ABSTRACT
Gliding motility is common in members of the phylum Bacteroidetes, including Flavobacterium johnsoniae and Cellulophaga algicola. F. johnsoniae gliding has been extensively studied and involves rapid movement of the cell surface adhesin SprB. Genetic analysis of C. algicola allowed a comparative analysis of gliding. Sixty-three HimarEm1-induced mutants that formed nonspreading colonies were characterized. Each had an insertion in an ortholog of an F. johnsoniae motility gene, highlighting similarities between the motility systems. Differences were also observed. C. algicola lacks orthologs of the F. johnsoniae motility genes gldA, gldF, and gldG that are thought to encode the components of an ATP-binding cassette (ABC) transporter. In addition, mutations in any of 12 F. johnsoniae gld genes result in complete loss of motility, whereas all C. algicola gld mutants retained slight residual motility. This may indicate that C. algicola has multiple motility systems, that the motility proteins exhibit partial redundancy of function, or that essential components of the motility machinery of both C. algicola and F. johnsoniae remain to be discovered.
IMPORTANCE The development of genetic tools for C. algicola and comparative analysis of F. johnsoniae and C. algicola motility mutants identified similarities and differences between their gliding motility machineries. Gliding motility is common in the phylum Bacteroidetes. Proteins that are important for gliding in both C. algicola and F. johnsoniae are potential core components of the Bacteroidetes gliding motility machinery.
INTRODUCTION
Many bacteria belonging to the phylum Bacteroidetes exhibit rapid gliding motility over surfaces (1). Gliding has been most well studied in Flavobacterium johnsoniae, in which the disruption of any of 11 gld genes (gldA, gldB, gldD, gldF, gldG, gldH, gldI, gldJ, gldK, gldL, and gldM) or deletion of gldN and its paralog gldO results in a complete loss of motility (2–10). These gld mutants form nonspreading colonies on agar, and individual cells exhibit no movement on glass or agar. Mutations in other F. johnsoniae genes (sprA, sprB, sprC, sprD, sprE, sprF, and sprT) result in partial motility defects (11–15). These spr mutants form nonspreading colonies composed of cells that exhibit some limited ability to glide on glass surfaces.
sprB encodes an adhesin that is propelled rapidly along the cell surface and is thought to account for much of the gliding movement of F. johnsoniae (11, 16). The F. johnsoniae genome encodes other SprB-like proteins, such as RemA, which explains why sprB mutants exhibit some residual motility (17). Seven proteins (GldK, GldL, GldM, GldN, SprA, SprE, and SprT) comprise the type IX secretion system (T9SS) that is required for the delivery of SprB to the cell surface and for the secretion of dozens of other cell surface and extracellular proteins (14, 15, 18, 19). SprF is also needed for the secretion of SprB but not for the secretion of other proteins targeted to the T9SS (13). The motor that propels SprB along the cell surface is not known, but it may consist of some of the Gld and Spr proteins.
Genetic analysis of Bacteroidetes gliding motility has been primarily confined to F. johnsoniae. In this study, we developed techniques to genetically manipulate the marine gliding bacteroidete Cellulophaga algicola and used these to conduct a comparative analysis of the genes and proteins involved in C. algicola and F. johnsoniae gliding. C. algicola was selected for three reasons. First, it is distantly related to F. johnsoniae within the class Flavobacteriia (see Fig. S1 in the supplemental material). Second, comparative genome analysis revealed that unlike almost all other gliding members of the phylum Bacteroidetes, C. algicola lacks orthologs of gldA, gldF, and gldG (1). GldA, GldF, and GldG are required for F. johnsoniae gliding and are thought to constitute a novel ATP-binding cassette (ABC) transporter (2, 5). The function of this transporter in gliding is not known, and comparative analysis of C. algicola and F. johnsoniae gliding might help reveal this function. Finally, C. algicola is a member of the large and diverse marine clade of the Flavobacteriaceae (20, 21). These bacteria are abundant in marine environments. They have received attention because of their ability to digest algal polysaccharides, but until now, genetic manipulations were not possible. The genetic analyses of C. algicola reported here demonstrate the importance of the C. algicola orthologs of the F. johnsoniae gld and spr genes in motility. They also suggest that an ABC transporter may not be central to Bacteroidetes gliding. Analyses of the motility phenotypes of gld and spr mutants revealed differences between C. algicola and F. johnsoniae and suggest that additional unidentified genes may be important in C. algicola, and perhaps F. johnsoniae, cell movement.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
C. algicola DSM 14237T, originally isolated from the surface of the Antarctic sea ice diatom Melosira (20), was the wild-type strain used in this study. The original culture contained two colony types that differed in the degree of colony spreading. We selected a single colony of the faster-spreading variety for our studies and refer to this as C. algicola strain CA2. C. algicola strains were grown at 15°C in Cytophaga medium (DSMZ medium 172; 1.0 g/liter yeast extract, 1.0 g/liter tryptone, 24.7 g/liter NaCl, 0.7 g/liter KCl, 6.3 g/liter MgSO4·7H2O, 4.6 g/liter MgCl2·6H2O, 1.2 g/liter CaCl2·2H2O, 0.2 g/liter NaHCO3 [pH 7.2]) or Cytophaga medium containing 15 g agar/liter (Cytophaga agar). Marine conjugation medium consisted of 1.0 g/liter yeast extract, 1.0 g/liter tryptone, 5 g/liter NaCl, 0.35 g/liter KCl, 3.15 g/liter MgSO4·7H2O, 2.3 g/liter MgCl2·6H2O, 0.6 g/liter CaCl2·2H2O, and 0.1 g/liter NaHCO3 (pH 7.2). Marine conjugation agar was identical except for the addition of 15 g agar/liter. Marine conjugation medium has salt concentrations tolerated by both Escherichia coli and C. algicola and was developed to allow conjugative transfer of DNA from E. coli into C. algicola and other marine bacteria. E. coli strains were grown in Luria-Bertani (LB) medium at 37°C. Antibiotics were used at the following concentrations when needed: ampicillin, 100 μg/ml; erythromycin, 50 μg/ml; kanamycin, 35 μg/ml; and streptomycin, 2 mg/ml. The strains used in this study are listed in Table 1, and the plasmids and primers are listed in Table S1 and S2, respectively, in the supplemental material.
TABLE 1.
Strains used in this study
| Strain | Descriptiona | Source or reference(s) |
|---|---|---|
| E. coli | ||
| DH5αmcr | Strain used for general cloning | Life Technologies (Grand Island, NY) |
| EC100D pir | Strain used to clone HimarEm1 and adjacent DNA from C. algicola Himar mutants | Epicentre (Madison, WI) |
| S17-1 λ pir | Strain used for conjugation | 51 |
| HB101 | Strain used with pRK2013 for triparental conjugation | 22, 52 |
| F. johnsoniae | ||
| CJ1827 | rpsL2 Smr; wild-type F. johnsoniae strain used in construction of deletion mutants | 26 |
| CJ2157 | rpsL2 ΔgldL Smr | 18 |
| C. algicola | ||
| CA2 | Wild-type DSM 14237T | DSMZ |
| CA4 | HimarEm1 insertion mutation in sprE (Celal_1548) of strain CA2; Emr | This study |
| CA19 | HimarEm1 insertion mutation in lolA (Celal_3622) of strain CA2; Emr | This study |
| CA24 | HimarEm1 insertion mutation in sprT (Celal_2247) of strain CA2; Emr | This study |
| CA32 | HimarEm1 insertion mutation in gldM (Celal_0882) of strain CA2; Emr | This study |
| CA33 | HimarEm1 insertion mutation in gldJ (Celal_0751) of strain CA2; Emr | This study |
| CA51 | HimarEm1 insertion mutation in sprA (Celal_2075) of strain CA2; Emr | This study |
| CA65 | HimarEm1 insertion mutation in gldK (Celal_0884) of strain CA2; Emr | This study |
| CA69 | HimarEm1 insertion mutation in gldN (Celal_0881) of strain CA2; Emr | This study |
| CA72 | HimarEm1 insertion mutation in sprF (Celal_1477) of strain CA2; Emr | This study |
| CA89 | HimarEm1 insertion mutation in sprC (Celal_1480) of strain CA2; Emr | This study |
| CA92 | HimarEm1 insertion mutation in gldL (Celal_0883) of strain CA2; Emr | This study |
| CA94 | HimarEm1 insertion mutation in gldB (Celal_2202) of strain CA2; Emr | This study |
| CA97 | HimarEm1 insertion mutation in gldI (Celal_2755) of strain CA2; Emr | This study |
| CA119 | gldD (Celal_1091) disruption in strain CA2; Emr | This study |
| CA121 | gldH (Celal_1514) disruption in strain CA2; Emr | This study |
| CA124 | sprD (Celal_1479) disruption in strain CA2; Emr | This study |
| CA130 | ΔgldD in strain CA2 | This study |
| CA135 | rpsLA168G in strain CA2; Smr wild-type strain used for construction of deletion mutations | This study |
| CA143 | ΔsprBI (Celal_1478) rpsLA168G Smr | This study |
| CA149 | ΔgldB rpsLA168G Smr | This study |
| CA150 | ΔgldL rpsLA168G Smr | This study |
| CA179 | ΔgldB ΔgldL rpsLA168G Smr | This study |
| CA184 | Δ(sprC-sprD) rpsLA168G Smr | This study |
| CA185 | Δ(sprC-sprD) ΔgldL rpsLA168G Smr | This study |
| CA239 | ΔsprBII (Celal_0324) rpsLA168G Smr | This study |
| CA240 | ΔsprBI ΔsprBII rpsLA168G Smr | This study |
| CA241 | Δ(gldK-gldN) rpsLA168G Smr | This study |
Emr, erythromycin resistant; Smr, streptomycin resistant.
Conjugation.
The donor strain of E. coli used for conjugative transfer of plasmids was S17-1 λ pir. E. coli strains containing mobilizable plasmids were grown overnight in LB at 37°C. C. algicola was grown 42 h to mid-exponential phase in Cytophaga medium at 15°C. C. algicola cells (5 ml) were harvested (centrifugation at 3,700 × g, 10 min) and washed once with Cytophaga medium. E. coli cells (1 ml) were harvested (centrifugation at 3,700 × g, 10 min) and washed once with LB medium. C. algicola and E. coli cells were suspended in marine conjugation medium and mixed together (approximately 1:1 ratio), spotted on marine conjugation agar, and allowed to dry. Following overnight incubation at 25°C, cells were scraped off the agar, diluted in Cytophaga medium, and plated on Cytophaga agar containing erythromycin. The plates were incubated for 10 to 14 days at 15°C. Triparental conjugation was carried out in the same way, with approximately equal amounts of recombinant E. coli DH5αmcr, C. algicola, and E. coli HB101 carrying the conjugative helper plasmid pRK2013 (22).
Transposon mutagenesis and identification of sites of insertion.
pHimarEm1 was introduced into wild-type C. algicola strain CA2 by conjugation from E. coli S17-1 λ pir. Mutants that formed nonspreading colonies were chosen for further study. Identification of the sites of HimarEm1 insertion was performed as previously described by cloning the disrupted region and determining the DNA sequence near the site of insertion (3). Southern blotting was performed using the DIG-High Prime DNA labeling and detection kit I (Roche, Indianapolis, IN, USA). Chromosomal DNA was isolated from C. algicola HimarEm1 mutants, digested with HindIII and XbaI, separated by gel electrophoresis, and transferred to nylon membranes. A 1.0-kbp fragment spanning ermF was amplified using primers 575 and 576, labeled with digoxigenin-dUTP, and hybridized with the fragments on the membrane. Labeled bands were detected with anti-digoxigenin conjugated to alkaline phosphatase using the colorimetric substrates 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium chloride.
Disruption of gldD, gldH, and sprD by plasmid insertion.
For the disruption of gldD (Celal_1091), a 405-bp internal fragment was amplified using primers 1410 and 1411. The fragment was digested with BamHI and PstI and inserted into pLYL03 that had been digested with BamHI and PstI to generate pYT174. pYT174 was introduced into C. algicola strain CA2 by triparental conjugation, and the mutant CA119 containing the plasmid inserted in gldD by recombination was isolated on Cytophaga agar containing 50 μg/ml erythromycin. The disruption of gldD was confirmed by PCR using primer 1500, which binds 446 bp upstream of the gldD translational start site, and primer 737, which is specific for pLYL03. For the disruption of gldH (Celal_1514), a 336-bp internal fragment was amplified using primers 1412 and 1413. The fragment was digested with BamHI and PstI and inserted into pLYL03 that had been digested with BamHI and PstI to generate pYT175. pYT175 was introduced into C. algicola strain CA2, as described above, to obtain the gldH mutant CA121. For the disruption of sprD (Celal_1479), a 565-bp internal fragment was amplified using primers 1447 and 1448. The fragment was digested with BamHI and PstI and inserted into pLYL03 that had been digested with BamHI and PstI to generate pYT181. pYT181 was introduced into C. algicola strain CA2, as described above, to obtain the sprD mutant CA124.
Development of shuttle vector pYT172.
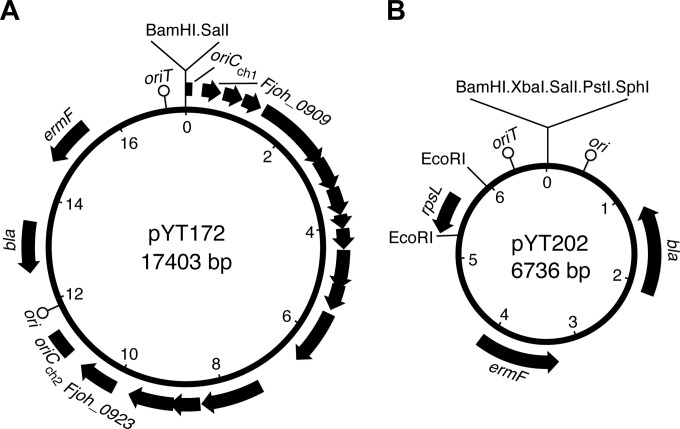
Plasmids used for complementation in F. johnsoniae, such as pCP11 (23), pCP23 (2), and pCP29 (24), were not maintained by C. algicola. A plasmid that allows complementation analysis in C. algicola was developed by modifying the E. coli-Cytophaga hutchinsonii shuttle vector pYT162 (25). pYT162, which contains the C. hutchinsonii chromosomal origin of replication (oriCCh), was introduced into F. johnsoniae and C. algicola by triparental conjugation. It did not replicate in either organism, but a single erythromycin-resistant colony of F. johnsoniae containing a modified form of the plasmid, pYT172, was obtained (Fig. 1A). pYT172 isolated from E. coli was analyzed by restriction enzyme digestion, gel electrophoresis, and DNA sequence analysis. pYT172 had a 10.9-kbp insert from the F. johnsoniae genome, spanning Fjoh_0909 to Fjoh_0923 (nucleotides 1027035 to 1037961 of the F. johnsoniae genome) inserted into oriCCh. This plasmid no longer replicated in C. hutchinsonii but was maintained by F. johnsoniae and by C. algicola. pYT172 was used for complementation of C. algicola mutants in this study.
FIG 1.
Maps of pYT172 used for complementation experiments and pYT202 used for construction of chromosomal gene deletions. (A) pYT172 was derived from the C. hutchinsonii-E. coli shuttle vector pYT162. Transfer to F. johnsoniae resulted in the recombinant plasmid, pYT172, which had incorporated 10.9 kbp of DNA from the F. johnsoniae chromosome. (B) The rpsL-containing suicide vector pYT202 was constructed by cloning the wild-type C. algicola rpsL gene into the EcoRI site of the Bacteroidetes suicide vector pLYL03. Integration of pYT202 into the genome of the streptomycin-resistant C. algicola strain CA135 results in streptomycin sensitivity, and loss of the plasmid from the cell results in streptomycin resistance. The numbers immediately inside the ring refer to kilobase pairs of sequence. ori refers to the origin of replication that functions in E. coli but not in C. algicola. oriCCh (split into oriCCh1 and oriCCh2) refers to the origin of replication of the C. hutchinsonii chromosome. oriT refers to the conjugative origin of transfer. bla confers ampicillin resistance on E. coli but not on C. algicola. ermF confers erythromycin resistance on C. algicola but not on E. coli.
Construction and complementation of a gldD deletion mutant.
A 2.1-kbp fragment spanning Celal_1090 and the final 33 bp of gldD was amplified using primers 1451 (introducing a SalI site) and 1452 (introducing a SphI site). The fragment was digested with SalI and SphI and ligated into pLYL03, which had been digested with the same enzymes, to generate pYT190. A 2.2-kbp fragment spanning Celal_1093, Celal_1092, and the first 54 bp of gldD was amplified using primers 1449 (introducing a BamHI site) and 1450 (introducing a SalI site). The fragment was digested with BamHI and SalI and fused to the upstream region of gldD by ligation with pYT190, which had been digested with the same enzymes, to generate the deletion construct pYT191.
Plasmid pYT191 was introduced into wild-type C. algicola strain CA2 by triparental conjugation and grown on Cytophaga agar containing erythromycin to select for integration of the plasmid into the genome by homologous recombination. Colonies were streaked for isolation at least once on medium containing erythromycin to eliminate background cells that had not recombined the plasmid into the chromosome. An erythromycin-resistant clone was grown in Cytophaga medium in the absence of antibiotics to allow for loss of the plasmid by a second recombination event. This involved incubation for 2 days, followed by dilution into fresh medium. This was repeated 5 times for a total of 10 days of incubation. The cells were diluted and plated on Cytophaga agar. Colonies that exhibited poor spreading were streaked for isolation to eliminate background cells that had not lost the plasmid and were screened by PCR using primers 1500 and 1501, which flank gldD, to identify the deletion mutant CA130.
The gldD deletion mutant was complemented by cloning wild-type gldD in pYT172. A 1.0-kbp fragment spanning gldD and its putative promoter was amplified using primers 1500 (introducing a BamHI site) and 1501 (introducing an SalI site). The fragment was digested with BamHI and SalI and ligated into pYT172, which had been digested with the same enzymes, to generate pYT200. pYT200 was introduced into the gldD deletion mutant CA130 by conjugation.
Development of an rpsL-based method for isolating gene deletions in C. algicola.
The method used to delete gldD (described above) was laborious and was only successful because we suspected that a distinct colony phenotype would result from the deletion of gldD. A more general method to construct targeted gene deletions was developed based on the use of a streptomycin resistance-conferring rpsL locus as a counterselectable marker, as was done in F. johnsoniae (26).
Streptomycin-resistant C. algicola cells were obtained by plating wild-type cells on Cytophaga agar containing 2 mg/ml streptomycin. Many clones were obtained, and the rpsL (Celal_0630) genes from three of these were amplified and sequenced using primers 1510 and 1511. Point mutations in rpsL were identified in each streptomycin-resistant strain. C. algicola CA135, which had an adenine-to-guanine point mutation at bp 168 of the rpsL coding sequence (rpsLA168G), resulting in a K43R substitution in RpsL, was used as the streptomycin-resistant strain for the generation of gene deletion mutants. CA135 was indistinguishable from its parent strain (CA2) in morphology, growth rate, colony spreading, and cell movement over glass and agar.
An rpsL-containing suicide vector was constructed to allow generation of unmarked mutations. The wild-type C. algicola rpsL gene was cloned into the suicide vector pLYL03 (27). Primers 1510 and 1511 were designed with engineered EcoRI restriction sites and used to amplify a 732-bp fragment spanning rpsL. The fragment was digested with EcoRI and cloned into the EcoRI site of pLYL03 to generate the rpsL-containing suicide vector pYT202 (Fig. 1B). Orientation of the rpsL fragment in pYT202 was determined by DNA sequencing.
Construction of strains with deletions in sprBI, sprBII, gldB, and gldL and strains with deletions spanning sprC-sprD and gldK-gldN.
A 2.0-kbp fragment spanning Celal_1476, sprF (Celal_1477), and the final 36 bp of sprBI (Celal_1478) was amplified using primers 1525 (introducing a SalI site) and 1526 (introducing a SphI site). The fragment was digested with SalI and SphI and ligated into pYT202, which had been digested with the same enzymes, to generate pYT205. A 2.2-kbp fragment upstream of and spanning the first 81 bp of sprBI was amplified using primers 1523 (introducing a BamHI site) and 1524 (introducing a SalI site). The fragment was digested with BamHI and SalI and fused to the downstream region of sprBI by ligation with pYT205, which had been digested with the same enzymes, to generate the deletion construct pYT207.
pYT207 was introduced into the streptomycin-resistant wild-type C. algicola strain CA135 by triparental conjugation and grown on Cytophaga agar containing erythromycin to select for integration of the plasmid into the genome by homologous recombination. Colonies were streaked for isolation at least once on medium containing erythromycin to eliminate background cells that had not recombined the plasmid into the chromosome. An erythromycin-resistant (streptomycin-sensitive) clone was selected and grown for 2 days in Cytophaga liquid in the absence of antibiotics to allow loss of the plasmid by a second recombination event. These cells were plated on Cytophaga agar containing streptomycin to select for cells that had lost the plasmid. Streptomycin-resistant colonies were streaked for isolation to eliminate background cells that had not lost the plasmid and were screened by PCR using primers 1447 and 1526, which flank sprBI, to identify the deletion mutant CA143. Streaking colonies for isolation on selective medium at both the plasmid integration and plasmid loss steps was critical to the elimination of nonselected cells.
Strains with deletions in sprBII (Celal_0324), gldB (Celal_2202), and gldL (Celal_0883), deletions spanning the adjacent genes sprC (Celal_1480) and sprD, and deletions spanning gldK (Celal_0884), gldL, gldM (Celal_0882), and gldN (Celal_0881) were constructed in the same way using the plasmids and primers listed in Table S1 and S2, respectively, in the supplemental material. ΔgldB and ΔgldL mutants were complemented by cloning the wild-type genes into pYT172, as described above for gldD, except that primers specific to each gene were used (see Table S2), resulting in the complementation plasmids listed in Table S1. Strains with multiple deletions [ΔsprBI ΔsprBII, ΔgldB ΔgldL, and Δ(sprC-sprD) ΔgldL strains] were constructed by sequential use of the deletion constructs mentioned above.
Microscopic observations of cell movement.
Wild-type and mutant cells of C. algicola, grown on Cytophaga agar at 15°C for 4 days, were examined for movement over glass and agar by phase-contrast microscopy. Motility on glass was analyzed using liquid-filled tunnel slides prepared essentially as described previously (1), using Nichiban NW-5 double-sided tape (Nichiban Co., Tokyo, Japan) to hold a glass coverslip over a glass slide. Cells suspended in Cytophaga medium were introduced into tunnel slides and incubated for 3 min. Unattached cells were removed by rinsing with Cytophaga medium three times, and the attached cells were observed for motility at 20°C. Movement on agar was examined by spotting cells on a pad of Cytophaga medium solidified with 1% agar on a glass slide, allowing the spot to dry, and covering it with an O2-permeable Teflon membrane (Yellow Springs Instrument Co., Yellow Springs, OH) that prevented dehydration and served as a coverslip. In some experiments, 20 μM carbonyl cyanide m-chlorophenyl hydrazine (CCCP) was added to inhibit motility. Cell movements over glass or agar were observed using an Olympus BH-2 phase-contrast microscope. Images were recorded using a Photometrics Cool-SNAPcf2 camera and analyzed using MetaMorph software (Molecular Devices, Downingtown, PA). Rainbow traces of cell movements were made using ImageJ version 1.45s (http://rsb.info.nih.gov/ij/) and the macro Color FootPrint (16).
RESULTS
Isolation of C. algicola motility mutants.
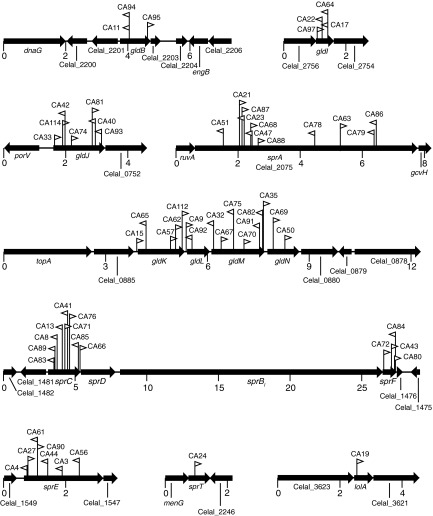
C. algicola was mutagenized with the transposon HimarEm1, and 63 independent HimarEm1-induced motility mutants that formed nonspreading colonies were isolated from approximately 50,000 erythromycin-resistant transconjugants (Fig. 2 and 3). Southern blot analyses demonstrated that each mutant had a single unique transposon insertion site (see Fig. S2 in the supplemental material). Mutants containing transposon insertions in gldB, gldI (Celal_2755), gldJ (Celal_0751), gldK, gldL, gldM, gldN, sprA (Celal_2075), sprC, sprE (Celal_1548), sprF, and sprT (Celal_2247), which are orthologs of F. johnsoniae gliding motility genes, were identified. The level of similarity ranged from 32% identity over 422 amino acids for SprC to 69% identity over 459 amino acids for GldK (see Table S3 in the supplemental material). Each mutant formed nonspreading colonies, indicating a motility defect (Fig. 3). A mutant with an insertion in lolA (Celal_3622), which encodes a protein related to E. coli LolA that delivers lipoproteins to the outer membrane, also formed nonspreading colonies (Fig. 3). Mutations in F. johnsoniae lolA also result in motility defects (14). lolA mutants could mislocalize lipoproteins, such as GldB, GldD, GldH, GldI, GldJ, GldK, and SprE, that are involved in motility. No novel C. algicola motility genes were found by transposon mutagenesis that were not previously identified in F. johnsoniae. F. johnsoniae gldA, gldF, and gldG, which are thought to encode components of an ABC transporter, are required for F. johnsoniae gliding motility. The C. algicola genome lacks orthologs of these genes (1) but does contain other ABC transporter genes. The failure to identify any of these in our transposon mutagenesis screen suggests that an ABC transporter may not be required for C. algicola gliding motility, although the absence of insertions in such genes might result from the nonrandomness of transposition or redundancy among transporters involved in motility.
FIG 2.
Maps of the HimarEm1 insertion sites in C. algicola motility mutants. The numbers below the maps refer to kilobase pairs of sequence. Orientations of HimarEm1 insertions are indicated by the triangles. Triangles pointing to the right indicate insertions with inverted repeat 2 (IR2) on the right side and the kanamycin resistance gene of the transposon reading toward the right.
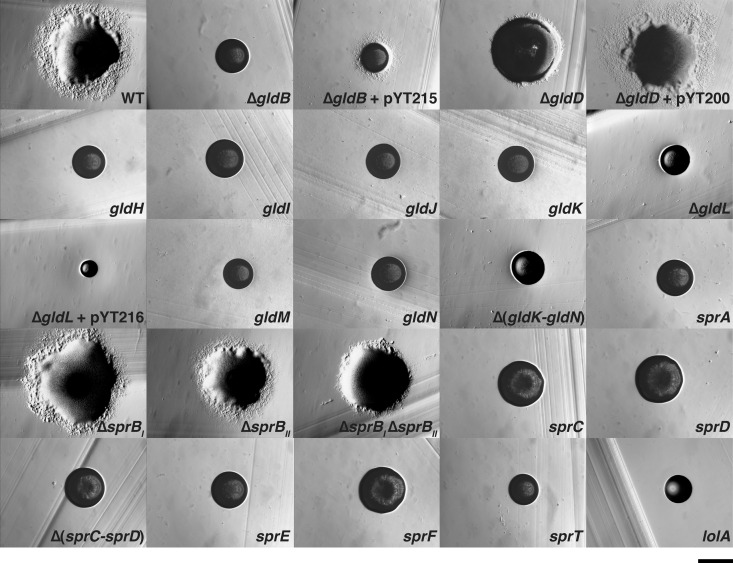
FIG 3.
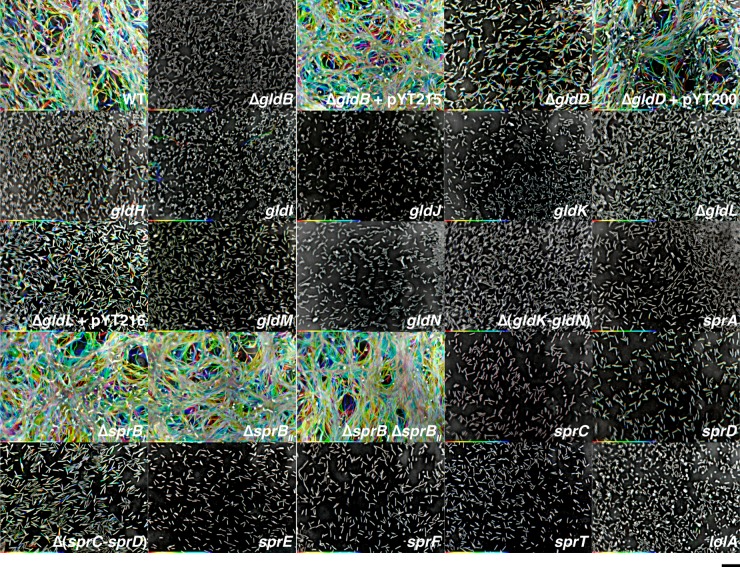
Photomicrographs of wild-type and mutant C. algicola colonies. Colonies were grown for 6 days at 15°C on Cytophaga agar, except for the lolA mutant, which was grown for 7 days. Photomicrographs were taken with a Photometrics Cool-SNAPcf2 camera mounted on an Olympus IMT-2 phase-contrast microscope. The scale bar indicates 0.5 mm and applies to all panels. WT is wild-type C. algicola strain CA135, ΔgldB is CA149, ΔgldD is CA130, gldH is CA121, gldI is HimarEm1 mutant CA97, gldJ is HimarEm1 mutant CA33, gldK is HimarEm1 mutant CA65, ΔgldL is CA150, gldM is HimarEm1 mutant CA32, gldN is HimarEm1 mutant CA69, Δ(gldK-gldN) is CA241, sprA is HimarEm1 mutant CA51, ΔsprBI is CA143, ΔsprBII is CA239, ΔsprBI ΔsprBII is CA240, sprC is HimarEm1 mutant CA89, sprD is CA124, Δ(sprC-sprD) is CA184, sprE is HimarEm1 mutant CA4, sprF is HimarEm1 mutant CA72, sprT is HimarEm1 mutant CA24, and lolA is HimarEm1 mutant CA19. pYT200, pYT215, and pYT216 carry gldD, gldB, and gldL, respectively, and were used to complement the appropriate mutants.
The C. algicola counterparts of F. johnsoniae gldD, gldH, and sprD were not identified by HimarEm1 mutagenesis. gldD and gldH are small (561 bp and 489 bp, respectively), which may explain the absence of HimarEm1 insertions in these genes. Targeted gene disruptions or deletions were generated in each of these genes. Cells with mutations in gldH and sprD formed nonspreading colonies (Fig. 3), as was previously observed for F. johnsoniae cells with mutations in the same genes (9, 13). In contrast, colonies of the gldD mutant exhibited slight spreading (Fig. 3), unlike F. johnsoniae gldD mutants, which are nonmotile and form nonspreading colonies (7).
F. johnsoniae sprB is required for movement on agar, and sprB mutants form nonspreading colonies (11). F. johnsoniae SprB is the major cell surface motility adhesin that is rapidly propelled along the cell surface. C. algicola has four genes (Celal_1478, Celal_0324, Celal_2797, and Celal_1125) that encode proteins with similarity over multiple regions to F. johnsoniae SprB. The primary regions of similarity are related to domains belonging to pfam13573 (SprB repeat). These domains are repeated several times in each protein. Each SprB-like protein also has a C-terminal region predicted to target the protein for secretion by the T9SS. The C. algicola gene most similar to F. johnsoniae sprB (sprBI) lies immediately downstream of sprC and sprD and immediately upstream of sprF (Fig. 2). This gene arrangement is identical to that found in F. johnsoniae. The coding region of C. algicola sprBI is 18,243 bp. In spite of the large size of this target, no transposon insertions were identified in C. algicola sprBI (Fig. 2). An sprBI deletion mutant was constructed. The ΔsprBI mutant formed spreading colonies that were similar to those formed by wild-type cells (Fig. 3). This explains why transposon insertions in C. algicola sprBI were not detected, since only colonies that were defective for spreading were examined. The second closest homolog to F. johnsoniae sprB, sprBII (Celal_0324), was also targeted for deletion. Cells of the ΔsprBII mutant spread similar to the wild type, whereas cells of the ΔsprBI ΔsprBII double mutant formed colonies on agar that spread slightly less than those of the wild type (Fig. 3). The presence of additional sprB-like genes (Celal_2797 and Celal_1125) may explain the ability of the mutants to form spreading colonies on agar.
Motility defects of individual cells of C. algicola gld and spr mutants.
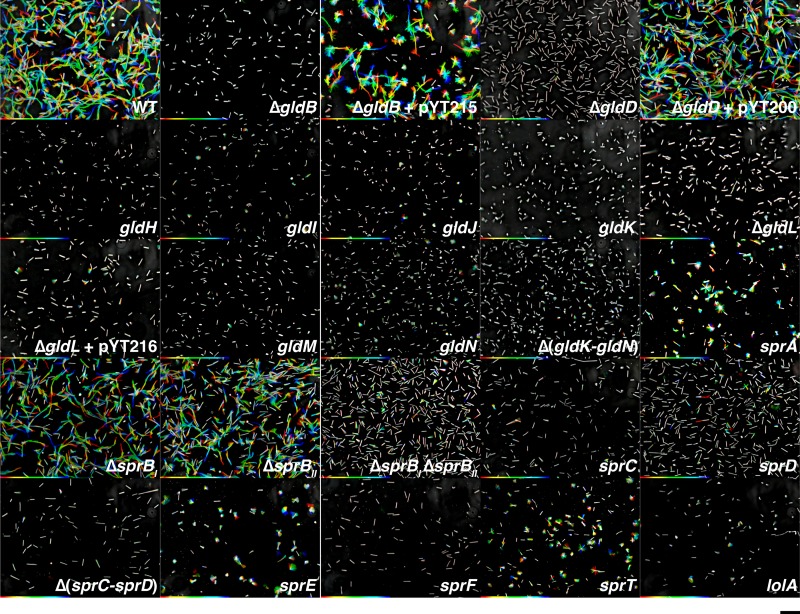
Cells of each of the gld and spr mutants were examined for movement on glass and on agar (Fig. 4 and 5; see also Movies S1 to S4 in the supplemental material). Cells were examined on glass in real time, whereas those on agar were analyzed by time-lapse (speeded up 30-fold) because wild-type cells move more slowly on agar. The data are presented as “rainbow traces” that illustrate the movements of individual cells on glass over 20 s (Fig. 4) or on agar over 150 s (Fig. 5). Under these conditions, cells with mutations in gldB, gldJ, gldK, gldL, gldM, and gldN exhibited no noticeable motility on glass or agar, which is identical to what was reported for F. johnsoniae strains with mutations in the same genes (3, 4, 6, 10). Cells of the C. algicola gldD, gldH, and gldI mutants were also defective for motility on glass (Fig. 4; see also Movie S1 in the supplemental material). Examination of cells of gldD, gldH, and gldI mutants on agar revealed active motility, although they were each less motile than were wild-type cells (Fig. 5; see also Movie S2 in the supplemental material). This contrasts with F. johnsoniae gldD, gldH, and gldI mutants, which exhibit no motility on glass or agar (7–9). GldI is a predicted periplasmic peptidyl-prolyl isomerase that may be involved in protein folding (8). C. algicola encodes several other predicted periplasmic peptidyl-prolyl isomerases that may account for the residual motility of the gldI mutants, although other explanations are also possible. C. algicola does not have any other genes related to gldD or gldH that might explain the residual motility of the gldD and gldH mutants. Several mutants had unusual cell morphology. Cells of each of four gldI mutants and of each of seven gldJ mutants of C. algicola had curved cells (see Movies S1 and S2 in the supplemental material), a trait not shared with the other motility mutants. This phenotype was not observed for F. johnsoniae gldI or gldJ mutants (4, 8) or for any other F. johnsoniae or C. algicola motility mutants.
FIG 4.
Gliding of wild-type and mutant cells on glass. Cells were grown on Cytophaga agar at 15°C for 4 days, suspended in Cytophaga medium, introduced into glass tunnel slides, and observed for motility at 20°C using an Olympus BH-2 phase-contrast microscope. A series of images were taken for 20 s using a Photometrics Cool-SNAPcf2 camera. Individual frames were colored from red (time zero) to yellow, green, cyan, and finally blue (20 s) and integrated into one image, resulting in rainbow traces of gliding cells. The rainbow traces correspond to the sequences shown in Movies S1, S3, and S5, and cells at time zero are shown in Fig. S3 in the supplemental material. Strains and plasmids are as listed in Fig. 3. White cells correspond to cells that exhibited little net movement. Multicolored “stars” (such as those seen for the sprA, sprE, and sprT mutants) indicate cells attached to the glass by one pole that rotate or flip. The scale bar indicates 10 μm and applies to all panels.
FIG 5.
Gliding of wild-type and mutant cells on agar. Cells were grown on Cytophaga agar at 15°C for 4 days, suspended in Cytophaga medium, and spotted on Cytophaga medium solidified with 1% agar on a glass slide. After drying, the cells were covered with an O2-permeable Teflon membrane that prevented dehydration and served as a coverslip. Cells were observed for motility at 20°C using an Olympus BH-2 phase-contrast microscope. A series of images were taken for 150 s using a Photometrics Cool-SNAPcf2 camera. Individual frames were colored from red (time zero) to yellow, green, cyan, and finally blue (150 s) and integrated into one image, resulting in rainbow traces of gliding cells. The rainbow traces correspond to the sequences shown in Movies S2, S4, and S6, and cells at time zero are shown in Fig. S4 in the supplemental material. Strains and plasmids are as listed in Fig. 3. White cells correspond to cells that exhibited little net movement. The scale bar indicates 10 μm and applies to all panels.
F. johnsoniae spr mutants exhibit severe motility defects but retain some limited ability to move, in contrast to F. johnsoniae gld mutants, which are completely nonmotile (11–14). Most C. algicola spr mutants were similar to those of F. johnsoniae and exhibited limited movement on glass, agar, or both (Fig. 4 and 5; see also Movies S3 and S4 in the supplemental material).
C. algicola sprBI and sprBII exhibit partial redundancy.
As demonstrated above, cells of the ΔsprBI and ΔsprBII mutants formed spreading colonies on agar, and cells of the ΔsprBI ΔsprBII double mutant were only slightly defective in spreading on agar. Cells of these mutants were examined for motility on glass and agar. Cells of the ΔsprBI and ΔsprBII mutants moved rapidly over glass and agar surfaces (Fig. 4 and 5; see also Movies S5 and S6 in the supplemental material). Cells of the ΔsprBI ΔsprBII double mutant also retained the ability to glide on agar but were severely defective in movement on glass. SprBI and SprBII may function as redundant cell surface adhesins involved in movement over glass. One or both of the remaining SprB-like proteins (Celal_2797 and/or Celal_1125) may contribute to the movement of cells on agar.
C. algicola gliding is reversibly inhibited by the proton uncoupler CCCP.
The proton motive force (PMF) has been shown to be involved in gliding of F. johnsoniae and other gliding members of the Bacteroidetes (16, 28–31). To determine whether this extends to C. algicola, we exposed wild-type cells to the proton uncoupler CCCP. The addition of CCCP resulted in the cessation of cell movement, and the removal of CCCP allowed cells to recover and glide, suggesting that the PMF may be required for C. algicola gliding (see Movie S7 in the supplemental material).
F. johnsoniae gld mutants are completely nonmotile, but all C. algicola gld mutants exhibit limited residual motility.
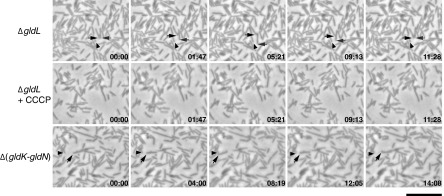
F. johnsoniae gld mutants are completely nonmotile (2–9). Preliminary experiments suggested that this was also true for the C. algicola gldB, gldJ, gldK, gldL, gldM, and gldN mutants (Fig. 3 to 5; see also Movies S1 and S2 in the supplemental material). However, more extensive observations revealed that cells of each of the C. algicola gld mutants (deletion and transposon) exhibited limited residual motility (Fig. 6; see also Movie S8 in the supplemental material). For these experiments, movement on agar was analyzed by time-lapse (speeded up 180-fold). Cells of gldB, gldJ, gldK, gldL, gldM, and gldN mutants moved at approximately 0.5 μm/min on agar, whereas wild-type cells moved at 36 μm/min on this surface. The mutant cells exhibited back-and-forth movements, traveling at most one cell length from their point of origin. For the ΔgldL mutant, 105 of 600 randomly selected cells exhibited obvious movement within the time of observation (30 min) that could not be explained by passive flow or by cell growth. In contrast, cells of an F. johnsoniae ΔgldL mutant exhibited no such movements (see Movie S8). Similarly, cells of F. johnsoniae gldA, gldB, gldD, gldF, gldG, gldH, gldI, gldJ, gldK, gldM, and gldNO mutants exhibited no obvious motility when examined in the same way (data not shown). The only movements observed for cells of the F. johnsoniae gld mutants appeared to be passive movement as a result of colony growth, wherein growing cells exert force on their neighbors. The motility phenotypes of wild-type and mutant C. algicola and F. johnsoniae cells are summarized in Fig. 7. The residual motility of the C. algicola gld mutants was stopped by addition of the uncoupler CCCP (Fig. 6; see also Movie S8), suggesting that it required metabolic energy. Several strains lacking multiple motility genes were constructed and analyzed in an attempt to eliminate the residual motility. CA241 [Δ(gldK-gldN)], CA179 (ΔgldB ΔgldL), and CA185 [Δ(sprC-sprD) ΔgldL] each exhibited limited cell movements similar to those of the ΔgldL mutant CA150 (Fig. 6; see also Movie S9 in the supplemental material).
FIG 6.
Residual motility of C. algicola ΔgldL and Δ(gldK-gldN) mutants on agar. Cells in Cytophaga medium were spotted on Cytophaga medium solidified with 1% agar on a glass slide. After drying, the cells were covered with an O2-permeable Teflon membrane that prevented dehydration and served as a coverslip. Cells were observed for motility at 20°C using an Olympus BH-2 phase-contrast microscope. Individual frames are shown corresponding to C. algicola ΔgldL mutant CA150 (top row; taken from Movie S8 in the supplemental material), the ΔgldL mutant incubated with 20 μM CCCP to dissipate the proton gradient (middle row; taken from Movie S8), and Δ(gldK-gldN) mutant CA241 (bottom row; taken from Movie S9 in the supplemental material). Arrows indicate moving cells, and arrowheads indicate stationary reference points. Arrows and arrowheads were not added to the images of the ΔgldL mutant with CCCP because no cell movements were observed. The numbers indicate time in minutes:seconds. The scale bar indicates 10 μm and applies to all panels.
FIG 7.
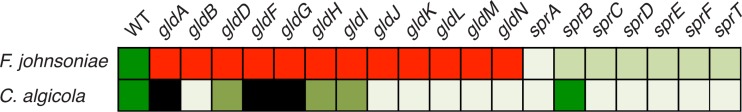
Motility of wild-type and mutant F. johnsoniae and C. algicola cells on agar. Red, nonmotile; lightest green (C. algicola gldB, for example), very slight motility; light green (F. johnsoniae sprB, for example), slight motility; medium green (C. algicola gldD, for example), moderate motility; dark green, wild-type (WT) motility; black, genes absent in the wild type. The F. johnsoniae ΔgldNO mutant was used for gldN, and the C. algicola sprBI mutant was used for sprB. C. algicola mutants are those described in the legends to Fig. 3 and 5. The F. johnsoniae mutants used were as follows: gldA, CJ101-288 (2); gldB, CJ569 (6); gldD, CJ282 (7); gldF, CJ787 (5); gldG, CJ776 (5); gldH, CJ1043 (9); gldI, UW102-41 (8); gldJ, UW102-80 (4); gldK, CJ2122 (18); gldL, CJ2157 (18); gldM, CJ2262 (18); ΔgldNO, CJ2090 (17); sprA, CJ2302 (18); ΔsprB, CJ1922 (26); sprC, UW102-91 (13); sprD, CJ1695 (13); sprE, Fj149 (14); sprF, CJ1814 (13); and sprT, KDF001 (15).
DISCUSSION
Gliding motility is common in the large and diverse phylum Bacteroidetes but has been studied in detail only in F. johnsoniae (2–4, 11, 16, 19, 32). Much has been learned from these studies, but uncertainties remain, such as the nature of the gliding motor. The degree to which information regarding F. johnsoniae gliding can be extrapolated to other members of the Bacteroidetes is also not known. Genetic analysis of C. algicola gliding was performed to allow a comparative analysis of Bacteroidetes gliding. Transposon mutagenesis of C. algicola revealed orthologs of motility genes that were previously identified in F. johnsoniae, confirming the importance of these genes in motility. No new motility genes were identified in C. algicola, suggesting that most of the Bacteroidetes gliding motility genes that can easily be identified by this approach may have already been found in F. johnsoniae.
While the F. johnsoniae and C. algicola motility systems exhibit similarities, many differences were also detected. Perhaps the most obvious was the absence in C. algicola of the ABC transporter composed of GldA, GldF, and GldG. While it remains possible that some other ABC transporter is required for C. algicola gliding, transposon insertions resulting in motility defects were not identified in genes encoding such proteins. These results suggest the possibility that an ABC transporter is not central to Bacteroidetes gliding motility.
Mutations in most orthologs of F. johnsoniae motility genes resulted in motility defects in C. algicola. An exception was sprB. The deletion of F. johnsoniae sprB results in defects in gliding on agar, and the mutants thus form nonspreading colonies (13, 26). In contrast, the deletion of C. algicola sprBI resulted in no noticeable defect in gliding. C. algicola has four genes that are similar in sequence to F. johnsoniae sprB, and these might have overlapping functions that compensate for the loss of a single gene. sprBI and sprBII appear to function in this way. C. algicola mutants lacking either sprBI or sprBII moved well on agar and glass. Cells of a double mutant lacking both genes also moved on agar, but they were severely deficient in gliding on glass. C. algicola SprBI and SprBII may be semiredundant cell surface adhesins that allow movement on glass. The remaining SprB-like proteins may contribute to the movement of cells on agar. Multiple sprB-like genes are also found in F. johnsoniae, and a partial redundancy of function has been demonstrated between two of them, sprB and remA (17). F. johnsoniae SprB and RemA are each rapidly propelled along the outer membrane, apparently facilitating movement over different surfaces (11, 16, 17). The presence of multiple motility adhesins may be a common property of gliding members of the Bacteroidetes that allows them to move over diverse surfaces.
gldD, gldH, and gldI mutants of C. algicola also exhibited significant differences from those of F. johnsoniae. F. johnsoniae gldD, gldH, and gldI mutants are completely nonmotile (7–9), whereas C. algicola gldD, gldH, and gldI mutants were only partially deficient in motility. Cells of the C. algicola mutants were deficient in gliding on glass but exhibited obvious motility on agar, although much less than wild-type cells. The results indicate that GldD, GldH, and GldI may have less critical roles than some of the other Gld proteins in Bacteroidetes gliding.
Perhaps most surprisingly, whereas F. johnsoniae gld mutants are completely nonmotile under all conditions that we have examined, all C. algicola gld mutants retained very limited ability to move. C. algicola gldB, gldJ, gldK, gldL, gldM, and gldN mutants moved approximately 75 times slower than wild-type cells. C. algicola lacks obvious redundant copies of any gld genes to account for these movements. Further, GldL and GldM are the only known C. algicola motility proteins predicted to span the cytoplasmic membrane (Fig. 8). Proteins that span the cytoplasmic membrane are presumably needed to harvest the proton gradient to power cell movement. F. johnsoniae GldL and GldM are thought to power the secretion of SprB and other proteins by the T9SS (18). They have also been suggested as candidates for motor proteins that harvest the PMF and propel SprB and related motility adhesins along the cell surface (16, 18, 33). The residual motility of the C. algicola Δ(gldK-gldN) mutant could be explained in two ways. GldL and GldM perhaps function as motor proteins for gliding, but C. algicola may have a second independent system that allows much slower cell movements. Alternatively, GldL and GldM may function solely in secretion in C. algicola and perhaps also in F. johnsoniae, and the motor proteins for gliding may have eluded detection by genetic analysis. In this case, the motility defects of the gldL and gldM mutants would be entirely explained by the failure to secrete motility adhesins to the cell surface. The motor may still function properly in the mutants, but without the cell surface motility adhesins, it results in slow intermittent cell movements in C. algicola. We do not know which of these possibilities is correct, but they both suggest an unidentified motor capable of at least limited cell movement. Genome analysis failed to identify genes involved in the formation and function of obvious motility structures, such as flagella or type IV pili, so the motor may be novel (1).
FIG 8.
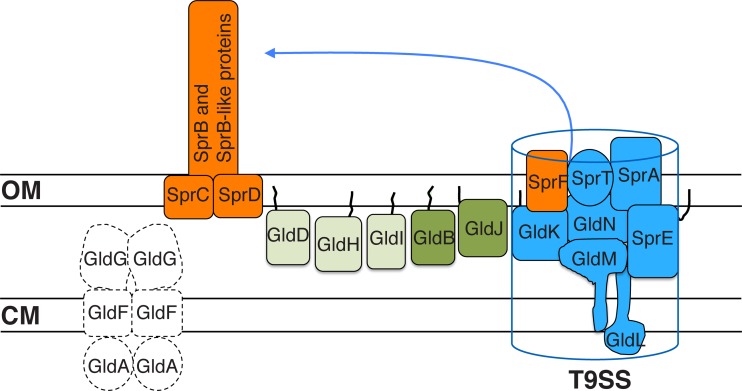
Conserved proteins involved in gliding motility of both C. algicola and F. johnsoniae. SprB and related motility adhesins are propelled along the cell surface. SprC and SprD support SprB function. Proteins in blue constitute the T9SS required for secretion of SprB and other proteins and may also have more direct roles in motility. SprF is required for the secretion of SprB but not for the secretion of other proteins targeted to the T9SS. Proteins in green are involved in motility and are not thought to be components of the T9SS. Black lines are lipid tails on lipoproteins. Proteins in light green are less important for motility of C. algicola than are proteins in dark green. Proteins in white are present in F. johnsoniae but not in C. algicola. GldA, GldB, GldD, GldF, GldG, GldH, GldI, GldJ, GldK, GldL, GldM, and GldN are essential for the motility of F. johnsoniae. In contrast, C. algicola mutants lacking any of these proteins exhibit limited residual motility. Proteins are not drawn to scale, and the stoichiometry of the components is not known.
The well-studied gliding proteobacterium Myxococcus xanthus uses type IV pili for its social motility (S-motility) (34, 35) and uses a motility apparatus composed of at least 14 proteins (11 Glt proteins, the ExbB-like protein AglR, and the ExbD-like proteins AglQ and AglS) for its adventurous motility (A-motility) (36–39). C. algicola does not have orthologs of type IV pilus genes, including pilT and pilB, which encode the pilus motor proteins, and it lacks orthologs of any of the M. xanthus A-motility glt genes (see Table S4 in the supplemental material). It also lacks orthologs of aglR, aglQ, and aglS, but it does have two exbBD-like operons (Celal_2032-Celal_2035 and Celal_0762-Celal_0764). Most Gram-negative bacteria have ExbB and ExbD proteins, and these typically function together to facilitate PMF-driven transport of molecules across the outer membrane (40). The C. algicola ExbB-like and ExbD-like proteins probably function in transport, but it remains possible that some of them have roles in cell movement. The C. algicola ExbB-like proteins Celal_0764 and Celal_2032 have orthologs in the well-studied members of the Bacteroidetes, F. johnsoniae, Bacteroides thetaiotaomicron, and Porphyromonas gingivalis (see Table S5 in the supplemental material). For B. thetaiotaomicron and P. gingivalis, these were bidirectional best hits that exhibited 47 to 53% amino acid identity over the entire sequences. Orthologs of each of the three C. algicola ExbD-like proteins Celal_0763, Celal_2034, and Celal_2035 were also identified in B. thetaiotaomicron and P. gingivalis. The genes encoding these proteins were clustered with the exbB-like genes mentioned above. The apparent orthologous exbBD operons in C. algicola, B. thetaiotaomicron, and P. gingivalis may have similar functions. Since B. thetaiotaomicron and P. gingivalis are nonmotile, this function is probably not gliding motility. However, additional experiments are needed to conclusively determine if GldL and GldM, ExbB and ExbD, or some other unidentified proteins are components of the Bacteroidetes gliding motor(s). Regardless, the Bacteroidetes and myxobacterial motility machineries have many differences. C. algicola, F. johnsoniae, and other gliding Bacteroidetes lack orthologs to the components of the M. xanthus S-motility and A-motility machines, and M. xanthus lacks orthologs to the Gld and Spr proteins involved in Bacteroidetes gliding (1, 3, 41).
Comparative analysis of F. johnsoniae and C. algicola gliding identified conserved proteins involved in motility (Fig. 8). Cell surface components, such as SprB, appear to have roles in motility in both organisms. The T9SS components (GldK, GldL, GldM, GldN, SprA, SprE, SprF, and SprT) and motility proteins GldB, GldD, GldH, GldI, and GldJ are also involved in gliding in both organisms. The motility phenotypes of cells of C. algicola gldD, gldH, and gldI mutants (Fig. 7) indicate that these genes may be less important for motility than are the other gld genes. Further, the residual movement displayed by all C. algicola motility mutants suggests that additional motility genes may await discovery. Further research is needed to determine if C. algicola has multiple motility machines accounting for the residual motility or if some essential components of the Bacteroidetes gliding motor remain to be identified.
In addition to uncovering novel aspects of Bacteroidetes gliding motility, the genetic tools developed here for C. algicola will be useful for studies of other aspects of the physiology of C. algicola. They are currently being used to study its T9SS to provide a better understanding of the common features of this secretion system across the phylum Bacteroidetes. C. algicola belongs to a large group of marine bacteria that has been referred to as the marine clade of the family Flavobacteriaceae (21). These bacteria are abundant in many marine environments and have received attention because of their abilities to rapidly digest and modify diverse algal polysaccharides. They are of value as sources of novel enzymes and sugars for the pharmaceutical and chemical industries and for their potential to aid in the conversion of algal polysaccharides into biofuels (20, 42–47). Many of these bacteria exhibit other unusual properties, such as colony iridescence (48, 49) and light-harvesting bacterial rhodopsins (50), which may be important for survival in their marine habitats. The genetic tools developed here allow an exploration of these and other aspects of the biology of these common bacteria.
Supplementary Material
Funding Statement
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01020-15.
REFERENCES
- 1.McBride MJ, Zhu Y. 2013. Gliding motility and Por secretion system genes are widespread among members of the phylum Bacteroidetes. J Bacteriol 195:270–278. doi: 10.1128/JB.01962-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S, Hunnicutt DW, McBride MJ. 1997. Cloning and characterization of the Flavobacterium johnsoniae (Cytophaga johnsonae) gliding motility gene, gldA. Proc Natl Acad Sci U S A 94:12139–12144. doi: 10.1073/pnas.94.22.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun TF, Khubbar MK, Saffarini DA, McBride MJ. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J Bacteriol 187:6943–6952. doi: 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun TF, McBride MJ. 2005. Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility. J Bacteriol 187:2628–2637. doi: 10.1128/JB.187.8.2628-2637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunnicutt DW, Kempf MJ, McBride MJ. 2002. Mutations in Flavobacterium johnsoniae gldF and gldG disrupt gliding motility and interfere with membrane localization of GldA. J Bacteriol 184:2370–2378. doi: 10.1128/JB.184.9.2370-2378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunnicutt DW, McBride MJ. 2000. Cloning and characterization of the Flavobacterium johnsoniae gliding motility genes, gldB and gldC. J Bacteriol 182:911–918. doi: 10.1128/JB.182.4.911-918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunnicutt DW, McBride MJ. 2001. Cloning and characterization of the Flavobacterium johnsoniae gliding motility genes gldD and gldE. J Bacteriol 183:4167–4175. doi: 10.1128/JB.183.14.4167-4175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride MJ, Braun TF. 2004. GldI is a lipoprotein that is required for Flavobacterium johnsoniae gliding motility and chitin utilization. J Bacteriol 186:2295–2302. doi: 10.1128/JB.186.8.2295-2302.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride MJ, Braun TF, Brust JL. 2003. Flavobacterium johnsoniae GldH is a lipoprotein that is required for gliding motility and chitin utilization. J Bacteriol 185:6648–6657. doi: 10.1128/JB.185.22.6648-6657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes RG, Samarasam MN, Shrivastava A, van Baaren JM, Pochiraju S, Bollampalli S, McBride MJ. 2010. Flavobacterium johnsoniae gldN and gldO are partially redundant genes required for gliding motility and surface localization of SprB. J Bacteriol 192:1201–1211. doi: 10.1128/JB.01495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson SS, Bollampalli S, McBride MJ. 2008. SprB is a cell surface component of the Flavobacterium johnsoniae gliding motility machinery. J Bacteriol 190:2851–2857. doi: 10.1128/JB.01904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson SS, Glocka PP, Agarwal S, Grimm DP, McBride MJ. 2007. Flavobacterium johnsoniae SprA is a cell-surface protein involved in gliding motility. J Bacteriol 189:7145–7150. doi: 10.1128/JB.00892-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes RG, Nelson SS, Pochiraju S, McBride MJ. 2011. Flavobacterium johnsoniae sprB is part of an operon spanning the additional gliding motility genes sprC, sprD, and sprF. J Bacteriol 193:599–610. doi: 10.1128/JB.01203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes RG, Samarasam MN, Van Groll EJ, McBride MJ. 2011. Mutations in Flavobacterium johnsoniae sprE result in defects in gliding motility and protein secretion. J Bacteriol 193:5322–5327. doi: 10.1128/JB.05480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato K, Naito M, Yukitake H, Hirakawa H, Shoji M, McBride MJ, Rhodes RG, Nakayama K. 2010. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc Natl Acad Sci U S A 107:276–281. doi: 10.1073/pnas.0912010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakane D, Sato K, Wada H, McBride MJ, Nakayama K. 2013. Helical flow of surface protein required for bacterial gliding motility. Proc Natl Acad Sci U S A 110:11145–11150. doi: 10.1073/pnas.1219753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrivastava A, Rhodes RG, Pochiraju S, Nakane D, McBride MJ. 2012. Flavobacterium johnsoniae RemA is a mobile cell-surface lectin involved in gliding. J Bacteriol 194:3678–3688. doi: 10.1128/JB.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrivastava A, Johnston JJ, van Baaren JM, McBride MJ. 2013. Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell-surface gliding motility adhesins SprB and RemA. J Bacteriol 195:3201–3212. doi: 10.1128/JB.00333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBride MJ, Nakane D. 2015. Flavobacterium gliding motility and the type IX secretion system. Curr Opin Microbiol 28:72–77. doi: 10.1016/j.mib.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Bowman JP. 2000. Description of Cellulophaga algicola sp. nov., isolated from the surfaces of Antarctic algae, and reclassification of Cytophaga uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Cellulophaga uliginosa comb. nov. Int J Syst Evol Microbiol 50:1861–1868. [DOI] [PubMed] [Google Scholar]
- 21.McBride MJ. 2014. The family Flavobacteriaceae, p 643–676. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes: other major lineages of bacteria and the archaea, 4th ed, vol 11 Springer; Berlin Heidelberg, Berlin, Germany. doi: 10.1007/978-3-642-38954-2_130. [DOI] [Google Scholar]
- 22.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride MJ, Kempf MJ. 1996. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J Bacteriol 178:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempf MJ, McBride MJ. 2000. Transposon insertions in the Flavobacterium johnsoniae ftsX gene disrupt gliding motility and cell division. J Bacteriol 182:1671–1679. doi: 10.1128/JB.182.6.1671-1679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, McBride MJ. 2014. Deletion of the Cytophaga hutchinsonii type IX secretion system gene sprP results in defects in gliding motility and cellulose utilization. Appl Microbiol Biotechnol 98:763–775. doi: 10.1007/s00253-013-5355-2. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes RG, Pucker HG, McBride MJ. 2011. Development and use of a gene deletion strategy for Flavobacterium johnsoniae to identify the redundant motility genes remF, remG, remH, and remI. J Bacteriol 193:2418–2428. doi: 10.1128/JB.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L-Y, Shoemaker NB, Salyers AA. 1995. Location and characterization of the transfer region of a Bacteroides conjugative transposon and regulation of the transfer genes. J Bacteriol 177:4992–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duxbury T, Humphrey BA, Marshall KC. 1980. Continuous observations of bacterial gliding motility in a dialysis microchamber: the effects of inhibitors. Arch Microbiol 124:169–175. [Google Scholar]
- 29.Dzink-Fox JL, Leadbetter ER, Godchaux W III. 1997. Acetate acts as a protonophore and differentially affects bead movement and cell migration of the gliding bacterium Cytophaga johnsonae (Flavobacterium johnsoniae). Microbiology 143:3693–3701. doi: 10.1099/00221287-143-12-3693. [DOI] [PubMed] [Google Scholar]
- 30.Pate JL, Chang L-YE. 1979. Evidence that gliding motility in prokaryotic cells is driven by rotary assemblies in the cell envelopes. Curr Microbiol 2:59–64. doi: 10.1007/BF02601737. [DOI] [Google Scholar]
- 31.Ridgway HF. 1977. Source of energy for gliding motility in Flexibacter polymorphus: effects of metabolic and respiratory inhibitors on gliding movement. J Bacteriol 131:544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrivastava A, Berg HC. 2015. Towards a model for Flavobacterium gliding. Curr Opin Microbiol 28:93–97. doi: 10.1016/j.mib.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrivastava A, Lele PP, Berg HC. 2015. A rotary motor drives Flavobacterium gliding. Curr Biol 25:338–341. doi: 10.1016/j.cub.2014.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H, Zusman DR, Shi W. 2000. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr Biol 10:1143–1146. doi: 10.1016/S0960-9822(00)00705-3. [DOI] [PubMed] [Google Scholar]
- 35.Wall D, Kaiser D. 1999. Type IV pili and cell motility. Mol Microbiol 32:1–10. doi: 10.1046/j.1365-2958.1999.01339.x. [DOI] [PubMed] [Google Scholar]
- 36.Nan B, Chen J, Neu JC, Berry RM, Oster G, Zusman DR. 2011. Myxobacteria gliding motility requires cytoskeleton rotation powered by proton motive force. Proc Natl Acad Sci U S A 108:2498–2503. doi: 10.1073/pnas.1018556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nan B, Bandaria JN, Moghtaderi A, Sun IH, Yildiz A, Zusman DR. 2013. Flagella stator homologs function as motors for myxobacterial gliding motility by moving in helical trajectories. Proc Natl Acad Sci U S A 110:E1508–E1513. doi: 10.1073/pnas.1219982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Wartel M, Cascales E, Shaevitz JW, Mignot T. 2011. Motor-driven intracellular transport powers bacterial gliding motility. Proc Natl Acad Sci U S A 108:7559–7564. doi: 10.1073/pnas.1101101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam ST, Mignot T. 2015. The mysterious nature of bacterial surface (gliding) motility: a focal adhesion-based mechanism in Myxococcus xanthus. Semin Cell Dev Biol 46:143–154. doi: 10.1016/j.semcdb.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 40.Noinaj N, Guillier M, Barnard TJ, Buchanan SK. 2010. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol, Vol 64, 2010 64:43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McBride MJ, Xie G, Martens EC, Lapidus A, Henrissat B, Rhodes RG, Goltsman E, Wang W, Xu J, Hunnicutt DW, Staroscik AM, Hoover TR, Cheng YQ, Stein JL. 2009. Novel features of the polysaccharide-digesting gliding bacterium Flavobacterium johnsoniae as revealed by genome sequence analysis. Appl Environ Microbiol 75:6864–6875. doi: 10.1128/AEM.01495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Gómez B, Richter M, Schüler M, Pinhassi J, Acinas SG, González JM, Pedrós-Alió C. 2013. Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J 7:1026–1037. doi: 10.1038/ismej.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gómez-Pereira PR, Schüler M, Fuchs BM, Bennke C, Teeling H, Waldmann J, Richter M, Barbe V, Bataille E, Glöckner FO, Amann R. 2012. Genomic content of uncultured Bacteroidetes from contrasting oceanic provinces in the North Atlantic Ocean. Environ Microbiol 14:52–66. doi: 10.1111/j.1462-2920.2011.02555.x. [DOI] [PubMed] [Google Scholar]
- 44.Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. 2010. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 45.Ma S, Tan YL, Yu WG, Han F. 2013. Cloning, expression and characterization of a new iota-carrageenase from marine bacterium, Cellulophaga sp. Biotechnol Lett 35:1617–1622. doi: 10.1007/s10529-013-1244-0. [DOI] [PubMed] [Google Scholar]
- 46.Rebuffet E, Groisillier A, Thompson A, Jeudy A, Barbeyron T, Czjzek M, Michel G. 2011. Discovery and structural characterization of a novel glycosidase family of marine origin. Environ Microbiol 13:1253–1270. doi: 10.1111/j.1462-2920.2011.02426.x. [DOI] [PubMed] [Google Scholar]
- 47.Williams TJ, Wilkins D, Long E, Evans F, DeMaere MZ, Raftery MJ, Cavicchioli R. 2013. The role of planktonic flavobacteria in processing algal organic matter in coastal East Antarctica revealed using metagenomics and metaproteomics. Environ Microbiol 15:1302–1317. doi: 10.1111/1462-2920.12017. [DOI] [PubMed] [Google Scholar]
- 48.Kientz B, Agogué H, Lavergne C, Marié P, Rosenfeld E. 2013. Isolation and distribution of iridescent Cellulophaga and other iridescent marine bacteria from the Charente-Maritime coast, French Atlantic. Syst Appl Microbiol 36:244–251. doi: 10.1016/j.syapm.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Kientz B, Ducret A, Luke S, Vukusic P, Mignot T, Rosenfeld E. 2012. Glitter-like iridescence within the Bacteroidetes especially Cellulophaga spp.: optical properties and correlation with gliding motility. PLoS One 7:e52900. doi: 10.1371/journal.pone.0052900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Consarnau L, Gonzalez JM, Coll-Llado M, Gourdon P, Pascher T, Neutze R, Pedros-Alio C, Pinhassi J. 2007. Light stimulates growth of proteorhodopsin-containing marine flavobacteria. Nature 445:210–213. doi: 10.1038/nature05381. [DOI] [PubMed] [Google Scholar]
- 51.de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol 235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 52.Bolivar F, Backman K. 1979. Plasmids of E. coli as cloning vectors. Methods Enzymol 68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.