ABSTRACT
African green monkeys (AGMs) are natural hosts of simian immunodeficiency virus (SIVAGM). Because these animals do not develop simian AIDS despite maintaining high viral loads, there is considerable interest in determining how these animals have evolved to avoid SIV disease progression. Unlike nonnatural hosts of SIV, adult AGMs maintain low levels of CD4+ T cells at steady states and also have a large population of virus-resistant CD8αα T cells that lack CD4 expression despite maintaining T helper cell functionalities. In recent work, we have shown that homeostatic cytokines can induce CD4 downregulation in AGM T cells in vitro. Through administering therapeutic doses of recombinant human interleukin-2 (IL-2) to AGMs, we show here that this mechanism is operative in vivo. IL-2 therapy induced transient yet robust proliferation in all major T cell subsets. Within the CD4+ T cell population, those that were induced into cycle by IL-2 exhibited characteristics of CD4-to-CD8αα conversion. In all animals receiving IL-2, circulating CD4+ T cell counts and proportions tended to be lower and CD4− CD8αα+ T cell counts tended to be higher. Despite reductions in circulating target cells, the viral load was unaffected over the course of study.
IMPORTANCE The data in this study identify that homeostatic cytokines can downregulate CD4 in vivo and, when given therapeutically, can induce AGMs to sustain very low levels of circulating CD4+ T cells without showing signs of immunodeficiency.
INTRODUCTION
African green monkeys (AGMs) are natural hosts of simian immunodeficiency virus (SIVagm) that progress to AIDS very rarely despite maintaining high viral loads in plasma (1). Yet, SIVagm is pathogenic in pigtail macaques when they are experimentally infected, implying a coevolution of SIVagm with its natural host (2, 3). Adult AGMs have low numbers of CD4 T cells and a large population of memory T cells that lack CD4 expression and express CD8α homodimers (4, 5). These CD8αα T cells are uniquely different from classical CD8αβ T cells in that they retain characteristics of CD4 T cells, such as major histocompatibility complex class II (MHC-II) restriction, expression of Foxp3, and production of interleukin-2 (IL-2) and IL-17 (4, 6, 7). We have previously shown that these cells arise from downregulation of CD4 by canonical CD4 T cells (8). Importantly, downregulation of CD4 protects these cells from infection by SIVagm in vivo (4, 9). The maintenance of immunological function by cells that are resistant to infection is thought to, in part, underlie the nonprogressive nature of SIVagm infection in AGM.
The exact mechanisms of CD4-to-CD8α conversion in AGMs are not entirely understood, although cellular division is likely important for this process. It is clear that cellular division through antigenic stimuli can induce CD4 downregulation in vitro and infection of AGMs with SIV can accelerate CD4-to-CD8α conversion in vivo (8). Yet, the conversion of CD4 to CD4− CD8α+ T cells during infection is not entirely limited to those that are SIV specific, which make up less than 1% of the total T cell pool (4). Moreover, a particular AGM who lacks CD4 T cells can mount an MHC-II-restricted neoantigenic response within the CD4− CD8αα+ T cell pool, implying that CD4 downregulation can occur through antigen-independent mechanisms in vivo (8). Homeostatic T cell division may also contribute to CD4 downregulation, and we have previously observed that AGM T cells downregulate CD4 when stimulated in vitro with the common gamma chain cytokines IL-2, IL-7, and IL-15 (8). It is unknown whether this same process occurs in vivo or whether CD4 downregulation can be accelerated with therapeutic intervention.
The homeostatic cytokine IL-2 is an autocrine cell growth factor that binds the high-affinity IL-2 receptor, CD25, to promote T cell proliferation, differentiation, and survival (10). T regulatory (Treg) cells that express high levels of CD25 are particularly responsive to this cytokine, and they depend on IL-2 for their homeostasis (11, 12). Recombinant IL-2 has been given therapeutically in large clinical trials to human immunodeficiency virus (HIV)-infected patients on antiretroviral therapy to boost reconstitution of CD4 T cells (13). Yet despite a significant and sustained increase in CD4 T cell counts, no clinical benefit was observed compared to patients treated with antiretroviral therapy alone (13). IL-2 therapy has also been used in untreated SIV-infected rhesus macaques, yet here too, administration did not improve prognoses (14).
Given these previous studies using IL-2 to expand CD4 T cells in HIV/SIV-infected individuals coupled with our previous studies demonstrating that IL-2 can cause proliferation and downregulation of CD4 by CD4 T cells in AGM T cells in vitro, we hypothesized that IL-2 administration could lead to cellular proliferation and downregulation of CD4 in AGMs in vivo. This, we hypothesized, might lead to decreased target cells for SIV and corresponding decreases in plasma viremia. Thus, we treated 4 AGMs with 8 rounds of recombinant human IL-2 and asked whether administration could drive these animals to CD4 downregulation with concomitant reduction in viremia. We find that proportions and absolute numbers of circulating conventional CD4 T cells tended to be lower in all 4 animals and absolute numbers of circulating conventional CD8αα T cells that lacked CD4 expression tended to be higher. Interestingly, viremia remained relatively unchanged over the course of administration. Thus, a very limited number of CD4 T cells may be sufficient to support ongoing viral replication in AGMs, similar to what is seen in sooty mangabeys (15).
MATERIALS AND METHODS
Ethics.
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Office of Animal Welfare, and the U.S. Department of Agriculture (16). All animal work was approved by the NIAID Division of Intramural Research Animal Care and Use Committees (IACUC) in Bethesda, MD (protocols LMM-12 and LMM-6). The animal facility is accredited by the American Association for Accreditation of Laboratory Animal Care. All procedures were carried out under ketamine anesthesia by trained personnel under the supervision of veterinary staff, and all efforts were made to maximize animal welfare and to minimize animal suffering in accordance with the recommendations of the Weatherall report on the use of nonhuman primates (17). Animals were housed in adjoining individual primate cages, allowing social interactions, under controlled conditions of humidity, temperature, and light (12-h light/12-h dark cycles). Food and water were available ad libitum. Animals were monitored twice daily and fed commercial monkey chow, treats, and fruit twice daily by trained personnel.
Animals.
We housed a total of 4 vervet African green monkeys (Chlorocebus pygerythrus) (AGMs). The AGMs included 3 SIV+ adults that were infected intravenously with the SIVagm90 strain. A 4th AGM was born to SIV+ parents yet maintained undetectable viral loads at the time of study. All relevant animal information is summarized in Table 1.
TABLE 1.
Characteristics of study animalsa
| Animal ID | DOB | Date inoculated | Inoculation route | SIV strain |
|---|---|---|---|---|
| AG7 | 12/3/01 | 11/19/06 | IV | SIVagm90 |
| AG13 | 1/14/02 | 11/19/06 | IV | SIVagm90 |
| AG26 | 9/25/06 | Born to SIV+ parents | NA | NA |
| 302 | 2/27/01 | 4/22/07 | IV | SIVagm90 |
DOB, date of birth. NA, not applicable. Dates are in the month/day/year format.
Interleukin-2 administration.
Recombinant human interleukin-2 was purchased under the commercial name Proleukin (Prometheus Laboratories, San Diego, CA) at the NIH Clinical Center Pharmacy. Proleukin vials were reconstituted in sterile water, and animals were injected subcutaneously twice daily for 5 days at 600,000 units per kilogram of body weight. The IL-2 dosage was previously estimated from a maximum nontoxic dose of 300,000 to 600,000 U/kg body weight defined for humans (18). Previous reports have shown that these concentrations are well-tolerated in rhesus macaques (19). Proleukin administration was performed for a total of 8 rounds with 56 days between rounds.
Absolute cell counts.
Absolute cell counts were calculated from flow cytometry frequencies and complete blood count (CBC) absolute lymphocyte counts at days 7, 14, 21, 30, and 53 after the start of each round of IL-2 administration.
Flow cytometry.
Cellular frequency and activation status were determined through ex vivo staining of isolated peripheral blood mononuclear cells (PBMCs). Cells were washed once with cold phosphate-buffered saline (PBS) and incubated with the Live/Dead fixable Aqua dead cell stain (Invitrogen, Carlsbad, CA) for 5 min at room temperature. Cells then were stained with the fluorescently conjugated monoclonal antibody to CCR7 (clone 3D12, conjugated to Cy7PE; BD Biosciences, Carlsbad, CA) and incubated for 15 min at 37°C, after which antibodies to CD3 (clone SP34-2, conjugated to Alexa Fluor 700; BD Biosciences), CD4 (clone L200, conjugated to Percp Cy5.5; BD Biosciences), CD8 (clone RPA-T8, conjugated to Pacific Blue), CD28 (clone 28.2, conjugated to energy-coupled dye [ECD]; Beckman Coulter, Brea, CA), CD95 (clone DX2, conjugated to Cy5-phycoerythrin [Cy5-PE]; BD Biosciences), HLA-DR (clone L243, conjugated to H7-allophycocyanin [H7-APC]; BD Biosciences), CD25 (clone 2A3, conjugated to brilliant violet 605; BD Biosciences), and α4β7 (conjugated to APC; NIH AIDS Reagent Program) were added and incubated for an additional 30 min at 4°C. Cells were washed once with cold PBS, fixed for 1 h with 1× fixation/permeabilization solution (eBioscience, San Diego, CA) at 4°C, and subsequently permeabilized with 1× permeabilization buffer (eBioscience). For intracellular staining, cells were incubated at 4°C for 30 min with monoclonal antibodies directed against ki67 (clone B56, conjugated to fluorescein isothiocyanate [FITC]; BD Biosciences) and Foxp3 (clone 236A, conjugated to PE; eBioscience). After intracellular staining, cells were washed in 1× permeabilization buffer (eBioscience) and fixed in 1% paraformaldehyde solution (Electron Microscopy Sciences, Hatfield, PA). Flow cytometric acquisition was performed on a BD Fortessa (BD Biosciences) flow cytometer, and data were analyzed with Flowjo software (v9.8.3; Tree Star Inc., Ashland, OR).
Quantitation of plasma viral RNA levels.
Viral RNA levels in plasma were determined by real-time reverse transcription-PCR (ABI Prism 7700 sequence detection system; Applied Biosystems, Foster City, CA) as previously reported, using reverse-transcribed viral RNA in plasma samples from SIVagm-inoculated African green monkeys (20).
Statistics.
Given the low number of samples used in this study, we used the paired Student t test to compare means among different time points. This statistical test has been used in other settings with a similar number of animals and in most cases results in type I error rates close to the 5% nominal value (21, 22).
RESULTS
Robust responses to recombinant human IL-2 in AGMs.
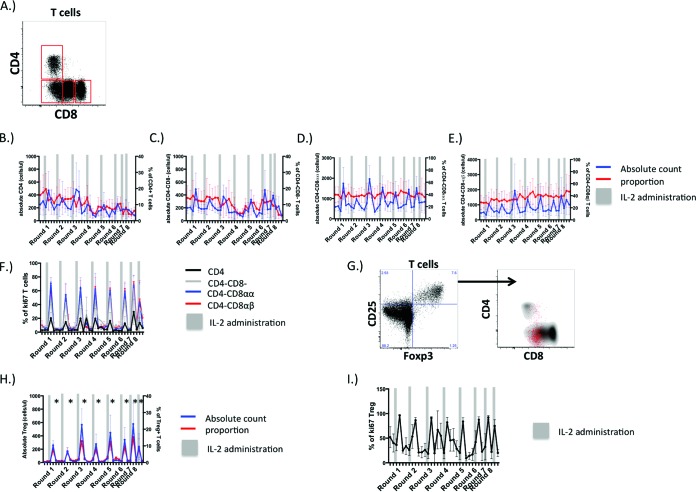
Unlike what occurs in Asian macaques, humans, or mice, 4 distinct T cell populations can be defined by CD4 and CD8 expression in AGMs (4). While CD4+ T cells are present at low frequencies in adult AGMs (4), the majority of T cells lack CD4 surface expression and either do not express CD8, are CD8αdim T cells that have downregulated CD4 expression postthymically, or are classical CD8+ T cells that express the CD8 α and β chains (Fig. 1A). We have previously shown in AGM T cells that cytokines that induce homeostatic T cell proliferation, such as IL-2, IL-7, and IL-15, induce CD4 downregulation in vitro (8). To determine if the same mechanism is operative in vivo, we administered recombinant human IL-2 to 4 adult AGMs. A total of eight rounds of IL-2 were given at 56-day intervals, and the frequency, absolute numbers, and phenotypes of circulating T cell populations in AGMs were determined at various time points relative to IL-2 administration. In each round of administration, IL-2 therapy induced transient increases in absolute numbers of circulating CD4+, CD4− CD8−, CD8αα+, and CD8αβ+ T cells, which were most apparent 7 days after the start of IL-2 administration and normalized after 56 days posttreatment (Fig. 1B to E). Changes in the proportions of these T cell populations in AGMs were less dramatic (Fig. 1B to E). Expression of the cell cycle protein ki67 was found to parallel periods of peak T cell expansion induced by IL-2, as ki67 was expressed in some CD4 T cells and much greater proportions of distinct T cell populations that lacked CD4 expression (Fig. 1F). These data indicate that IL-2 may expand T cells that lack CD4 expression in AGMs.
FIG 1.
Recombinant human IL-2 induces robust, well-tolerated, proliferative responses in AGM T cells in vivo. (A) Representative expression of CD4 and CD8 by adult AGM T cells. (B to F) Absolute numbers (blue lines) and proportions (red lines) of CD4+ T cells (B), CD4− CD8− T cells (C), CD4− CD8αα+ T cells (D), CD4− CD8αβ+ T cells (E) over 8 rounds of IL-2 administration. (F) Proportions of CD4+, CD4− CD8−, CD4− CD8αα+, and CD4− CD8αβ+ T cells that are cycling over 8 rounds of IL-2 administration. (G and H) CD4 and CD8 surface expression on circulating AGM “T regulatory-like” cells (CD25+ Foxp3+) (G) with their associated proportions and absolute numbers (H). (I) Frequencies of CD25+ Foxp3+ cells that are ki67+; significant expansions of Treg-like cells are denoted by an asterisk (*).
We next assessed the dynamics of T regulatory cells in response to IL-2 therapy, as these cells can respond preferentially to IL-2 compared to other T cell subsets. While expression of Foxp3 can be a general consequence of T cell activation and Foxp3 expression does not always confer regulatory T cell function (23), we do find that a fraction of circulating Treg-like T cells are present in AGMs that coexpress Foxp3 and CD25. Unlike in other species, the majority of Foxp3+ CD25+ T cells in AGMs do not express CD4 and express low levels of the CD8αα homodimer (Fig. 1G). Absolute numbers of circulating CD25+ Foxp3+ T cells expanded acutely with each round after the start of IL-2 therapy yet normalized thereafter, and this was apparent in CD25+ Foxp3+ T cell proportions as well (Fig. 1H). Additionally, IL-2 administration induced ki67 expression in nearly all circulating CD25+ Foxp3+ T cells at the peak of T cell expansion (Fig. 1I). Taken together, these data suggest that AGMs do not mount a humoral immune response to the recombinant human IL-2 that might hinder its effectiveness.
Cycling in AGM CD4+ T cells promotes transition to a CD4− CD8αα+ memory phenotype.
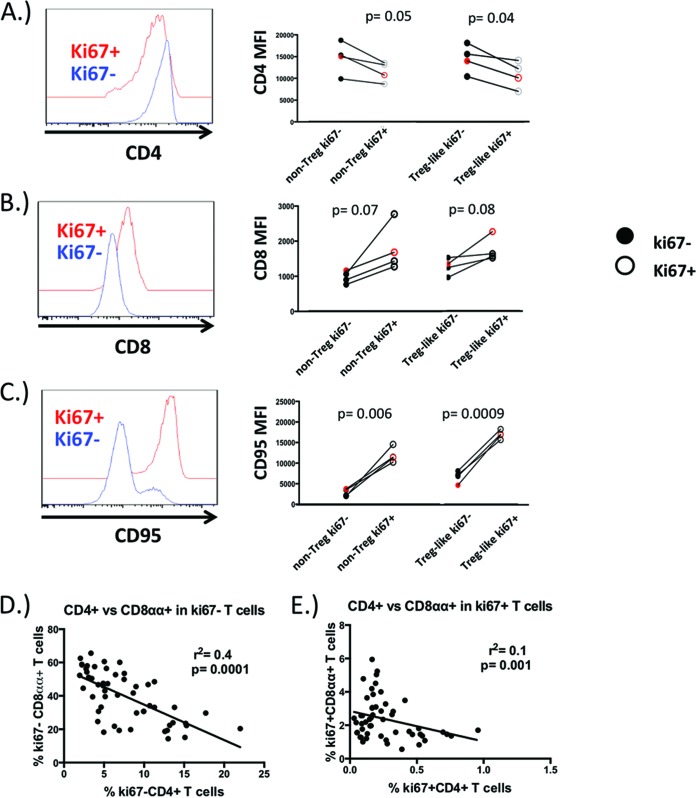
In previous work, we had found that a large proportion of divided CD4+ T cells downregulated CD4 expression in response to IL-2 stimulation in vitro and that CD4 surface densities were lower for each generation of divided cells (8). As this suggests that cellular division is likely important for CD4 downregulation, we reasoned that those CD4+ T cells found to be in cycle immediately after IL-2 administration in AGMs would exhibit some characteristics of memory CD4− CD8αα+ T cells. We compared CD4 expression in cycling and noncycling CD4+ T cells at 7 days after the first round of IL-2 administration and found that surface densities were significantly lower on both T regulatory and non-T-regulatory subsets that were found to be in cycle (Fig. 2A). Surface densities of CD8 on cycling CD4+ T cells tended to be higher in both Treg-like and non-Treg subsets, yet these did not reach statistical significance (Fig. 2B). Cycling CD4+ T cells acquired some characteristics of a memory phenotype and CD95 was dramatically upregulated in cycling Treg and non-Treg populations (Fig. 2C). These results suggest that in AGMs, homeostatic signals can induce a memory phenotype on CD4+ T cells and that cell cycle entry may be important for this process.
FIG 2.
Evidence of CD4-to-CD8αα conversion in cycling CD4+ T cells. Surface densities of CD4 (A), CD8α (B), and the memory marker CD95 (C) were assessed in CD25− Foxp3- and CD25+ Foxp3+ CD4+ T cells that were induced into cell cycle by IL-2 and compared to those CD4+ T cells not in cycle. Black circles correspond to SIV+ AGMs, and the red circle denotes the SIV- AGM used in this study. Correlation of circulating CD4+ T cells and CD4− CD8αα+ T cells that are not cycling (D) or are in cell cycle (E) in 4 adult AGMs over the course of IL-2 therapy. Surface densities of CD4, CD8, and CD95 were compared by the Student t test. A Spearman rank correlation was calculated for panels D and E.
Because IL-2 appeared to drive cycling CD4 T cells to become more “CD8α-like,” we reasoned that the disappearance of CD4+ T cells would be related to increases in the CD4− CD8αα+ T cell compartment. Throughout the course of the study, we found a significant negative correlation between noncycling CD4+ T cells and CD8αα+ T cells, yet this relationship was less apparent in CD4+ T cells and CD8αα+ T cells that were found to be in cycle (Fig. 2D). Thus, CD4-to-CD8α conversion may be a transitory process that is driven by cycling, yet once CD4 T cells divide, they stably downregulate CD4 expression and upregulate CD8α. These data are in accordance with previous work demonstrating a lack of CD4 gene transcripts in mature CD4− CD8αα+ T cells (4).
Intermittent IL-2 administration tends to reduce CD4 T cell numbers in AGMs.
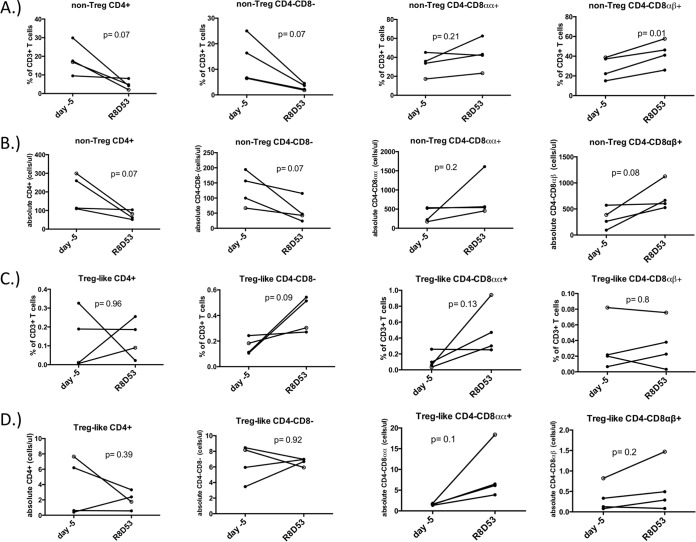
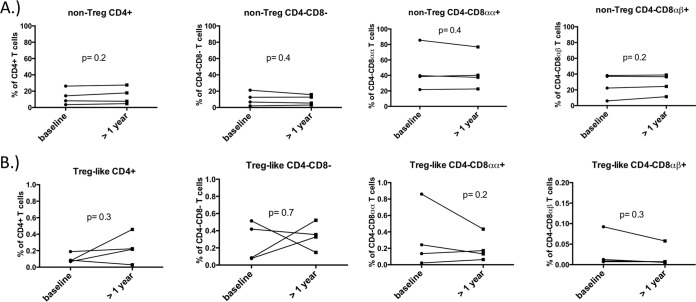
In many species, CD4+ T cells that divide through homeostatic stimuli such as IL-2 largely retain their phenotype. AGMs are unique in that homeostatic cytokine-induced proliferation in vitro induces CD4+ T cells to acquire a phenotype resembling that of memory CD8αα T cells that lack CD4 expression. Thus, while IL-2 therapy durably increases CD4+ T cell counts in many species, we hypothesized that AGMs would exhibit CD4+ T cell losses and concomitant rises in CD8αα+ T cell numbers over the course of treatment. After 8 rounds of IL-2, frequencies of CD4+ T cell subsets that lacked CD25 and Foxp3 expression tended to be lower whereas classical CD25− Foxp3− CD8αβ T cells were significantly higher (Fig. 3A). When we assessed the absolute numbers of these same CD25− Foxp3− T cell populations after 8 rounds of IL-2 administration, circulating CD25− Foxp3− CD4+ T cell numbers were lower and numbers of CD25− Foxp3−, CD4− CD8αα+, and CD4− CD8αβ+ T cells increased, yet these changes did not reach statistical significance (Fig. 3B). When assessing frequencies and absolute numbers of circulating Treg-like cells in AGMs, only absolute numbers of CD25+ Foxp3+ CD4− CD8αα+ T cells tended to be higher after 8 rounds of IL-2 (Fig. 3C and D). Taken together, all animals receiving IL-2 exhibited similar trends of decreasing CD4+ T cell counts and proportions. Concomitant increases in CD4− CD8αα+ T cell counts were observed as well. In contrast, proportions of circulating Foxp3− CD25− and Treg-like Foxp3+ CD25+ CD4+, CD4− CD8−, CD4− CD8αα+, and CD4− CD8αβ+ T cells did not change over a similar time course in a cohort of AGMs not receiving IL-2 (Fig. 4A and B). Given that only 4 animals were administered IL-2 in this study, it is striking that these differences in the IL-2-treated AGMs approached statistical significance.
FIG 3.
IL-2 therapy tends to decrease the size of the CD4+ T cell pool in AGMs. Proportions (A) and absolute numbers (B) of circulating CD25− Foxp3- T cells that were CD4+, CD4− CD8αα+, or CD4− CD8αβ+ were compared just prior to the start of IL-2 therapy (day −5) to 53 days after the 8th round of IL-2 administration in 4 adult AGMs. Proportions and absolute numbers of CD25+ Foxp3+ T cells that were CD4+, CD4− CD8αα+, or CD4− CD8αβ+ were compared in a similar fashion in these animals. Closed circles represent SIV+ AGMs. The open circle represents the one SIV− AGM used in this study. A Student t test was used for all comparisons.
FIG 4.
Circulating proportions of CD4 and CD8 T cells are stable in AGMs not treated with IL-2. Frequencies of circulating nonregulatory (A) or regulatory (B) T cells based on CD4 and CD8 expression were compared in a group of AGMs that did not receive IL-2 over a time period of >1 year. All animals in this cohort were SIV positive. Comparisons were made by paired Student's t test.
Recombinant IL-2 therapy does not reduce viremia in AGMs.
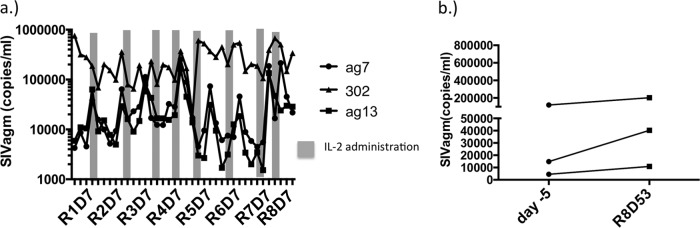
In nonnatural hosts of SIV such as rhesus macaques as well as HIV+ humans that are not receiving antiretroviral therapy (20), single or intermittent rounds of IL-2 do not affect the SIV or HIV load (14, 24). Because homeostatic proliferation induces CD4 T cells to downregulate CD4 in AGMs and become resistant to SIV infection, we asked whether the loss of target cells induced by IL-2 might reduce viremia in these animals. Prior to treatment, 3 of the 4 animals displayed persistent viremia whereas the 4th animal was SIV uninfected despite being born to SIV+ parents. IL-2 therapy induced transient spikes of viremia that were observed in all 3 of the viremic animals (Fig. 5A); however, no diminishing trends in viral loads were seen after the completion of 8 rounds of therapy (Fig. 5B). These data indicate that viremia persists in animals that are incompletely depleted of CD4 T cells and that viral replication can be sustained in a relatively low number of target cells.
FIG 5.
IL-2 therapy does not alter viremia in SIV+ adult AGMs. (a) Plasma viral load over the course of IL-2 therapy in SIV+ AGMs. (b) Comparison of viral loads just prior to the start of therapy (day −5) and 53 days after the 8th round of IL-2 administration in SIV+ AGMs.
DISCUSSION
In previous work, we established that homeostatic signals can downregulate CD4 expression on AGM T cells in vitro and may be important in driving the generation of the CD8αα T cell pool, a population that displays functional similarity to CD4 T cells but is resistant to SIV infection (8). Here, we sought to determine whether treatment of AGMs with therapeutic doses of the homeostatic cytokine IL-2 could accelerate CD4− CD8αα+ T cell conversion and resultantly lower viremia. Although host-derived immune responses against recombinant human IL-2 were not specifically examined in this study, the treatment was generally well tolerated and it is unlikely that strong anti-IL-2 antibodies developed given the continued bioactivity of IL-2 after multiple administrations in vivo. We find that recombinant human IL-2 was well tolerated, with no apparent immune response directed against the cytokine despite being administered to AGMs. IL-2 induced a transient yet robust proliferation in all major AGM T cell populations, and within the CD4 T cell population, those that were induced into cycle by IL-2 displayed characteristics of CD4-to-CD8α T cell conversion. After 8 rounds of administration, exogenous IL-2 tended to reduce the proportions and absolute numbers of circulating CD4 T cells and concomitantly increase the absolute numbers of CD4− CD8αα+ T cells, whereas these proportions remained constant in a group of AGMs not receiving IL-2. Changes in T cell proportions, however, did not appear to influence viral replication. These data demonstrate that the CD4− CD8αα+ T cell pool can be generated in vivo through homeostatic signals and that relatively few numbers of target cells can sustain viral replication.
It is not entirely surprising that AGMs could be driven toward CD4 depletion experimentally, as this has occurred under natural circumstances in some animals of the Cercopithecini tribe (which includes all species of AGMs and the closely related patas monkeys). One AGM, AG346, has sustained very low numbers of circulating CD4 T cells for several years at steady state and has concomitantly become aviremic, suggesting that a lack of target cells may prevent sustained viral replication in this animal (7). Additionally, a similar report of a patas monkey that maintains very low numbers of CD4 T cells has been described (25). In both of these cases, nearly complete CD4 T cell depletion did not result in immunodeficiency. Similarly, immunodeficiency was not observed in the 4 AGMs treated with IL-2 despite circulating T cell counts that would be AIDS defining in nonnatural hosts. Although IL-2 tended to decrease CD4 T cells over the course of therapy in these animals, there did not appear to be the homeostatic proliferation and increase in numbers of residual CD4 T cells that occurs with rhesus macaques who are depleted of CD4 T cells experimentally (26). Thus, it appears that AGMs can maintain negligible numbers of CD4 T cells, and this can represent a steady state for these animals.
Other primate species who are natural hosts of SIV exhibit similar characteristics of severe CD4+ T cell depletion without showing any clinical signs of AIDS. When infected with SIV, a subset of sooty mangabeys develop a large population of virus-resistant CD4− CD8− double negative T cells that maintain many functional qualities of CD4+ T cells (27, 28). The precursors of this double negative population or the signals responsible for their development are not known. However, it is worth noting that MHC-II-restricted immune responses against neoantigens, such as those of influenza virus, can develop within the CD4− CD8− T cell pool, which bear striking similarity to a CD4 T cell helper response (27), suggesting that these T cells can develop independent of antigen exposure, just as the CD4− CD8αα+ T cell pool can develop in AGMs.
In either species of natural hosts, the uncoupling of adaptive immunity from T cell populations that support viral replication may be important in avoiding disease progression. Other mechanisms unique to AGMs that may contribute to disease nonprogression have also been described, including a robust but transient type I interferon response and non-CCR5 entry pathways of SIVagm (29, 30). Our findings that homeostatic signals can drive CD4 downregulation give key insights on how AGMs can preserve these essential immune functions while still maintaining a diverse T cell repertoire, as one would expect a skewed representation with some T cell clones more represented than others if the CD4− CD8αα+ T cell pool were driven solely by antigen.
It is worth noting that in our study increases in proportions or absolute numbers of CD4− CD8αα+ T cells were not always similar in magnitude compared to the loss of CD4+ T cells in some of the animals receiving IL-2. It is possible that expansion of CD4− CD8αα+ T cells may have occurred at other anatomical sites such as the gastrointestinal (GI) tract, where CD4− CD8αα+ T cells represent the vast majority of the T cell pool in AGMs (4). Another possibility is that because CD4− CD8αα+ T cells vastly outnumber the CD4+ T cell pool in AGMs, CD4− CD8αα+ T cell conversion induced by IL-2 may make statistically insignificant impacts on the size of the existing CD4− CD8αα+ T cell pool. Nevertheless, we do find evidence of a “CD8α-like” memory phenotype in cycling CD4+ T cells, and once divided, CD4− CD8αα+ T cell proportions correlate inversely with CD4 T cells, suggesting that some of the CD4 T cells induced into cycle by IL-2 may go on to become CD4− CD8αα+ memory T cells.
Interestingly, while IL-2 therapy induced circulating numbers of target cells that were CD4+ to decrease in AGMs, we did not observe any significant changes in viremia in these animals. These findings might contrast to some instances stated earlier whereby parallel decreases in CD4 T cells and viral loads have been observed in some particular animals who are natural hosts of SIV (8). Although we did not assess SIVagm replication at tissue sites, it remains a possibility that IL-2 administration could affect viral loads locally but not systemically. It is worth noting, however, that there are instances where viral load is not always coupled to the quantity of total CD4+ T cells. For example, memory CD4+ T cells represent the main targets of viral replication in both juvenile and adult AGMs, yet juvenile AGMs maintain viral loads similar to those of adults despite being immunologically inexperienced (4, 8). The same is true of SIV+ rhesus macaques who are experimentally depleted of CD4 T cells yet maintain viral loads comparable to those of SIV+ rhesus macaques with ample numbers of CD4+ T cells (26). In humans, recombinant IL-7 therapy induces cell division predominantly in CD4+ memory T cells (31). Because most viral replication in AGMs occurs in CD4+ memory T cells, IL-7 may affect the dynamics of viral replication differently from IL-2, which preferentially induces T regulatory cells to divide (12).
One limitation of this study is that only a limited number of AGMs were treated with IL-2. Thus, while reductions in circulating CD4 T cells induced by IL-2 approached statistical significance in these 4 animals, this study is underpowered and we cannot statistically conclude that the response to IL-2 is representative of all AGMs. However, based on previous in vitro studies of IL-2-induced CD4 downregulation and the broad T cell receptor (TCR) diversity that exists in the CD4− CD8αα+ T cell pool, we can be reasonably certain that larger studies would recapitulate these findings.
We also cannot conclude in this study that CD25+ Foxp3+ T cells induced by IL-2 therapy are true T-regulatory cells. In HIV-infected humans, IL-2 induced CD25+ Foxp3+ CD4 T cell expansion, yet these exerted only weak suppressive capability (32, 33). It is worth noting that peaks in Treg-like expansion did not correspond with reduced effector function of conventional T cell subsets in our study (data not shown), arguing that the suppressive function of CD25+ Foxp3+ T cells induced by IL-2 is limited.
In four AGMs, we have shown that the homeostatic cytokine IL-2 can induce CD4 downregulation in vivo and lower CD4+ T cell counts. Regardless of whether CD4 downregulation is induced by homeostatic or antigenic signals, there are likely commonalities in the mechanism that drives this phenomenon in AGMs. A better understanding of this mechanism may pave the way for potential therapies in HIV+ humans that could uncouple adaptive immune processes to viral replication.
ACKNOWLEDGMENTS
The content of this publication does not necessarily reflect the views or policies of DHHS, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding Statement
Funding for this study was provided in part by the Division of Intramural Research/NIAID/NIH.
REFERENCES
- 1.VandeWoude S, Apetrei C. 2006. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin Microbiol Rev 19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, Hirsch VM. 2005. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J Virol 79:5153–5162. doi: 10.1128/JVI.79.8.5153-5162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol 69:955–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med 15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol 179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murayama Y, Amano A, Mukai R, Shibata H, Matsunaga S, Takahashi H, Yoshikawa Y, Hayami M, Noguchi A. 1997. CD4 and CD8 expressions in African green monkey helper T lymphocytes: implication for resistance to SIV infection. Int Immunol 9:843–851. doi: 10.1093/intimm/9.6.843. [DOI] [PubMed] [Google Scholar]
- 7.Murayama Y, Mukai R, Inoue-Murayama M, Yoshikawa Y. 1999. An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm. Clin Exp Immunol 117:504–512. doi: 10.1046/j.1365-2249.1999.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins MR, Briant JA, Calantone N, Whitted S, Vinton CL, Klatt NR, Ourmanov I, Ortiz AM, Hirsch VM, Brenchley JM. 2014. Homeostatic cytokines induce CD4 downregulation in African green monkeys independently of antigen exposure to generate simian immunodeficiency virus-resistant CD8alphaalpha T cells. J Virol 88:10714–10724. doi: 10.1128/JVI.01331-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz JE, Ma ZM, Hagan EA, Wilks AB, Furr KL, Linde CH, Zahn RC, Brenchley JM, Miller CJ, Permar SR. 2012. Memory CD4(+) T lymphocytes in the gastrointestinal tract are a major source of cell-associated simian immunodeficiency virus in chronic nonpathogenic infection of African green monkeys. J Virol 86:11380–11385. doi: 10.1128/JVI.01556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayer AL, Pugliese A, Malek TR. 2013. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunol Res 57:197–209. doi: 10.1007/s12026-013-8452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadzadeh M, Rosenberg SA. 2006. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.INSIGHT-ESPRIT Study , SILCAAT Scientific Committee, Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. 2009. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garibal J, Laforge M, Silvestre R, Mouhamad S, Campillo-Gimenez L, Levy Y, Estaquier J. 2012. IL-2 immunotherapy in chronically SIV-infected Rhesus macaques. Virol J 9:220. doi: 10.1186/1743-422X-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, Mayne A, Dunham RM, Lawson B, Ratcliffe SJ, Sodora DL, Else J, Reimann K, Staprans SI, Haase AT, Estes JD, Silvestri G, Ansari AA. 2008. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest 118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Council NR. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. doi: 10.17226/12910. [DOI] [PubMed] [Google Scholar]
- 17.Weatherall D. 2006. The use of non-human primates in research. [Google Scholar]
- 18.Jacobson EL, Pilaro F, Smith KA. 1996. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci U S A 93:10405–10410. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacsa J, Edghill-Smith Y, Tsai WP, Venzon D, Tryniszewska E, Hryniewicz A, Moniuszko M, Kinter A, Smith KA, Franchini G. 2005. Contrasting effects of low-dose IL-2 on vaccine-boosted simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cells in macaques chronically infected with SIVmac251. J Immunol 174:1913–1921. doi: 10.4049/jimmunol.174.4.1913. [DOI] [PubMed] [Google Scholar]
- 20.Endo Y, Igarashi T, Nishimura Y, Buckler C, Buckler-White A, Plishka R, Dimitrov DS, Martin MA. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J Virol 74:6935–6945. doi: 10.1128/JVI.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassena L, Miao H, Cimbro R, Malnati MS, Cassina G, Proschan MA, Hirsch VM, Lafont BA, Morre M, Fauci AS, Lusso P. 2012. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS Pathog 8:e1002636. doi: 10.1371/journal.ppat.1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Winter JCF. 2013. Using the Student's t-test with extremely small sample sizes. Practical Assess Res Eval 18(10). http://pareonline.net/getvn.asp?v=18&n=10. [Google Scholar]
- 23.Tran DQ, Ramsey H, Shevach EM. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy Y, Thiebaut R, Gougeon ML, Molina JM, Weiss L, Girard PM, Venet A, Morlat P, Poirier B, Lascaux AS, Boucherie C, Sereni D, Rouzioux C, Viard JP, Lane C, Delfraissy JF, Sereti I, Chene G, ILIADE Study Group. 2012. Effect of intermittent interleukin-2 therapy on CD4+ T-cell counts following antiretroviral cessation in patients with HIV. AIDS 26:711–720. doi: 10.1097/QAD.0b013e3283519214. [DOI] [PubMed] [Google Scholar]
- 25.Apetrei C, Gaufin T, Gautam R, Vinton C, Hirsch V, Lewis M, Brenchley J, Pandrea I. 2010. Pattern of SIVagm infection in patas monkeys suggests that host adaptation to simian immunodeficiency virus infection may result in resistance to infection and virus extinction. J Infect Dis 202(Suppl 3):S371–S376. doi: 10.1086/655970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, Sternberg L, Lawson B, Carnathan PM, Cramer EM, Engram JC, Little DM, Ryzhova E, Gonzalez-Scarano F, Paiardini M, Ansari AA, Ratcliffe S, Else JG, Brenchley JM, Collman RG, Estes JD, Derdeyn CA, Silvestri G. 2011. Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest 121:4433–4445. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, Reeves JD, Anton E, O'Neill E, Butler E, Hancock K, Cole KS, Brenchley JM, Else JG, Silvestri G, Sodora DL. 2011. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Invest 121:1102–1110. doi: 10.1172/JCI44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, Miamidian JL, Paiardini M, Barry AP, Staprans SI, Silvestri G, Sodora DL. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol 179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 29.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riddick NE, Wu F, Matsuda K, Whitted S, Ourmanov I, Goldstein S, Goeken RM, Plishka RJ, Buckler-White A, Brenchley JM, Hirsch VM. 2015. Simian immunodeficiency virus SIVagm efficiently utilizes non-CCR5 entry pathways in African green monkey lymphocytes: potential role for GPR15 and CXCR6 as viral coreceptors. J Virol 90:2316–2331. doi: 10.1128/JVI.02529-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, Asmuth DM, Tenorio AR, Altman JD, Fox L, Moir S, Malaspina A, Morre M, Buffet R, Silvestri G, Lederman MM, ACTG 5214 Study Team. 2009. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sereti I, Martinez-Wilson H, Metcalf JA, Baseler MW, Hallahan CW, Hahn B, Hengel RL, Davey RT, Kovacs JA, Lane HC. 2002. Long-term effects of intermittent interleukin 2 therapy in patients with HIV infection: characterization of a novel subset of CD4(+)/CD25(+) T cells. Blood 100:2159–2167. [PubMed] [Google Scholar]
- 33.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]