Abstract
Chronic viruses, such as herpesviruses, shape host physiology. These viruses modulate the inflammatory state of the immune system and have evolved to harness inflammation as a mechanism to regulate viral latency and reactivation. In this review, I examine some of the recent work demonstrating the important role of inflammation in the regulation of the herpesvirus life cycle and discuss recent work that implicates coinfection in the regulation of herpesvirus latency.
INTRODUCTION
Herpesviruses are a family of viruses that have closely evolved with their host species. Every vertebrate examined has at least one herpesvirus, and each herpesvirus is closely associated with one host species (1). In humans, more than 90% of the population is infected with at least one herpesvirus (2). This suggests that these viruses have evolved with their hosts over a long period of time and are well adapted to them. It also suggests that our immune systems have evolved in the company of these infections and are shaped by their durable presence.
Herpesviruses establish lifelong chronic infections with periods of limited viral gene expression and no viral progeny production. This noncytopathic infection is termed latency. In the case of gammaherpesviruses, which are the focus of this review, latency is established primarily in B cells but also in macrophages and dendritic cells (1).
Rather than being a static event, latency is quite dynamic. Even though chronic infection is usually associated with little to no disease, these viruses are undergoing brief periods of reactivation during which small amounts of virus are produced. Production of virus during latency is likely important for spread of the virus to new hosts, as well as maintenance of a viral reservoir in the host. However, these reactivation events are tightly controlled by the immune system, which forces the virus to return to latency. Both the delicate balance between viral reactivation and latency and the complex relationship between the host immune system and herpesviruses are important for understanding how viruses influence host physiology.
We recently demonstrated not only that a mouse gammaherpesvirus (murine gammaherpesvirus 68 [MHV68; also known as γHV68 or MHV4]) modulates the immune system but also that subsequent infections with particular pathogens induce gammaherpesvirus reactivation from latency (3). These findings add another layer of complexity to our understanding of host-virus interactions and suggest that this relationship is modulated by coinfections. This has important implications for how herpesviruses shape immunity.
GAMMAHERPESVIRUSES AS SHAPERS OF HOST IMMUNITY
Gammaherpesviruses can be both beneficial and harmful to the host. We and others have shown that latent infection leads to a heightened state of immune activation that promotes protection against lethal challenges with bacteria in both wild-type and immunodeficient mice (4–6). Latent infection also protects mice from a lethal lymphoma challenge (7). However, gammaherpesvirus infection can also exacerbate disease. For example, latent infection with MHV68 increases the severity of experimental autoimmune encephalomyelitis (EAE), as well as the mortality rate of mice infected with malaria parasites (1, 8, 9). The mechanisms underlying these differences in outcomes are not well understood; however, different inflammatory responses are a likely explanation.
The mechanisms by which gammaherpesviruses change immunity to secondary challenges are diverse. In the case of cross-protection from a lethal bacterial challenge, it appears that enhanced macrophage activation and elevated levels of gamma interferon (IFN-γ) and tumor necrosis factor alpha in serum promote a heightened innate immune activation that is similar to the classical cross-protection described by Mackaness decades ago (10). What distinguishes it from classical cross-protection is that the effects of herpesvirus infection are more prolonged. In the case of lymphoma protection, it was noted that the mouse natural killer (NK) cells isolated from the peripheral blood of herpesvirus-infected mice are primed for expression of granzyme B and perforin. Usually mouse NK cells, in contrast to human NK cells, require a priming step ex vivo to attain full effector function. Latent infection with MHV68 protects against a lethal lymphoma challenge in an NK cell-dependent manner, likely because of the enhanced priming of NK cells found in latently infected mice compared to that in uninfected mice (7). The signals from herpesvirus infection that arm NK cells are still unknown. However, because latency activates macrophages and activated macrophages make interleukin-15 (IL-15) and other cytokines that are able to prime NK cells, it is possible that macrophages play a role in latency-mediated NK cell priming.
If we examine antigen-specific T cell responses during latency, we see that although herpesviruses spend much of their time dormant in the host, they are continuously stimulating the immune system. Whether that stimulation occurs only through low-level reactivation is unclear, but there is a broad repertoire of CD8+ and CD4+ T cells capable of producing cytokines during latency (1, 11–13). Some of these T cells respond to antigen early in infection and contract quickly, while others decline more slowly and maintain a response for a longer time (1, 11–13).
Latent gammaherpesvirus infection also induces activation of bystander (non-MHV68-specific) T cells. In the EAE model, there is increased T cell infiltrate and more T cells making IFN-γ in the brains and spinal cords of mice with EAE and MHV68 infection (8). These data point to an important question; i.e., if latency changes the basal level of activation of macrophages, NK cells, and possibly other cell types, then how are T cells changed by latent infection? Recent work examining the primary and secondary T cell responses to a lymphocytic choriomeningitis virus (LCMV) challenge of latently MHV68-infected mice found that the differentiation of CD4+ and CD8+ T cells specific to LCMV was different in latently infected animals. The effector T cell responses to LCMV were enhanced; however, the memory T cell responses were decreased in MHV68-coinfected mice (14). This work suggests that latent infection with MHV68 alters both the activation of naive T cells and the maintenance of memory cells specific to coinfecting pathogens.
Together, these data suggest a model whereby virus stimulates virus-specific T cells, perhaps through low-level reactivation, to become activated and produce cytokines, including IFN-γ. IFN-γ potently activates macrophages to become antimicrobicidal. Activated macrophages produce cytokines and possibly other immune activators that promote priming of NK cells, thus making them armed for potent and rapid innate responses to other challenges. Importantly, activated NK cells are also a significant source of IFN-γ (15), and IFN-γ is critical for controlling persistent replication and reactivation from latency (reviewed in reference 1). Therefore, NK cells, along with T cells, may play a critical role in maintaining viral latency (Fig. 1A).
FIG 1.
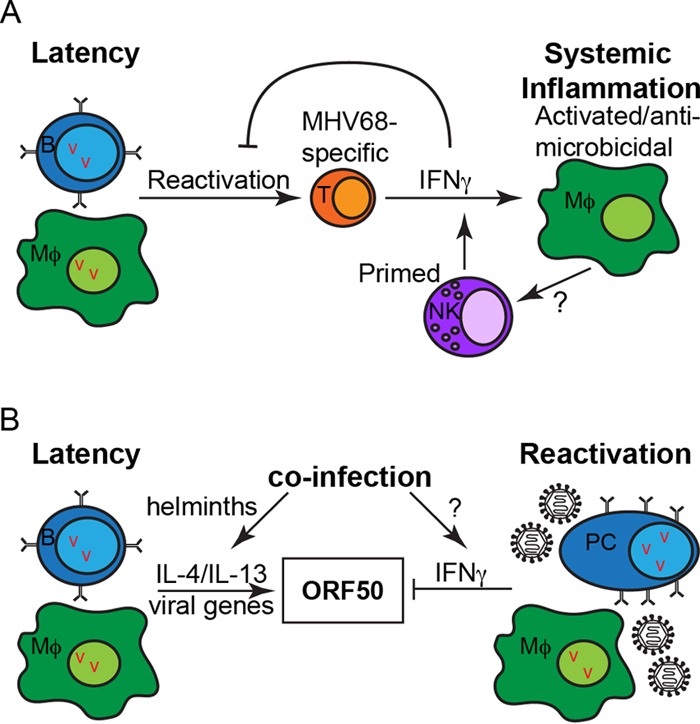
Systemic inflammation (A) and coinfection-mediated regulation of herpesvirus latency and reactivation (B). Abbreviations: B, B cell; Mϕ, macrophage; T, T cell; NK, NK cell; PC, plasma cell; v, viral episome.
SYSTEMIC INFLAMMATION AND REACTIVATION
If herpesviruses lead to systemic inflammation, then how is that inflammation controlled and how does the virus reactivate? In other words, what genes and pathways promote reactivation and dampen inflammation? While there has been much research over the years into the question of viral reactivation, this discussion focuses on more recent findings.
Autophagy and autophagy-related (Atg) genes are important for controlling many pathogens, including viruses (16). Autophagy is an essential cellular process whereby cytoplasmic cargo, and sometimes a pathogen, is engulfed in a double-membrane-bound vesicle, termed the autophagosome, that fuses to the lysosome for degradation. We recently reported that multiple Atg genes in myeloid cells regulate systemic inflammation, and especially IFN-γ, thus inhibiting viral reactivation (17). In the absence of Atg genes, MHV68 fails to reactivate efficiently, and neutralization of IFN-γ partially reverses this defect. We did not find a cell-intrinsic role for autophagy genes in viral reactivation but rather identified a role for autophagy-mediated control of systemic inflammation.
HOIL-1 is a member of the linear ubiquitin chain assembly complex, important for activation of the NF-κB transcription factor downstream of many receptor-signaling complexes. Patients with a defect in HOIL-1 exhibit hyperinflammation, among other phenotypes (18). We found that mice deficient in HOIL-1 do not reactivate MHV68 efficiently, despite normal lytic replication and establishment of latency (6). Similar to the Atg-deficient mice described above, HOIL-1-deficient mice infected with MHV68 have hyperinflammation and, most notably, an increase in IFN-γ. Because of this elevated IFN-γ level, we challenged latently infected HOIL-1-deficient mice with Listeria monocytogenes (4) and found that the latently infected mice were more resistant to the bacterial challenge.
Herpesviruses have evolved mechanisms to promote reactivation by manipulating the cellular environment. MHV68 expresses an open reading frame (ORF), M2, that is required for efficient reactivation from latency in B cells (reviewed in reference 1). M2 activates NFAT signaling in B cells, driving plasma cell differentiation and expression of IRF4 and IL-10 (19), and plasma cell differentiation was previously shown to drive viral reactivation from B cells (reviewed in reference 1). This is an interesting mechanism for viral reactivation, because it is an example of a viral protein that does not directly participate in virus replication but instead manipulates the cell in such a way as to promote reactivation. Additionally, this pathway ties in with the induction of another viral protein, M1, in reactivated plasma cells. M1 acts as a superantigen to drive Vβ4+ CD8+ T cells to produce IFN-γ (20), which blocks reactivation in macrophages (reviewed in reference 1), thus dampening reactivation in another latently infected cell type.
How does coinfection relate to reactivation? Chronic herpesvirus infection in humans likely evolved in the context of other coinfections, including helminth infections. Although helminth infections are rare in developed countries in the modern day, they are common in developing countries and were prevalent in most human populations until recently. There is growing evidence that they represent a piece of the evolutionary puzzle of immune systems and that they modulate host immune responses to coinfections with other microbial pathogens (21). We showed that infection with a helminth parasite, which induces T helper type 2 (Th2) inflammation, induces viral reactivation in a Stat6-dependent manner. The Th2 cytokines IL-4 and IL-13 signal through Stat6, promote virus replication in vitro in bone marrow-derived macrophages, and induce virus reactivation in vivo (in conjunction with blockade of IFN-γ) (3). We defined a direct mechanism for IL-4-mediated reactivation through Stat6 binding to the viral ORF50 promoter of the latent-to-lytic switch gene, though we do not exclude possible indirect mechanisms that modulate inflammation in the host. Of note, IFN-γ also modulates the activity of this gene by inhibiting the promoters, thus illustrating the important nature of this particular gene in sensing the immune status of the host (22). These data suggest that coinfection can either promote or inhibit viral reactivation, depending on the type of systemic inflammation it induces in the host (Fig. 1B).
PERSPECTIVES
Together, these data reveal that cytokines and the inflammatory state of the host directly and indirectly control latency and reactivation. In the case of IL-4/IL-13 and IFN-γ, there are data to suggest that the transcription factors immediately downstream of these cytokines have direct effects on the ORF50 gene (1, 3). In addition, recent work suggests that perturbations of the immune system that alter autophagy in macrophages, HOIL-1 expression, or viral M2 expression in B cells also regulate viral reactivation and latency through indirect effects on host inflammation. This raises the possibility that other coinfections that change the inflammatory state of the host will also modulate latency and reactivation.
The data highlighted by this review focus on recent advances made in the mouse model of gammaherpesvirus infection. However, similar mechanisms are likely true for the human gammaherpesviruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus (KSHV). We have demonstrated that IL-4 treatment of a KSHV-infected cell line induces viral genome and lytic transcript production (3). Whether Atg genes or HOIL-1 play a role in the human herpesviruses remains to be determined. The cell-intrinsic role of autophagy has been studied by others, but the systemic effects of autophagy on inflammation have not been demonstrated in humans. Intriguingly, HOIL-1 deficiency in humans is associated with hyperinflammation and mild immunodeficiency, whereas barrier-raised HOIL-1-deficient mice are severely immunodeficient (6). MacDuff et al. demonstrated that infection of HOIL-1-deficient mice with MHV68 leads to hyperinflammation, similar to what is seen in HOIL-1-deficient humans. Latent infection with MHV68 protects HOIL-1-deficient mice from an otherwise lethal bacterial challenge. These data suggest that the disease caused by HOIL-1 deficiency in humans could be modified by the presence of a chronic herpesvirus infection, thus driving variability between individuals with the same immunodeficiency, as well as between humans and barrier-raised mice.
A final remaining question is whether this systemic inflammation induced by gammaherpesvirus infection is really inflammation or if it actually represents the normal, basal state of the immune system. If 90% or more of the human population is infected with herpesviruses, then I would argue that they are a normal part of our virome (2) and that this basal level of inflammation reflects, instead, the “normal” inflammatory set point. This novel paradigm has important implications for how we model diseases and pathogenic challenges in mice. Many chronic viral infections have been eliminated from specific-pathogen-free mice in barrier facilities. In doing so, we may have artificially lowered the basal state of inflammation in mouse immune systems, thus making these mice less predictive of human immune responses (23).
ACKNOWLEDGMENTS
Thank you to Megan Baldridge and Donna MacDuff for critically reviewing the manuscript.
I apologize for the omission of many primary references because of length constraints.
I am the W. W. Caruth, Jr., Scholar in Biomedical Research at the University of Texas Southwestern Medical School, and my lab is supported by the Endowed Scholars program.
REFERENCES
- 1.Barton E, Mandal P, Speck SH. 2011. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 2.Virgin HW, Wherry EJ, Ahmed R. 2009. Redefining chronic viral infection. Cell 138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, Buck MD, Jezewski A, Kambal A, Liu CY, Goel G, Murray PJ, Xavier RJ, Kaplan MH, Renne R, Speck SH, Artyomov MN, Pearce EJ, Virgin HW. 2014. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 345:573–577. doi: 10.1126/science.1254517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 5.Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, Blackman MA. 2009. γ-Herpesvirus-induced protection against bacterial infection is transient. Viral Immunol 22:67–71. doi: 10.1089/vim.2008.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDuff DA, Reese TA, Kimmey JM, Weiss LA, Song C, Zhang X, Kambal A, Duan E, Carrero JA, Boisson B, Laplantine E, Israël A, Picard C, Colonna M, Edelson BT, Sibley LD, Stallings CL, Casanova J-L, Iwai K, Virgin HW. 2015. Phenotypic complementation of genetic immunodeficiency by chronic herpesvirus infection. eLife 4:e04494. doi: 10.7554/eLife.04494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White DW, Keppel CR, Schneider SE, Reese TA, Coder J, Payton JE, Ley TJ, Virgin HW, Fehniger TA. 2010. Latent herpesvirus infection arms NK cells. Blood 115:4377–4383. doi: 10.1182/blood-2009-09-245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casiraghi C, Shanina I, Cho S, Freeman ML, Blackman MA, Horwitz MS. 2012. Gammaherpesvirus latency accentuates EAE pathogenesis: relevance to Epstein-Barr virus and multiple sclerosis. PLoS Pathog 8:e1002715. doi: 10.1371/journal.ppat.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matar CG, Anthony NR, O'Flaherty BM, Jacobs NT, Priyamvada L, Engwerda CR, Speck SH, Lamb TJ. 2015. Gammaherpesvirus co-infection with malaria suppresses anti-parasitic humoral immunity. PLoS Pathog 11:e1004858. doi: 10.1371/journal.ppat.1004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackaness GB. 1964. The immunological basis of acquired cellular resistance. J Exp Med 120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman ML, Lanzer KG, Cookenham T, Peters B, Sidney J, Wu T-T, Sun R, Woodland DL, Sette A, Blackman MA. 2010. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J Virol 84:2881–2892. doi: 10.1128/JVI.02229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman ML, Burkum CE, Lanzer KG, Jensen MK, Ahmed M, Yager EJ, Flaño E, Winslow GM, Woodland DL, Blackman MA. 2011. Cutting edge: activation of virus-specific CD4 T cells throughout γ-herpesvirus latency. J Immunol 187:6180–6184. doi: 10.4049/jimmunol.1102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman ML, Burkum CE, Cookenham T, Roberts AD, Lanzer KG, Huston GE, Jensen MK, Sidney J, Peters B, Kohlmeier JE, Woodland DL, van Dyk LF, Sette A, Blackman MA. 2014. CD4 T cells specific for a latency-associated γ-herpesvirus epitope are polyfunctional and cytotoxic. J Immunol 193:5827–5834. doi: 10.4049/jimmunol.1302060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton ES, Rajkarnikar S, Langston PK, Price MJ, Grayson JM. 2014. Gammaherpesvirus latency differentially impacts the generation of primary versus secondary memory CD8+ T cells during subsequent infection. J Virol 88:12740–12751. doi: 10.1128/JVI.02106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang Z-E, Gapin L, Kronenberg M, Locksley RM. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Buck MD, Desai C, Zhang X, Loginicheva E, Martinez J, Freeman ML, Saitoh T, Akira S, Guan J-L, He Y-W, Blackman MA, Handley SA, Levine B, Green DR, Reese TA, Artyomov MN, Virgin HW. 2016. Autophagy genes enhance murine gammaherpesvirus 68 reactivation from latency by preventing virus-induced systemic inflammation. Cell Host Microbe 19:91–101. doi: 10.1016/j.chom.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, Abhyankar A, Israël L, Trevejo-Nunez G, Bogunovic D, Cepika A-M, MacDuff D, Chrabieh M, Hubeau M, Bajolle F, Debré M, Mazzolari E, Vairo D, Agou F, Virgin HW, Bossuyt X, Rambaud C, Facchetti F, Bonnet D, Quartier P, Fournet J-C, Pascual V, Chaussabel D, Notarangelo LD, Puel A, Israël A, Casanova J-L, Picard C. 2012. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol 13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangaswamy US, Speck SH. 2014. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog 10:e1003858. doi: 10.1371/journal.ppat.1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Flaherty BM, Soni T, Wakeman BS, Speck SH. 2014. The murine gammaherpesvirus immediate-early Rta synergizes with IRF4, targeting expression of the viral M1 superantigen to plasma cells. PLoS Pathog 10:e1004302. doi: 10.1371/journal.ppat.1004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salgame P, Yap GS, Gause WC. 2013. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol 14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin MM, Canny S, Steed A, Virgin HW. 2010. Murine gammaherpesvirus 68 has evolved gamma interferon and Stat1-repressible promoters for the lytic switch gene 50. J Virol 84:3711–3717. doi: 10.1128/JVI.02099-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reese TA, Bi K, Kambal A, Filali-Mouhim A, Beura LK, Bürger MC, Pulendran B, Sekaly R, Jameson SC, Masopust D, Haining WN, Virgin HW. 2016. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe doi: 10.1016/j.chom.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


