffold seeded with autologous chondrocytes, was implanted in 21 patients with grade III chondral defects in the distal femur. These patients were randomly compared to 9 patients who received microfracture treatment for the same lesion. At 2-years follow-up, patients noted that they had significantly reduced pain scores than prior to surgery, improved function, and increased motion compared to the microfracture group.20
Carbohydrate-based polymers
Carbohydrates, such as hylauronan, alginate, chitosan, agarose, and polyethylene glycol (PEG), have been also used in hydrogel scaffolds. These scaffolds are comprised of crosslinked polymers that absorb a great deal of water, which is similar to the properties of cartilage extracellular matrix. They are also efficient in cell encapsulation, allowing chondrocytes to maintain their spherical morphology within the scaffold.21 Hydrogel scaffolds may be modified by their mechanism of gelation, the inclusion of synthetic materials, and the addition of growth factors to enhance chondrogenesis.
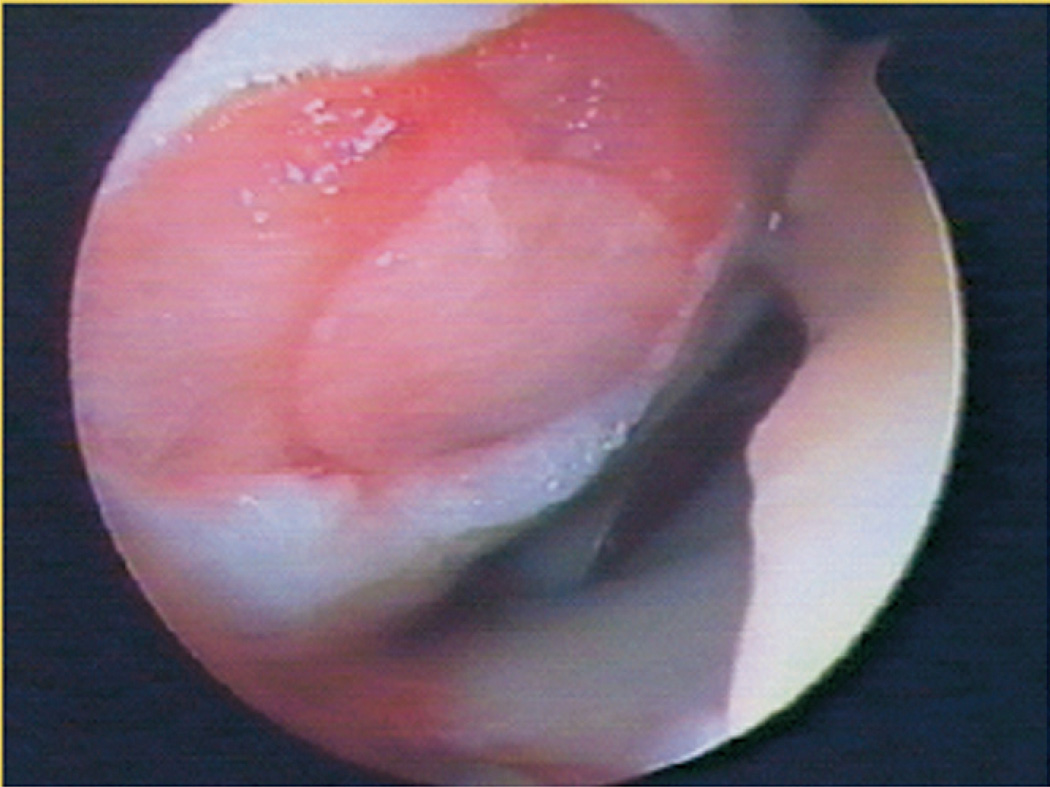
One hyaluronic acid-based scaffold (Hyalograft C Autograft, Anika Therapeutics, Bedford, MA) was tested in patients with 2-year follow-up that demonstrated improved functional scores.22 At 7-year follow-up, 62 patients who were treated with this scaffold for cartilage defects that were an average of 2.5 cm2 were clinically and radiographically evaluated.23 Significant improvement in function and decrease in pain was seen in study patients, and post-operative MRI evaluation showed complete filling of the cartilage defect (57%) and complete integration of the scaffold (62%). An example of an implanted Hyalograft C autograft can be seen in Figure 1, but this product is not yet available in the United States.
Figure 1. Hyalograft C Autograft.
(Anika Therapeutics, Bedford, MA). Implantation of a hyaluronic acid-based scaffold used to treat cartilage defects that showed cartilage integration and significant improvement in patient function. (Reprinted with permission from Marcacci M, Kon E, Zaffagnini S, Iacono F, Filardo G, and Delcogilano M. Autologous Chondrocytes in a Hyaluronic Acid Scaffold. Operative Techniques in Orthopaedics. Oct 2006: 16(4), 266–70.)
In addition to hyaluronic acid, alginate has also been used in scaffolds seeded with adult allogenic chondrocytes and implanted in 21 patients.24 At a mean of 6.1 years follow-up, clinical scores improved (Western Ontario and McMaster Universities Osteoarthritis Index – WOMAC and visual analog scale – VAS), and MRI imaging remained stable. Four failures were reported, including periosteal flap loosening, delamination of repair tissue, decline of clinical function, and MRI thinning of the repair tissue. Despite these failures, there is promise in the development of carbohydrate-based polymers as scaffolding material for cartilage.
Synthetic polymers
Synthetic polymer-based scaffolds using polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), and polylactic-co-glycolic acid (PLGA) are the most common, and the materials may be woven or made into electrospun nanofibers.25 A synthetic scaffold containing PLGA, PGA, and calcium sulfate was implanted in patients with patellofemoral cartilage defects and was followed post-operatively up to 2 years.26 In contrast to other scaffolds, the results of this study demonstrated that there were improved short-term results, but without the restoration of subchondral bone with the hyaline cartilage, the authors could not endorse the use of this scaffolding construct. PLGA was also combined with calcium sulfate in the commercially available TruFit Plug (Smith & Nephew, Nashville, TN), which is a synthetic, resorbable biphasic implant that encourages the growth of cartilage and bone.27 PLA serves as the scaffold for the Kensey Nash Cartilage Repair Device (CRD) (Kensey Nash, Exton, PA), which contains β-tricalcium phosphate to stimulate bone growth and a collagen type I matrix to stimulate the growth of cartilage.28 The Kensey Nash CRD only received approved for use in Europe in 2010 and is not yet available in the USA.
Other synthetic scaffolding materials include polybutyric acid, carbon fiber, Dacron®, and Teflon®. Ceramics, such as hydroxyapatite (HA), tricalcium phosphate, and bioactive glass, are also considered when developing scaffolds for cartilage replantation, since these ceramic materials promote the growth of a bone-like apatite layer to anchor the overlying cartilage scaffold to the existing bed of the osteochondral defect. A recent study was conducted evaluating the treatment of knee chondral or osteochondral defects using a 3D scaffold (MaioRegen, Fin-Ceramica Faenza S.p.A.) with layered collagen type I fibrils and HA nanoparticles to form a synthetic cartilage and bone scaffold.29 The size of the treated lesions ranged from 1.5 cm2 to 6.0 cm2, and at two-year follow-up, patient clinical scores improved, especially in active patients. MRI results demonstrated complete graft integration in 70% of patients. A large, multicenter clinical trial is currently being conducted in Europe to further study the use of this scaffold in treating osteochondral defects.
Growth Factors
While cells and scaffolds comprise the network by which cartilage is regenerated, growth factors are biologically active polypeptides that are endogenous molecules that can be applied to stimulate cell growth, enhance chondrogenesis, and augment treatment of cartilage defects.
Transforming Growth Factor (TGF) superfamily
Members of the TGF-β superfamily are often used to stimulate cartilage repair, including TGF-β1, BMP-2, and BMP-7, TGF-β3, and cartilage-derived morphogenetic proteins (CDMP-1 and CDMP-2), to induce chondrogenic differentiation and stimulate production of cartilage extracellular matrix.30 The most common growth factor used for stimulating chondrogenesis is TGF-β, which stimulates extracellular matrix (ECM) synthesis, chondrogenesis in the synovial lining, and of BMSCs, while decreasing the catabolic activity of IL-1. BMP-2 has been used in other orthopaedic applications for stimulating bone growth, either in the setting of fracture healing or the formation of a fusion mass, but has the potential to stimulate matrix synthesis and reverse chondrocyte dedifferentiation. Finally, BMP-7 helps to stimulating cartilage matrix synthesis, acts synergistically with other anabolic growth factors, and also inhibits catabolic factors, such as matrix metalloproteinase-1 (MMP-1), MMP-13, IL-1, Il-6, and IL-8.
Fibroblast growth factor (FGF) family
Members of the fibroblast growth factor (FGF) family, specifically FGF-2 (basic FGF, bFGF) and FGF-18, act by binding to cell surface receptors, promote anabolic pathways, and decrease the activity of the catabolic enzyme, aggrecanase. In mouse, subcutaneous administration of FGF-2 suppressed OA, while FGF-2 knock-out mice were found to have accelerated OA.31 However, caution must be used when using FGF-2, as higher doses of FGF-2 may promote increased inflammation by antagonizing insulin-like growth factor (IGF)-1 and upregulating MMPs.
Insulin-like growth factor (IGF)
IGF-1 is another growth factor that helps to maintain articular cartilage integrity and induces anabolic effects for cartilage repair while decreasing catabolic ones. IGF-1 works better in combination with other growth factors, such as TGF-β and BMP-7. Mice with chronic IGF-1 deficiency are more likely to develop articular cartilage lesions, and increased IGF-1 results in increased protection of the synovial membrane.32
Platelet derived growth factor (PDGF)
PDGF is a chemotactic factor for mesenchymal cells, and has been shown to stimulate wound healing and promote the formation of cartilage with increased proteoglycan production and cell proliferation.33 PDGF has also been shown to suppress IL-1β induced cartilage degradation by downregulating NF-κB signaling.
Platelet-rich plasma (PRP)
PRP is also considered a potential source of growth factors, given its role in wound healing and in treating other musculoskeletal diseases. Clinical studies have been conducted evaluating the role of intraarticular injections of PRP as a treatment for OA. One study by Spaková et.al. demonstrated that patients with knee OA who received three intraarticular injections of PRP had improved clinical function and decreased pain compared to knee OA patients who received three intraarticular injections of hyaluronic acid.34 Treatment of hip OA patients with ultrasound-guided injection of PRP into the affected hip demonstrated improved patient assessment scores (WOMAC and Harris Hip score) and decreased pain at 6 months follow-up.35
Gene Therapy
The concept of utilizing gene therapy to treat musculoskeletal conditions was first proposed for the treatment of rheumatoid arthritis.36 Biologic factors applied to suppress cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), have been integral for the treatment of rheumatoid arthritis. The search for therapeutic targets to treat cartilage degradation through viral or non-viral vectors by in vivo or ex vivo means are important topics of research that are currently being investigated for clinical application.
Therapeutic targets
For osteoarthritis, five gene therapeutic targets that enhance chondrogenesis have been extensively studied: (1) growth factors – including TGF-β, BMP, FGF, IGF-1, and epidermal growth factor (EGF); (2) transcription factors – SOX9; (3) signal transduction molecules – SMADs; (4) pro-inflammatory cytokine inhibition – TNF-α and IL-1β; and (5) apoptosis or senescence inhibition – Bcl-2, Bcl-XL, and inducible nitric oxide synthase (iNOS). Of these molecules, only TGF-β1 has been studied in the clinical setting. Phase I of a clinical trial examining the treatment of knee arthritis using TissueGene-C (TG-C, TissueGene Inc.), a cell-mediated gene therapy system where allogenic chondrocytes express TGF-β1, has been completed.37 The safety of the product was established with minor local reactions from administration of the injection. Further studies are being conducted to determine functional improvements, clinical results, and radiographic parameters used to evaluate the treatment of osteoarthritis.
Vectors
The vectors used to deliver gene therapy treatment include viral and non-viral constructs. Non-viral delivery methods, using naked DNA, liposomes, and complexed DNA have the advantage that they are non-infectious, but they are also transient. Viral vectors, including adenovirus, adeno-associated virus, herpes simplex virus, foamyvirus, and lentivirus, are beneficial since they allow for stable gene expression, since the DNA is inserted into the host chromosome. However, by altering the host DNA, there is the potential for insertional mutagenesis. In addition, there is also the possibility of host immune reaction to viral proteins.
Delivery
Gene delivery can be conducted in vivo or ex vivo, depending on the location of delivery. Gene therapy delivery to synovium is more ideal than cartilage, since synovium has a larger surface area with a thin lining of synoviocytes. Delivery to both tissues using an ex vivo approach is beneficial in that gene transfer can be used to augment cartilage repair. However, in vivo approaches are less labor intensive and costly than cell culture and maintenance ex vivo. Using gene constructs of appropriate chondrogenesis enhancing factors, gene therapy delivery to chondrocytes and MSCs can also enhance cartilage formation.38
Bioreactors
The engineered cartilage tissue construct, consisting of cells, scaffold, and growth factors must be cultured in a controlled manner that facilitates nutrient supply, metabolite exchange, and generation of a three-dimensional construct within a contained environment that mimics physiological conditions. This is generally accomplished by using automated bioreactors that are capable of delivering mechanobiological activation to cell-loaded scaffolds that are used to develop ex vivo cartilage tissue. Automated processing using bioreactors also increases reproducibility and decreases contamination.39 There are three main types of bioreactors that have been used for cartilage tissue constructs: (1) hydrostatic bioreactors, (2) dynamic loading bioreactors, and (3) hydrodynamic bioreactors.
Hydrostatic bioreactors
Hydrostatic bioreactors are medium-filled chambers that can administer hydrostatic pressure to enhance chondrogenesis of MSCs and to condition engineered tissue constructs by mimicking the hydrostatic load in joints.40
Dynamic loading bioreactors
Dynamic loading bioreactors are motorized to generate mechanical loading to cells or tissue constructs, either in confined or unconfined conformations, at specific frequencies and magnitudes of strain.40 This type of dynamic loading also mimics certain aspects of physiologic weight-bearing and has been found to improve MSC chondrogenesis and mechanical properties of engineered cartilage.
Hydrodynamic bioreactors
Hydrodynamic bioreactors generally consist of instrumentations that rotate or agitate to enhance nutrient transport, gas exchange, and metabolite removal to engineered constructs either suspended in medium or fixed in place. Use of hydrodynamic bioreactors on chondrogenic constructs has been reported to enhance matrix proteoglycan production, resulting in constructs with compressive properties more similar to native cartilage.4
Conclusions
The field of cartilage tissue engineering has advanced quickly over the last decade and a large number of novel approaches, exemplified by those described above, have been developed. However, while early results of these approaches have been promising, engineered cartilage with properties identical to those of native cartilage is currently unavailable. Significant obstacles remain, and the future of cartilage engineering lies in addressing issues such as ensuring optimal and stable chondrogenic cellular phenotype and cartilage matrix production, preventing matrix and cellular degradation, promoting appropriate cartilage integration, and delivering antioxidant and anti-inflammatory factors to provide durable cartilage constructs. Regulatory hurdles, as well as safety, viability, and potential immunogenicity of the engineered tissue are all outstanding challenges. The variety and depth of emerging technologies have the potential to revolutionize the field of cartilage regeneration, which will continue to develop and flourish over the next decade.
Table 3.
Common growth factors used in cartilage regeneration
| Growth Factor | General effects on chondrocytes/cartilage |
|---|---|
| BMP-2 | Stimulates ECM production Increase ECM turnover Increases aggregan degradation |
| BMP-7 | Stimulates ECM production Inhibits cartilage degradation by decreasing ILs and MMPs |
| FGF-2 | Increases aggregan degradation Inhibits proteoglycan synthesis Upregulates MMPs |
| FGF-18 | Stimulates ECM (injured joints) production Increases chondrocyte proliferation |
| IGF-1 | Stimulates ECM production Decreases ECM catabolism |
| PDGF | Chemotactic factor for mesenchymal cells Suppresses IL-1 induced cartilage degradation |
| PRP | Biological cocktail of multiple growth factors and cytokines |
| TGF-β1 | Stimulates ECM production Inhibits cartilage degradation by decreasing ILs and MMPs |
Abbreviations: BMP, Bone morphogenetic protein; ECM, Extracellular matrix; FGF, Fibroblast growth factor; IGF, Insulin-like growth factor; IL, Interleukin; MMP, Matrix metalloproteinase; PGDF, Platelet-derived growth factor; PRP, platelet-rich plasma; and TGF, Transforming growth factor
Table 4.
Gene delivery targets used for cartilage regeneration
| Gene targets | |
|---|---|
| Growth Factors | TGF-β |
| BMP | |
| FGF | |
| IGF-1β | |
| EGF | |
| Transcription factors | SOX9 |
| Signal transduction molecules | SMADs |
| Pro-inflammatory cytokine inhibition | TNF-α |
| IL-1 | |
| Apoptosis | Bcl-2, Bcl-XL |
| iNOS |
Abbreviations: Bcl, B-cell lymphoma; BMP – Bone morphogenetic protein; TGF, Transforming growth factor; FGF, Fibroblast growth factor; IGF – Insulin-like growth factor; EGF, Epidermal growth factor; SOX, Sry-related HMG box; IL, Interleukin; iNOS, inducible nitric oxide synthase
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Wood JJ, Malek MA, Frassica FJ, et al. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am. 2006;88:503–507. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]
- 4.Kuo CK, Li WJ, Mauck RL, Tuan RS. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 5.Adkisson HDt, Martin JA, Amendola RL, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 7.Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toh WS, Lee EH, Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. 2011;7:544–559. doi: 10.1007/s12015-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 9.Tuan RS. Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis Rheum. 2006;54:3075–3078. doi: 10.1002/art.22148. [DOI] [PubMed] [Google Scholar]
- 10.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem Cells. 2007;25:2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 13.Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 14.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 15.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 16.Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. 2010 Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530–2540. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 18.Safran MR, Kim H, Zaffagnini S. The use of scaffolds in the management of articular cartilage injury. J Am Acad Orthop Surg. 2008;16:306–311. doi: 10.5435/00124635-200806000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Ruoslahti E. RGD and other recognition sequences for integrins. Annual review of cell and developmental biology. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 20.Crawford DC, DeBerardino TM, Williams RJ., 3rd NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94:979–989. doi: 10.2106/JBJS.K.00533. [DOI] [PubMed] [Google Scholar]
- 21.Reddi AH, Becerra J, Andrades JA. Nanomaterials and hydrogel scaffolds for articular cartilage regeneration. Tissue Eng Part B Rev. 2011;17:301–305. doi: 10.1089/ten.TEB.2011.0141. [DOI] [PubMed] [Google Scholar]
- 22.Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763–1773. doi: 10.1177/0363546506288853. [DOI] [PubMed] [Google Scholar]
- 23.Filardo G, Kon E, Di Martino A, Iacono F, Marcacci M. Arthroscopic Second-Generation Autologous Chondrocyte Implantation: A Prospective 7-Year Follow-up Study. Am J Sports Med. 2011;39:2153–2160. doi: 10.1177/0363546511415658. [DOI] [PubMed] [Google Scholar]
- 24.Dhollander AA, Verdonk PC, Lambrecht S, et al. Midterm Results of the Treatment of Cartilage Defects in the Knee Using Alginate Beads Containing Human Mature Allogenic Chondrocytes. Am J Sports Med. 2011 doi: 10.1177/0363546511423013. [DOI] [PubMed] [Google Scholar]
- 25.Li WJ, Tuli R, Okafor C, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Joshi N, Reverte-Vinaixa M, Diaz-Ferreiro EW, Dominguez-Oronoz R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients: magnetic resonance imaging and clinical evaluation. Am J Sports Med. 2012;40:1289–1295. doi: 10.1177/0363546512441585. [DOI] [PubMed] [Google Scholar]
- 27.Williams RJ, Gamradt SC. Articular cartilage repair using a resorbable matrix scaffold. Instr Course Lect. 2008;57:563–571. [PubMed] [Google Scholar]
- 28.Elguizaoui S, Flanigan DC, Harris JD, Parsons E, Litsky AS, Siston RA. Proud osteochondral autograft versus synthetic plugs - Contact pressures with cyclical loading in a bovine knee model. The Knee. 2012 doi: 10.1016/j.knee.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Kon E, Delcogliano M, Filardo G, Busacca M, Di Martino A, Marcacci M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. Am J Sports Med. 2011;39:1180–1190. doi: 10.1177/0363546510392711. [DOI] [PubMed] [Google Scholar]
- 30.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chia SL, Sawaji Y, Burleigh A, et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 32.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. The Journal of bone and joint surgery British volume. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2012;91:411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez M, Guadilla J, Fiz N, Andia I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford) 2012;51:144–150. doi: 10.1093/rheumatology/ker303. [DOI] [PubMed] [Google Scholar]
- 36.Evans CH, Robbins PD, Ghivizzani SC, et al. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A. 2005;102:8698–8703. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14:247–256. doi: 10.3109/14653249.2011.629645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinert AF, Noth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury. 2008;39(Suppl 1):S97–S113. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hildner F, Albrecht C, Gabriel C, Redl H, van Griensven M. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.386. [DOI] [PubMed] [Google Scholar]
- 40.Partap S, Blunkett N, O'Brien F. Tissue Engineering. Vukovar, Croatia: In-Tech; 2010. [Google Scholar]
Ref
- Koyama N, Miura M, Nakao K, Kondo E, Fujii T, Taura D, Kanamoto N, Sone M, Yasoda A, Arai H, Bessho K, Nakao K. Human Induced Pluripotent Stem Cells Differentiated into Chondrogenic Lineage Via Generation of Mesenchymal Progenitor Cells. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0127. In press. [DOI] [PubMed] [Google Scholar]