ABSTRACT
RNA silencing acts as a defense mechanism against virus infection in a wide variety of organisms. Here, we investigated inductions of RNA silencing against encapsidated double-stranded RNA (dsRNA) fungal viruses (mycoviruses), including a partitivirus (RnPV1), a quadrivirus (RnQV1), a victorivirus (RnVV1), a mycoreovirus (RnMyRV3), and a megabirnavirus (RnMBV1) in the phytopathogenic fungus Rosellinia necatrix. Expression profiling of RNA silencing-related genes revealed that a dicer-like gene, an Argonaute-like gene, and two RNA-dependent RNA polymerase genes were upregulated by RnMyRV3 or RnMBV1 infection but not by other virus infections or by constitutive expression of dsRNA in R. necatrix. Massive analysis of viral small RNAs (vsRNAs) from the five mycoviruses showed that 19- to 22-nucleotide (nt) vsRNAs were predominant; however, their ability to form duplexes with 3′ overhangs and the 5′ nucleotide preferences of vsRNAs differed among the five mycoviruses. The abundances of 19- to 22-nt vsRNAs from RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1 were 6.8%, 1.2%, 0.3%, 13.0%, and 24.9%, respectively. Importantly, the vsRNA abundances and accumulation levels of viral RNA were not always correlated, and the origins of the vsRNAs were distinguishable among the five mycoviruses. These data corroborated diverse interactions between encapsidated dsRNA mycoviruses and RNA silencing. Moreover, a green fluorescent protein (GFP)-based sensor assay in R. necatrix revealed that RnMBV1 infection induced silencing of the target sensor gene (GFP gene and the partial RnMBV1 sequence), suggesting that vsRNAs from RnMBV1 activated the RNA-induced silencing complex. Overall, this study provides insights into RNA silencing against encapsidated dsRNA mycoviruses.
IMPORTANCE Encapsidated dsRNA fungal viruses (mycoviruses) are believed to replicate inside their virions; therefore, there is a question of whether they induce RNA silencing. Here, we investigated inductions of RNA silencing against encapsidated dsRNA mycoviruses (a partitivirus, a quadrivirus, a victorivirus, a mycoreovirus, and a megabirnavirus) in Rosellinia necatrix. We revealed upregulation of RNA silencing-related genes in R. necatrix infected with a mycoreovirus or a megabirnavirus but not with other viruses, which was consistent with the relatively high abundances of vsRNAs from the two mycoviruses. We also showed common and different molecular features and origins of the vsRNAs from the five mycoviruses. Furthermore, we demonstrated the activation of RNA-induced silencing complex by mycoviruses in R. necatrix. Taken together, our data provide insights into an RNA silencing pathway against encapsidated dsRNA mycoviruses which is differentially induced among encapsidated dsRNA mycoviruses; that is, diverse replication strategies exist among encapsidated dsRNA mycoviruses.
INTRODUCTION
RNA silencing is a sequence-specific RNA degradation mechanism that is widely conserved among eukaryotic organisms, including animals, plants, and fungi (1–4). This mechanism is triggered by the processing of double-stranded RNA (dsRNA) into small 20- to 30-nucleotide (nt) RNA duplexes (sRNA) by an RNase III domain-containing dicer protein. The sRNAs are incorporated into an Argonaute protein, which is a core component of the RNA-induced silencing complex (RISC). The RISC is activated by removal of one strand of sRNA, and the activated RISC containing another strand of sRNA degrades target RNAs in a sequence-specific manner (5, 6). In some organisms, including nematodes, plants, and fungi, the effect of RNA silencing is amplified by a host RNA-dependent RNA polymerase (RdRp), which produces dsRNA from single-stranded RNA (ssRNA) using the primary sRNA as a primer by which secondary sRNA is generated (7, 8). One of the biological roles of RNA silencing is antiviral defense (9–12). Accumulated viral dsRNA as viral genomes or replication intermediates, and/or highly structured viral ssRNA, are thought to be processed into virus-derived sRNA (vsRNA) by a dicer protein (13–15). The resulting vsRNA is thought to be involved in the degradation of viral ssRNA by RISC and amplification of RNA silencing by an endogenous RdRp. To counteract RNA silencing, many viruses have evolved RNA silencing suppressor genes (RSSs) to inhibit various steps in the RNA silencing machinery (16–18). This host defense-viral counterdefense interaction strongly supports the view that virus propagation is inhibited in multiple steps in the RNA silencing machinery.
dsRNA viruses are found in a wide variety of organisms, including animals, insects, plants, fungi, and bacteria (19). Many dsRNA viruses are encapsidated and replicate inside their virions (19); therefore, their genome and replicating dsRNAs are never exposed to the cytoplasm. This mode of replication raises an interesting question: do dsRNA viruses escape from dsRNA-mediated antiviral responses, including RNA silencing? Some studies have detected vsRNAs, which are a hallmark of RNA silencing, from dsRNA viruses in insects using next-generation sequencing (NGS), including reoviruses (20–22), a totivirus (23), and entomobirnaviruses (24, 25). These data suggested that encapsidated dsRNA viruses are also a target of RNA silencing.
In fungi, most fungal viruses (mycoviruses) are encapsidated dsRNA viruses belonging to the Totiviridae, Chrysoviridae, Partitiviridae, Reoviridae, Megabirnaviridae, and Quadriviridae families, while some mycoviruses are naked ssRNA viruses belonging to the Hypoviridae, Narnaviridae, and Endornaviridae families (26–29). A naked ssRNA mycovirus, Cryphonectria hypovirus 1 (CHV1) in Cryphonectria parasitica, is the most well-studied virus/fungus system for RNA silencing against mycovirus infection, and the requirements for a dicer-like (dcl-2) and an Argonaute-like (agl-2) gene for antiviral silencing have been demonstrated by reverse-genetic studies (11, 30, 31). Importantly, it has been reported that dcl-2 and agl-2 are upregulated by CHV1, and constitutive expression of exogenous dsRNA in C. parasitica and the upregulation of DCL-2 and AGL-2 are repressed by P29 protein of CHV1 (31, 32). Moreover, vsRNAs have been detected from CHV1 in C. parasitica (32). However, generally, RNA silencing against encapsidated dsRNA mycoviruses is not well understood. In C. parasitica, some encapsidated mycoviruses, including a mycoreovirus (MyRV1), a partitivirus (Rosellinia necatrix partitivirus 2; RnPV2), and a victorivirus (Rosellinia necatrix victorivirus 1; RnVV1), more effectively replicated in an RNA silencing-defective, dicer-like protein 2 knockout mutant strain of C. parasitica (Δdcl-2) than in the wild-type strain (11, 33, 34). This suggests that these encapsidated dsRNA mycoviruses are also sensitive to RNA silencing in C. parasitica. Himeno et al. (35) detected vsRNA from an encapsidated dsRNA mycovirus belonging to the Totiviridae, Magnaporte oryzae virus 2 (MoV2), in M. oryzae. Recently, vsRNAs were detected from encapsidated dsRNA mycoviruses belonging to Partitiviridae, an unassigned dsRNA mycovirus, and an ssRNA mycovirus belonging to the Narnaviridae in Heterobasidion (36). These studies suggested that encapsidated dsRNA mycoviruses also induce RNA silencing. Notably, the accumulated level of vsRNA from MoV2 (approximately 0.2% [35]) was significantly lower than that from CHV1 in C. parasitica (approximately 70% [32]). This finding led to the hypothesis that encapsidated dsRNA mycoviruses, including MoV2, are sequestered from the RNA silencing machinery. To address this hypothesis, comparative analysis of inductions of RNA silencing against encapsidated dsRNA mycoviruses in the same host fungus is required.
The white root rot fungus R. necatrix is a soilborne fungus. It may be infected with several dsRNA mycoviruses (37, 38), including partitiviruses (RnPV1 and RnPV2), a mycoreovirus (Rosellinia necatrix mycoreovirus 3; RnMyRV3), a megabirnavirus (Rosellinia necatrix megabirnavirus 1; RnMBV1), a quadrivirus (Rosellinia necatrix quadrivirus 1; RnQV1), a victorivirus (RnVV1), and a novel ssRNA virus (Rosellinia necatrix fusarivirus 1; RnFV1) (28, 29, 33, 34, 39–42). Among them, RnPV1, RnMyRV3, RnMBV1, RnQV1, and RnVV1 were successfully introduced into the same R. necatrix strain W97, in which RnMyRV3 and RnMBV1 affect mycelial growth and virulence on apples but RnPV1, RnQV1, and RnVV1 were asymptomatic (28, 29, 34, 43, 44). Moreover, we showed that the RNA silencing mechanism is present in R. necatrix and is suppressed by RnMyRV3 but not by RnPV1, RnQV1, RnMBV1 (45), or RnVV1 (our unpublished data). Consequently, our mycovirus/R. necatrix system is suitable for the comparative analysis of inductions of RNA silencing among encapsidated dsRNA mycoviruses. In this study, we investigated inductions of RNA silencing against five unrelated encapsidated dsRNA mycoviruses in R. necatrix, including RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1. Our data revealed that RNA silencing-related genes, including a dicer-like (RnDCL-2), an Argonaute-like (RnAGL-2), and two RNA-dependent RNA polymerase (RnRdRP-1 and RnRdRP-2) genes, were upregulated by RnMyRv3 or RnMBV1 infection in R. necatrix but not by other virus infections or by constitutive expression of exogenous dsRNA. NGS analysis of vsRNA populations from the mycoviruses suggested diverse vsRNA generation pathways among the five mycoviruses. Furthermore, we observed, for the first time, activation of RISC by RnMBV1 infection in R. necatrix. Based on the presented data, we propose differential inductions of RNA silencing among encapsidated dsRNA mycoviruses.
MATERIALS AND METHODS
Fungal strains.
Rosellinia necatrix W97 strains infected with Rosellinia necatrix partitivirus 1 (RnPV1)-W8 (43), Rosellinia necatrix mycoreovirus 3 (RnMyRV3)-W370 (44), Rosellinia necatrix megabirnavirus 1 (RnMBV1)-W779 (28), Rosellinia necatrix quadrivirus 1 (RnQV1)-W1075 (29), Rosellinia necatrix victorivirus (RnVV1)-W1029 (34), and the transgenic W97 strain expressing dsRNA of the green fluorescent protein (irGFP) (45) were used in this study. These strains were cultured on potato dextrose agar (PDA; Difco, Becton Dickinson, Sparks, MD, USA) plates overlaid with sterilized cellophane (6 cm2) for 3 and 6 days at 25°C in the dark. The cellophanes containing the mycelia were peeled from the PDA plate and used for RNA extraction.
RNA extraction.
Total RNA was extracted from mycelial samples as described previously, with minor modifications (46). Mycelial samples were homogenized with dsRNA extraction buffer (0.15 M sodium acetate [pH 5.2], 0.1 M LiCl, 4% SDS, 10 mM EDTA [pH 8.0], and 20 mM β-mercaptoethanol) using a Multi-beads shocker (Yasui Kikai, Osaka, Japan). Total RNA was purified from these extracts by two rounds of phenol-chloroform extraction and precipitated with isopropanol. After digestion with 30 U of DNase I at 37°C for 30 min, total RNA was extracted with phenol-chloroform and concentrated by ethanol precipitation. Total RNA was dissolved with distilled water and prepared at 200 ng/μl. Fifty microliters (10 μg) of total RNA was digested with S1 nuclease at 37°C for 2 h, and dsRNA was extracted by phenol-chloroform and concentrated by ethanol precipitation. dsRNA was dissolved in 50 μl of distilled water.
Quantitative real-time reverse-transcription PCR (qRT-PCR).
To analyze the relative expression levels of RNA silencing-related genes, including RnDCL-2, RnAGL-1, RnAGL-2, RnRdRP-1, RnRdRP-2, RnRdRP-3, and RnRdRP-4, 400 ng of total RNA was denatured at 65°C for 10 min and chilled on ice. The denatured RNA was used for a reverse transcription (RT) reaction using SuperScript VILO (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. The resulting cDNA was diluted 20 times with distilled water. Standard curves for the actin gene and RNA silencing-related genes were prepared by serial dilutions of cDNA from the virus-free W97 strain, and the levels of the actin gene in each sample were used for normalization among samples. The cDNAs and standard samples were subjected to real-time PCR using a Power SYBR green master mix (Applied Biosystems, Thermo Fisher Scientific) and specific primer pairs (available upon request). The primer pairs for RnRdRP-1, RnRdRP-2, RnRdRP-3, and RnRdRP-4 were from a previous study (47). Two replicates in each of three RNA samples from independent experiments were analyzed on an ABI7300 real-time PCR system (Applied Biosystems) according to the manufacturer's instructions.
For absolute quantification of viral RNA from genome segments, 1 μl of total RNA or dsRNA was heat denatured at 95°C for 15 min and chilled on ice. The denatured RNA was used for an RT reaction as described above. The resulting cDNA was diluted 200 times with distilled water and used for real-time PCR. To prepare a standard curve, serial dilutions (10−18, 10−19, 10−20, and 10−21 mol/μl) of a standard plasmid, p21seg, were used. p21seg was synthesized by Fasmac Co., Ltd. (Kanagawa, Japan), to contain 21 viral cDNA sequences (100 bp each; total of 2,100 bp) corresponding to target regions for real-time PCR in each of the viral genome segments (RnPV1-S1, RnPV1-S2, RnQV1-S1, RnQV1-S2, RnQV1-S3, RnQV1-S4, RnVV1, RnMyRV3-S1, RnMyRV3-S2, RnMyRV3-S3, RnMyRV3-S4, RnMyRV3-S5, RnMyRV3-S6, RnMyRV3-S7, RnMyRV3-S8, RnMyRV3-S9, RnMyRV3-S10, RnMyRV3-S11, RnMyRV3-S12, RnMBV1-S1, and RnMBV1-S2). Real-time PCR using specific primer pairs (available upon request) was carried out as described above. Two replicates in each of three RNA samples from independent experiments were analyzed.
Next-generation sequencing.
The mycelial samples were homogenized using a Multi-beads shocker. Low-molecular-weight (LMW) RNA samples were extracted using a mirVana miRNA isolation kit (Ambion, Thermo Fisher Scientific) according to the manufacturer's instructions. Purification of small RNA fractions (18 to 30 bases) from LMW RNA samples, adapter ligations, cDNA synthesis, and sequencing by the single-read method using an Illumina Hiseq2000 were carried out by the small RNA sequencing service of InfoBio Inc. (Tokyo, Japan).
All computational informatics analyses of the sequence data were carried out by InfoBio Inc., including removing the adapter sequence, discarding the low-quality reads, mapping to RnPV1-S1 and -S2 (accession no. AB113347 and AB113348), RnQV1-S1 to -S4 (accession no. AB620061 to AB620064), a dsRNA genome of RnVV1 (accession no. AB742454), RnMyRV3-S1 to -S12 (accession no. AB073276 to AB732783, AB098022, AB098023, AB102674, and AB102675), and RnMBV1-S1 and -S2 (accession no. AB512282 and AB512283), and other calculations of mapped reads. Mapping of the reads to genome segment(s) of the mycoviruses was carried out using an original program provided by InfoBio Inc. (Tokyo, Japan) with settings that allow only exact matches and multiple hits to different regions of genome segments in each mycovirus. Analysis of 3′ overhangs between sense and antisense vsRNA reads was carried out by InfoBio Inc. according to the algorithm described by Li et al. (48).
Detection of vsRNAs. (i) Northern blot analysis.
LMW RNAs were purified from mycelium samples using a mirVana miRNA isolation kit (Ambion) according to the manufacturer's instructions, and 5 μg of LMW-RNA was used for Northern blotting of vsRNA. Northern blot analyses of vsRNAs were carried out as described previously (45). The cDNA fragments corresponding to nucleotides 1 to 600 or 1680 to 2279 of RnPV1-S2 or nucleotides 1 to 600, 3000 to 3600, or 8332 to 8931 of RnMBV1-S1 were amplified by RT-PCR using a PrimeScript one-step RT-PCR kit (TaKaRa Bio, Shiga, Japan) with specific primer pairs (available upon request). The cDNA fragments were ligated into pGEM-T Easy vector (Promega, Madison, WI, USA). The resulting recombinant plasmids were linearized and used for in vitro transcription of digoxigenin (DIG)-labeled RNA probes corresponding to the sense or antisense sequence using a DIG RNA labeling mix (Roche, Mannheim, Germany) and T7 or SP6 polymerase (Promega). To analyze the detection efficacy of each RNA probe, dot blot analyses of in vitro-synthesized RNA complementary to each probe were carried out. One hundred or 10 ng of the RNAs was blotted onto a Hybond N+ nylon membrane (GE Healthcare, Buckinghamshire, United Kingdom), and the membrane was hybridized with each corresponding RNA probe. Detection of the chemiluminescent signal was performed according to the recommended protocol for the DIG system (Roche).
All Northern and dot blot analyses were done simultaneously. The exposure times showing similar signal strengths among the dot blot analyses were determined and were used for each vsRNA Northern blot analysis.
For Northern blot analysis using the vsRNA probe, the LMW RNA sample was separated by 6 M urea–15% polyacrylamide gel electrophoresis, from which an approximately 18- to 26-nt fraction (estimated using 14 to 30 ssRNA markers [TaKaRa Bio]) was recovered using a ZR small-RNA PAGE recovery kit (Zymo Research, Irvine, CA, USA) according to the manufacturer's instructions. The recovered 2 μg of 18- to 26-nt RNAs were directly labeled with digoxin using a Label IT digoxin labeling kit (TaKaRa Bio) according to the manufacturer's instructions. Five hundred nanograms of in vitro-synthesized sense and antisense RNA corresponding to nt 1 to 600 or 1680 to 2279 of RnPV1-S2 and nt 1 to 600, 3000 to 3600, or 8332 to 8931 of RnMBV1-S1 were dot blotted onto Hybond N+ (GE Healthcare) and hybridized with the DIG-labeled 18- to 26-nt RNA probe.
(ii) Stem-loop RT-PCR.
To test the accumulation of vsRNAs at the 3′ termini on the sense strand of all four segments of RnQV1 (nt 4023 to 4942 for S1, nt 4333 to 4352 for S2, nt 4080 to 4099 for S3, and nt 3666 to 3685 for S4; consensus sequence, 5′-AUUGAYCAUGAGAAUAUUCR-3′) and at the 3′ region on the sense strand of RnMBV1-S2 (nt 7018 to 7037; 5′-UGACGUGUGGACGACAUGGA-3′), stem-loop RT-PCR was conducted according to the protocol by Varkonyi-Gasic et al. (49). The heat-denatured (65°C, 5 min) LMW RNA sample (500 ng) was used for a pulsed RT reaction using SuperScript III reverse transcriptase (Invitrogen) with the following steps: incubation at 16°C for 30 min; 60 cycles at 30°C for 30 s, 42°C for 30 s, and 50°C for 1 s; and incubation at 85°C for 5 min. The resulting cDNA was used for PCR using Ex Taq polymerase (TaKaRa Bio) with the following steps: incubation at 94°C for 5 min and then 20 cycles at 94°C for 15 s and 60°C for 1 min. Specific primers were designed for these vsRNA sequences and their complementary sequences (available upon request). The PCR products were electrophoresed through a readymade gradient polyacrylamide gel (5 to 20%; ATTO, Tokyo, Japan) in Tris-glycine buffer (25 mM Tris, 192 mM glycine), and the gel was stained by ethidium bromide.
(iii) GFP sensor assay.
The cDNA fragments corresponding to nucleotides 6920 to 7160 of RnMBV1-S2 and to nucleotides 580 to 718 of RnPV1-S1 were amplified by RT-PCR using a PrimeScript high-fidelity one-step RT-PCR kit (TaKaRa Bio) and specific primer pairs (available upon request). The resulting PCR products were ligated into the StuI site in the reverse direction downstream of the green fluorescent protein (GFP) sequence of pCPXHY1-eGFP (49) using an In-Fusion HD cloning kit (TaKaRa Bio). The resulting plasmids were designated pCPXHYGFP-MB2 and pCPXHYGFP-PV1. Transformation of R. necatrix W97 with pCPXHYGFP-MB2 or pCPXHYGFP-PV1 was carried out as described previously (50). The resulting transformed strains, W97/GFP-MB2 and W97/GFP-PV1, were dual cultured with W97 strains infected with RnPV1 or RnMBV1 on PDA for 10 to 14 days. Mycelial plugs then were transferred from the counterpart of W97/GFP-MB2 or W97/GFP-PV1 to PDA containing 100 μg/ml of hygromycin overlaid with cellophane (3 cm2), and virus infection in W97/GFP-MB2 or W97/GFP-PV1 was confirmed by total RNA extraction as described above. The virus-free and virus-infected transformants were subcultured twice and stored at −80°C in 10% glycerol until use. The fungal colony was photographed under bright-field illumination using a digital camera (EOS1000D; Canon, Tokyo, Japan), and a fluorescent image of the colony was obtained using a LAS4000 image analyzer (GE Healthcare).
Virus-free and virus-infected transformants were cultured on PDA plates containing 100 μg/ml of hygromycin overlaid with sterilized cellophane (6 cm2) for 6 days at 25°C in the dark. Total RNA extraction, the RT reaction, and real-time PCR were performed as described above. Standard curves for the actin gene and GFP-MB2 or GFP-PV1 were prepared by serial dilutions of cDNA from each virus-free transformant, and the levels of the actin gene in each sample were used for normalization among samples. Specific primers used are available upon request. Extraction of LMW-RNA and Northern blot analyses of sRNA were carried out as described above. Digoxigenin-labeled RNA probes corresponding to the sense or antisense sequence of GFP, the antisense sequence of nucleotides 6920 to 7160 of RnMBV1-S2, and the sense sequence of nucleotides 580 to 718 of RnPV1-S1 were used.
Sequencing data.
The total sRNA reads from W97 strains infected with each of the five mycoviruses were deposited in the DNA Databank of JAPAN (accession numbers DRR055291 to DRR055295).
RESULTS
Upregulation of silencing-related genes by mycovirus infections in R. necatrix.
In the draft genome sequence of Rosellinia necatrix strain W97, two dicer-like (RnDCL-1 and -2), three Argonaute-like (RnAGL-1, -2, and -3), and four RNA-dependent RNA polymerase (RnRdRP-1, -2, -3, and -4) genes were found (T. Shimizu, H. Yaegashi, S. Kanematsu, unpublished data). The expression of the four RdRp genes in mycelia were confirmed previously (47). RT-PCR analysis of total RNA from the W97 strain showed that RnDCL-2, RnAGL-1, and RnAGL-2 were expressed in mycelia, but RnDCL-1 and RnAGL-3 were not (data not shown). Similar results were found in the W97 strain and other strains, including W370T1 and W1015, infected with RnMBV1 (data not shown). The relative expression levels of RnDCL-2, RnAGL-1, RnAGL-2, RnRdRP-1, RnRdRP-2, RnRdRP-3, and RnRdRP-4 in W97 strains infected with RnPV1, RnQV1, RnVV1, RnMyRV3, or RnMBV1 were analyzed by qRT-PCR. The results showed that the expression levels of RnDCL-2, RnAGL-2, RnRdRP-1, and RnRdRP-2 in W97 strains infected with RnMyRV3 or RnMBV1 were 2- to 4-fold higher than those in virus-free strains at both 3 and 6 days of mycelial growth (Fig. 1A, C, D, and E). In addition, the expression level of RnAGL-1 in the W97 strain infected with RnMBV1 was upregulated at 6 days of mycelial growth (Fig. 1B). The expression levels of RnRdRP-3 and RnRdRP-4 in strains infected with each mycovirus were comparable to those in the virus-free W97 strain (Fig. 1F and G). In other fungi (Cryphonectria and Neurospora), expression of dsRNA induces upregulation of DCL and AGL genes (31, 51). Analysis of the expression levels of RNA silencing-related genes in the R. necatrix strain expressing dsRNA of GFP (irGFP) (45) showed that none of the genes in the irGFP strain were upregulated at 6 days of mycelial growth (Fig. 1A to G). Collectively, these results suggested that the RNA silencing machinery is upregulated in response to RnMyRV3 and RnMBV1 infections but not to RnPV1, RnQV1, and RnVV1 infections or constitutive expression of dsRNA.
FIG 1.
Relative expression levels of RnDCL-2 (A), RnAGL-1 (B), RnAGL-2 (C), RnRdRP-1 (D), RnRdRP-2 (E), RnRdRP-3 (F), and RnRdRP-4 (G) in R. necatrix W97 strains. Mycelial samples from virus-free strain W97 (VF) or W97 strains infected with each mycovirus (RnPV1, RnQV1, RnVV1, RnMyRV3, or RnMBV1) and grown for 3 (gray bar) or 6 (white bar) days, as well as a mycelial sample from a GFP-dsRNA-expressing transgenic W97 strain (irGFP) (45) grown for 6 days, were used for the analyses. (H) Relative expression levels of RnDCL-2, RnAGL-2, and RnRdRP-2 in mycelial samples from virus-free strain W97 (white bar) or W97 strains infected with RnMBV1 (gray bar), coinfected with RnMBV1 and RnPV1 (dotted bar), or infected with RnQV1 (slashed bar) or RnVV1 (light gray bar) and grown for 6 days. The level of mRNA of each gene was normalized to the level of the actin mRNA in each sample. Averages and standard deviations from three independent experiments are shown.
To investigate whether RnPV1, RnQV1, and RnVV1 have the ability to repress upregulation of the silencing-related genes, W97 strains coinfected with RnMBV1 and RnPV1, RnQV1, or RnVV1 were generated. The expression levels of RnDCL-2, RnAGL-2, and RnRdRP-2 were not significantly different among W97 strains infected with RnMBV1 and those coinfected with RnMBV1 and RnPV1, RnQV1, or RnVV1 (Fig. 1H), indicating that RnPV1, RnQV1, and RnVV1 have no ability to repress the RNA silencing-related genes upregulated by RnMBV1 infection.
Next-generation sequencing of vsRNAs.
The accumulation of sRNAs is a hallmark of induction of RNA silencing. NGS analysis of sRNA populations from W97 strains infected with each of RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1 was carried out independently. As shown in Fig. 1, upregulation of some RNA silencing-related genes in W97 strains infected with RnMyRV3 or RnMBV1 was observed at 3 and 6 days of mycelial growth; therefore, sRNA fractions from cultures grown for 6 days were used for NGS analysis. The NGS results are summarized in Table 1. Approximately 40 to 60 million redundant 17- to 25-nt reads were obtained in each sample from W97 infected with RnPV1, RnQV1, RnVV1, RnMyRV3, or RnMBV1. The proportions of redundant 17- to 25-nt vsRNA reads mapped to the genome segment(s) of RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1 in each library were 4.3%, 1.2%, 0.3%, 9.5%, and 17.6%, respectively. In contrast, those of redundant 19- to 22-nt vsRNA reads from RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1 were 6.8%, 1.2%, 0.3%, 13.0%, and 24.9%, respectively.
TABLE 1.
Summary of next-generation sequencing of the small RNA populations
| Virus | No. of reads by sequence size (nt) |
|||||
|---|---|---|---|---|---|---|
| Redundanta (total) |
Redundant vsRNAb |
Unique vsRNA |
||||
| 17–25 | 19–22 | 17–25 | 19–22 | 17–25 | 19–22 | |
| RnPV1 | 42,227,672 | 23,488,805 | 1,819,063 (4.3) | 1,605,039 (6.8) | 49,642 | 26,014 |
| RnQV1 | 49,842,939 | 25,257,510 | 584,445 (1.2) | 300,967 (1.2) | 151,909 | 70,794 |
| RnVV1 | 50,883,139 | 28,112,819 | 130,070 (0.3) | 83,601 (0.3) | 34,580 | 17,733 |
| RnMyRV3 | 47,915,660 | 24,587,825 | 4,533,723 (9.5) | 3,186,501 (13.0) | 290,634 | 141,668 |
| RnMBV1 | 63,062,194 | 39,524,783 | 11,105,170 (17.6) | 9,824,348 (24.9) | 185,723 | 94,978 |
Total reads after removing the adapter sequence.
The reads perfectly matched to a viral double-stranded genome(s) in each virus. Percentages of redundant viral reads among total redundant reads in each library (17 to 25 or 19 to 22 nt) are in parentheses.
Molecular characterization of vsRNAs.
Size distributions of the 17- to 25-nt redundant vsRNA reads in each virus showed a predominance of 19- to 22-nt vsRNAs in all mycoviruses (Fig. 2). In particular, 20- and 21-nt reads were apparent in the vsRNA libraries of RnPV1 and RnMBV1. Strand polarities of vsRNA reads differed among the mycoviruses, i.e., vsRNA reads from the antisense strand were abundant in RnPV1. Conversely, vsRNA reads from the sense strand were abundant in RnMBV1. In RnQV1, RnVV1, and RnMyRV3, vsRNA reads were distributed almost equally on both strands. Based on the data for size preference, 19- to 22-nt vsRNA reads were used for subsequent analyses.
FIG 2.
Size distributions of vsRNAs from RnPV1 (A), RnQV1 (B), RnVV1 (C), RnMyRV3 (D), and RnMBV1 (E). Gray and black bars indicate redundant vsRNA reads from the sense and antisense strands, respectively, of viral genome dsRNA.
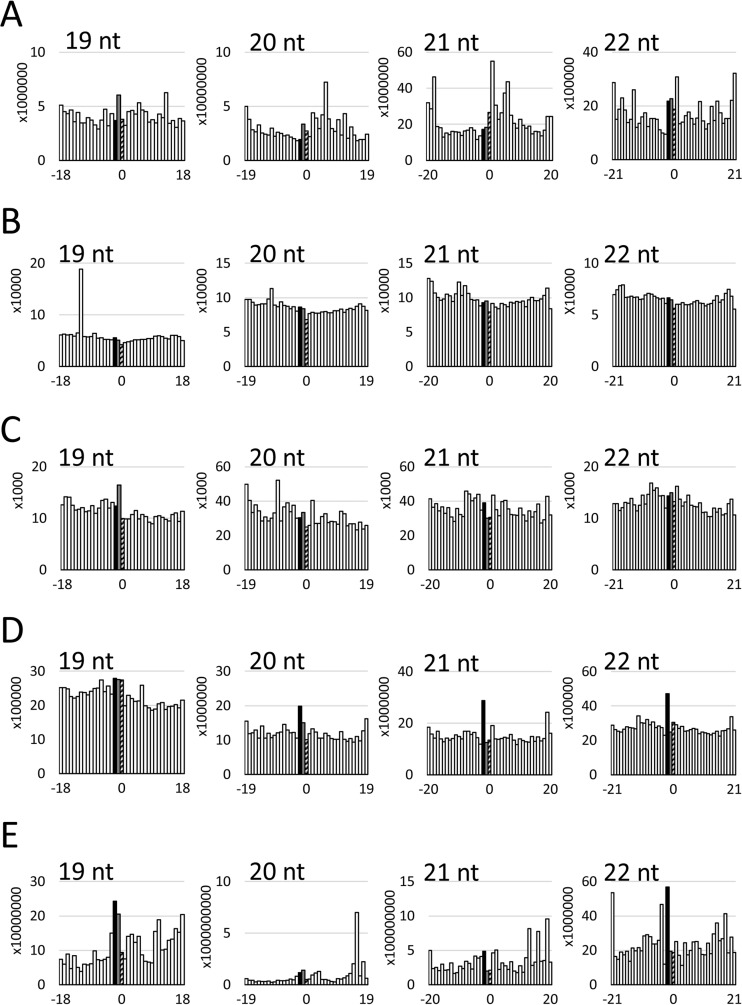
To analyze whether the obtained vsRNA reads could form duplexes with 3′ overhang(s), a bioinformatics analysis was carried out according to previous studies (48, 52). We could not detect specific enrichment of vsRNA duplexes with 1- or 2-nt 3′ overhangs in 19- to 22-nt vsRNA reads from RnPV1, RnQV1, and RnVV1 (Fig. 3A to C). In contrast, specific enrichment of vsRNA duplexes with 2-nt 3′ overhangs was found in the 20-, 21-, and 22-nt vsRNA reads from RnMyRV3 and in the 18- and 22-nt vsRNA reads from RnMBV1 (Fig. 3D and E). Except for the enrichment of vsRNA duplexes with 1- or 2-nt 3′ overhangs found in 23-nt vsRNA reads from RnPV1 and in 18-nt vsRNA reads from RnMBV1, there was no enrichment of vsRNA duplexes with 1- or 2-nt 3′ overhangs in other size classes of vsRNA reads from the five mycoviruses (data not shown).
FIG 3.
Analysis of duplex formation ability between sense and antisense strands in the 19-, 20-, 21-, or 22-nt samples of vsRNAs from RnPV1 (A), RnQV1 (B), RnVV1 (C), RnMyRV3 (D), and RnMBV1 (E). The x axis indicates distance from the 5′ terminus of the sense strand to the 3′ terminus of the antisense strand. The y axis indicates counts of pairs. Black, gray, and striped bars indicate −2 (2-nt 3′ overhangs), −1 (1-nt 3′ overhang), and 0 (no overhang), respectively.
The nucleotide species at the 5′ termini of vsRNAs is an important feature for its functions, including selective incorporation into an Argonaute family protein, which is a core component of the RISC (53–55). We analyzed the nucleotide species at the 5′ termini of redundant and unique 19- to 22-nt vsRNA reads in each of the five mycoviruses. Among the redundant 19- to 22-nt vsRNA reads, a strong bias toward A and U were found in RnPV1 (90 to 93%) and RnMBV1 (88 to 92%), and slight biases toward A and U were found in RnQV1 (57 to 66%), RnVV1 (58 to 71%), and RnMyRV3 (68 to 77%) (Fig. 4). There was no significant bias at the 5′-terminal nucleotide in unique vsRNA reads from the five mycoviruses; in addition, there was no bias at the 3′-terminal nucleotide in the redundant and unique vsRNA reads of all five mycoviruses (Fig. 4). These data strongly suggested that vsRNAs with 5′ A/U are selectively enriched after dicing. We also confirmed that the degrees of bias toward the 5′ A/U in each mycovirus did not differ among the genome segments or between sense and antisense strands (data not shown).
FIG 4.
Nucleotide species at the 5′ and 3′ ends of vsRNAs. Proportions of four nucleotide species (A, U, G, or C) at the 5′ or 3′ position of 19- to 22-nt vsRNA reads from RnPV1, RnQV1, RnVV1, RnMyRV3, or RnMBV1. R and U indicate redundant or unique vsRNA reads, respectively. Black, gray, white, and striped bars indicate proportions of A, U, G, or C at the 5′ or 3′ position of vsRNAs, respectively.
vsRNA abundances are not always dependent on viral RNA levels.
The abundances of redundant 19- to 22-nt vsRNA reads of RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1 were 6.8%, 1.2%, 0.3%, 13.0%, and 24.9%, respectively (Table 1). To analyze whether the differences correlated with accumulation levels of viral RNAs among the five mycoviruses, the weights of viral ss/dsRNA from genome segments (including both single-stranded and double-stranded forms of viral RNA) in 1 μg of total RNAs (including both host and viral RNAs) from W97 strains infected with RnPV1, RnQV1, RnVV1, RnMyRV3, or RnMBV1 were determined by a qRT-PCR assay. It is believed that the virion of a reovirus contains all of the viral genome segments in equimolar amounts (19). To verify our qRT-PCR assay using the plasmid DNA as standard samples, dsRNA was extracted from the purified virions of RnMyRV3 (43) and subjected to the qRT-PCR assay. The result showed that molar ratios were almost equal among the 12 genome segments of RnMyRV3 (data not shown), confirming the reliability of our qRT-PCR assay. The accumulation levels of viral ss/dsRNA and dsRNA (sum of weights of genome segments; picograms of viral RNA/1 μg of total RNA) of RnQV1, RnMyRV3, and RnMBV1 were apparently higher than those of RnPV1 and RnVV1 (Fig. 5A). This indicated that vsRNA abundances and viral RNA accumulation levels did not always correlate among the five mycoviruses, i.e., for RnPV1, the vsRNA abundance was higher than that of RnQV1, but the viral ss/dsRNA level apparently was lower than that of RnQV1. For RnQV1, the vsRNA abundance was lower than that of RnPV1, RnMyRV3, and RnMBV1; however, their viral ss/dsRNA levels were similar to those of RnMyRV3 and RnMBV1. For RnVV1, both the abundance of vsRNA and the viral ss/dsRNA level were the lowest among the five mycoviruses. Between RnMyRV3 and RnMBV1, vsRNA abundances differed, but the viral ss/dsRNA levels were similar.
FIG 5.
Accumulation levels of viral RNA of the five mycoviruses. (A) Comparison of accumulation levels of viral ss/dsRNA or dsRNA among RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1. For RnPV1, RnQV1, RnMyRV3, and RnMBV1, the sum of weights (picograms in 1 μg of total RNA) of ss/dsRNA (gray bar) or dsRNA (white bar) of multipartite genome segments are shown. The standard deviations were calculated by the square-root-of-sum-of-squares method. (B) Ratios of viral ss/dsRNA, dsRNA, and vsRNA among genome segments in RnPV1, RnQV1, RnMyRV3, or RnMBV1. The proportions of the weights of ss/dsRNA (dark gray bar) or dsRNA (white bar) of each genome segment in total weight (shown in panel A) are shown. The proportions of the number of redundant vsRNA reads from each genome segment are shown (light gray bar).
The four mycoviruses RnPV1, RnQV1, RnMyRV3, and RnMBV1 have 2, 4, 12, or 2 genome segments, respectively. The ratios of vsRNA, viral ss/dsRNA, or dsRNA among the genome segments in each virus were analyzed. As shown in Fig. 5B, the abundance of vsRNAs positively correlated with the accumulation levels of viral RNA among genome segments in each mycovirus, i.e., the proportions of vsRNA, viral ss/dsRNA, and dsRNA of RnPV1-S1 were higher than those of RnPV1-S2. The ratios of vsRNA, viral ss/dsRNA, and dsRNA were almost equal among the four genome segments of RnQV1. The ratios of vsRNA, viral ss/dsRNA, and dsRNA were similar among the 12 genome segments of RnMyRV3; however, the proportion of vsRNA from RnMyRV3-S6 was relatively large. The proportions of vsRNA, viral ss/dsRNA, and dsRNA of RnMBV1-S2 were greater than those of RnMBV1-S1.
Spatial distribution of vsRNAs along the viral genome.
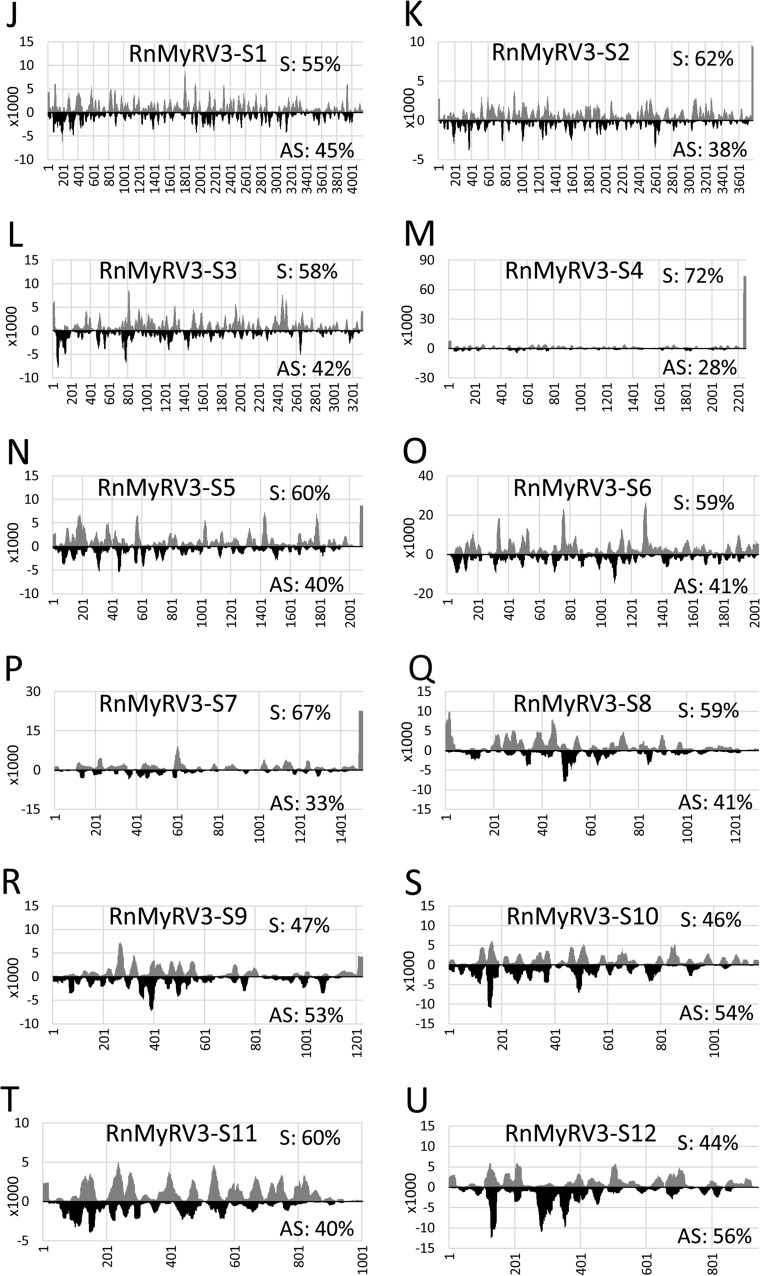
The distribution patterns of vsRNAs along the viral genome segment(s) in each mycovirus were analyzed. As expected, vsRNAs were distributed on both strands in all segments of the five mycoviruses; however, their distribution patterns appeared to be different. In RnPV1, higher antisense vsRNA peaks tended to be clustered in the 5′ region in both genome segments (Fig. 6A and B). In RnQV1, a higher sense vsRNA peak was found in the 3′-terminal region of all four genome segments (Fig. 6C to F). In RnVV1, the sense and antisense vsRNA peaks were distributed throughout, and a higher sense vsRNA peak was found at the 3′ terminus of the genome. In RnMBV1, the sense and antisense vsRNA peaks apparently were clustered in the 5′ and 3′ regions of the two genome segments (Fig. 6G and H). In particular, the highest sense vsRNA peak was found in the 3′ region of RnMBV1-S2. In RnMyRV3, the sense and antisense vsRNA peaks were distributed throughout the genome segments (Fig. 6I to T), and higher sense vsRNA peaks were found in the 5′-terminal region of RnMyRV3-S8 and in the 3′-terminal regions of RnMyRV3-S2, -S4, -S5, and -S7.
FIG 6.
Spatial distributions of vsRNAs along viral genome segments of RnPV1 (A and B), RnQV1 (C to F), RnVV1 (G), RnMBV1 (H and I), and RnMyRV3 (J to U). Redundancies (vertical axis) at a nucleotide position (horizontal axis) on the sense and antisense strands are shown by gray and black bars, respectively. The percentages shown in each graph indicate the proportion of redundant vsRNA reads from the sense and antisense strands, respectively. The two direction arrows in panels B and H indicate the region corresponding to the strand-specific RNA probes that were used for Northern blot analysis of vsRNAs shown in Fig. 7. The asterisks indicate the vsRNA peaks subjected to stem-loop RT-PCR in Fig. 7.
The percentages of redundant viral reads on each strand of the genome segments of RnPV1, RnQV1, RnVV1, RnMyRV3, and RnMBV1 confirmed the distribution patterns of vsRNAs in the genome segments of the five mycoviruses. For example, the proportions of antisense vsRNAs in RnPV1-S1 and -S2 were approximately 1.9- and 3.8-fold higher than those of sense vsRNAs, respectively. In contrast, the proportions of sense vsRNAs in RnMBV1-S1 and -S2 were approximately 1.5- and 3.2-fold higher than those of antisense vsRNAs, respectively.
The distribution patterns of vsRNAs in RnPV1-S2 and RnMBV1-S1 were validated by Northern blot analyses using strand-specific RNA probes. The dot blot analyses of in vitro-synthesized RNAs showed different detection efficacies among the RNA probes (Fig. 7A), and therefore different exposure times were used for each Northern blot analysis of vsRNAs. In RnPV1-S2, sense and antisense vsRNAs from the 5′ region were detected, but faint signals were detected in sense and antisense vsRNAs from the 3′ region (Fig. 7B). In RnMBV1-S1, sense and antisense vsRNAs from the 5′ and 3′ regions were readily detected, but those from the central region were not (Fig. 7B). To confirm the different vsRNA distribution patterns among the genome regions and between sense and antisense strands in RnPV1-S2 and RnMBV1-S1, we carried out dot blot analysis of in vitro-synthesized RNA corresponding to different regions on each strand of RnPV1-S2 and RnMBV1-S1 using 18- to 26-nt sRNAs from the W97 strain infected with RnPV1 or RnMBV1 as a probe. To exclude nonspecific signals by cross-reaction of each RNA with a host sRNA, we analyzed the relative value of the signal strength in each RNA after deducting the signal strength for each RNA by sRNA probes from virus-free strain W97. In RnPV1-S2, similar signals were detected for all RNAs; however, the relative values of the 5′- and 3′-sense RNAs were higher than those for the 5′- and 3′-antisense RNAs (Fig. 7C), indicating the higher accumulation of antisense vsRNAs. In RnMBV1-S1, strong signals were detected in 5′- and 3′-antisense RNAs (Fig. 7C), and their relative values were higher than those of other RNAs, indicating higher accumulation of sense vsRNAs at 5′ and 3′ regions. These results are consistent with the vsRNA distribution patterns produced by NGS analysis.
FIG 7.
Detection of vsRNAs. (A) Dot blot analyses of in vitro-synthesized RNAs (100 or 10 ng) by sense (S) and antisense (AS) RNA probes corresponding to the 5′ (nt 1 to 600) or 3′ (nt 1680 to 2279) region of RnPV1-S2 and the 5′ (nt 1 to 600), central (C; nt 3000 to 3600), or 3′ (nt 8332 to 8931) region of RnMBV1-S1. The exposure times were different among blots to obtain similar signal strengths. (B) Northern blot analyses of vsRNAs from RnPV1-S2 and RnMBV1-S1. Sense (S) and antisense (AS) RNA probes corresponding to each region of RnPV1-S2 (5′ or 3′) and RnMBV1-S1 (5′, central [C], or 3′) were used. Five micrograms of low-molecular-weight (LMW) samples of virus-free (VF) and RnPV1- or RnMBV1-infected (VI) W97 strains were used. Ethidium bromide staining of tRNA is shown as a loading control. Arrowheads indicate the positions of the 21- and 25-nt synthetic RNAs. (C) Dot blot analysis of in vitro-synthesized RNAs using vsRNA probes. Eighteen- to 26-nt sRNA fractions from virus-free W97 strain (VF) or W97 strains infected with RnPV1 (PV) or RnMBV1 (MBV) were directly labeled with digoxigenin and used for hybridization with 500 ng of in vitro-transcribed RNAs used for panel A. The relative values of the signal strength of each RNA were calculated by deducting the signal strength in each RNA generated by sRNA probes from that of the virus-free W97 strain. The relative value of the 3′-sense RNA of RnPV1-S2 or central-antisense RNA of RnMBV1-S2 were set to 1 in each analysis. (D) Stem-loop RT-PCR of vsRNAs from 3′ termini on the sense strand of all four segments of RnQV1 (nt 4023 to 4942 for S1, nt 4333 to 4352 for S2, nt 4080 to 4099 for S3, and nt 3666 to 3685 for S4; consensus sequence, 5′-AUUGAYCAUGAGAAUAUUCR-3′) and from the 3′ region on the sense strand of RnMBV1-S2 (nt 7018 to 7037; 5′-UGACGUGUGGACGACAUGGA-3′). Five hundred nanograms of LMW samples of virus-free (VF) and RnQV1- or RnMBV1-infected (VI) W97 strains were used. Arrows indicate the positions of specific bands. An arrowhead indicates the position of the 125-bp DNA marker.
Stem-loop RT-PCR analysis was conducted to verify accumulation of the specific vsRNA peaks. We selected apparent vsRNA peaks, including 3′ termini on the sense strands of four genome segments of RnQV1 (Fig. 6C to F, indicated by asterisks) and the 3′ region on the sense strand of RnMBV1-S2 (Fig. 6H, indicated by an asterisk). The results showed that the PCR product from sense vsRNAs at the 3′ termini in all four segments of RnQV1 and at the 3′ region in RnMBV1-S2 were readily detected (Fig. 7D). In contrast, the PCR products from each complementary antisense vsRNAs were detected as a faint band (Fig. 7D).
Functional analysis of vsRNAs in R. necatrix.
As shown in Fig. 4, the 5′-terminal nucleotide of 19- to 22-nt vsRNAs was strongly biased to A/U, suggesting the specific association of vsRNAs with an Argonaute protein and activation of RISC. We mapped the 5′ position of vsRNAs on the viral genome at which redundancies of the four nucleotide species and their correspondences to vsRNA peaks were analyzed. As shown in Fig. 8A, high accumulation of a single redundant U nucleotide position was found at the 5′ end of the vsRNA peak on the sense strand of RnMBV1-S2 (nt 7018 to 7037). Moreover, accumulation of the 20-nt vsRNA was validated by stem-loop RT-PCR (Fig. 7D). We also confirmed the association between the redundant 5′-A/U nucleotide position and the 5′ end of the vsRNA peak in other vsRNA peaks in the genome segment(s) of the other four mycoviruses (data not shown).
FIG 8.
Green fluorescent protein (GFP)-based sensor assay in Rosellinia necatrix. (A) Redundancies of four nucleotide species at the 5′ position of vsRNAs along the region of nt 6920 to 7120 on RnMBV1-S2 (MB2). Gray bars indicate redundancy at a nucleotide position. Blue, green, yellow, and red bars indicate redundancy of A, G, C, and U at the 5′ positions of vsRNAs, respectively. Also shown is a schematic representation of the plasmid expressing the sensor gene, based on pCPXHY-eGFP (48). A pentagon, green box, red line, and open box indicate the A. nidulans glyceraldehydes-3-phosphate dehydrogenase gene (gpd) promoter, full-length GFP gene, MB2 antisense sequence, and gpd terminator, respectively. (B) GFP fluorescence of colonies of virus-free (VF) or RnPV1- or RnMBV1-infected W97/GFP-MB2 strains cultured for 6 days. (C) Relative expression levels of GFP-MB2 mRNA. The level of GFP-MB2 mRNA was normalized by the level of the actin mRNA in each sample. Averages and standard deviations from three independent experiments are shown. (D) Detection of dsRNA and sRNA. The upper panel represents agarose gel electrophoresis of total RNA from each strain. Asterisks indicate the positions of viral dsRNAs. The bottom panel represents Northern blotting of MB2-sRNA using the RNA probe corresponding to the antisense MB2 sequence. Five micrograms of LMW RNA samples were used, and ethidium bromide staining of tRNA is shown as a loading control. (E) Northern blotting of GFP-sRNA using the RNA probe corresponding to sense or antisense sequence of full-length GFP. Five-microgram LMW RNA samples from W97/GFP-MB2 strains were used. As a positive control, 1 μg of LMW-RNA sample from the GFP silencing strain RiGFP was used. Ethidium bromide staining of tRNA is shown as a loading control.
To investigate whether vsRNAs from RnMBV1 activate the RISC in R. necatrix, we designed a GFP-based sensor assay in R. necatrix. The antisense sequence from the 3′ region of RnMBV1-S2 (nt 6920 to 7160) was used as a target sequence (MB2), because MB2 contains sequence complementary to the abundant vsRNA with 5′-U (nt 7018 to 7037) (Fig. 8A). We generated an R. necatrix strain expressing the sensor gene consisting of the full-length GFP gene and MB2 (Fig. 8A), which was infected with RnPV1 or RnMBV1 by hyphal anastomosis. The R. necatrix strain expressing the sensor gene (W97/GFP-MB2) showed strong GFP fluorescence. In contrast, GFP fluorescence disappeared in the W97/GFP-MB2 strain infected with RnMBV1 but not with RnPV1 (Fig. 8B). qRT-PCR analysis showed that the GFP-MB2 mRNA level in the W97/GFP-MB2 strain infected with RnMBV1 was approximately 4-fold lower than that in the virus-free strain (Fig. 8C). Northern blot analysis using an RNA probe corresponding to the MB2 antisense sequence readily detected the sense MB2-sRNA in the W97/GFP-MB2 strain infected with RnMBV1 (Fig. 8D), indicating that vsRNAs from RnMBV1 activated the RISC and guided it to degrade GFP-MB2 mRNA. In some organisms, including plants and nematodes, secondary sRNAs are generated from new dsRNA synthesized by a host RdRp using the primary sRNA as a primer, which amplifies the effect of silencing (8). To investigate whether RnMBV1 infection induces amplification of silencing, Northern blot analysis of GFP-sRNA was carried out. If amplification of silencing was induced, secondary GFP-sRNAs would be detected. The result showed that neither the sense nor antisense GFP-sRNA was detected in the W97/GFP-MB2 strain infected with RnMBV1 (Fig. 8E), suggesting that vsRNAs from RnMBV1 do not induce amplification of silencing. A GFP sensor assay designed for RnPV1 also showed that RnPV1 but not RnMBV1 infection induces silencing of the target GFP-sensor gene (Fig. 9).
FIG 9.
GFP-based sensor assay for a partitivirus (RnPV1) in Rosellinia necatrix. (A) Redundancies of four nucleotide species at the 5′ position of vsRNAs along the region of nt 580 to 718 on RnPV1-S1 (PV1). Gray bars indicate redundancy at a nucleotide position. Blue, green, yellow, and red bars indicate redundancy of A, G, C, and U at the 5′ position of vsRNAs, respectively. Schematic representation of the plasmid expressing the sensor gene, based on pCPXHY-eGFP (48). A pentagon, green box, red line, and open box indicate the A. nidulans glyceraldehyde-3-phosphate dehydrogenase gene (gpd) promoter, full-length GFP gene, PV1 sense sequence, and gpd terminator, respectively. (B) GFP fluorescence of colonies of virus-free (VF) and RnPV1- or RnMBV1-infected W97/GFP-PV1 strains cultured for 6 days. (C) Relative expression levels of GFP-PV1 mRNA. The level of GFP-PV1 mRNA was normalized by the level of the actin mRNA in each sample. Averages and standard deviations from three independent experiments are shown. (D) Detection of dsRNA and sRNA. The upper panel represents agarose gel electrophoresis of total RNA from each strain. Asterisks indicate the positions of viral dsRNAs. The bottom panel represents Northern blotting of GFP- and PV1-sRNA using the RNA probe corresponding to GFP or the sense PV1 sequence. Five-microgram LMW RNA samples were used, and ethidium bromide staining of tRNA is shown as a loading control.
DISCUSSION
RNA silencing is a fundamental antiviral mechanism in a wide variety of eukaryotes. In fungi, previous studies demonstrated an antiviral role of RNA silencing in C. parasitica by a reverse genetics approach. For example, several mycoviruses, including a hypovirus (CHV1), a mycoreovirus (MyRV1), a partitivirus (RnPV2), and a victorivirus (RnVV1), more effectively replicated in an RNA silencing-defective, dicer-like protein 2 knockout mutant strain of C. parasitica (Δdcl-2) than in the wild-type strain of the fungus (11, 33, 34). Notably, both dcl-2 and agl-2 are upregulated by infection with CHV1 and MyRV1 and also by constitutive expression of dsRNA in C. parasitica (31, 32). In this study, we showed that infections of R. necatrix by RnMyRV3 or RnMBV1, but not RnPV1, RnQV1, and RnVV1, induce upregulation of a dicer-like (RnDCL-2), an Argonaute-like (RnAGL-2), and two RNA-dependent RNA polymerase (RnRdRP-1 and RnRdRP-2) genes in R. necatrix (Fig. 1). In contrast to C. parasitica and Neurospora crassa (31, 51), a transgenic R. necatrix strain expressing GFP-dsRNA (irGFP) did not show upregulation of the RNA silencing-related genes (Fig. 1). We previously confirmed that silencing of GFP is induced in the irGFP strain by detecting GFP-sRNA (45); therefore, the upregulation of the RNA silencing-related genes is not necessary to induce RNA silencing. Nevertheless, the abundances of vsRNA from RnMyRV3 and RnMBV1 were relatively higher than those from the other mycoviruses (Table 1), suggesting that the two mycoviruses would induce RNA silencing strongly. Previously, we revealed that RnMyRV3 has RNA silencing suppression (RSS) ability in R. necatrix (45). In contrast to the observation that P29 of CHV1 represses upregulation of DCL-2 and AGL-2 in C. parasitica (11, 30–32), the putative RSS of RnMyRV3 (S10 gene) would not repress the upregulation of the RNA silencing-related genes in R. necatrix but rather interferes with another step(s), as discussed below. It is unclear how the RNA silencing-related genes are upregulated in R. necatrix infected with RnMyRV3 or RnMBV1. RnMyRV3 and RnMBV1 affect mycelial growth and virulence of R. necatrix (28, 44); therefore, there is a possibility that the phenotypic alterations are involved in the upregulation of the RNA silencing-related genes. A future study will investigate the biological significance and mechanism of upregulation of the RNA silencing-related genes in R. necatrix.
NGS analysis showed accumulation of vsRNAs from the five mycoviruses (Table 1) in which the 19- to 22-nt vsRNAs were enriched (Fig. 2). Interestingly, we detected enrichment of vsRNA duplexes between sense and antisense strands with 2-nt 3′ overhangs in 20-, 21-, and 22-nt vsRNA reads from RnMyRV3 (Fig. 3D), suggesting that vsRNAs from RnMyRV3 are generated by the canonical RNA silencing pathway. In contrast, such enrichment was detected in 19- and 22-nt, but not in 20- and 21-nt, vsRNA reads from RnMBV1 (Fig. 3E) and not in the 19- to 22-nt vsRNA reads from RnPV1, RnQV1, and RnVV1 (Fig. 3A to C). Importantly, we found that the 5′-terminal nucleotides of redundant 19- to 22-nt vsRNA reads from RnPV1 and RnMBV1 were strongly biased to A and U, which were more apparent than those among unique vsRNA reads (Fig. 4), indicating that vsRNAs with 5′-A/U must be enriched after dicing. In N. crassa, the 5′-terminal nucleotides of sRNAs associated with the Argonaute-like protein (QDE-2) are strongly biased toward U (56). Additionally, the sRNA duplex incorporated into QDE-2 is converted into a single strand by removing one strand (57). In accordance with this scenario, 19- to 22-nt (especially 20- and 21-nt) vsRNAs with 5′-A/U would be incorporated into an Argonaute-like protein as a duplex form in R. necatrix, in which one strand of vsRNA duplex with 5′-A/U must be enriched by removal of the other, complementary strand. This idea is a possible explanation for the inconsistency between the size preferences and duplex formation ability with 3′ overhangs in 19- to 22-nt vsRNA reads from RnPV1 and RnMBV1. In contrast, selective enrichment of vsRNAs from RnMyRV3 dependent on their size and 5′ nucleotide species seems to be suppressed compared with those of RnPV1 and RnMBV1 (Fig. 2D and 4), despite the enrichment of vsRNA duplexes with 3′ overhangs (Fig. 3D). RnMyRV3 might affect the association of vsRNAs with an Argonaute protein in addition to inhibiting vsRNA generation, as reported previously (45). We could not exclude the possibility that some vsRNAs from the five mycoviruses are generated by nonspecific random degradation of viral RNAs. Alternatively, a dicer-independent small interfering RNA generation pathway might exist and be involved in vsRNA generation in R. necatrix, as reported for N. crassa (56). In RnQV1 and RnVV1, enrichment of vsRNAs dependent on size (19 to 22 nt), duplex formation ability with 3′ overhangs, and 5′ nucleotide species (A or U) were repressed compared with the other three mycoviruses. Therefore, such nonspecific degradation or noncanonical pathway(s) might be involved in vsRNA generation from RnQV1 and RnVV1. We have not yet succeeded in disrupting the RNA silencing-related genes; therefore, the pathway(s) involved in vsRNA generation from the five mycoviruses in R. necatrix remain to be identified.
Significantly low levels of vsRNAs have been reported from mycoviruses in Aspergillus nidulans and MoV2 in M. oryzae (35, 58). Encapsidated dsRNA mycoviruses retain their dsRNA genome and replicate inside their virions (19); therefore, it has been suggested that their dsRNA would be sequestered from dicing into vsRNA. However, we showed that vsRNA abundances apparently are different among the five encapsidated dsRNA mycoviruses, and the vsRNA abundances did not always correlate with their viral RNA levels (Table 1 and Fig. 5A). In addition, in multisegmented mycoviruses, including RnPV1, RnQV1, RnMyRV3, and RnMBV1, the ratios of vsRNA and viral RNA among genome segments were consistent (Fig. 5B), indicating that vsRNA generation is dose dependent among the genome segments in each mycovirus but not among the mycoviruses. These combined data indicated that the differences in vsRNA abundances among the five mycoviruses reflect their replication and/or counterdefense strategies. Notably, the ratio of vsRNAs from RnMyRV3-S6 among 12 genome segments was relatively high (Fig. 5B), suggesting that RnMyRV3-S6 is preferentially diced. The protein encoded by RnMyRV3-S6 has conserved mu2-like nucleoside triphosphate (NTP)-binding motifs (59), and the mu2 protein of mammalian orthoreoviruses is associated with viral RNA synthesis (60). It is unclear whether the relatively high vsRNA abundance is associated with the potential gene function of RnMyRV3-S6.
The vsRNA abundances of RnMyRV3 and RnMBV1 were different (13.0% versus 24.9%); however, their viral RNA levels were similar (Fig. 5A). The lower vsRNA abundances of RnMyRV3 reflected its RSS ability, which inhibits sRNA generation (45). Nevertheless, the accumulation of vsRNAs from RnMyRV3 implied that its RSS ability does not completely inhibit vsRNA generation. On the other hand, the significantly lower vsRNA abundance of RnVV1 (0.3%) corresponded with the lower viral RNA level (Fig. 5A). In contrast, the vsRNA abundance of RnQV1 was significantly low (1.2%), but its viral RNA level was similar to those of RnMyRV3 and RnMBV1 (Fig. 5A). A previous study could not detect any RSS ability of RnQV1 (45), and this study showed that RnQV1 did not repress upregulation of RNA silencing-related genes by RnMBV1 infection (Fig. 1H). Therefore, RnQV1 may sequester its RNA from dicing by encapsidation, as suggested for MoV2 (35), or the virus may have other, unknown counterdefense mechanism(s). The vsRNA abundance of RnPV1 (6.8%) was higher than that of RnQV1 (1.2%), but the viral RNA level of RnPV1 apparently was lower than that of RnQV1 (Fig. 5A), suggesting that RnPV1 also effectively induces RNA silencing, like RnMyRV3 and RnMBV1. This notion was also supported by the fact that vsRNAs from RnPV1 showed strong size preferences (19 to 22 nt) and 5′-terminal nucleotides (A/U) similar to those of RnMBV1 (Fig. 2A and 4A).
It has been suggested that the five mycoviruses replicate inside their virions like other encapsidated dsRNA viruses (19). Which types of RNAs are diced into vsRNA? The sense-strand RNA of dsRNA viruses functions as the mRNA, which is released into the cytoplasm for protein expression (19); therefore, it may be diced into vsRNA if it forms a secondary structure(s). Nandety et al. (21) suggested that the panhandle structures on sense-strand RNA of each genome segment are sources of sense-strand-specific vsRNA for a reovirus in insects. We found apparent sense-strand-specific vsRNA peaks at the 3′ termini of all segments of RnQV1 (Fig. 6C to F), and this finding was confirmed by stem-loop RT-PCR (Fig. 7D). Similarly, sense-strand-specific vsRNA peaks were also found at the 3′ termini of RnVV1 (Fig. 6G) and RnMyRV3-S2, -S4, -S5, and -S7 (Fig. 6K, M, N, and P) and at the 5′ terminus of RnMyRV3-S8 (Fig. 6Q). It has been predicted that sense-strand-specific inverted repeat and panhandle structures exist between the 5′ and 3′ termini of genome segments of RnQV1, RnVV1, and RnMyRV3 (29, 34, 40, 61); therefore, these secondary structures might be diced. In contrast, we found that vsRNA peaks were densely distributed at the 5′ region of both RnPV1-S1 and RnPV1-S2 (Fig. 6A and B). In addition, sense and/or antisense vsRNA peaks were densely distributed at both 5′ and 3′ regions of RnMBV1-S1 and -S2 (Fig. 6H and I), and these distribution patterns were confirmed by Northern and dot blot analyses of vsRNAs for RnPV1-S2 and RnMBV1-S1 (Fig. 7B and C). These data suggested another possibility in which incomplete dsRNAs generated during transcription and/or replication might be exposed to dicing. Alternatively, an endogenous host RdRp might be involved in the dsRNA generation, as reported for plant viruses (62, 63). As described above, upregulation of two RdRp genes (RnRdRP-1 and -2) by RnMyRV3 or RnMBV1 infection was observed (Fig. 1); however, we could not detect any secondary sRNA that would be generated if endogenous RdRp(s) was active (Fig. 8). This result is consistent with a previous study, which showed that hairpin RNA-induced silencing did not induce dsRNA synthesis effectively in R. necatrix (47). Thus, the role of the RdRps in vsRNA generation remains unclear. In C. parasitica, disruptions of four RdRp genes did not affect CHV1 propagation (64). Overall, the mechanisms of vsRNA generation from encapsidated dsRNA mycoviruses seem to be more complex. Further dissection of the replication cycle of encapsidated dsRNA mycoviruses is needed to clarify this issue.
Incorporation of an sRNA into an Argonaute protein activates the RISC for degradation of the target RNA (5, 6, 65). We showed that RnMBV1 infection induced degradation of an artificial RNA (GFP-MB2) comprising GFP and the partial RnMBV1 sequence (MB2) in R. necatrix (Fig. 8). Degradation of GFP-MB2 was not found after RnPV1 infection, and the MB2-sRNA was detected after RnMBV1, but not RnPV1, infection. A similar assay designed for RnPV1 also showed that the artificial RNA comprising GFP and the partial sequence of RnPV1-S1 was silenced by RnPV1 infection (Fig. 9). Thus, we concluded that vsRNAs from the mycoviruses could activate the RISC and guide it toward the sequence-specific degradation of target RNA. To the best of our knowledge, this is the first evidence that vsRNAs from mycoviruses are functional in the RNA silencing pathway. Consequently, replication of encapsidated dsRNA mycoviruses would be repressed not only by dicing of viral dsRNAs but also by RISC-mediated degradation of viral ssRNA. In other words, these data imply that a vsRNA-RISC also targets a host mRNA if it contains sequence complementary to a vsRNA, as reported for plant and nematode (66–68).
In conclusion, this study revealed differential responses of RNA silencing against five unrelated encapsidated dsRNA mycoviruses. Moreover, we provided insights into the molecular features and function of vsRNAs from mycoviruses in the RNA silencing pathway in R. necatrix. The present data indicated that encapsidated dsRNA mycoviruses interact differentially with antiviral RNA silencing, i.e., there are differences in replication strategies among encapsidated dsRNA mycoviruses. Consequently, this study deepened our knowledge of the replication strategies of dsRNA viruses.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Young Scientists (B) (JSPS Kakenhi grant number 23780045 to H.Y.) and a Grant-in-Aid for Scientific Research (B) (JSPS Kakenhi grant number 25292031 to S.K. and H.Y.).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Cogoni C. 2001. Homology-dependent gene silencing mechanisms in fungi. Annu Rev Microbiol 55:38–1406. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ. 2002. RNA interference. Nature 418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 3.Zamore PD. 2002. Ancient pathways programmed by small RNAs. Science 296:1265–1269. doi: 10.1126/science.1072457. [DOI] [PubMed] [Google Scholar]
- 4.Baulcombe DC. 2004. RNA silencing in plants. Nature 431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 5.Meister G, Tuschl T. 2004. Mechanisms of gene silencing by double-stranded RNA. Nature 431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 6.Carthew RW, Sontheimer EJ. 2009. Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassenegger M, Krczal G. 2006. Nomenclature and functions of RNA-directed RNA polymerases. Trends Plant Sci 11:142–151. doi: 10.1016/j.tplants.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Dang Y, Yang Q, Xue Z, Liu Y. 2011. RNA interference in fungi: pathways, functions, and applications. Eukaryot Cell 10:1148–1155. doi: 10.1128/EC.05109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang MB, Metzlaff M. 2005. RNA silencing and antiviral defense in plants. Curr Opin Plant Biol 8:216–222. doi: 10.1016/j.pbi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Ding SW, Voinnet O. 2007. Antiviral immunity directed by small RNAs. Cell 130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segers GC, Zhang X, Deng F, Sun Q, Nuss DL. 2007. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc Natl Acad Sci U S A 104:12902–12906. doi: 10.1073/pnas.0702500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aliyari R, Ding SW. 2009. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol Rev 227:176–188. doi: 10.1111/j.1600-065X.2008.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaire L, Wang Y, Gonzalez-Ibeas D, Mayer KF, Aranda MA, Llave C. 2009. Deep-sequencing of plant viral small RNAs reveals effective and widespread targeting of viral genomes. Virology 392:203–214. doi: 10.1016/j.virol.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Aregger M, Borah BK, Seguin J, Rajeswaran R, Gubaeva EG, Zvereva AS, Windels D, Vazquez F, Blevins T, Farinelli L, Pooggin MM. 2012. Primary and secondary siRNAs in geminivirus-induced gene silencing. PLoS Pathog 8:e1002941. doi: 10.1371/journal.ppat.1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blevins T, Rajeswaran R, Aregger M, Borah BK, Schepetilnikov M, Baerlocher L, Farinelli L, Meins F Jr, Hohn T, Pooggin MM. 2011. Massive production of small RNAs from a non-coding region of Cauliflower mosaic virus in plant defense and viral counter-defense. Nucleic Acids Res 39:5003–5014. doi: 10.1093/nar/gkr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voinnet O. 2005. Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet 6:206–220. doi: 10.1038/nrg1555. [DOI] [PubMed] [Google Scholar]
- 17.Bivalkar-Mehla S, Vakharia J, Mehla R, Abreha M, Kanwar JR, Tikoo A, Chauhan A. 2011. Viral RNA silencing suppressors (RSS): novel strategy of viruses to ablate the host RNA interference (RNAi) defense system. Virus Res 155:1–9. doi: 10.1016/j.virusres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csorba T, Kontra L, Burgyán J. 2015. Viral silencing suppressors: tools forged to fine-tune host-pathogen coexistence. Virology 479–480C:85–103. [DOI] [PubMed] [Google Scholar]
- 19.Mertens P. 2004. The dsRNA viruses. Virus Res 101:3–13. doi: 10.1016/j.virusres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Andika IB, Shen J, Lv Y, Ji Y, Sun L, Chen J. 2013. Characterization of rice black-streaked dwarf virus- and rice stripe virus-derived siRNAs in singly and doubly infected insect vector Laodelphax striatellus. PLoS One 8:e66007. doi: 10.1371/journal.pone.0066007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandety RS, Fofanov VY, Koshinsky H, Stenger DC, Falk BW. 2013. Small RNA populations for two unrelated viruses exhibit different biases in strand polarity and proximity to terminal sequences in the insect host Homalodisca vitripennis. Virology 442:12–19. doi: 10.1016/j.virol.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Schnettler E, Ratinier M, Watson M, Shaw AE, McFarlane M, Varela M, Elliott RM, Palmarini M, Kohl A. 2013. RNA interference targets arbovirus replication in Culicoides cells. J Virol 87:2441–2454. doi: 10.1128/JVI.02848-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Luo Y, Lu R, Lau N, Lai EC, Li WX, Ding SW. 2010. Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc Natl Acad Sci U S A 107:1606–1611. doi: 10.1073/pnas.0911353107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Cleef KW, van Mierlo JT, Miesen P, Overheul GJ, Fros JJ, Schuster S, Marklewitz M, Pijlman GP, Junglen S, van Rij RP. 2014. Mosquito and Drosophila entomobirnaviruses suppress dsRNA- and siRNA-induced RNAi. Nucleic Acids Res 42:8732–8744. doi: 10.1093/nar/gku528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vodovar N, Goic B, Blanc H, Saleh MC. 2011. In silico reconstruction of viral genomes from small RNAs improves virus-derived small interfering RNA profiling. J Virol 85:11016–11102. doi: 10.1128/JVI.05647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson MN, Beever RE, Boine B, Arthur K. 2009. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol 10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghabrial SA, Suzuki N. 2009. Viruses of plant pathogenic fungi. Annu Rev Phytopathol 47:353–384. doi: 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- 28.Chiba S, Salaipeth L, Lin YH, Sasaki A, Kanematsu S, Suzuki N. 2009. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: molecular and biological characterization, taxonomic considerations, and potential for biological control. J Virol 83:12801–12812. doi: 10.1128/JVI.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YH, Chiba S, Tani A, Kondo H, Sasaki A, Kanematsu S, Suzuki N. 2012. A novel quadripartite dsRNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix. Virology 426:42–50. doi: 10.1016/j.virol.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Nuss DL. 2008. A host dicer is required for defective viral RNA production and recombinant virus vector RNA instability for a positive sense RNA virus. Proc Natl Acad Sci U S A 105:16749–16754. doi: 10.1073/pnas.0807225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Q, Choi GH, Nuss DL. 2009. A single Argonaute gene is required for induction of RNA silencing antiviral defense and promotes viral RNA recombination. Proc Natl Acad Sci U S A 106:17927–17932. doi: 10.1073/pnas.0907552106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Segers GC, Sun Q, Deng F, Nuss DL. 2008. Characterization of hypovirus-derived small RNAs generated in the chestnut blight fungus by an inducible DCL-2-dependent pathway. J Virol 82:2613–2619. doi: 10.1128/JVI.02324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. 2013. Effects of defective-interfering RNA on symptom induction by, and replication of, a novel partitivirus from a phytopathogenic fungus Rosellinia necatrix. J Virol 87:2330–2341. doi: 10.1128/JVI.02835-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. 2013. A novel victorivirus from a phytopathogenic fungus, Rosellinia necatrix, is infectious as particles and targeted by RNA silencing. J Virol 87:6727–6738. doi: 10.1128/JVI.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Himeno M, Maejima K, Komatsu K, Ozeki J, Hashimoto M, Kagiwada S, Yamaji Y, Namba S. 2010. Significantly low level of small RNA accumulation derived from an encapsidated mycovirus with dsRNA genome. Virology 396:69–75. doi: 10.1016/j.virol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Vainio EJ, Jurvansuu J, Streng J, Rajamäki ML, Hantula J, Valkonen JP. 2015. Diagnosis and discovery of fungal viruses using deep sequencing of small RNAs. J Gen Virol 96:714–725. doi: 10.1099/jgv.0.000003. [DOI] [PubMed] [Google Scholar]
- 37.Arakawa M, Nakamura H, Uetake Y, Matsumoto N. 2002. Presence and distribution of double-stranded RNA elements in the white root rot fungus Rosellinia necatrix. Mycoscience 43:21–26. doi: 10.1007/s102670200004. [DOI] [Google Scholar]
- 38.Ikeda K, Nakamura H, Matsumoto N. 2005. Comparison between Rosellinia necatrix isolates from soil and diseased roots in terms of hypovirulence. FEMS Microbiol Ecol 54:307–315. doi: 10.1016/j.femsec.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki A, Miyanishi M, Ozaki K, Onoue M, Yoshida K. 2005. Molecular characterization of a partitivirus from the plant pathogenic ascomycete Rosellinia necatrix. Arch Virol 150:1069–1083. doi: 10.1007/s00705-005-0494-0. [DOI] [PubMed] [Google Scholar]
- 40.Osaki H, Wei CZ, Arakawa M, Iwanami T, Nomura K, Matsumoto N, Ohtsu Y. 2002. Nucleotide sequences of double-stranded RNA segments from a hypovirulent strain of the white root rot fungus Rosellinia necatrix: possibility of the first member of the Reoviridae from fungus. Virus Genes 25:101–107. doi: 10.1023/A:1020182427439. [DOI] [PubMed] [Google Scholar]
- 41.Yaegashi H, Nakamura H, Sawahata T, Sasaki A, Iwanami Y, Ito T, Kanematsu S. 2013. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol Ecol 83:49–62. doi: 10.1111/j.1574-6941.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Liu S, Chiba S, Kondo H, Kanematsu S, Suzuki N. 2014. A novel single-stranded RNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix, with similarity to hypo-like viruses. Front Microbiol 5:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki A, Kanematsu S, Onoue M, Oyama Y, Yoshida K. 2006. Infection of Rosellinia necatrix with purified viral particles of a member of Partitiviridae (RnPV1-W8). Arch Virol 151:697–707. doi: 10.1007/s00705-005-0662-2. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki A, Kanematsu S, Onoue M, Oikawa Y, Nakamura H, Yoshida K. 2007. Artificial infection of Rosellinia necatrix with purified viral particles of a member of the genus mycoreovirus reveals its uneven distribution in single colonies. Phytopathology 97:278–286. doi: 10.1094/PHYTO-97-3-0278. [DOI] [PubMed] [Google Scholar]
- 45.Yaegashi H, Yoshikawa N, Ito T, Kanematsu S. 2013. A mycoreovirus suppresses RNA silencing in the white root rot fungus, Rosellinia necatrix. Virology 444:409–416. doi: 10.1016/j.virol.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Yaegashi H, Sawahata T, Ito T, Kanematsu S. 2011. A novel colony-print immunoassay reveals differential patterns of distribution and horizontal transmission of four unrelated mycoviruses in Rosellinia necatrix. Virology 409:280–289. doi: 10.1016/j.virol.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu T, Yaegashi H, Ito T, Kanematsu S. 2015. Systemic RNA interference is not triggered by locally-induced RNA interference in a plant pathogenic fungus, Rosellinia necatrix. Fungal Genet Biol 76:27–35. doi: 10.1016/j.fgb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Lu J, Han Y, Fan X, Ding SW. 2013. RNA interference functions as an antiviral immunity mechanism in mammals. Science 342:231–234. doi: 10.1126/science.1241911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. 2007. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12. doi: 10.1186/1746-4811-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pliego C, Kanematsu S, Ruano-Rosa D, de Vicente A, López-Herrera C, Cazorla FM, Ramos C. 2009. GFP sheds light on the infection process of avocado roots by Rosellinia necatrix. Fungal Genet Biol 46:137–145. doi: 10.1016/j.fgb.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Choudhary S, Lee HC, Maiti M, He Q, Cheng P, Liu Q, Liu Y. 2007. A double-stranded-RNA response program important for RNA interference efficiency. Mol Cell Biol 27:3995–4005. doi: 10.1128/MCB.00186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, Heissmeyer V, Einav S, Jackson W, Doukas T, Paranjape S, Polacek C, dos Santos FB, Jalili R, Babrzadeh F, Gharizadeh B, Grimm D, Kay M, Koike S, Sarnow P, Ronaghi M, Ding SW, Harris E, Chow M, Diamond MS, Kirkegaard K, Glenn JS, Fire AZ. 2010. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog 6:e1000764. doi: 10.1371/journal.ppat.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, Chen S, Hannon GJ, Qi Y. 2008. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133:116–127. doi: 10.1016/j.cell.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montgomery TA, Howell MD, Cuperus JT, Li D, Hansen JE, Alexander AL, Chapman EJ, Fahlgren N, Allen E, Carrington JC. 2008. Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133:128–141. doi: 10.1016/j.cell.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 55.Takeda A, Iwasaki S, Watanabe T, Utsumi M, Watanabe Y. 2008. The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol 49:493–500. doi: 10.1093/pcp/pcn043. [DOI] [PubMed] [Google Scholar]
- 56.Lee HC, Li L, Gu W, Xue Z, Crosthwaite SK, Pertsemlidis A, Lewis ZA, Freitag M, Selker EU, Mello CC, Liu Y. 2010. Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol Cell 38:803–814. doi: 10.1016/j.molcel.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiti M, Lee HC, Liu Y. 2007. QIP, a putative exonuclease, interacts with the Neurospora Argonaute protein and facilitates conversion of duplex siRNA into single strands. Genes Dev 21:590–600. doi: 10.1101/gad.1497607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond TM, Andrewski MD, Roossinck MJ, Keller NP. 2008. Aspergillus mycoviruses are targets and suppressors of RNA silencing. Eukaryot Cell 7:350–357. doi: 10.1128/EC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nibert ML, Kim J. 2004. Conserved sequence motifs for nucleoside triphosphate binding unique to turreted reoviridae members and coltiviruses. J Virol 78:5528–5530. doi: 10.1128/JVI.78.10.5528-5530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J, Parker JS, Murray KE, Nibert ML. 2004. Nucleoside and RNA triphosphatase activities of orthoreovirus transcriptase cofactor mu2. J Biol Chem 279:4394–4403. [DOI] [PubMed] [Google Scholar]
- 61.Wei CZ, Osaki H, Iwanami T, Matsumoto N, Ohtsu Y. 2004. Complete nucleotide sequences of genome segments 1 and 3 of Rosellinia anti-rot virus in the family Reoviridae. Arch Virol 149:773–777. doi: 10.1007/s00705-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 62.Qi X, Bao FS, Xie Z. 2009. Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS One 4:e4971. doi: 10.1371/journal.pone.0004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llave C. 2010. Virus-derived small interfering RNAs at the core of plant-virus interactions. Trends Plant Sci 15:701–707. doi: 10.1016/j.tplants.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Zhang DX, Spiering MJ, Nuss DL. 2014. Characterizing the roles of Cryphonectria parasitica RNA-dependent RNA polymerase-like genes in antiviral defense, viral recombination and transposon transcript accumulation. PLoS One 9:e108653. doi: 10.1371/journal.pone.0108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. 2004. A protein sensor for siRNA asymmetry. Science 306:1377–1380. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 66.Shimura H, Pantaleo V, Ishihara T, Myojo N, Inaba J, Sueda K, Burgyán J, Masuta C. 2011. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog 7:e1002021. doi: 10.1371/journal.ppat.1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith NA, Eamens AL, Wang M-B. 2011. Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog 7:e1002022. doi: 10.1371/journal.ppat.1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo X, Li WX, Lu R. 2012. Silencing of host genes directed by virus-derived short interfering RNAs in Caenorhabditis elegans. J Virol 86:11645–11653. doi: 10.1128/JVI.01501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]