Abstract
Extensive research supports the role of striatal dopamine in pursuing and responding to reward, and that eye-blink rate is a valid indicator of striatal dopamine. This study tested whether phasic changes in blink rate could provide an index of reward pursuit. This hypothesis was tested in people with bipolar I disorder (BD; a population with aberrations in reward responsivity), and in those without BD. Thirty-one adults with BD and 28 control participants completed a laboratory task involving effort towards monetary reward. Blink rate was recorded using eye-tracking at baseline, reward anticipation, and post-reward. Those in the BD group completed self-report measures relating to reward and ambition. Results showed that across all participants, blink rates increased from reward anticipation to post-reward. In the BD group, reward-relevant measures were strongly correlated with variation in blink rate. These findings provide validation for phasic changes in blink rate as an index of reward response.
In both humans and other animals, the reward system is a key network of neurobiological pathways that help an organism to identify, pursue, and achieve environmental rewards, and to learn from these rewarded experiences (Haber & Knutson, 2010). Research further shows that the reward system is multifaceted, with differential biological and psychological mechanisms responsible for the hedonic response to a reward, sometimes termed “liking,” and the engagement of effort in order to achieve rewards, broadly termed “wanting,” or incentive salience (Berridge & Robinson, 1998; Berridge, Robinson, & Aldridge, 2009).
Regarding this second process, extensive research in animal and human studies supports a key role for mesolimbic dopamine (DA) in guiding pursuit of rewards (Berridge, 2007; Berridge et al., 2009; Salamone, Correa, Farrar, Nunes, & Pardo, 2009). Though studies clearly show a role for DA in reward anticipation (cf. Knutson, Fong, Adams, Varner, & Hommer, 2001; Schott et al., 2008), animal (Salamone et al., 2009) and human research (Treadway et al., 2012) have identified that individual differences in dopaminergic activity in the striatum are particularly correlated with willingness to expend effort for reward. In keeping with the idea that DA supports motivation to pursue rewards, greater activation in the nucleus accumbens (a DA-rich area of the striatum) while anticipating effort for reward has been shown to predict the degree of effort an individual will expend on reward (Kroemer, Guevara, Ciocanea Teoderescu, Wuttig, Kobiella, & Smolka, 2014). Transdiagnostic studies increasingly show evidence for the importance of differences in willingness to expend effort for reward across different forms of psychopathology (Salamone, Koychev, Correa, & McGuire, 2014; Whitton, Treadway, & Pizzagalli, 2015). Hence, our focus is on willingness to expend effort toward reward. In the present study, we aimed to test whether an indicator of striatal DA, spontaneous eye-blink rate, is an effective marker of effort for reward.
Aim 1: Phasic Blink Rate as an Index of Reward Response
The role of DA in reward has been well-documented in neuroimaging research. In human studies, much of what is known about DA transmission stems from PET imaging studies (e.g., Davis et al., 2003; Drevets et al., 2001; Zald et al., 2004; Volkow, Fowler, & Wang, 1999; see Haber & Knutson, 2010, for review). Ligand and metabolic PET imaging studies provide a wealth of information on neurotransmitter action, but are limited by several practical factors such as high cost and exposure to radioactive isotopes. Given this, there is a need to consider other ways to approximate striatal DA levels, and spontaneous eye-blink rate has been used as one such measure (Karson, 1983).
Several decades of research have validated eye-blink rate as a reliable proxy for dopaminergic functioning. In an important validational study, Taylor and colleagues (1999) found that blink rate in monkeys correlated strongly with DA concentration in the ventromedial caudate nucleus. This relationship between eye-blink rate and DA is supported by studies of clinical populations known to be affected by altered dopaminergic functioning, such as increased eye-blink rate among people with schizophrenia (Chen, Lam, Chen, & Nguyen, 1996; Freed et al., 1980; Karson, 1983; Mackert et al., 1990; Mackert et al., 1991) and decreased blink rates among those with Parkinson’s disease (Deuschl & Goddemeier, 1998; Karson, 1983; Karson, LeWitt, Calne, & Wyatt, 1982) and among cocaine users (Colzato, van den Wildenberg, & Hommel, 2008). Blink rate has also been found to be associated with a genetic polymorphism relevant to DA transmission, the DRD4 genotype (Dreisbach et al., 2005). More recently, tonic eye-blink rate has been shown to correlate with personality traits (Barbato, della Monica, Costanzo, & de Padova, 2012; Colzato, Slagter, van den Wildenberg, & Hommel, 2012) as well as cognitive flexibility in humans (Akbari Chermahini & Hommel, 2010, 2012; Colzato, van den Wildenberg, van Wouwe, Pannebakker, & Hommel, 2009; Dreisbach et al., 2005; Müller, Dreisbach, Brocke, Lesch, Strobel, & Goschke, 2007; Tharp & Pickering, 2011).
While tonic blink rate has helped to classify specific populations characterized by altered dopaminergic functioning and predicted behavioral performance, strong evidence from studies that manipulate DA suggest that phasic changes in midbrain DA are particularly relevant for mobilizing effort towards reward (Salamone et al., 2009). Beyond evidence that tonic DA is correlated with eye-blink rates, several studies have tested how phasic changes in DA relate to eye-blink rates. Across multiple studies of humans and non-human primates, increases in eye blink rate have generally been documented after administration of a DA agonist (Blin, Masson, Azulay, Fondarai, & Serratrice, 1990; Elsworth et al., 1991; Jutkiewicz & Bergman, 2004; Kleven & Koek, 1996; though see van der Post et al., 2004, for a non-replication). In addition to pharmacological evidence, Akbari Chermahini and Hommel (2012) studied phasic changes in blink rate as corresponding with a behavioral task. Specifically, they found that eye-blink rates increased significantly after a positive mood induction (but not a negative mood induction), particularly for those participants with low tonic dopamine levels. Thus, changes in eye-blink rate may be a viable measure of individual fluctuations in DA. Further evidence regarding positive stimuli and eye-blink rate shows that variation in tonic blink rate interacts with the valence of pictures (positive or neutral) to predict participants’ reported self-agency on a laboratory task (Aarts et al., 2012). This finding, combined with those of Akbari Chermahini and Hommel (2012) suggest that responses to positive stimuli and positive mood inductions each may relate to eye-blink rate.
In sum, an abundance of evidence supports using eye-blink rate as a sensitive indicator of striatal DA function, and more importantly, as an index of phasic shifts in DA levels. Given the large literature suggesting that phasic shifts in striatal DA are central in mobilizing effort for the pursuit of anticipated rewards, that individual differences in striatal DA are associated with degree of effort for reward (Treadway et al., 2012), and that activation of the nucleus accumbens is associated with anticipating effort for reward (Kroemer et al., 2014), it is surprising that studies to date have not used blink rate to study the reward anticipation processes more directly. That is the goal of this study. We hypothesized that eye-blink rate should increase during anticipation of reward. The first goal of this study was to test whether changes in eye-blink rate correspond with a specific component of the reward process: preparing to expend effort towards reward.
Aim 2: Blink Rate and Reward in Psychopathology
As numerous studies have supported alterations in eye-blink rate in clinical populations characterized by dopaminergic dysregulation, we also tested an extension of this hypothesis in a clinical sample: adults with bipolar disorder. Converging evidence suggests a central role for DA dysfunction in bipolar disorder, in that those with the disorder show evidence of a possible hyper-sensitization of DA receptors (Cousins, Butts, & Young, 2009). Neuroimaging studies as well suggest heightened striatal activation during reward anticipation in bipolar disorder (Caseras, Lawrence, Murphy, Wise, & Phillips, 2013; Nusslock et al., 2012; Nusslock, Young, & Damme, 2014; though see Abler, Greenhouse, Ongur, Walter, & Heckers, 2008; Chase, Nusslock, Almeida, Forbes, LaBarbara, & Phillips, 2013), and striatal activation during reward anticipation is correlated with self-report measures of reward responsivity in people with bipolar disorder (Caseras et al., 2013). Thus, while some evidence suggests differences in anticipating reward in bipolar disorder, it is unknown if these differences would also manifest in differences in anticipating effort for reward.
More specifically, we studied the link between blink rate and reward in this population because of well-documented dysregulation in the reward system in people with bipolar disorder (Johnson, Edge, Holmes, & Carver, 2012). People with bipolar disorder also describe themselves on self-report measures as more sensitive to reward (Johnson, Edge, et al., 2012; Meyer, Johnson, & Winters, 2001). Specifically, those with the disorder or at risk for the disorder have been shown to plan for pursuing more ambitious goals (Johnson & Carver, 2006; Johnson, Eisner, & Carver, 2009; Johnson, Carver, & Gotlib, 2012), to sustain greater engagement during pursuit of difficult goals (Harmon-Jones et al., 2008), to be less likely to reduce effort towards goals after attaining their goals (Fulford, Johnson, Llabre, & Carver, 2010), and to demonstrate greater increases in confidence after success on self-report and laboratory-based measures (Eisner, Johnson, & Carver, 2008; Johnson & Jones, 2009; Meyer, Baron, Baur, & Jordan, 2010; Stern & Berrenberg, 1979). Some people with bipolar disorder also endorse experiencing mania after achieving reward (Edge et al., 2013), a finding that has also been substantiated by longitudinal research with life event interviews (Johnson et al., 2000; 2008).
Based on these studies, we predicted that people with bipolar disorder would show evidence of a potentiated blink response before and after engaging in the pursuit of reward. Given previous evidence that self-reported sensitivity to reward correlates with striatal activation during reward anticipation (Caseras et al., 2013), as well as research showing elevations in ambition, reward sensitivity, and confidence in bipolar disorder (Johnson, Edge, et al., 2012), we also hypothesized that eye-blink rate during a reward task should correspond with self-reported parameters of ambition, reward-triggered mania, and confidence.
Despite significant evidence implicating reward dysregulation and altered DA functioning, very little research has considered eye-blink rate in people with bipolar disorder, and no research to date has explored the potential link between eye-blink rate and reward in this population. In a small sample, Depue and colleagues (1990) found that people with a seasonal affective course of bipolar II disorder showed elevated tonic blink rates compared to healthy control participants, but not as compared to persons with unipolar depression with a seasonal course. To our knowledge, no research to date has considered blink rate in bipolar I disorder, nor have studies considered phasic changes in eye blink in those with bipolar disorder, or how eye-blink rate relates to measures relevant to reward function within a bipolar sample.
Hypotheses
The primary goals of this study were to investigate whether eye-blink rate increased as a function of preparing to pursue a reward and upon receiving reward; to investigate if people with bipolar disorder show elevations in blink rate during reward anticipation and reward receipt, relative to controls; and to test whether blink rate is related to validated measures of goal striving and reward responsivity. To investigate these hypotheses, we measured blink rate in healthy adults with no history of a mood disorder, and in adults with remitted bipolar I disorder. Blink rate was tested during a baseline condition, and at two phases (preparing to pursue a reward and after receiving reward) of a previously validated reward engagement paradigm (Harmon-Jones et al., 2008). Participants with bipolar disorder also completed self-report measures of reward sensitivity, confidence, and ambition.
Method
All study procedures were approved by the university Institutional Review Board. Participants provided consent before study procedures, and all participants were financially compensated for their time. All data were collected as part of a larger study also described elsewhere (Edge, Lwi, & Johnson, in press; Ng & Johnson, 2013).
Participants
Participants were recruited in the San Francisco Bay Area using online advertisements, and for the bipolar group, additional participants were recruited via advertising with local support groups and treatment centers. Effort was made to recruit demographically comparable control participants who were comparable on age, employment status, and education history through advertising in unemployment centers and through targeted ads in online media. Potential participants were screened initially by phone to ensure eligibility. Only participants who were fluent in the English language and between the age of 18 and 60 were invited to participate. Exclusion criteria included history of severe head trauma, vision problems that would interfere with eye-tracking (e.g., glaucoma, cataracts), central nervous system illness (e.g., Alzheimer’s disease), or learning disabilities that would interfere with understanding consent and study procedures.
Potential participants were invited to the university to complete the Structured Clinical Interview for DSM-IV (SCID; First et al., 1997; described in more detail below). Participants in the bipolar disorder group (n = 32) were included if they met criteria for a lifetime history of bipolar I disorder; those in the control group (n = 31) were included only if they did not meet criteria for a lifetime mood disorder (bipolar disorder, major depressive disorder, dysthymic disorder, or cyclothymic disorder). In both participant groups, those who met criteria for a primary psychotic disorder or a current substance use disorder (abuse or dependence) were excluded. Participants reporting regular cannabis use were also excluded regardless of whether they met DSM-IV criteria for a substance use disorder, given evidence for significantly altered blink rates in heavy cannabis users (Kowal, Colzato, & Hommel, 2011).
Measures
Structured Clinical Interview for DSM-IV (SCID, First et al., 1997)
The SCID is a well-validated semi-structured clinical interview that evaluates for the presence of DSM-IV (APA, 1994) Axis I disorders. Interviews were conducted by trained clinical psychology graduate students; interviewers achieved strong inter-rater reliability based on a random sample of audiotaped interviews: for current and lifetime manic episodes and lifetime major depressive episodes, ICCs ranged from 0.88 to 0.89, for current major depressive episode ratings, the ICC was 0.99.
Modified Hamilton Rating Scale for Depression (MHRSD; Miller et al., 1985)
The MHRSD is a clinician-administered interview designed to assess severity of current depression symptoms. The semi-structured interview has achieved good reliability and validity in previous studies (Miller et al., 1985), and has been validated for use in bipolar I disorder (Johnson et al., 2008). MHRSD scores range from 0 to 52, with score below 7 indicating remission, and those above 17 indicate the presence of a depressive episode. Interviews were conducted by trained graduate students who achieved strong interrater reliability on the basis of randomly selected interviews (ICC > .99).
Young Mania Rating Scale (YMRS; Young et al., 1978)
The YMRS is an 11-item interview designed to assess the severity of current symptoms of mania. This scale shows good reliability and consistency with other mania ratings (Young et al., 1978). As with the other clinical scales, ratings were conducted by trained graduate students. Randomly selected tapes of interviews suggested strong inter-rater reliability (ICC > 0.99).
Mood State and Confidence Rating
Participants completed 6 items regarding their current mood state and arousal using a 5-item Likert scale (1 = “Very Slightly or Not at All” to 5 = “Extremely”) for positive emotion (enthusiasm, confidence), negative emotions (sad, nervous, frustrated), as well as rating how tired they currently felt.
Medication Coding
For participants in the bipolar group, medication information and adherence was assessed using the Somatotherapy Index (Bauer, McBride, Shea, Gavin, Holden, & Kendall, 1997). Dosages were adjusted for adherence. Medications were converted to standard equivalent dosages for mood stabilizers, antiseizure medications, lamotragine, antidepressants (imipramine equivalents), atypical antipsychotic medication (risperidone equivalents) and for benzodiazepines (diazepam equivalents). Participants who were not taking a medication were coded as “0”.
Reward Responses Inventory (RRI, Edge et al., 2013)
The RRI is a 21-item self-report measure designed to evaluate responses to reward in people with bipolar disorder. The RRI contains two subscales: Reward-Triggered Mania, in which participants rate the extent to which they have experienced a manic episode following a rewarding or goal-pursuit related event, and Reward Avoidance, in which participants rate the degree to which they limit or avoid rewarding activities in order to prevent mania. Responses are rated on a 4-point scale ranging from 1 “Very false for me” to 4 “Very true for me.” Only participants in the bipolar group completed this scale, as it is specific to experiences of mania. Prior research using this scale in bipolar I disorder show good internal consistency for each subscale and previous validational work shows that a many individuals with bipolar I disorder endorse items relating to reward-triggered mania (Edge et al., 2013). In the present study, internal consistency was good for both subscales (Reward-Triggered Mania, α = 0.87, Reward Avoidance: α = 0.87).
Willingly Approached Set of Statistically Unlikely Pursuits (WASSUP, Johnson & Carver, 2006)
The WASSUP is a self-report measure of ambitious goal-setting. On this scale, participants read a series of extreme goals and rate the degree to which they expect this goal to occur for them, ranging from 1 (“No chance of occurring”) to 5 (“Definitely will occur”). For the present study, participants only completed two of the factor-analytically supported subscales: Popular Fame (measuring extreme ambitions for fame, for example, “You will appear regularly on TV”) and Finances (extreme ambitions for wealth, e.g. “You will have 20 million dollars or more”), as these two subscales have been found to be elevated in bipolar I disorder (Johnson et al., 2009), and to predict symptoms of mania (Johnson, Carver, et al., 2012) and onset of bipolar spectrum disorders (Alloy et al., 2012). These WASSUP subscales have demonstrated good internal consistency and reliability with other mania-relevant measures (Johnson & Carver, 2006; Johnson et al., 2009; Johnson, Carver, et al., 2012). In the present study, internal consistency was moderate for Financial Success (α = 0.60)1 and for Popular Fame (α = 0.74).
Procedure
After the phone screening, potential participants were invited to the laboratory to complete a diagnostic interview (SCID). Those who met criteria were invited to participate in the experimental session. Self-report questionnaires (WASSUP and RRI) were also completed at this time. Before the experimental session, participants in the bipolar group also completed mood interviews (MHRSD and YMRS) over the phone with a trained interviewer. Those whose mood rating scores fell below 7 on the YMRS or below 10 on the MHRSD were scheduled to complete the experimental session. Participants whose symptoms exceeded the cutoff range on either the MHRSD or the YMRS were followed monthly with phone interviews until they reached remission, and then given the opportunity to complete the experimental session.
To minimize confounds in blink rate, participants were asked to refrain from nicotine and caffeine use during the 12 hours leading up to the experimental session, and not to wear contact lenses for the session (e.g., to wear glasses). All sessions took place between 10am and 5pm, as eye-blink rates have been shown to increase in the evening (Barbato, Ficca, Muscettola, Fichele, Beatrice, & Rinaldi, 2000). All procedures took place in a windowless room with constant overhead lighting; temperature and humidity were also monitored for consistency throughout all recording sessions (Doughty, 2001).
Participants completed a five-minute baseline eye-blink recording, in which they sat in front of a fixation cross displayed in the center of the eye-tracking computer and were instructed not to look away from the screen. Eye-blink rate was recorded continuously during these five minutes, and blink rate per minute was calculated by dividing the total number of blinks by five. After the baseline, participants completed the Mood and Confidence Rating and then began the Anagrams reward task.
Anagrams Reward Task
The reward task was adapted from a previously validated reward paradigm used in a study of effort towards reward in bipolar disorder (Harmon-Jones et al., 2008). In the version of this paradigm used in the present study, participants completed a computerized reward task in which they were given five minutes to solve as many anagrams as possible, for the chance to win 50 cents per correctly solved anagram (incorrect responses did not result in any money won, but were not penalized). Before the task, participants read instructions on-screen that were also provided verbally by an experimenter, and then given the chance to practice solving anagrams to ensure they understood the goals of the task.
For each trial, a scrambled word appeared at the center of the screen, and participants used a keyboard to type their solution under the scrambled word. Trials advanced after each participant either entered a solution word or pressed the space bar to move on without providing a response. Immediately before each trial, participants received a cue on-screen that stated whether the next trial was easy, medium, or difficult; all trials were randomized so that easy, medium, and hard trials were interspersed throughout the task. Participants were not informed of their accuracy on each individual trial, and did not receive feedback about their total winnings until the end of the five-minute task. After five minutes had elapsed, participants saw a screen that showed their actual monetary winnings on the task.
To enhance reward anticipation and to encourage participants to mobilize effort for the task at hand, the anagrams task was preceded by a “countdown” phase lasting one minute. During this minute, participants saw the phrase “Get Ready!” displayed onscreen, adjacent to a digital countdown display counting down the seconds remaining until the task began (60 to 1). To further enhance anticipation, participants heard a clip of upbeat music during the count-down minute (“Vamos a Bailar,” recorded by the Gipsy Kings; this recording has previously been used in the context of positive affect inductions in the laboratory, cf. Edge et al., 2013). The “countdown” phase occurred only once, immediately before the start of the five-minute anagrams task. While participants viewed their winnings on-screen after the task, the same music was played again for 60 seconds, to enhance the positive effects of winning money on the task.
Eye-blink rate was recorded during the 60-second “countdown” phase immediately before the task (reward anticipation), as well as during the 60-second “reward” phase immediately after the task, when participants saw their winnings displayed onscreen (post-reward). Eye-blink rate was not calculated during the anagram task due to the potential confounding nature of reading words (cf. Doughty, 2001) and sustained cognitive effort (cf. Caplan, Guthrie, & Komo, 1996; Stern, Walrath, & Goldstein, 1984) during the task itself. Therefore, dependent variables of interest were limited to the eye-blink rate during reward anticipation (the count-down) and post-reward (the feedback screen).
Apparatus and Data Reduction
Eye-blink rate was captured using a Tobii T-120 infrared eye-tracker (Tobii Technologies, Dandyred, Sweden), synchronized with a secondary computer used to display stimuli. The Anagrams task and baseline phase were programmed with E-Prime Professional, Version 2.0, linked with the eye-tracker using E-Prime Extensions for Tobii (Psychology Software Tools, Pittsburgh, PA). Eye-tracking was recorded at 120 Hz, providing one sample per 8.3 msc. Stimuli were displayed on a 17-inch computer monitor with 1280 × 1024 screen resolution. A nine-point calibration was conducted before the baseline recording and again before the reward task. Based on previously established infrared eye tracking norms, eye blinks were coded as continuous periods of time of at least 100 msec and < 500 msec in which the coordinates and pupil diameter for both left and right eyes were not recorded (cf. Aarts et al., 2012; den Daas, Häfner, & de Wit, 2013; Smilek, Carriere, & Cheyne, 2010).
Results
Analyses were conducted with SPSS version 22.0 (IBM, Chicago, IL). Four participants (3 control, 1 bipolar) were excluded from data analyses because of eye-tracking equipment failure during the anagrams reward task (3 participants) or eye injuries not disclosed during screening (1 participant). Baseline data was additionally not available for 3 BD participants. As this was the result of equipment or experimenter error, the baseline data were imputed, resulting in a final sample of 31 in the bipolar group and 28 in the control group. Within the bipolar group, three MHRSD and two YMRS scores were missing, and these data were imputed (using linear regression) as well. As would be expected, analyses were parallel with and without the imputed scores. Before analyzing eye-blink rates, distributions of blink rates were analyzed for normality. Observed outliers, defined as blink rate values greater than 2.5 standard deviations from the sample mean, were Winsorized to the next highest non-outlying value for each task phase (Baseline: 2 participants, reward anticipation and post-reward phases: 1 participant each); amounting to less than 2.5% of all blink measurements. Blink rate estimates throughout the baseline and reward task phases were comparable to previously established norms (Doughty, 2001).
As shown in Table 1, diagnostic groups were well matched and did not differ on age, gender, employment status, race, or years of education. Bipolar and control groups did not differ on self-reported enthusiasm, confidence, or fatigue (all ps > .06); however, those in the bipolar disorder group reported experiencing significantly more frustration, t(45.38) = 2.21, p = .03, and sadness, t(32.96) = 2.44, p = .02. Eighty-four percent of participants in the bipolar group reported taking at least one psychotropic medication, the most common of which were atypical antipsychotic medication (13/31), lithium (11/31), and lamotrigine (7/31).
Table 1.
Sample Characteristics: Demographic Variables and Self-Report Measures
| Demographic or Mood Variable |
BP I Mean (SD) or % n = 31 |
Control Mean (SD) or % n = 28 |
Test statistic (t or chi-square) |
|---|---|---|---|
| Age | 38.16 (11.13) | 34.11 (13.51) | t(57) = 1.26; p = .21 |
| Gender (% Female) | 51.6 | 42.9 | χ2 (1) = 0.45, p = .50 |
| Race (% Minority Race) | 19.4 | 30.8 | χ2 (1) = 0.99, p = .32 |
| Years of Education | 15.26 (1.59) | 15.07 (2.21) | t(48.61) = 0.37, p =.71 |
| % Employed | 41.9 | 50 | χ2 (1) = 0.39, p = .54 |
| MHRSD | 2.42 (2.86) | -- | |
| YMRS | 1.19 (1.55) | -- | |
| WASSUP – Popular Fame | 10.52 (3.67) | -- | |
| WASSUP – Financial Success | 7.22 (3.04) | -- | |
| RRI-Reward Avoidance | 16.41 (6.53) | ||
| RRI – Reward Triggered Mania | 27.95 (7.29) | -- |
Note. n= 29 for RRI.
Before testing hypotheses, bivariate Pearson correlations were used to consider potential confounds influencing eye-blink rates. Across the sample as a whole (n = 59), mood and arousal variables (enthusiasm, frustration, sadness, and tiredness) were not significantly correlated with baseline or task-related blink rate, rs < .22, ps > .09. Correlations with confidence are presented in tests of hypotheses below. Because bipolar and control groups differed on two negative emotion variables (frustration and sadness), these variables were also compared to eye-blink rates in the bipolar group alone; frustration and sadness were not significantly correlated with eye-blink at any timepoint (rs < −.17, ps > .20). Within the bipolar group (n = 31), medication dosages were unrelated to eye-blink rate for any medication class at baseline (all rs < .18, ps > .33) or during reward anticipation or reward receipt (all rs < .31, ps > .09). Current mood symptoms in the bipolar group were also unrelated to baseline or reward-task blink rates (MHRSD: rs < .04, ps > .83; YMRS: rs < .10, ps > .59). Given the possibility that blink rate would shift with perceived task difficulty, Pearson correlations were used to examine the relationship between blink rate at each task phase and accuracy on the anagrams task. Accuracy was not significantly correlated with baseline blink rate, (r = .05, p = .69), reward anticipation blink rate (r = .11, p = .41), or reward receipt blink rate (r = .10, p = .46). Further, there was no evidence that variability in the difficulty of anagrams was related to blink rate: correlational analyses between eye blink rate at each time point and accuracy within each difficulty level showed no association between performance on easy anagrams and blink rates (rs < .11, ps > .42), nor with medium-difficulty (rs < .13, ps > .32) or hard anagrams (rs < .15, ps > .26).
Behavioral Data
In the sample as a whole, participants correctly solved more than half of the anagram trials (56.64%, SD = 20.58). A 3 (Trial Difficulty: easy, medium, or hard) by 2 (Group: bipolar or control) ANOVA with task accuracy as the dependent variable showed a large main effect of trial difficulty, F(2, 112) = 134.18, p < .001, ηp2 = 0.71. Planned contrasts showed that participants were significantly more accurate on Easy trials (89.09%, SD = 22.62) than Medium trials (56.56%, SD = 32.29), F(1, 56) = 57.27, p < .001, and significantly more accurate on Medium trials than on Hard trials (26.48%, SD =23.44), F(1, 56) = 69.2, p < .001. There was no main effect of group, F(1, 56) = .11, p = .74, ηp2 = .002, nor evidence of a Group x Difficulty interaction, F (2, 112) = .40, p = .67, ηp2 = .007.
Overall, participants won an average of $4.65 (SD= $2.68) and completed 15.36 trials (SD = 4.13). In parallel to the task accuracy findings, bipolar and control groups did not differ on overall money won, t(57) = −1.55, p = .13, nor on number of completed trials (regardless of success), t(57) = −1.87, p = .07. Bipolar and control groups were therefore matched on behavioral performance of the reward task.
Eye-Blink Rate
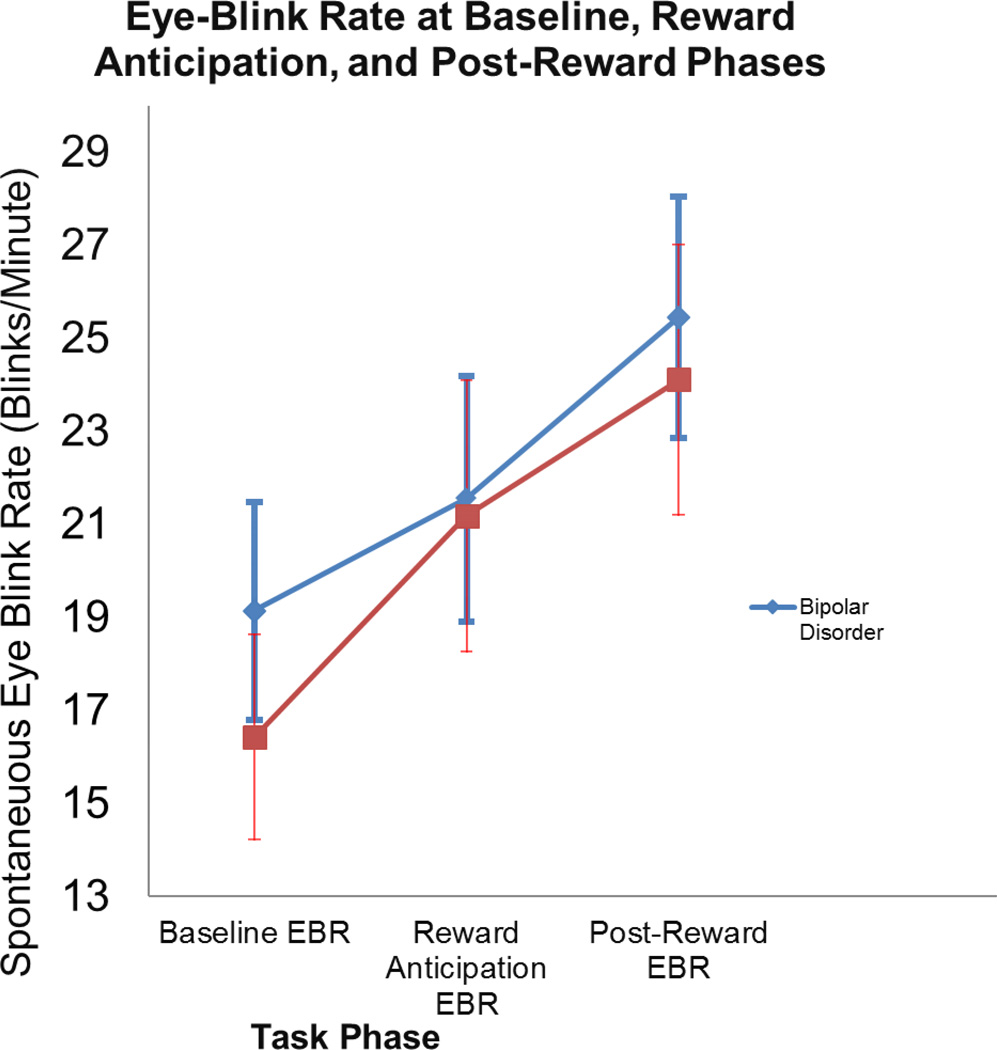
To assess change in eye-blink rate as a function of reward and diagnostic category, a 3 (Task phase: baseline, reward anticipation, and reward receipt) by 2 (Group: Bipolar or Control) ANOVA was conducted, with spontaneous eye-blink rate (blinks per minute) as the dependent variable. Figure 1 shows the results for eye-blink rate across these time points. This analysis yielded a significant main effect of time (task phase), F(2, 114) = 6.88, p < .01, ηp2 = .11. Planned contrasts showed that eye-blink rate marginally increased from the baseline phase to the reward anticipation phase, F(1, 57) = 3.15, p = .08, ηp2 = .05, and then significantly increased from the reward anticipation phase to the reward receipt phase, F(1, 57) = 4.35, p = .04, ηp2 = .07. There was no significant main effect of diagnostic group, F(1, 57) = 0.25, p = .62, ηp2 = .004, nor evidence of a significant Task phase x Diagnostic group interaction, F(2, 114) = .20, p = 0.82, ηp2= .003.2
Figure 1.
Error bars indicate 1 standard error of the mean. EBR= Spontaneous Blink Rate.
Correlations of Blink Rate with Measures of Reward, Ambition, and Confidence
Blink rate during reward anticipation and reward receipt were compared against self-report measures of reward, confidence, and goal-setting, using partial correlations controlling for baseline blink rate. As shown in Table 2, blink rate during the reward anticipation phase was significantly correlated with ambitious goal-setting (WASSUP Popular Fame subscale) and confidence ratings in those with bipolar disorder, while blink rate during reward receipt was correlated with higher scores on the reward-triggered mania scale of the RRI. In contrast, confidence ratings were not significantly correlated with either reward anticipation (partial r = .18, p = .37) or post-reward blink rate (partial r = .25, p = .22) in the control group, although the strength of the correlations between blink rate and confidence did not significantly differ between bipolar and control groups (Fisher’s Z test; reward anticipation p = .36; post-reward p = .68).
Table 2.
Partial Correlations of Confidence and Reward Measures with Eye-Blink Rate, Controlling for Baseline Eye-Blink Rate
| Eye Blink Task Phase |
||
|---|---|---|
| Measure | Reward Anticipation Eye Blink Rate |
Post-Reward Eye-Blink Rate |
| WASSUP-Popular Fame | .43* | .24 |
| WASSUP-Financial Success | .11 | .04 |
| RRI-Reward Triggered Mania | .11 | .38* |
| RRI – Reward Avoidance | .18 | .12 |
| Pre-Task Confidence Rating | .41* | .14 |
Note.
p < .05. n= 29 for RRI; n = 31 for WASSUP and Confidence.
Discussion
Eye-blink rate is a validated, though indirect, measure of striatal dopamine. The present study contributes one of the first investigations of repeated measurement of eye-blink rates on a behavioral task, and the first to evaluate changes in eye-blink rate as a function of striving towards reward. We tested whether preparing to expend effort for reward and receiving a reward would be associated with increases in eye-blink rate in both healthy adults and adults with bipolar disorder. Eye-blink rate across both groups of participants marginally but non-significantly increased while preparing to expend effort on a difficult, rewarding task; blink rate further and significantly increased upon receipt of reward. These findings suggest that eye-blink rate may provide a psychophysiological marker of response to receiving reward. Although no group differences between those with and without bipolar disorder emerged, measures of confidence, ambitious goal-setting, and reward-triggered mania were strongly correlated with blink responses in people with bipolar disorder, suggesting a potential role for eye blink rate to index individual differences in reward sensitivity.
Neuroimaging studies show consistent evidence for heightened activity in the striatum during reward anticipation (Haber & Knutson, 2010; Knutson et al., 2001), and mounting evidence shows the importance of mesolimbic dopamine for effort towards reward (Salamone et al., 2009). Though speculative, the trend towards elevated eye-blink rate during the reward anticipation phase in the present study is consistent with these findings.
It is less clear why participants showed additional elevations in eye-blink rates (both compared to baseline and to the pre-reward phase) immediately after receiving reward. One possibility is that the elevated blink rate post-reward may reflect a residual signal of prior effort towards reward. Evidence suggests that among people with and without bipolar disorder, many do not reduce effort after achieving a goal (Fulford et al., 2010). Thus, the sustained increase in blink rate after goal attainment may reflect continued striving. Another possibility is that participants experienced the reward as unexpected. Striatal activation is associated with receiving unanticipated rewards (Haber & Knutson, 2010; Schulz, 1998), and although participants were informed that they would have five minutes to work on the reward task to win as much money as possible, their actual winnings were not displayed during the task and they did not receive feedback along the way. Thus, the sudden appearance of monetary reward feedback after five minutes may have served to function as an unexpected reward, and thus could index a phasic dopamine response.
It will be important for future studies to validate this method of testing effort towards reward. In particular, measurement of eye-blink during the actual expenditure of effort towards reward was not explored in the present study, so future research is needed to test eye blink responses during goal-striving behavior. This next step of measurement is particularly important in validating a behavioral index of dopaminergic responses to reward, given research suggesting that striatal dopamine is tightly linked to the actual expenditure of effort towards reward (Salamone et al., 2009, Berridge, 2007). The present findings suggest that receiving a reward and to a lesser extent preparing to expend effort towards reward are each tied to increased blink rate—essentially, two time periods that “bookend” the reward pursuit process. We eagerly await future applications of this paradigm that will fill in these bookends.
Also, we cannot rule out the possibility that other the observed increase in eye-blink rate was also influenced by other cognitive or biological influences, rather than reward processing. It is possible that other components involved in solving anagrams, such as verbal fluency or cognitive flexibility, also contributed to the increase in eye-blink rate. Given that performance on the anagrams task was unrelated to eye-blink rate, it seems unlikely cognitive effort alone was responsible for this increase in blinking. Similarly, it is possible that blink rate was influenced by music during the measurement periods before and after the reward task. However, this too seems unlikely to explain the observed increase in blink rate, as other studies have found no differences in eye-blink rate measured with and without music (cf. Lichtenberg, Even-Or, Bachner-Melman, Levin, Brin, & Heresco-Levy, 2008). Finally, although eye-blink rate was unrelated to medication dosages and other confounds (such as caffeine and nicotine use) were controlled to the best of the experimenters’ abilities, it is possible that other biological mechanisms influencing ocular or dopaminergic systems could have contributed to the increase in eye-blink rate or might have differed by group.
The hypothesis that bipolar disorder would be linked to a larger increase in blink rate on a reward task was not confirmed. This is somewhat surprising, as many studies have documented evidence for increased reward sensitivity in people with bipolar disorder (Johnson, Edge et al., 2012). Given some previous evidence showing enhanced striatal activation during passive reward anticipation in bipolar disorder (Nusslock et al., 2012), it is possible that group differences in blink rate would emerge during a passive reward anticipation paradigm, rather than anticipating reward in the context of effort. Further, although the task used in the present study was designed to maximize ability to test blink rates before and after reward, this design precluded the ability to test group differences in responses to reward on individual trials. In a previous study using a similar anagrams paradigm, Harmon-Jones and colleagues (2008) found evidence for increased preparatory effort for difficult trials in bipolar disorder, suggesting that future studies of blink rate and reward could benefit from studying changes in blink rate before and after individual trials. This is a goal for future studies.
The present findings also showed that baseline eye-blink rate did not differ in people with and without bipolar disorder, in contrast to early findings in this population (Depue et al., 1990). Depue and colleagues’ early findings of elevated blink rate in bipolar disorder were observed in individuals with bipolar II disorder, a subtype that has been found to show a different neural reward profile than bipolar I disorder (Caseras et al., 2013). It is possible that the difference in subtypes contributed to this divergent finding. Bipolar disorder is a highly heterogenous disorder, and gaining specificity in identifying specific facets of reward that are disrupted, as well as individual differences in response to reward is needed.
Consistent with the importance of individual differences, measures of confidence, extreme ambition, and reward-triggered mania were strongly linked to eye-blink response to the reward task in bipolar disorder. Given our finding that the strength of blink rate-confidence correlations did not differ between bipolar and control groups, it seems possible that the observed links between individual difference measures and eye-blink rate are indicative of general individual differences rather than a disorder-specific mechanism. However, given the strong correlations between blink rate and individual differences on the two bipolar-specific measures, more research is needed in this domain in order to understand where psychopathology-specific differences may emerge. These findings are compatible with research showing links between individual differences in reward responsivity and neural response to reward (Hahn et al., 2009; Linke et al., 2010; Simon et al., 2010; Tomer et al., 2014), including recent findings within a bipolar sample (Caseras et al., 2013).
Importantly, we observed a dissociation between the effects of ambition and confidence (relating to reward anticipation blink rate) and reward-triggered mania (relating to post-reward blink rate). That is, the questionnaires showed expected temporal patterns in their links with pre and post-goal blink rates. Across multiple studies, heightened ambition has been documented among those diagnosed with bipolar disorder (Johnson et al., 2009; Johnson, Carver, et al., 2012) and has been consistently found to be present before onset among those at risk for the disorder (Alloy et al., 2012; Carver & Johnson, 2009; Fulford, Johnson, & Carver, 2008; Gruber & Johnson, 2009; Johnson & Carver, 2006; Johnson & Jones, 2009). Heightened ambition also predicts a more severe course of mania (Johnson, Carver, et al., 2012) and the onset of bipolar spectrum disorder (Alloy et al., 2012). To date, this literature has rested entirely on self-report measures. These findings are novel in providing a window into a potential biological mechanism, in that blink rate may capture one aspect of biological sensitivity to goal striving in bipolar disorder.
The finding that higher blink rates when receiving a modest monetary reward significantly correlated with more frequent instances of experiencing mania after a rewarding life event also seems highly relevant. Several studies have suggested that life events involving reward can trigger mania (Johnson et al., 2000; 2008), and recent research suggests that those with bipolar disorder show considerable variability in whether they have observed this process in their own course of symptoms (Edge et al., 2013). Findings in this study suggest that persons who reported reward-triggered mania demonstrated a stronger physiological response to the reward task, raising the possibility that eye-blink rate in response to reward could potentially be relevant for predicting the course of mania. Longitudinal studies are needed to test this possibility.
On the whole, the current findings suggest a new approach to measuring reward sensitivity for basic research, and for understanding how this sensitivity might differ among those with psychopathology. Increasingly, researchers have emphasized the need to incorporate neuroscience-informed methods to inform diagnosis and treatment of psychological disorders (Craske, 2014; Holmes, Craske, & Graybiel, 2014; Siegle, 2011; Siegle, Ghinassi, & Thase, 2007), though one observed challenge is the cost and difficulty involved in using neuroimaging to guide this process (Nusslock et al., 2014). Given the comparably low cost of eye-blink rate, future studies could explore the utility of this methodology as a putative index of individual differences in reward response.
The present study has important limitations. Previous literature has often tested eye-blink rate over time periods lasting 3–5 minutes (Doughty, 2001), so it is possible that the 60-second measurement periods before and after the reward task may be less reliable than a longer measurement period. However, good reliability has been observed for one-minute blink rate measurement periods (Deuschl & Goddemeier, 1998). Another limitation is the relatively small sample size, which may have reduced the ability to test between-group differences in eye-blink rate; regarding statistical power, though, we would note that between group effect sizes were extremely small. Finally, it is unclear the extent to which between-groups differences in blink rate were influenced by medication usage in the bipolar group: although group differences remained non-significant when controlling for medication dosage, correlational analyses suggest that some degree of variability in blink rate is associated with medication dosage.
In sum, this study provides initial evidence that phasic changes in eye-blink rate are tied to receiving reward, and marginally related to preparing to expend effort towards reward. As spontaneous eye-blink rate is a validated index of dopaminergic activity in the striatum, and given the importance of striatal dopamine in effort towards reward, it is possible that increases in striatal dopamine are driving the observed blink response during the reward task in the present study. Clearly, more research is needed to test this hypothesis, such as PET imaging and animal paradigms of reward response and eye-blink rate. The correlational findings showing links between self-reported confidence and reward response in bipolar disorder indicate a potential window into measuring individual differences in reward responsivity in psychopathology. It is hoped that future research continues to investigate both of these early findings.
Acknowledgments
Preparation of this manuscript was supported by NIMH grant T32-MH089919 (to ADP). The authors thank Eddie Harmon-Jones for sharing the stimuli for the anagrams reward task. We thank Silvia Bunge, Carter Wendelken, and Jesse Niebaum for assisting with development of blink rate analysis strategies, and Jordan Tharp, Anna Feiss, Steven Wandrey, and Sant Kumar for help with data collection and compiling blink rate data files.
Footnotes
Alpha for the Financial Success scale was .77 if one item was excluded; with this item excluded, however, effect sizes for tests of hypotheses were comparable. Results here present effects with the item included, to be consistent with previous research.
A separate repeated-measures ANOVA was also conducted including medication dosages for each of the classes of medication described entered simultaneously as covariates. As in the original tests of hypotheses, this analysis revealed a significant main effect of time, F(2,102) = 4.38, p = .02, ηp2 = .08. Parallel to the initial analyses, there was no significant main effect of diagnostic group, F(1, 51) = 0.06, p = .81, ηp2 = .001, nor evidence of a significant Task phase x Diagnostic group interaction, F(2, 102) = .008, p = 0.992, ηp2 < .001. There were no significant main effects of medication classes (Fs < 2.54; ps < .12), nor interactions with medication and time (Fs < 1.81; ps < .17).
Author Contributions
ADP and SLJ jointly developed the study concept and design. ADP conducted statistical analyses under the guidance of SLJ, and wrote the first draft of the manuscript. SLJ provided critical revisions.
References
- Aarts H, Bijleveld E, Custers R, Dogge M, Deelder M, Schutter D, Van Haren NEM. Positive priming and intentional binding: Eye-blink rate predicts reward information effects on the sense of agency. Social Neuroscience. 2012;7:105–112. doi: 10.1080/17470919.2011.590602. [DOI] [PubMed] [Google Scholar]
- Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari Chermahini SA, Hommel B. The (b)link between creativity and dopamine: Spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition. 2010;115:458–465. doi: 10.1016/j.cognition.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Akbari Chermahini SA, Hommel B. More creative through positive mood? Not everyone! Frontiers in Human Neuroscience. 2012;6(319):1–7. doi: 10.3389/fnhum.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Abramson LY. High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. Journal of Abnormal Psychology. 2012;121:339–51. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Barbato G, della Monica C, Costanzo A, De Padova V. Dopamine activation in Neuroticism as measured by spontaneous eye blink rate. Physiology & Behavior. 2012;105:332–336. doi: 10.1016/j.physbeh.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, Rinaldi F. Diurnal variation in spontaneous eye-blink rate. Psychiatry Research. 2000;93:145–151. doi: 10.1016/s0165-1781(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Bauer MS, McBride L, Shea N, Gavin C, Holden F, Kendall S. Impact of an easy-access VA clinic-based program for patients with bipolar disorder. Psychiatric Services. 1997;48:491–496. doi: 10.1176/ps.48.4.491. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: “liking”, “wanting”, and learning. Current Opinion in Pharmacology. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin O, Masson G, Azulay JP, Fondarai J, Serratrice G. Apomorphine-induced blinking and yawning in healthy volunteers. British Journal of Clinical Pharmacology. 1990;30:769–773. doi: 10.1111/j.1365-2125.1990.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan R, Guthrie D, Komo S. Blink rate in children with attention-deficit-hyperactivity disorder. Biological Psychiatry. 1996;39:1032–1038. doi: 10.1016/0006-3223(95)00315-0. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL. Tendencies toward mania and tendencies toward depression have distinct motivational, affective, and cognitive correlates. Cognitive Therapy and Research. 2009;33:552–569. doi: 10.1007/s10608-008-9213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: Differences between bipolar I and II disorders. American Journal of Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders. 2013;15:839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EYH, Lam LCW, Chen RYL, Nguyen DGH. Blink rate, neurocognitive impairments, and symptoms in schizophrenia. Biological Psychiatry. 1996;40:597–603. doi: 10.1016/0006-3223(95)00482-3. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Slagter HA, van den Wildenberg WPM, Hommel B. Closing one’s eyes to reality: Evidence for a dopaminergic basis of Psychoticism from spontaneous eye blink rates. Personality and Individual Differences. 2009;46:377–380. [Google Scholar]
- Colzato LS, van den Wildenberg WPM, Hommel B. Reduced spontaneous eye blink rates in recreational cocaine users: evidence for dopaminergic hypoactivity. PloS One. 2008;3:e3461. doi: 10.1371/journal.pone.0003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WPM, van Wouwe N, Pannebakker MM, Hommel B. Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Experimental Brain Research. 2009;196:467–474. doi: 10.1007/s00221-009-1862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins D, Butts K, Young A. The role of dopamine in bipolar disorder. Bipolar Disorders. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- Craske MG. Introduction to special issue: How does neuroscience inform psychological treatment? Behaviour Research and Therapy. 2014;62:1–2. doi: 10.1016/j.brat.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Davis MR, Votaw JR, Bremner JD, Byas-smith MG, Faber TL, Voll RJ, Goodman MM. Initial human PET imaging studies with the dopamine transporter ligand 18 F-FECNT. Journal of Nuclear Medicine. 2003;44:855–861. [PubMed] [Google Scholar]
- den Daas C, Häfner M, de Wit J. Out of sight, out of mind: cognitive states alter the focus of attention. Experimental Psychology. 2013;60:313–323. doi: 10.1027/1618-3169/a000201. [DOI] [PubMed] [Google Scholar]
- Depue RA, Arbisi P, Krauss S, Iacono WG, Leon A, Muir R, Allen J. Seasonal independence of low prolactin concentration and high spontaneous eye blink rates in unipolar and bipolar II seasonal affective disorder. Archives of General Psychiatry. 1990;47:356–361. doi: 10.1001/archpsyc.1990.01810160056009. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Goddemeier C. Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease, and in normal subjects. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64:320–324. doi: 10.1136/jnnp.64.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optometry and Vision Science. 2001;78:712–725. doi: 10.1097/00006324-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Müller J, Goschke T, Strobel A, Schulze K, Lesch K-P, Brocke B. Dopamine and cognitive control: The influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behavioral Neuroscience. 2005;119:483–490. doi: 10.1037/0735-7044.119.2.483. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Edge MD, Lwi S, Johnson SL. An assessment of emotional reactivity to frustration of goal pursuit in euthymic bipolar I disorder. Clinical Psychological Science. (in press). [Google Scholar]
- Edge MD, Miller CJ, Muhtadie L, Johnson SL, Carver CS, Marquinez N, Gotlib IH. People with bipolar I disorder report avoiding rewarding activities and dampening positive emotion. Journal of Affective Disorders. 2013;146:407–413. doi: 10.1016/j.jad.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner LR, Johnson SL, Carver CS. Cognitive responses to failure and success relate uniquely to bipolar depression versus mania. Journal of Abnormal Psychology. 2008;117:154–163. doi: 10.1037/0021-843X.117.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth D, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Redmond DE. D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. Journal of Pharmacology and Experimental Therapeutics. 1991;259:595–600. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV axis I disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Freed WJ, Kleinman J, Karson C, Potkin S, Murphy D, Wyatt R. Eye-blink rates and platelet monoamine oxidase activity in chronic schizophrenic patients. Biological Psychiatry. 1981;15:329–332. [PubMed] [Google Scholar]
- Fulford D, Johnson SL, Carver CS. Commonalities and differences in characteristics of persons at risk for narcissism and mania. Journal of Research in Personality. 2008;42:1427–1438. doi: 10.1016/j.jrp.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford D, Johnson SL, Llabre MM, Carver CS. Pushing and coasting in dynamic goal pursuit: Coasting is attenuated in bipolar disorder. Psychological Science. 2010;21:1021–1027. doi: 10.1177/0956797610373372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Johnson SL. Positive emotional traits and ambitious goals among people at risk for mania: The need for specificity. International Journal of Cognitive Therapy. 2009;2:179–190. doi: 10.1521/ijct.2009.2.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis A-C, Plichta MM, Heinzel S, Polak T, Fallgatter AJ. Neural response to reward anticipation is modulated by Gray’s impulsivity. NeuroImage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, Fearn M. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Holmes EA, Craske MG, Graybiel AM. A call for mental-health science. Nature. 2014;511:287–289. doi: 10.1038/511287a. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Carver CS. Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognitive Therapy and Research. 2006;30:377–395. doi: 10.1007/s10608-006-9044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, Gotlib IH. Elevated ambitions for fame among persons diagnosed with bipolar I disorder. Journal of Abnormal Psychology. 2012;121:602–609. doi: 10.1037/a0026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Cueller AK, Ruggero C, Winett-Perlman C, Goodnick P, White R, Miller I. Life events as predictors of mania and depression in bipolar I disorder. Journal of Abnormal Psychology. 2008;117:268–277. doi: 10.1037/0021-843X.117.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annual Review of Clinical Psychology. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eisner LR, Carver CS. Elevated expectancies among persons diagnosed with bipolar disorder. British Journal of Clinical Psychology. 2009;48:217–222. doi: 10.1348/014466509X414655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Jones S. Cognitive correlates of mania risk: Are responses to success, positive moods, and manic symptoms distinct or overlapping? Journal of Clinical Psychology. 2009;65:891–905. doi: 10.1002/jclp.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, Keitner G. Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Bergman J. Effects of dopamine D1 ligands on eye blinking in monkeys: Efficacy, antagonism, and D1/D2 interactions. The Journal of Pharmacology and Experimental Therapeutics. 2004;311:1008–1015. doi: 10.1124/jpet.104.071092. [DOI] [PubMed] [Google Scholar]
- Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Karson CN, LeWitt PA, Calne DB, Wyatt RJ. Blink rates in Parkinsonism. Annals of Neurology. 1982;12:580–583. doi: 10.1002/ana.410120614. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in Cynomolgus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1996;279:1211–1219. [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kowal MA, Colzato LS, Hommel B. Decreased spontaneous eye blink rates in chronic cannabis users: evidence for striatal cannabinoid-dopamine interactions. PloS One. 2011;6:e26662. doi: 10.1371/journal.pone.0026662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Guevara A, Ciocanea Teodorescu I, Wuttig F, Kobiella A, Smolka MN. Balancing reward and work: Anticipatory brain activation in NAcc and VTA predict effort differentially. NeuroImage. 2014;102:510–519. doi: 10.1016/j.neuroimage.2014.07.060. [DOI] [PubMed] [Google Scholar]
- Lichtenberg P, Even-Or E, Bachner-Melman R, Levin R, Brin A, Heresco-Levy U. Hypnotizability and blink rate: A test of the dopamine hypothesis. International Journal of Clinical and Experimental Hypnosis. 2008;56:37–41. doi: 10.1080/00207140802039474. [DOI] [PubMed] [Google Scholar]
- Linke J, Kirsch P, King AV, Gass A, Hennerici MG, Bongers A, Wessa M. Motivational orientation modulates the neural response to reward. NeuroImage. 2010;49:2618–2625. doi: 10.1016/j.neuroimage.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Mackert A, Flechtner K-M, Woyth C, Frick K. Increased blink rates in schizophrenics: influences of neuroleptics and psychopathology. Schizophrenia Research. 1991;4:41–47. doi: 10.1016/0920-9964(91)90008-f. [DOI] [PubMed] [Google Scholar]
- Mackert A, Woyth C, Flechtner KM, Volz HP. Increased blink rate in drug-naive acute schizophrenic patients. Biological Psychiatry. 1990;27:1197–1202. doi: 10.1016/0006-3223(90)90417-z. [DOI] [PubMed] [Google Scholar]
- Meyer TD, Barton S, Baur M, Jordan G. Vulnerability factors for bipolar disorders as predictors of attributions in ability-based and chance-based tests. Journal of Individual Differences. 2010;31:29–37. [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: Reliability and validity. Psychiatry Research. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Müller J, Dreisbach G, Brocke B, Lesch K-P, Strobel A, Goschke T. Dopamine and cognitive control: the influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Research. 2007;1131:155–162. doi: 10.1016/j.brainres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Ng TH, Johnson SL. Rejection sensitivity is associated with quality of life, psychosocial outcome, and the course of depression in euthymic patients with bipolar I disorder. Cognitive Therapy and Research. 2013;37:1169–1178. doi: 10.1007/s10608-013-9552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, Phillips ML. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Young CB, Damme K. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: Assessment and treatment implications. Behaviour Research and Therapy. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience. 2009;3:1–12. doi: 10.3389/neuro.08.013.2009. Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Koychev I, Correa M, McGuire P. Neurobiological basis of motivational deficits in psychopathology. European Neuropsychopharmacology. 2014 doi: 10.1016/j.euroneuro.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. Journal of Neuroscience. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Siegle GJ. Beyond Depression Commentary: Wherefore art thou, depression clinic of tomorrow? Clinical Psychology: Science and Practice. 2011;18:305–310. doi: 10.1111/j.1468-2850.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research. 2007;31:235–262. [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich H-C, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage. 2010;49:1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Smilek D, Carriere JSA, Cheyne JA. Out of mind, out of sight: Eye blinking as indicator and embodiment of mind wandering. Psychological Science. 2010;21:786–789. doi: 10.1177/0956797610368063. [DOI] [PubMed] [Google Scholar]
- Stern GS, Berrenberg JL. Skill-set, success outcome, and mania as determinants of the illusion of control. Journal of Research in Personality. 1979;13:206–220. [Google Scholar]
- Stern JA, Walrath LC, Goldstein R. The endogenouse eyeblink. Psychophysiology. 1984;21:22–33. doi: 10.1111/j.1469-8986.1984.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Lawrence MS, Sladek JR, Roth RH, Redmond DE. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Experimental Neurology. 1999;158:214–220. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- Tharp IJ, Pickering AD. Individual differences in cognitive-flexibility: the influence of spontaneous eyeblink rate, trait psychoticism and working memory on attentional set-shifting. Brain and Cognition. 2011;75:119–125. doi: 10.1016/j.bandc.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Tomer R, Slagter HA, Christian BT, Fox AS, King CR, Murali D, Davidson RJ. Love to win or hate to lose? Asymmetry of dopamine D2 receptor binding predicts sensitivity to reward vs. punishment. Journal of Cognitive Neuroscience. 2014;26:1039–1048. doi: 10.1162/jocn_a_00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. Journal of Neuroscience. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Post J, de Waal PP, de Kam ML, Cohen AF, van Gerven JMA. No evidence of the usefulness of eye blinking as a marker for central dopaminergic activity. Journal of Psychopharmacology. 2004;18:109–114. doi: 10.1177/0269881104042832. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. Journal of Psychopharmacology. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. The Journal of Neuroscience. 2004;24:4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]