Abstract
Interactions between proteins and cell membranes are critical for biological processes such as transmembrane signaling, and specific components of the membrane may play roles in helping to organize or mandate particular conformations of both integral and peripheral membrane proteins. One example of a signaling enzyme whose function is dependent on membrane binding and whose activity is affected by specific lipid components is G protein-coupled receptor (GPCR) kinase 2 (GRK2). Efficient GRK2-mediated phosphorylation of activated GPCRs is dependent not only on its recruitment to the membrane by heterotrimeric Gβγ subunits, but also on the presence of highly negatively charged lipids, in particular phosphatidylinositol-4',5'-bisphosphate (PIP2). We hypothesized that PIP2 may favor a distinct orientation of the GRK2-Gβγ complex on the membrane that is more optimal for function. In this study, we compared the possible orientations of GRK2-Gβγ and Gβγ alone on model cell membranes prepared with various anionic phospholipids as deduced from sum frequency generation (SFG) vibrational and attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopic methods. Our results indicate that PIP2 affects the membrane orientation of GRK2-Gβ1γ2, but not that of complexes form with anionic phospholipid-binding deficient mutations in the GRK2 pleckstrin homology (PH) domain. Gβ1γ2 exhibits a similar orientation on the lipid bilayer regardless of its lipid composition. The PIP2-induced orientation of the GRK2-Gβ1γ2 complex is therefore most likely caused by specific interactions between PIP2 and the GRK2 PH domain. Thus, PIP2 not only helps recruit GRK2 to the membrane, but also “fine tunes” the orientation of the GRK2-Gβγ complex so that it is better positioned to phosphorylate activated GPCRs.
Keywords: Sum frequency generation (SFG) vibrational spectroscopy, membrane orientation, PH domain, PIP2, G protein-coupled receptor kinases, heterotrimeric G proteins
Graphical abstract
Introduction
Protein orientation at interfaces plays critical roles in many research areas and in applications such as biocompatibility, biosensors, and cell-cell communication. However, the orientation of proteins at solid/liquid interfaces is difficult to determine, particularly in situ and with molecular level detail. One important system that likely depends on optimizing protein orientation at the plasma membrane is represented by the regulation of effector enzymes by heterotrimeric G proteins (Gαβγ), which regulate cell homeostasis in response to activation of G protein-coupled receptors (GPCRs).1–7 For example, Gβγ subunits, which are prenylated and dissociate from Gα subunits in response to GPCR activation, recruit GPCR kinase 2 (GRK2) to the cell membrane, an event that is required for GRK2 activity in cells and ultimately receptor downregulation.8–16 At the same time, GRK2 activity is also strongly dependent on certain anionic components of the cell membrane, in particular phosphatidylinositol-4',5'-bisphosphate (PIP2).3–5, 10–11 However, not all anionic lipids activate GRK2 equally well. Thus, it has been proposed that PIP2 has an allosteric effect on the structure of GRK2, or that its specific interactions with the PH domain lead to changes in the orientation of GRK2 at the membrane that are required for function.3, 10–11 In our previous studies, we demonstrated that the combined sum frequency generation (SFG) vibrational and attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopic method can be used to determine the orientation of G proteins and G protein complexes, such as Gαiβ1γ2, Gβ1γ2, GRK2-Gβ1γ2, and GRK5 when associated with model cell membranes.17–20 The same approach was applied to test if PIP2 has an effect on the membrane orientation of either Gβ1γ2 or its complex with GRK2.
In this study, we created model cell membranes containing a 9:1 mixture of the neutral lipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) with either the anionic lipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) lipid or 1,2-dioleoyl-sn-glycero-3-phospho-(1'-myo-inositol-4',5'-bisphosphate) (PIP2). Combined SFG and ATR-FTIR experiments showed that Gβ1γ2 adopts a similar membrane orientation on the two model cell membranes (9:1 POPC:POPG and 9:1 POPC:PIP2 lipid bilayers), whereas the orientation of the GRK2-Gβ1γ2 complex is distinct on each surface, suggesting that POPG and PIP2 differentially affect the orientation of this complex at the membrane. Conversely, anionic phospholipid-binding deficient mutants of GRK2 in complex with Gβ1γ2 exhibited no analogous lipid dependence in orientation. 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), another negatively charged phospholipid, has, like PIP2, been proposed to be a physiologically relevant regulator of GRK2 and induce conformational changes in the GRK2 kinase domain.11 Thus, we also studied the orientations of Gβ1γ2 and the GRK2-Gβ1γ2 complex associated with 9:1 POPC:POPS lipid bilayers, which we showed to be the same as when they are associated with 9:1 POPC:POPG bilayers. We therefore conclude that the distinct and specific interactions formed between the PIP2 head group and the PH domain of GRK2 have substantial impact not only on membrane binding but also on protein orientation in our model system, and, by extension, in living cells.
Materials and Methods
Protein Samples
GRK2 variants (all based on the bovine GRK2-S670A mutant background) and bovine Gβ1γ2 were purified as previously described,12–13 and frozen in liquid N2 until used. The GRK2-Gβ1γ2 heterotrimer was formed by mixing the two components in a 1:1 molar ratio and either using the resulting complex directly or after purification of the complex on a Superdex S200 gel filtration column equilibrated with 20 mM HEPES pH 8.0, 50 mM NaCl, 1 mM CHAPS, and 5 mM DTT. This buffer mixture without CHAPS was used as the liquid subphase for the lipid bilayer in SFG and ATR-FTIR studies. Protein complexes formed via either method yielded identical results in SFG experiments.
SFG Spectroscopy
SFG is a surface-sensitive nonlinear optical vibrational spectroscopic technique that can provide in situ structural information about molecules at an interface.21–26 Two input beams at frequencies ωvis (visible light) and ωIR (infrared light) mix in a medium and generate an output beam at the sum frequency ωsum=ωvis+ωIR.27–32 This process occurs only in media where inversion symmetry is broken, such as for peptides/proteins bound at biological membranes.33–38 SFG technique has been successfully applied to examine structural information of interface peptides/proteins at the molecular level.39–44 The design and theoretical background of our SFG spectrometer has been reported previously.45,46
POPC, PIP2, and POPS were purchased from Avanti Polar Lipids Inc. (AL) as a chloroform solution, and mixed to produce the desired lipid composition. Planar supported lipid bilayers (PSLBs) composed of a 9:1 mixture of POPC:POPG, a 9:1 mixture of POPC:PIP2, or a 9:1 mixture of POPC:POPS was prepared on clean right-angle CaF2 prisms (Altos Photonics, Bozeman, MT) using the Langmuir-Blodgett/Langmuir Schaefer (LB/LS) method, as described previously.23 Each protein sample (336 nM) was injected into the lipid subphase, and allowed to equilibrate with the lipid bilayer over the course of 1 hr. SFG spectra from interfacial proteins were collected at room temperature (24 °C) in a near total internal reflection geometry.23,45–46 SFG ssp (an s-polarized output sum frequency signal collected with an s-polarized input visible beam and a p-polarized input infrared beam) and ppp spectra were collected in this study.47–52 We have previously reported on the application of this methodology for the determination of the membrane orientation of proteins by using SFG technique.18–19 A computer program was developed to facilitate the protein orientation analysis by calculating the SFG signal ratios (e.g., ) as a function of the protein orientation defined by two angles, twist and tilt, assuming that the protein does not change conformation when it binds to membranes.18–19 The possible orientation angle regions can be determined by comparing the experimentally measured SFG polarized spectra and the calculated angle dependent SFG signals.
ATR-FTIR Spectroscopy
The lipid bilayers described above were prepared on clean ZnSe substrates (Specac, UK), using the same LB/LS method to prepare the lipid bilayers for SFG experiments. Because the vibrational signal of the water O-H bending mode overlaps with the protein amide I signal, we used D2O in the subphase in the ATR-FTIR experiments. Protein samples (336 nM in buffer solution with D2O as the solvent) were injected into the subphase and allowed to equilibrate for 2 hr prior to the collection of P and S polarized spectra using a Nicolet 6700 FTIR spectrometer with an ATR accessory. All the ATR-FTIR spectra are the average of 128 scans. In order to reduce the interference from the water vapor present in the air, the instrument was purged with dry N2 prior to use, and spectra were corrected for trace amounts of water vapor using an additional background correction based on the spectrum of pure water vapor in air at 24 °C. The background subtraction and a baseline correction in the amide I region were performed in OMNIC 7.2, after which spectra were fit to a Gaussian lineshape using a nonlinear curve fitting algorithm in Origin 8.53 Unlike SFG measurements, which only detect the secondary structure where inversion symmetry is broken (e.g. in α-helices), ATR-FTIR signals are generated by all secondary structures in the protein. Therefore, the ATR-FTIR spectra contain contributions from β-sheet, random coil/disordered, α-helical, and β-turn structures.19 The dichroic ratio RATR of the P and S polarized amide I signals contributed from the α-helices in the protein was deduced from the fitted spectra.19 We furthermore developed a computer program similar to the SFG data analysis program to analyze ATR-FTIR data.19 The likely orientation angles of a protein can be determined by comparing the experimentally measured dichroic ratio RATR and the predicted ratio RATR as a function of its twist and tilt angles.
Results and Discussion
Deduced Membrane Orientation of GRK2-Gβ1γ2
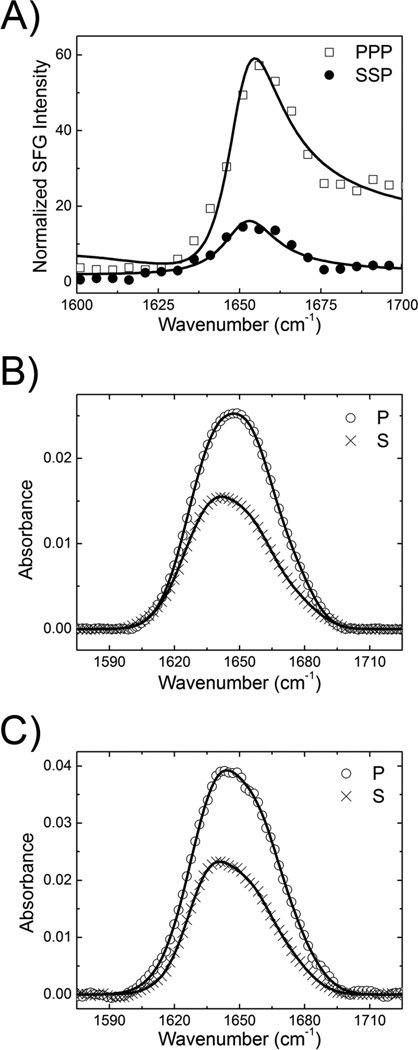
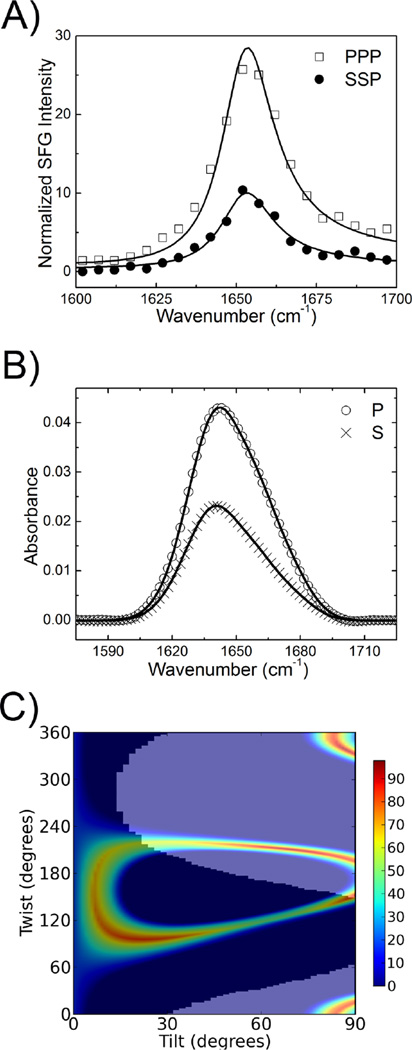
Because SFG and ATR-FTIR represent independent measurements, the final deduced possible range of protein membrane orientations should satisfy both SFG and ATR-FTIR data. We successfully applied this combined vibrational spectroscopic approach to more accurately determine membrane orientations of proteins in situ based on the overall orientation of α–helical components in each molecule.19–21 In this study, we applied this technique to compare the membrane orientation of GRK2-Gβ1γ2 on two negatively charged lipid bilayers composed of 9:1 POPC:PIP2 and 9:1 POPC:POPG. Figure 1A shows the SFG ssp and ppp amide I signals detected from the associated GRK2-Gβ1γ2 on the 9:1 POPC:PIP2 lipid bilayer. The fitting results indicated that SFG spectra contain strong contributions from the α-helices in the protein, with a major peak centered at 1652 cm−1. The ratio for the peak was 1.9 (Table S1). The P and S polarized ATR-FTIR spectra were also collected and fitted (Figure 1B) to determine a dichroic ratio RATR of 1.6 based on the α-helical contributions centered at 1658 cm−1 (Table S2).
Figure 1.
SFG and ATR-FTIR Amide I spectra for GRK2-Gβ1γ2 bound to phospholipid bilayers. (A) SFG specta for GRK2-Gβ1γ2 associated with a 9:1 POPC:PIP2 lipid bilayer. (B) ATR-FTIR spectra for GRK2-Gβ1γ2 associated with a 9:1 POPC:PIP2 lipid bilayer. (C) ATR-FTIR spectra for GRK2-Gβ1γ2 associated with a 9:1 POPC:POPG lipid bilayer. The circles and crosses are experimental data. The solid lines are the fitting results.
SFG spectra from the GRK2-Gβ1γ2 complex associated with a 9:1 POPC:POPG yielded a of 2.2 for the peak centered at 1652 cm−1, the same as reported previously.18 Polarized P and S ATR-FTIR spectra of GRK2-Gβ1γ2 on the 9:1 POPC:POPG lipid bilayer were collected and fitted to determine a dichroic ratio RATR of 1.5 for the peak centered at 1658 cm−1 (Figure 1C, Table S3). Thus the SFG and ATR-FTIR data collected from the GRK2-Gβ1γ2 complex associated with lipid bilayers containing PIP2 or POPG are distinct, likely reflecting different membrane orientations.
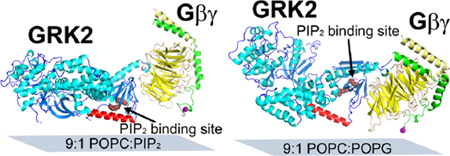
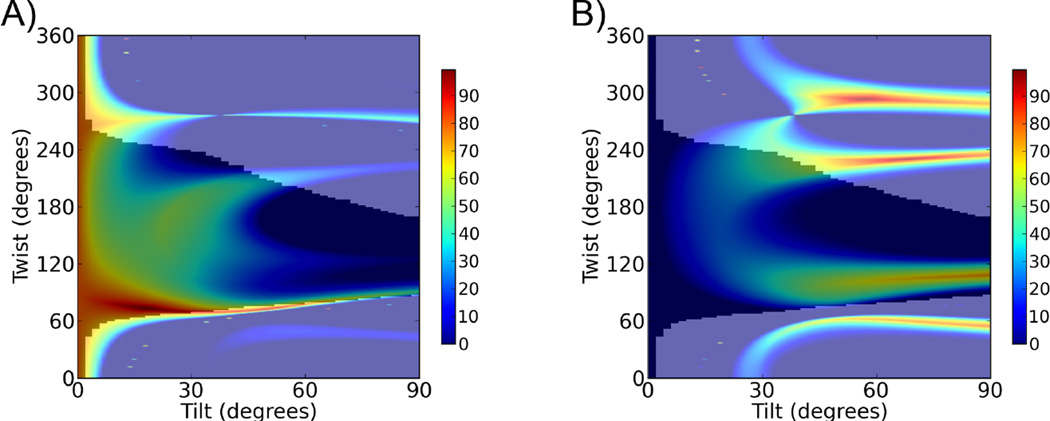
We first deduced the orientation of the GRK2-Gβ1γ2 complex by comparing the experimental and calculated SFG data as a function of protein orientation, using the crystal structure of GRK2-Gβ1γ2 in a standard pose as a reference.18 We then performed a similar operation with the measured and calculated ATR-FTIR data (Figure S1). The most likely orientations are those that optimally satisfy both SFG and ATR-FTIR measurements. Figure 2A shows that the most likely orientation range of GRK2-Gβ1γ2 associated with the 9:1 POPC:PIP2 lipid bilayer based on the combined data falls in a very narrow range of moderate twist (~75°) and low tilt (10–30°). The scores in the heat maps shown in Figure 2A include all the errors in experiments and data analysis. The details of the errors described in the heat maps were discussed in ref.18. Figure 3A depicts a representative likely orientation with twist of 75° and tilt of 15°. In this pose, the β1–β2 loop of the GRK2 PH domain, a known PIP2 binding determinant,2 the highly positively charged C-terminal helix of the regulator of G protein signaling homology (RH) domain, and the C-terminus of Gγ2, which is geranylgeranylated, would all be in reasonable proximity to a common membrane plane.
Figure 2.
Possible orientations of GRK2-Gβ1γ2 on bilayers with various compositions. (A) Possible orientations of the complex using a combination of SFG and ATR-FTIR (dichroic ratio RATR =1.6 ± 0.2) measurements on a 9:1 POPC:PIP2 lipid bilayer. (B) Possible orientations of the complex using a combination of SFG and ATR-FTIR (dichroic ratio RATR =1.5 ± 0.2) measurements on a 9:1 POPC:POPG lipid bilayer. These are identical to those calculated for the GRK2(K567E/R578E)-Gβ1γ2 and GRK2(K567A/R578A)-Gβ1γ2 complexes on a 9:1 POPC:PIP2 lipid bilayer because they have identical SFG and ATR-FTIR ratios. In each panel, the effect of experimental errors is accounted for using a coloring scheme based on how well the calculated and experimentally measured quantities agree for each possible orientation, within specified error bars (±10%).18 If the calculated ratio or RATR does not match the experimental value within ±10%, a score of 0 is assigned and show in blue.18–19 The total score is calculated as the product of the scores for all individual criteria. A score of 100% indicates an exact match for all experimental measurements and show in red color.18–19 The dark areas indicate orientations of GRK2-Gβ1γ2 that are considered to be physically reasonable, according to previously defined criteria (e.g. the Gβ1γ2 component has a membrane anchor that imposes some constraints on the possible orientations of the associated GRK2-Gβ1γ2 complex). Physically obtainable orientations fall within the shadowed region.
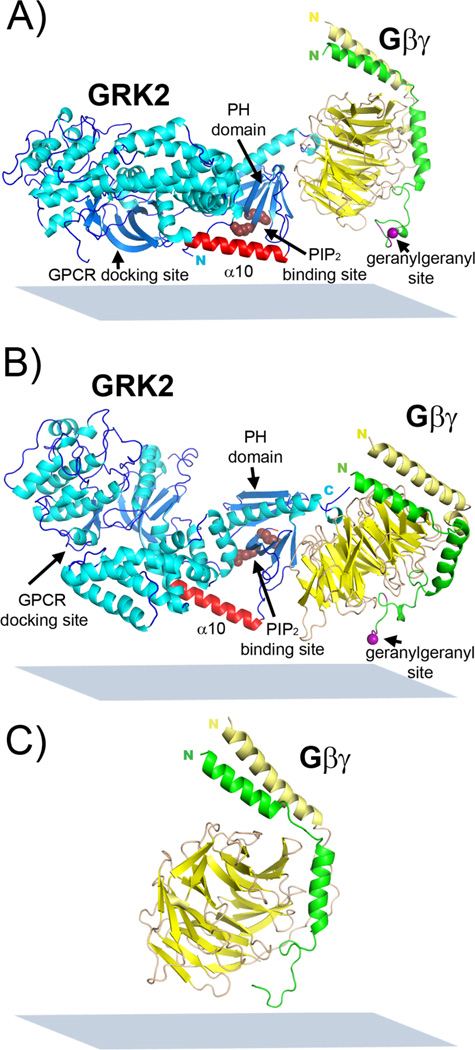
Figure 3.
Deduced possible membrane orientation of GRK2-Gβ1γ2 on (A) 9:1 POPC:PIP2 lipid bilayer (twist=75°, tilt=15°), and (B) 9:1 POPC:POPG lipid bilayer (twist=100°, tilt=65°). (C) Deduced possible membrane orientation of Gβ1γ2 on 9:1 POPC:PIP2 lipid bilayer (twist=94°, tilt=23°). GRK2 is colored blue with cyan helices, Gβ is yellow, Gγ is green, and the GRK2 α10 helix is red. The purple sphere indicates the geranylgeranyl site, and ruby spheres residues that bind anionic phospholipids. The plane of the membrane is shown as a blue-grey rectangle.
We similarly deduced the most likely orientation angle region for GRK2-Gβ1γ2 associated with the 9:1 POPC:POPG lipid bilayer (Figure 2B). In this case, the most likely orientations of GRK2-Gβ1γ2 fall in a region with twist of ~100° and tilt angles >40°. A twist of 100° and tilt of 65° yields an orientation in which only the RH domain C-terminus and Gγ2 prenyl group are adjacent to the membrane (Figure 3B). Choosing, for example, a higher tilt angle generates an orientation where these two features still remain in proximity to the membrane (Figure S2). We note that this orientation is different for GRK2-Gβ1γ2 than we reported previously18 although the experimental SFG values are essentially identical. This was due to a sign error in the program that oriented the complex for visual display,18 an error corrected in subsequent papers (see Supplementary Methods).19–22
Given that a key difference in the likely orientations of GRK2-Gβ1γ2 on the 9:1 POPC:POPG and 9:1 POPC:PIP2 membranes is how the PH domain is oriented with respect to the membrane, we speculated that specific interactions of PIP2 with the β1–β2 loop of the GRK2 PH domain might be responsible for the different poses. We therefore studied two anionic phospholipid-binding deficient mutants of GRK2: GRK2(K567E/R578E) and GRK2(K567A/R578A). The SFG and ATR-FTIR amide I signals from GRK2(K567E/R578E)-Gβ1γ2 and GRK2(K567A/R578A)-Gβ1γ2 complexes associated with the 9:1 POPC:PIP2 lipid bilayer were collected (Figure S3). The spectral fitting results indicate that the measured ratio and the ATR-FTIR dichroic ratio RATR were 2.2 (Table S4, S6) and 1.5 (Table S5, S7) respectively for both GRK2(K567E/R578E)-Gβ1γ2 and GRK2(K567A/R578A)-Gβ1γ2, identical to that measured for GRK2-Gβ1γ2 associated with a 9:1 POPC:POPG lipid bilayer. Figure 2B displays the likely membrane orientations of all these complexes. Because they are different from that of GRK2-Gβ1γ2 associated with a 9:1 POPC:PIP2 lipid bilayer (Figure 2A), the results are consistent with the hypothesis that specific interactions made by the PH domain have a substantial impact on the orientation of the GRK2-Gβ1γ2 complex when PIP2 is contained in the lipid bilayer.
It was shown previously that other negatively charged lipids besides POPG and PIP2 can also positively influence the activity of GRK2, and may induce conformational changes in the kinase domain of the enzyme, e.g. POPS.11 Therefore, we also studied the interaction of GRK2-Gβ1γ2 associated with a 9:1 POPC:POPS bilayer. As shown in Figure S4A, SFG ssp and ppp amide I signals were detected from the associated protein complex. The fitting results shown in Table S8 indicate that SFG spectra contain strong contributions from the α-helices in the protein at 1652 cm−1. The ratio for the α-helical peak centered at 1652 cm−1 was 2.2, identical to the ratio for the complex on a 9:1 POPC:POPG lipid bilayer. This result demonstrates that GRK2-Gβ1γ2 complex behaves similarly on both 9:1 POPC:POPG and 9:1 POPC:POPS lipid bilayers and that thus far only PIP2 seems capable of changing the orientation of the complex on membranes, at least at the concentrations used.
Orientation of Gβ1γ2
We also investigated whether Gβ1γ2 changes its orientation in the presence of PIP2, which may indirectly contribute to an altered orientation of bound GRK2. The likely membrane orientations of Gβ1γ2 associated with a 9:1 POPC:POPG lipid bilayer was previously deduced using combined SFG and ATR-FTIR and shown to yield a narrow range of possible orientations, with low tilt (15–35°) and moderate twist (60–90°).19 For comparison, we determined the orientation of Gβ1γ2 associated with a 9:1 POPC:PIP2 lipid bilayer. The polarized SFG (Figure 4A) and ATR-FTIR (Figure 4B) amide I signals of Gβ1γ2 yielded a ratio for the peak at 1652 cm−1 of 2.0 (Table S9), and a dichroic ratio RATR for the peak at 1657 cm−1 of 1.9 (Table S10), respectively. Figure 4C shows that most likely membrane orientations of Gβ1γ2 associated with the 9:1 POPC:PIP2 bilayer once again exhibit low tilt (10–35°) and moderate twist angles (80–110°). A similar Gβ1γ2 orientation on a 9:1 POPC:POPS lipid bilayer was also determined (Figure S4B, S5; Table S11, S12). Therefore the lipid components do not have a substantial impact on the orientation of Gβ1γ2. Figure 3C depicts representative membrane orientations of Gβ1γ2 on a 9:1 POPC:PIP2 lipid bilayer deduced from the above experimental measurements (twist 94°, tilt 23°). Furthermore, this result is consistent with our studies of the GRK2(K567E/R578E) and GRK2(K567A/R578A) mutants, whose complexes with Gβ1γ2 likewise failed to generate a different orientation on 9:1 POPC:PIP2 relative to 9:1 POPC:POPG.
Figure 4.
Membrane orientation of Gβ1γ2 as a function of lipid composition. (A) SFG Amide I spectra from Gβ1γ2 associated with a 9:1 POPC:PIP2 lipid bilayer. (B) ATR-FTIR Amide I spectra from Gβ1γ2 associated with a 9:1 POPC:PIP2 lipid bilayer. The symbols represent experimental data, and solid lines the fitting results. (C) The possible orientations of Gβ1γ2 determined by the combination of SFG and ATR-FTIR (dichroic ratio RATR =1.9 ± 0.2) measurements on 9:1 POPC:PIP2 lipid bilayer. The dark region demarks physically allowed orientations for Gβ1γ2.
Conclusions
In this study, we have evaluated the effect of PIP2 on a heterotrimeric G protein complex that has both PIP2 dependent and independent membrane binding determinants using a combination of SFG and ATR-FTIR spectroscopy. We showed that the membrane orientation of the GRK2-Gβ1γ2 complex is distinct on 9:1 POPC:PIP2 relative to 9:1 POPC:POPG or 9:1 POPC:POPS lipid bilayers in situ. Gβ1γ2, which lacks a specific PIP2 binding site, adopted the same orientation on all membranes tested. Analysis of GRK2 variants with site specific mutations in its PH domain indicated that the different orientation of the GRK2-Gβ1γ2 complex on PIP2 containing bilayers is likely caused by the specific interactions between PIP2 and the PH domain. This result is in stark contrast to GRK5, which adopts similar orientations on lipid bilayers whether they contain PIP2 or not.20 Thus a PIP2-dependent change in orientation is dependent on the specific GRK regardless of the presence a PIP2 binding sites in each protein.
But when a change of orientation does occur, what are the molecular consequences? There is extensive biochemical evidence that PIP2 interactions mediated by the PH domain are important for GRK2 activity on receptors such as the β2AR and the µ-opioid receptor.2,3,10,11 Thus we hypothesize that the distinct PIP2-mediated pose leads to an orientation of GRK2 that is more optimal for engaging activated GPCRs. Figure3A and 3B shows that the proposed receptor docking site of GRK2 is more oriented towards the membrane surface in the PIP2-dependent pose than in the POPG-dependent pose.14 Because anionic phospholipids such as POPG and POPS are known to also activate GRK2, it is therefor tempting to speculate that for GPCRs in non-cell based assay systems, the GRKs are used under nonphysiological conditions (e.g. vast excess of GRK at very low ionic strength) such that activation of receptor phosphorylation may only reflect bulk charge effects.4–5 Thus, even though 10% POPG and POPS membranes fail to mediate a distinct pose for the GRK2-Gβγ complex like PIP2, likely because they do not bind with high affinity or selectivity to the PH domain, they may promote receptor phosphorylation by simply driving more GRK2 to the membrane.
PIP2 and phosphatidyl-inositol-4'-phosphate (PIP) were previously shown to have a biphasic effect on GRK2 phosphorylation of muscarinic receptors reconstituted in phosphatidyl choline vesicles, with activation occurring up to 25 µM added PIP2/PIP, and gradually decreasing activity thereafter.5 This biphasic property helps to explain contradictory early reports wherein PIP2 was either shown to be activating or inhibitory, although differences in experimental protocols also likely contribute (e.g. use of mixed micelles versus reconstituted receptor preparations).3–4,10–11 A likely mechanism for the inhibition mediated by PIP2/PIP is via interactions of the head group with the kinase domain,5 which contains an unusually basic peptide binding cleft on its large lobe.15 Thus, an important question is whether the 10% mole fraction of PIP2 used in this study, which is above expected physiological levels in bulk membranes, is activating or inhibitory. Unfortunately, given profound differences in how membranes needed to be prepared in this versus the prior study,5 it is not possible to compare the molar concentration of PIP2 present in our membranes. If inhibitory, the different pose of the GRK-Gβ1γ2 complex we observed in the presence of PIP2 could reflect that of an inactive enzyme complex. Indeed, the kinase large lobe is positioned much closer to the membrane surface (cf. Fig. 3A vs. B). However, a ~10% PIP2 mole fraction in phosphatidyl choline vesicles was previously shown to be activating in the presence of Gβγ, suggesting that the conditions used in this study are conducive to phosphorylation of GPCRs, at least β2 adrenergic receptors.10 Furthermore, the fact that mutation of the anionic-phospholipid binding residues in the GRK2 PH domain reverts the unique pose mediated by the presence of PIP2 to that of POPS and POPG suggests that the unique pose is determined more by specific interactions of PIP2 with the PH domain than the large lobe of the kinase domain.
Another trivial explanation for the distinct spectral signals is that PIP2 binding mediates a conformational change in GRK2 that we cannot yet model. Although there is some biochemical data to support a conformational change in response to negatively charged lipids,11 structural variation among the GRK2-Gβγ complexes deposited in the PDB are small, including one that was determined in the presence of phosphoserine, an anionic phospholipid head group analog (PDB entry 1OMW).15 Of course, none were determined in the presence of an intact membrane. Therefore, although we believe the possibility for a dramatic structural change is low, it as of yet cannot be ruled out.
Disruption of the interaction between GRK2 and Gβγ is well known to block membrane recruitment of the enzyme, thereby abrogating GPCR phosphorylation in cells.2,4–5,16 The GRK2(K567E/R578E) mutation, which eliminates anionic phospholipid binding, likewise ablates receptor phosphorylation.2 However, neither of these interactions is required for GPCR phosphorylation in situ, where high concentrations of the kinase and/or receptor can be used to drive the reaction by mass action. It has been speculated that in cells, where the environment is much more complex and there are many competing interactions for GRK2, the synergistic action of Gβγ subunits and PIP2 enhances partitioning of much smaller amounts of the enzyme to appropriate regions of the membranes bearing activated receptors, and perhaps higher concentrations of PIP2.2 Our spectroscopic studies provide an additional explanation for why both Gβγ subunits and PIP2 are required for optimal GRK2 function. Gβγ, released by agonist-occupied receptors and constitutively membrane bound, recruits GRK2 to the vicinity of an activated receptors. Specific interactions between the PH domain and PIP2 head groups then stabilize a small range of orientations that allows the GRK2 docking site, formed largely by its N-terminal helix, to more productively engage an activated receptor. Of course, in cells both enhancement of membrane recruitment and stabilization of a particular orientation would operate simultaneously. We speculate that the need for such a system in the case of GRK2 may be a consequence of the fact that the enzyme must be able to recognize a very diverse and large number of receptor targets, and therefore cannot form highly specific (or high affinity) interactions with any specific GPCR. The combined action of enhancing membrane recruitment and dictating a specific membrane pose may help overcome this conundrum to ensure sufficiently rapid phosphorylation and timely desensitization of the appropriate receptor targets.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by NIH grant GM081655 to Z.C. and J.T., and by NIH grants HL071818 and HL086865 (to J.T.).
This work was supported by NIH grant GM081655 to Z.C. and J.T., and by NIH grants HL071818 and HL086865 (to J.T.).
Abbreviations
- GPCR
G protein-coupled receptor
- GRK2
G protein-coupled receptor kinase 2
- PH
pleckstrin homology
- PIP2
1,2-dioleoyl-sn-glycero-3-phospho-(1'-myo-inositol-4',5'-bisphosphate)
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol
- POPS
1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine
- SFG
sum frequency generation
- ATR-FTIR
attenuated total reflectance Fourier transform infrared.
Footnotes
Details of SFG and ATR-FTIR spectra fitting results and Supplementary Methods. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Homan KT, Glukhova A, Tesmer JJG. Regulation of G Protein-Coupled Receptor Kinases by Phospholipids. Curr. Med. Chem. 2013;20:39–46. [PubMed] [Google Scholar]
- 2.Carman CV, Barak LS, Chen C, Liu-Chen L, Onorato JJ, Kennedy SP, Caron MC, Benovic JL. Mutational analysis of Gβγ and phospholipid interaction with G protein-coupled receptor kinase 2. J. Biol. Chem. 2000;275:10443–10452. doi: 10.1074/jbc.275.14.10443. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher JA, Fredericks ZL, Stone WC, Premont RT, Stoffel RH, Koch WJ, Lefkowitz RJ. Phosphatidylinositol 4,5-Bisphosphate (PIP2)-enhanced G Protein-coupled Receptor Kinase (GRK) Activity. J. Biol. Chem. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- 4.Debburman SK, Ptasienski J, Boetticher E, Lomasney JW, Benovic JL, Hosey MM. Lipid-mediated regulation of G protein-coupled receptor kinases 2 and 3. J. Biol. Chem. 1995;270:5742–5747. doi: 10.1074/jbc.270.11.5742. [DOI] [PubMed] [Google Scholar]
- 5.DebBurman SK, Ptasienski J, Benovic JL, Hosey MM. G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein βγ subunits. J. Biol. Chem. 1996;271:22552–22562. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- 6.Inglese J, Koch WJ, Caron MG, Lefkowise RJ. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PK, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowiz RJ. Role of βγ Subunits of G Proteins in Targeting the β-Adrenergic Receptor Kinase to Membrane-Bound Receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 8.Evron T, Daigle TL, Caron MG. GRK2: multiple roles beyond G protein-coupled receptor desensitization. Trends Pharmacol. Sci. 2012;33:154–164. doi: 10.1016/j.tips.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJG. Snapshot of Activated G Proteins at the Membrane: The Gαq-GRK2-Gβγ Complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 10.Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ. Pleckstrin homology domain-mediated membrane association and activation of the-adrenergic receptor kinase requires coordinate interaction with G subunits and lipid. J. Biol. Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- 11.Onorato JJ, Gillis ME, Liu Y, Benovic JL, Ruoho AE. The β-adrenergic receptor kinase (GRK2) is regulated by phospholipids. J. Biol. Chem. 1995;270:21346–21353. doi: 10.1074/jbc.270.36.21346. [DOI] [PubMed] [Google Scholar]
- 12.Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, Sklar LA, Koch WY, Tesmer JJG. Paroxetine Is a Direct Inhibitor of G Protein-Coupled Receptor Kinase 2 and Increases Myocardial Contractility. ACS Chem. Biol. 2012;7:1830–1839. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homan KT, Wu E, Wilson MW, Singh P, Larsen SD, Tesmer JJ. Structural and Functional Analysis of G Protein-Coupled Receptor Kinase Inhibition by Paroxetine and a Rationally Designed Analog. Mol. Pharmacol. 2014;85:237–248. doi: 10.1124/mol.113.089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beautrait A, Michalski KR, Lopez TS, Mannix KM, McDonald DJ, Cutter AR, Medina CB, Hebert AM, Francis CJ, Bouvier M, Tesmer JJ, Sterne-Marr R. Mapping the Putative G Protein-coupled Receptor (GPCR) Docking Site on GPCR Kinase 2 : Insights from Intact Cell Phosphorylation and Recruitment Assays. J. Biol. Chem. 2014;289:25262–25275. doi: 10.1074/jbc.M114.593178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJG. Keeping G proteins at bay: A complex between G protein-coupled receptor kinase 2 and Gβγ. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 16.Boekhoff I, Inglese J, Schleicher S, Koch WJ, Lefkowitz RJ, Breer H. Olfactory desensitization requires membrane targeting of receptor kinase mediated by beta gamma-subunits of heterotrimeric G proteins. J. Biol. Chem. 1994;269:37–40. [PubMed] [Google Scholar]
- 17.Chen X, Boughton AP, Tesmer JJG, Chen Z. In Situ Investigation of Heterotrimeric G Protein βγ Subunit Binding and Orientation on Membrane Bilayers. J. Am. Chem. Soc. 2007;129:12658–12659. doi: 10.1021/ja075542w. [DOI] [PubMed] [Google Scholar]
- 18.Boughton AP, Yang P, Tesmer VM, Ding B, Tesmer JJ, Chen Z. Heterotrimeric G protein β1γ2 Subunits Change Orientation upon Complex Formation with G Protein-coupled Receptor Kinase 2 (GRK2) on a Model Membrane. Proc Natl Acad Sci USA. 2011;108:E667–E673. doi: 10.1073/pnas.1108236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang P, Boughton AP, Homan KT, Tesmer JJG, Chen Z. Membrane Orientation of Gαiβ1γ2 and Gβ1γ2Determined via Combined Vibrational Spectroscopic Studies. J. Am. Chem. Soc. 2013;135:5044–5051. doi: 10.1021/ja3116026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P, Glukhova A, Tesmer JJG, Chen Z. Membrane Orientation and Binding Determinants of G Protein-Coupled Receptor Kinase 5 as Assessed by Combined Vibrational Spectroscopic Studies. PLoS ONE. 2013;8:e82072. doi: 10.1371/journal.pone.0082072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Ogorzalek TL, Yang P, Marsh ENG, Chen Z. Molecular Orientation of Enzymes Attached to Surfaces Through Defined Chemical Linkages at the Solid/Liquid Interface. J. Am. Chem. Soc. 2013;135:12660–12669. doi: 10.1021/ja403672s. [DOI] [PubMed] [Google Scholar]
- 22.Shen L, Schroeder M, Ogorzalek TL, Yang P, Wu FG, Marsh ENG, Chen Z. Surface Orientation Control of Site-Specifically Immobilized Nitro-reductase (NfsB) Langmuir. 2014;30:5930–5938. doi: 10.1021/la5016862. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, Ramamoorthy A, Chen Z. Membrane Orientation of MSI-78 Measured by Sum Frequency Generation Vibrational Spectroscopy. Langmuir. 2011;27:7760–7767. doi: 10.1021/la201048t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye S, Li H, Wei F, Jasensky J, Boughton AP, Yang P, Chen Z. Observing a Model Ion Channel Gating Action in Model Cell Membranes in Real Time in Situ: Membrane Potential Change Induced Alamethicin Orientation Change. J. Am. Chem. Soc. 2012;134:6237–6243. doi: 10.1021/ja2110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang P, Wu FG, Chen Z. Dependence of Alamethicin Membrane Orientation on the Solution Concentration. J. Phys. Chem. C. 2013;117:3358–3365. doi: 10.1021/jp3099522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang P, Wu FG, Chen Z. Lipid Fluid–Gel Phase Transition Induced Alamethicin Orientational Change Probed by Sum Frequency Generation Vibrational Spectroscopy. J. Phys. Chem C. 2013;117:17039–17049. doi: 10.1021/jp4047215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu FG, Yang P, Zhang C, Li B, Han X, Song M, Chen Z. Molecular Interactions between Amantadine and Model Cell Membranes. Langmuir. 2014;30:8491–8499. doi: 10.1021/la501718n. [DOI] [PubMed] [Google Scholar]
- 28.Wu FG, Yang P, Zhang C, Han X, Song M, Chen Z. Investigation of Drug–Model Cell Membrane Interactions Using Sum Frequency Generation Vibrational Spectroscopy: A Case Study of Chlorpromazine. J. Phys. Chem C. 2014;118:17538–17548. [Google Scholar]
- 29.Shen YR. Surface Properties Probed by Second-Harmonic and Sum-Frequency Generation. Nature. 1989;337:519–525. [Google Scholar]
- 30.Eisenthal KB. Liquid Interfaces Probed by Second-Harmonic and Sum-Frequency Spectroscopy. Chem. Rev. 1996;96:1343–1360. doi: 10.1021/cr9502211. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Somorjai GA. Molecular Packing of Lysozyme, Fibrinogen, and Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces Studied by Infrared−Visible Sum Frequency Generation and Fluorescence Microscopy. J. Am. Chem. Soc. 2003;125:3150–3158. doi: 10.1021/ja028987n. [DOI] [PubMed] [Google Scholar]
- 32.Richmond GL. Molecular Bonding and Interactions at Aqueous Surfaces as Probed by Vibrational Sum Frequency Spectroscopy. Chem. Rev. 2002;102:2693–2724. doi: 10.1021/cr0006876. [DOI] [PubMed] [Google Scholar]
- 33.Bain CD. Sum-frequency Vibrational Spectroscopy of the Solid/Liquid Interface. J. Chem. Soc., Dalton Trans. 1995;91:1281–1296. [Google Scholar]
- 34.Haupert LM, Simpson GJ. Chirality in Nonlinear Optics. Annu. Rev. Phys. Chem. 2009;60:345–365. doi: 10.1146/annurev.physchem.59.032607.093712. [DOI] [PubMed] [Google Scholar]
- 35.Liljeblad JFD, Bulone V, Rutland MW, Johnson CM. Supported Phospholipid Monolayers. The Molecular Structure Investigated by Vibrational Sum Frequency Spectroscopy. J. Phys. Chem. C. 2011;115:10617–10629. [Google Scholar]
- 36.Engelhardt K, Rumpel A, Walter J, Dombrowski J, Kulozik U, Braunschweig B, Peukert W. Protein Adsorption at the Electrified Air–Water Interface: Implications on Foam Stability. Langmuir. 2012;28:7780–7787. doi: 10.1021/la301368v. [DOI] [PubMed] [Google Scholar]
- 37.Tong Y, Li N, Liu H, Ge A, Osawa M, Ye S. Mechanistic Studies by Sum-Frequency Generation Spectroscopy: Hydrolysis of a Supported Phospholipid Bilayer by Phospholipase A2. Angew. Chem. Int. Ed. 2010;49:2369–2373. doi: 10.1002/anie.200904950. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Conboy JC. Phase Transition of a Single Lipid Bilayer Measured by Sum-Frequency Vibrational Spectroscopy. J. Am. Chem. Soc. 2004;126:8894–8895. doi: 10.1021/ja031570c. [DOI] [PubMed] [Google Scholar]
- 39.Ye S, Liu G, Li H, Chen F, Wang X. Effect of Dehydration on the Interfacial Water Structure at a Charged Polymer Surface: Negligible χ(3) Contribution to Sum Frequency Generation Signal. Langmuir. 2012;28:1374–1380. doi: 10.1021/la203690p. [DOI] [PubMed] [Google Scholar]
- 40.Ma G, Liu DF, Allen HC. Piperidine Adsorption on Hydrated α-Alumina (0001) Surface Studied by Vibrational Sum Frequency Generation Spectroscopy. Langmuir. 2004;20:11620–11629. doi: 10.1021/la0487343. [DOI] [PubMed] [Google Scholar]
- 41.Weidner T, Breen NF, Li K, Drohny GP, Castner DG. Sum Frequency Generation and Solid-state NMR Study of the Structure, Orientation, and Dynamics of Polystyrene-adsorbed Peptides. Proc. Natl. Acad. Sci. U.S.A. 2010;107:13288–13293. doi: 10.1073/pnas.1003832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weidner T, Samuel NT, McCrea K, Gamble LJ, Ward RS, Castner DG. Assembly and Structure of α-helical Peptide Films on Hydrophobic Fluorocarbon Surfaces. Biointerphases. 2010;5:9–16. doi: 10.1116/1.3317116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu L, Ma G, Yan EC. In Situ Misfolding of Human Islet Amyloid Polypeptide at Interfaces Probed by Vibrational Sum Frequency Generation. J. Am. Chem. Soc. 2010;132:5405–5412. doi: 10.1021/ja909546b. [DOI] [PubMed] [Google Scholar]
- 44.Fu L, Liu J, Yan EC. Chiral Sum Frequency Generation Spectroscopy for Characterizing Protein Secondary Structures at Interfaces. J. Am. Chem. Soc. 2011;133:8094–8097. doi: 10.1021/ja201575e. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen KT, Le Clair SV, Ye S, Chen Z. Orientation Determination of Protein Helical Secondary Structures Using Linear and Nonlinear Vibrational Spectroscopy. J. Phys. Chem. B. 2009;113:12169–12180. doi: 10.1021/jp904153z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen KT, Le Clair SV, Ye S, Chen Z. Molecular Interaction between Magainin 2 and Model Membranes in Situ. J. Phys. Chem. B. 2009;113:12358–12363. doi: 10.1021/jp904154w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Flores SC, Lim SM, Zhang YJ, Yang TL, Kherb J, Cremer PS. Specific Anion Effects on Water Structure Adjacent to Protein Monolayers. Langmuir. 2010;26:16447–16454. doi: 10.1021/la1015862. [DOI] [PubMed] [Google Scholar]
- 48.Campen RK, Ngo TTM, Sovago M, Ruysschaert JM, Bonn M. Molecular Restructuring of Water and Lipids upon the Interaction of DNA with Lipid Monolayers. J. Am. Chem. Soc. 2010;132:8037–8047. doi: 10.1021/ja100838q. [DOI] [PubMed] [Google Scholar]
- 49.Wang HF, Troxler T, Yeh AG, Dai HL. Adsorption at a Carbon Black Microparticle Surface in Aqueous Colloids Probed by Optical Second-Harmonic Generation. J. Phys. Chem. C. 2007;111:8708–8715. [Google Scholar]
- 50.Ye H, Gu Z, Gracias DH. Kinetics of Ultraviolet and Plasma Surface Modification of Poly(dimethylsiloxane) Probed by Sum Frequency Vibrational Spectroscopy. Langmuir. 2006;22:1863–1868. doi: 10.1021/la052030r. [DOI] [PubMed] [Google Scholar]
- 51.Leung BO, Yang Z, Wu SSH, Chou KC. Role of Interfacial Water on Protein Adsorption at Cross-Linked Polyethylene Oxide Interfaces. Langmuir. 2012;28:5724–5728. doi: 10.1021/la204805x. [DOI] [PubMed] [Google Scholar]
- 52.Green AJ, Perry A, Moore PB, Space B. A theoretical study of the sum frequency vibrational spectroscopy of the carbon tetrachloride/water interface. J. Phys.: Condens. Matter. 2012;24:124108. doi: 10.1088/0953-8984/24/12/124108. [DOI] [PubMed] [Google Scholar]
- 53.Tamm LK, Tatulian SA. Infrared Spectroscopy of Proteins and Peptides in Lipid Bilayers. Quart. Rev. Biophys. 1997;30:365–429. doi: 10.1017/s0033583597003375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.