ABSTRACT
This study seeks to assess the ability of seasonal trivalent inactivated influenza vaccine (TIV) to induce nonneutralizing antibodies (Abs) with Fc-mediated functions in HIV-uninfected and HIV-infected subjects. Functional influenza-specific Ab responses were studied in 30 HIV-negative and 27 HIV-positive subjects immunized against seasonal influenza. All 57 subjects received the 2015 TIV. Fc-mediated antihemagglutinin (anti-HA) Ab activity was measured in plasma before and 4 weeks after vaccination using Fc-receptor-binding assays, NK cell activation assays, and phagocytosis assays. At baseline, the HIV-positive group had detectable but reduced functional Ab responses to both vaccine and nonvaccine influenza antigens. TIV enhanced Fc-mediated Ab responses in both HIV-positive and HIV-negative groups. A larger rise was generally observed in the HIV-positive group, such that there was no difference in functional Ab responses between the two groups after vaccination. The 2015 TIV enhanced functional influenza-specific Ab responses in both HIV-negative and HIV-positive subjects to a range of influenza HA proteins. The increase in functional Ab responses in the HIV-positive group supports recommendations to immunize this at-risk group.
IMPORTANCE Infection with HIV is associated with increasing disease severity following influenza infections, and annual influenza vaccinations are recommended for this target group. However, HIV-infected individuals respond relatively poorly to vaccination compared to healthy individuals, particularly if immunodeficient. There is therefore a need to increase our understanding of immunity to influenza in the context of underlying HIV infection. While antibodies can mediate direct virus neutralization, interactions with cellular Fc receptors may be important for anti-influenza immunity in vivo by facilitating antibody-dependent cellular cytotoxicity (ADCC) and/or antibody-dependent phagocytosis (ADP). The ability of seasonal influenza vaccines to induce antibody responses with potent Fc-mediated antiviral activity is currently unclear. Probing the ADCC and ADP responses to influenza vaccination has provided important new information in the quest to improve immunity to influenza.
INTRODUCTION
Seasonal influenza infections cause significant illness and deaths worldwide, with immunocompromised people such as those infected with human immunodeficiency virus (HIV) at high risk for developing influenza-related complications (1, 2). The main strategy to prevent seasonal influenza is through a trivalent inactivated influenza vaccine (TIV), and there is evidence that TIV shows some efficacy in HIV+ subjects (3). The TIV contains two influenza type A strains (H1N1, H3N2) and one influenza type B strain and is thought to act by primarily inducing neutralizing antibodies (NAbs) to the viral hemagglutinin (HA) (4). These NAbs are generally highly specific to the influenza virus strains in the vaccine, but because influenza viruses constitutively mutate, vaccines to seasonal influenza are updated yearly to maintain their effectiveness (5).
Antibodies (Abs) that induce Fc-dependent cellular functions are generated following influenza infections (6, 7). Fc-dependent functions include antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent phagocytosis (ADP). ADCC and ADP are initiated when Abs recognize and bind to antigens on the surface of an infected cell and engage effector cells bearing receptors for the Fc portion of IgG (FcγR). The effector cells, including natural killer (NK) cells and monocytes, may then be activated to kill the infected target cell (5, 8). In addition, free virions bound by Abs are a key target for ADP. ADCC is mediated primarily through FcγRIIIa (CD16a), which is present in higher-affinity (V158) and lower-affinity (F158) variants (9), whereas ADP is mediated primarily through FcγRIIa (CD32a) (10).
Studies in both mice and macaques suggest that ADCC may play an important role in the control of influenza (11, 12). However, the extent to which TIV induces ADCC and other Fc-mediated Ab functions such as ADP has not been well studied. A seasonal TIV vaccine failed to elicit ADCC Abs in influenza-naive pigtail macaques (13); however, studies in animal models do not recapitulate the complex immune landscape from multiple prior influenza exposures in human adults. Since HIV can cause widespread B cell dysfunction (14), there is a need to understand non-NAb responses to influenza in subjects with HIV infection and how these are modulated by vaccination.
Here we recruited two cohorts of HIV-negative and HIV-positive subjects and studied functional influenza-specific Ab responses employing a number of novel assays. Two new influenza virus strains were introduced into the 2015 TIV [A/Switzerland/9715293/2013 (H3N2)-like virus and B/Phuket/3073/2013-like virus] in the Southern Hemisphere, and this vaccine is now being used in the Northern Hemisphere (http://www.who.int/influenza/vaccines/virus/recommendations/en/). The introduction of new strains in the 2015 TIV allowed us to study functional Abs to antigenically variant influenza virus strains in human adults.
MATERIALS AND METHODS
Influenza vaccine cohort.
We concurrently recruited 30 healthy and 27 HIV-infected adults from the Doherty Institute for Infection and Immunity at the University of Melbourne, Parkville, Australia, and from the Melbourne Sexual Health Centre (MSHC) in Melbourne, Australia (Fig. 1 and Table 1). Based on data regarding the variability of influenza ADCC in HIV-uninfected subjects prior to vaccination, we predicted that 30 subjects per group would provide adequate power (80%, 2-alpha = 0.05) to detect 2-fold differences in ADCC responses between baseline responses in HIV-negative and HIV-positive subjects. Subjects received an intramuscular injection of TIV (Fluvax; bio-CSL, Parkville, Australia) containing 15 μg of hemagglutinin (HA) from A/California/7/2009 (H1N1), A/South Australia/55/2014 (H3N2), and B/Phuket/3073/2013. A/South Australia/55/2014 is an A/Switzerland/9715293/2013 (H3N2)-like virus selected for inclusion in trivalent influenza vaccines for the Southern Hemisphere, with only a a single K207R mutation (H3 numbering) differentiating these two antigenically similar strains. Blood samples were collected at baseline and a mean of 28 days (interquartile range, 27 to 29; range, 19 to 45) after vaccination. The study protocol and procedures were approved by the Alfred Health and University of Melbourne Human Ethics Committees (IDs 432/14 and 1443420). All subjects provided written informed consent.
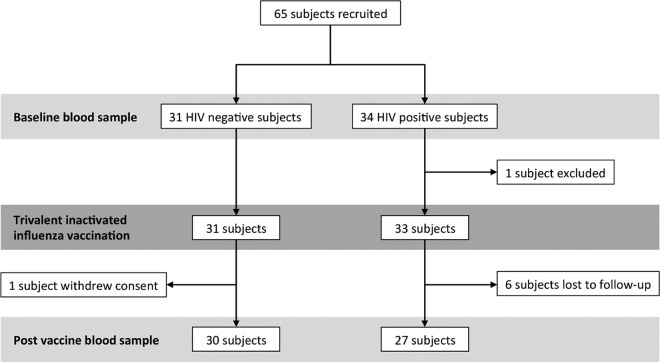
FIG 1.
Study enrollment flowchart.
TABLE 1.
Baseline characteristics of participants
| Characteristic | HIV negative (n = 30) | HIV positive (n = 27) |
|---|---|---|
| Age (yr) | ||
| Mean | 40.4 | 41.4 |
| Range | 22–55 | 26–54 |
| No. (%) of male gender | 13 (43.3) | 26 (96.3) |
| Duration of HIV infection (yr) | ||
| Mean | 8.1 | |
| Range | 1.0–20.8 | |
| No. (%) on antiretroviral therapy | 26 (96.3) | |
| CD4+ T cell count (per μl) | ||
| Mean | 729 | |
| Range | 257–1843 | |
| Nadir CD4+ T cell count (per μl) | ||
| Mean | 345 | |
| Range | 73–852 | |
| Viral load (HIV RNA copies/ml plasma) | <20a | |
| Days between Fluvax vaccine and 2nd blood sampling | ||
| Mean | 27 | 29 |
| Interquartile range | 22–29 | 27.5–31.5 |
| No. (%) who received influenza vaccinations in the last 5 yearsb | ||
| Unknown | 2 (6.7) | 3 (11.1) |
| 0 vaccinations | 2 (6.7) | 2 (7.4) |
| 1 vaccination | 6 (20.0) | 2 (7.4) |
| 2 vaccinations | 4 (13.3) | 5 (18.5) |
| ≥3 vaccinations | 16 (53.3) | 15 (53.3) |
Three subjects had detectable viral loads (25, 55, and 7,010 RNA copies/ml plasma).
Self-reported data and from medical records from MSHC if available.
Recombinant proteins.
Simian immunodeficiency virus (SIV) gp120 and purified HA from A/Switzerland/9715293/2013 (H3N2), A/Perth/16/2009 (H3N2), A/California/7/2009 (H1N1), A/New Caledonia/20/1999 (H1N1), B/Phuket/3073/2013 (Yamagata), and B/Florida/4/2006 (Yamagata) were purchased from Sinobiological, Beijing, China. SIV gp120 protein was included in each experiment as a negative control.
HI assays.
Hemagglutination inhibition (HI) assays were performed as previously described (15) using A/South Australia/55/2014 (H3N2), B/Phuket/3073/2013, A/California/7/2009 (H1N1), and A/New Caledonia/20/1999 (H1N1) viruses. Briefly, HI activity in serum samples was assessed using 1% turkey erythrocytes (H1N1 subtypes and type B influenza) or 1% guinea pig erythrocytes (H3N2 subtypes). Serum samples were titrated from a starting dilution of 1:10 to 1:1,280 in a phosphate-buffered saline (PBS) solution. HI titers were expressed as the reciprocal of the highest dilution of serum at which hemagglutination was completely inhibited.
FcγR dimer-binding assay.
When multiple IgG Abs bind simultaneously to antigen and present their Fc regions in close proximity to each other, they cluster cellular FcγRs, resulting in cell activation and effector function. A correlate of this “effector-activating” capacity of IgG Abs can be measured in vitro by novel assays that use dimers of the ligand binding domains of FcγRIIa or FcγRIIIa (see Fig. 3A) (B. D. Wines, H. A. Vanderven, S. E. Esparon, A. B. Kristensen, S. J. Kent, and P. M. Hogarth, unpublished data). Maxisorp enzyme-linked immunosorbent assay (ELISA) plates (96-well plates; Nunc, Rochester, NY) were coated with 50 ng/well of influenza HA or SIV gp120 (Sinobiological) in PBS overnight at 4°C. Wells were washed with PBS containing 0.05% Tween 20 (U-CyTech) and blocked with PBS containing 1 mM EDTA and 1% human serum albumin for 1 h at 37°C. Plates were washed and incubated with 1:20 diluted plasma (1:40 for A/New Caledonia/20/1999-coated wells) for 1 h at 37°C and then with 0.2 μg/ml of biotinylated FcγRIIa dimer or 0.1 μg/ml of biotinylated FcγRIIIa dimer diluted in PBS with 1 mM EDTA and 1% bovine serum albumin (PBS-EDTA-BSA; Sigma-Aldrich) for 1 h at 37°C. Subsequently, 100 ng/ml of Pierce high-sensitivity streptavidin-horseradish peroxidase (HRP; Thermo Scientific, Pittsburgh, PA) diluted in PBS-EDTA-BSA was added for 1 h at 37°C. Lastly, TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Sigma-Aldrich) was added, and color development was stopped with 1 M hydrochloric acid. Absorbance was read at 450 nm. Data were normalized using the signal from FcγR dimer binding to control wells containing 5 μg/ml human intravenous immunoglobulin (IVIG) (Sandoglobulin; CSL Behring, Australia) diluted in PBS-EDTA-BSA and directly absorbed to the plate.
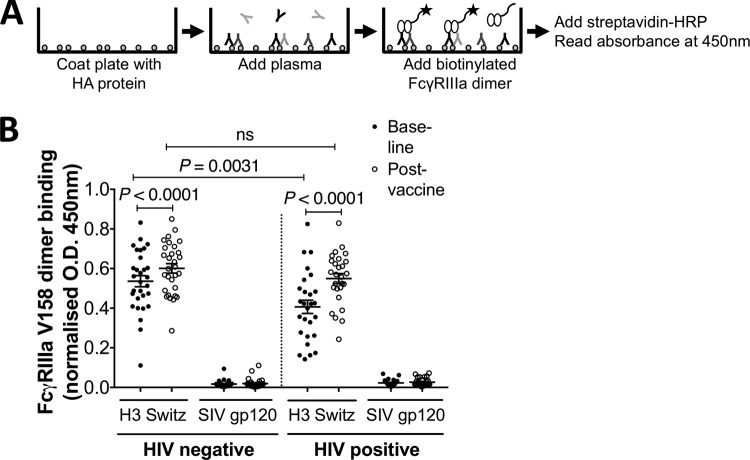
FIG 3.
FcγRIIIa dimer-binding assay. (A) Assay setup. Influenza HA protein (50 ng/well) was coated onto a 96-well plate before adding diluted plasma (containing IgGs). Subsequently, biotinylated FcγRIIIa dimers were added, and binding between Abs and FcγRIIIa dimers was measured using HRP-conjugated streptavidin, TMB, and HCl. Absorbance was measured at 450 nm. (B) FcγRIIIa-V158-binding Ab responses to HA from A/Switzerland/9715293/2013 (H3N2) (H3 Switz) and SIV gp120 protein. Responses are normalized to 5 μg/ml of IVIG. Plasma was diluted 20 times before analysis. Baseline and postvaccine data were compared using the Wilcoxon matched-pair test. HIV-negative and HIV-positive groups were compared using the Mann-Whitney test. P values of <0.05 were considered significant. Lines and error bars indicate means and SEM, respectively.
Plate-bound natural killer (NK) cell activation assay.
NK cell activation was measured by the ability of HA-specific Abs to induce NK-92 cell expression of CD107a (see Fig. 5A). The human NK-92 cell line (16) constitutively expressing the high-affinity variant of FcγRIIIa (V158) was cultured as previously described (17). Maxisorp ELISA plates (96-well; Nunc, Rochester, NY) were coated with 600 ng/well of influenza HA or SIV gp120 proteins in PBS overnight at 4°C. Wells were washed and blocked in PBS containing 5% bovine serum albumin and incubated with heat-inactivated 1:40 diluted plasma for 2 h at 37°C. NK-92 cells (2 × 105) were added to each well and incubated at 37°C for 5 h. Anti-human CD107a-allophycocyanin (clone H4A3; BD Biosciences) and 1 mM EDTA were added to the cells for 30 min at room temperature in the dark. NK-92 cells were washed twice with PBS, fixed with 1% formaldehyde, and acquired on the LSR Fortessa flow cytometer (BD Biosciences). Analysis was performed using FlowJo X 10.0.7r2 software (FlowJo LLC, Ashland, OR). Average background (SIV gp120-coated wells) was 1.5% NK-92 cells expressing CD107a.
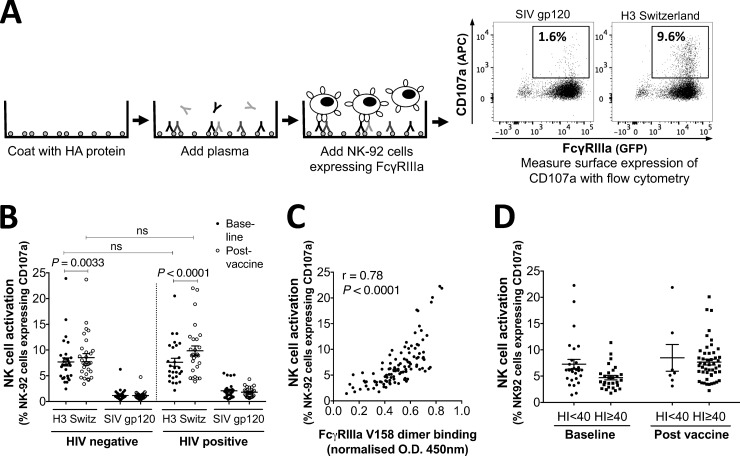
FIG 5.
NK cell activation assay with the NK-92 cell line. (A) Assay setup. A 96-well plate was coated with influenza HA protein (600 ng/well) from A/Switzerland/9715293/2013 (H3N2) before diluted plasma (containing IgGs) was added. The plate was then incubated with FcγRIIIa-expressing NK-92 cells (a human NK cell line) for 5 h at 37°C, after which NK-92 cell activation was analyzed with flow cytometry by measuring the percentage of CD107a-expressing NK-92 cells. SIV gp120 protein (600 ng/well) was included in each experiment as a negative control. (B) The NK-92 cell-activating Ab response to A/Switzerland/9715293/2013, a new antigen in the 2015 TIV, and to SIV gp120 protein. NK cell activation is measured as the percentage of CD107a-expressing NK-92 cells. Plasma was diluted 40 times before analysis. Baseline and postvaccine data were compared using the Wilcoxon matched-pair test. HIV-negative and HIV-positive groups were compared using the Mann-Whitney test. P values of <0.05 were considered significant. Lines and error bars indicate means and SEM, respectively. (C) Correlation between FcγRIIIa-V158-binding Abs and NK-92 cell-activating Abs to HA from A/Switzerland/9715293/2013, analyzed with nonparametric Spearman correlation. Background-subtracted data are presented in the graph. Results for all HIV-negative and HIV-positive subjects (baseline and postvaccine) are shown in the graph. (D) NK-92 cell-activating Ab response to HA from A/Switzerland/9715293/2013 in the absence (HI < 40) and presence (HI ≥ 40) of HI Abs to A/South Australia/55/2014, an A/Switzerland/9715293/2013-like virus. Background-subtracted data are presented in the graph.
ADP assay.
The antibody-dependent phagocytosis assay (10) was adapted for influenza virus (see Fig. 6A). Briefly, fluorescein isothiocyanate (FITC)-labeled NeutrAvidin FluoSpheres (1 μm; Invitrogen) were coated with a Cy5-fluorescent internalization probe (FIP) (5′-Cy5-TCAGTTCAGGACCCTCGGCT-3Bio-3′; Integrated DNA Technologies). After 10 min, excess FIP was washed. FIP-coated beads were incubated overnight with either 0.25 μg/μl biotinylated HA from A/Switzerland/9715293/2013 (H3N2) or biotinylated SIV gp120 (Sinobiological). Beads were washed to remove unbound protein and diluted 1:100 in PBS containing 2% BSA. Ten microliters coated beads was mixed with 10 μg/ml purified IgG from plasma for 2 h at 37°C. IgG was purified using the protein G HP Multitrap kits (GE Healthcare, Buckinghamshire, United Kingdom) as previously described (10). THP-1 cells (1 × 105), a monocytic cell line, were added to opsonized beads and cultured 16 h at 37°C. Cy5 fluorescence from surface-bound beads was blocked by adding the complementary quenching probe (5′-AGCCGAGGGTCCTGAACTGA-BHQ2-3′). Cells were fixed and analyzed by flow cytometry as described above. Average background (SIV gp120-coated beads) was 7.5% cells with internalized beads.
FIG 6.
ADP assay with THP-1 monocytic cell line. (A) Assay setup. Fluorescently labeled beads were coated with both biotinylated HA from A/Switzerland/9715293/2013 (H3N2) and a fluorescent internalization probe and then opsonized with Abs and incubated with a THP-1 monocytic cell line, known to express FcγRIIa. Fluorescence derived from internalization probes on surface-bound beads was quenched with a complementary quencher probe before internalization was measured with flow cytometry. SIV gp120 protein was included in each experiment as a negative control. (B) ADP Ab responses to HA from A/Switzerland/9715293/2013 and to SIV gp120 protein in HIV-negative and HIV-positive subjects. ADP activity is measured as the percentage of monocytes with internalized beads. Baseline and postvaccine data were compared using the Wilcoxon matched-pair test. HIV-negative and HIV-positive groups were compared using the Mann-Whitney test. P values of <0.05 were considered significant. Lines and error bars indicate means and SEM, respectively. (C) FcγRIIa-H131-binding Ab responses to A/Switzerland/9715293/2013 (H3N2) HA. Responses are with background subtracted (SIV gp120-coated wells) and normalized to 5 μg/ml of IVIG. Plasma was diluted 20 times before analysis. Baseline and postvaccine data were compared using the Wilcoxon matched-pair test. HIV-negative and HIV-positive groups were compared using the Mann-Whitney test. P values of <0.05 were considered significant. Lines and error bars indicate means and SEM, respectively. (D) Correlation between FcγRIIa-H131-binding Abs and ADP-stimulating Abs to HA from A/Switzerland/9715293/2013, analyzed with nonparametric Spearman correlation. Background-subtracted data are presented in the graph. Results for all HIV-negative and HIV-positive subjects (baseline and postvaccine) are shown in the graph. (E) Correlation between HI Ab titers and ADP activity to A/Switzerland/9715293/2013 (A/South Australia/55/2014 used in HI assay), analyzed with the nonparametric Spearman correlation. Background-subtracted ADP data are presented in the graph. Results for all HIV-negative and HIV-positive subjects (baseline and postvaccine) are shown in the graph.
Statistical analysis.
Statistical analysis was performed with Prism GraphPad version 6.0g (GraphPad Software, San Diego, CA). Data were analyzed by the Mann-Whitney U test, the Wilcoxon matched-pair signed-rank test, or the Spearman correlation coefficient as indicated in the figure legends. Data are presented as means ± standard errors of the means (SEM).
RESULTS
Influenza vaccination cohorts.
People infected with HIV are at greater risk for severe influenza infection and respond more poorly to influenza vaccination, particularly if immunodeficient (18, 19). To investigate if HIV infection alters ADCC and ADP responses to vaccination, we concurrently recruited cohorts of HIV-infected (n = 27) and HIV-uninfected (n = 30) subjects to receive the 2015 Southern Hemisphere TIV (Fig. 1). The cohorts were well matched for age and prior influenza vaccinations (Table 1). The HIV-positive subjects were more commonly male, and all but one was on effective antiretroviral therapy (ART) with a mean CD4 T cell count of 729/μl.
HI responses to 2015 TIV in HIV-positive and HIV-negative subjects.
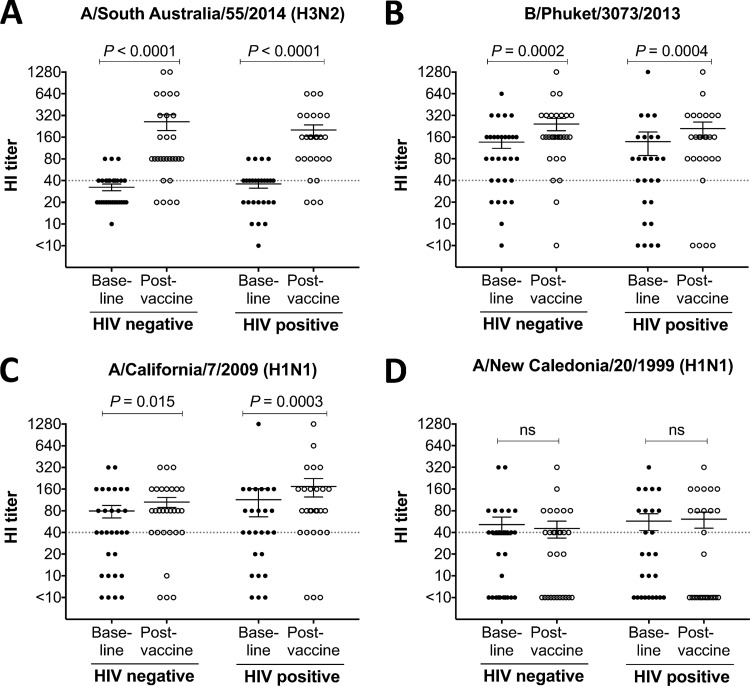
The 2015 Southern Hemisphere TIV saw the inclusion of two new circulating influenza virus strains, A/South Australia/55/2014 [an A/Switzerland/9715293/2013 (H3N2)-like virus] and a B/Phuket/3073/2013 virus. The 2015 TIV retained the A/California/7/2009, the seasonal H1N1 component since 2010 (http://www.who.int/influenza/vaccines/virus/recommendations/en/). Prior to vaccination, 43% (13/30) of HIV-negative subjects and 58% (15/26) of HIV-positive subjects were HI seropositive (HI titer, ≥40) to A/South Australia/55/2014 (Fig. 2A), 80% (24/30) and 69% (18/26) to B/Phuket/3073/2013 (Fig. 2B), and 67% (20/30) and 69% (18/26) to A/California/7/2009 (Fig. 2C). After vaccination, we detected a significant increase in HI titers against all three influenza virus strains in the TIV vaccine in both the HIV-positive and HIV-negative groups (Fig. 2A to C, paired analyses). No change in HI responses were observed against a heterologous nonvaccine H1N1 strain, A/New Caledonia/20/1999 (Fig. 2D), confirming that TIV induces primarily strain-specific NAbs. There was no significant difference between HIV-negative and HIV-positive cohorts in HI titers to any of the four influenza virus strains tested either at baseline or after vaccination. This finding contrasts with previous studies on HI responses in HIV-infected subjects (18, 20–23) and likely reflects the generally immunocompetent nature and high CD4 T cell counts of our HIV-positive cohort (24, 25).
FIG 2.
Hemagglutination inhibition (HI) assay. Serum HI titers against vaccine influenza virus strains A/South Australia/55/2014 (H3N2) (A), B/Phuket/3073/2013 (B), and A/California/7/2009 (H1N1) (C) and a nonvaccine strain, A/New Caledonia/20/1999 (H1N1) (D). Baseline and postvaccine data were compared using the Wilcoxon matched-pair test. Lines and error bars indicate means and SEM, respectively. A cutoff HI titer of ≥40 (dashed line) was used to assess positive responses.
Induction of HA-specific FcγRIIIa-binding Abs by TIV.
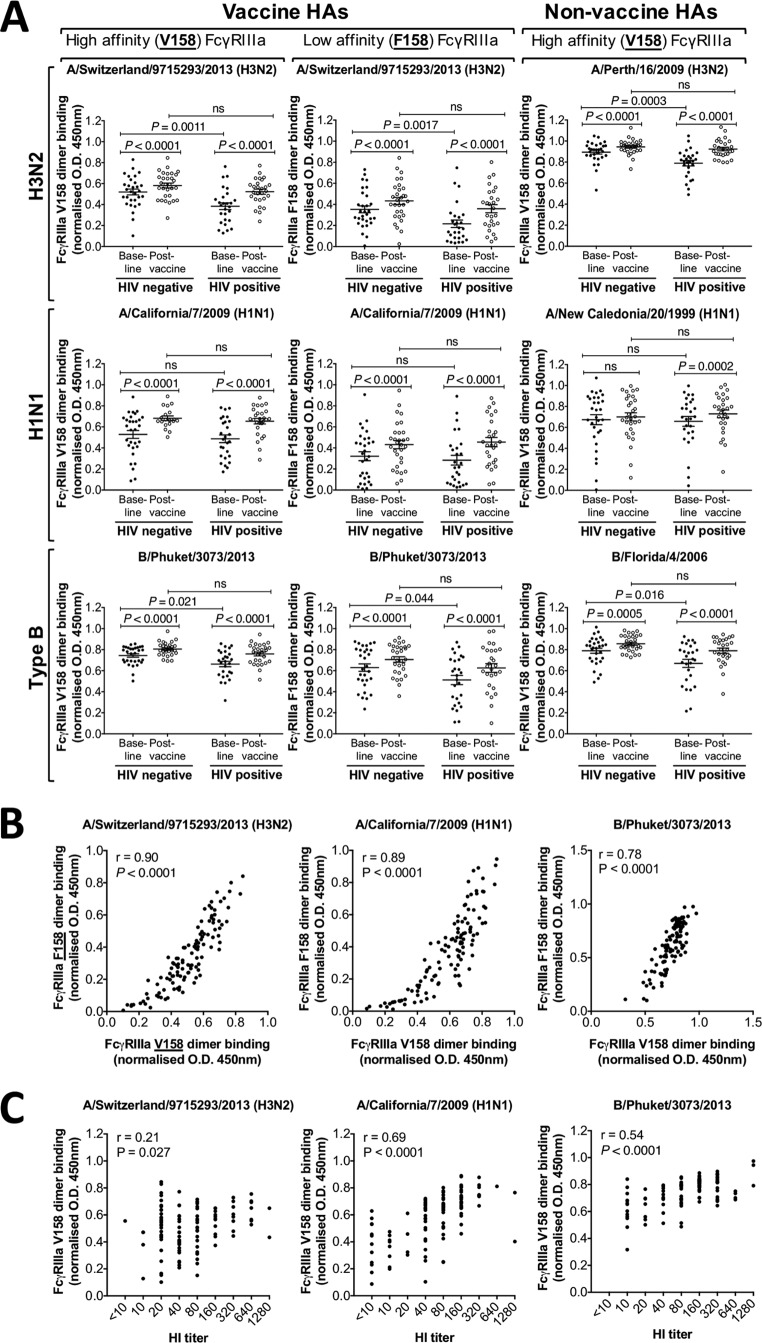
There is an increasing interest in functional Ab responses to influenza, such as ADCC and ADP, although the extent to which TIV induces ADCC or ADP in humans has not been well studied. Measuring Fc-mediated Ab function can be difficult to standardize because of genotypic differences and variable activation statuses of effector cells. Further, many flow cytometry-based assays are low throughput and are not well suited for studying multiple antigens in larger cohorts. To allow ready quantification of influenza-specific Abs with Fc-mediated functions, we recently developed a high-throughput ELISA-based assay that measures the ability of HA-specific Abs to bind to a dimer of FcγR ectodomains (Wines et al., unpublished) (Fig. 3A). This FcγR dimer-binding ELISA correlates strongly with measures of Fc-mediated NK cell function (Wines et al., unpublished). We first assessed the capacity of HA-specific Abs to bind to the high-affinity FcγRIIIa-V158 dimer in vaccine recipients (Fig. 3B). Background Fc dimer-binding responses to the SIV gp120 control were low (mean, 0.02; range, 0.001 to 0.1) in both cohorts and at both time points compared to the responses to A/Switzerland/9715293/2013 HA. Background was similarly and consistently low for all other antigens tested (mean, <0.01), and binding results are subsequently presented with the background subtracted. Interestingly, baseline responses to the two new vaccine strains, A/Switzerland/9715293/2013 (antigenically matched to A/South Australia/55/2014) and B/Phuket/3073/2013, were significantly lower in the HIV-positive group than in the HIV-negative group (P = 0.0011 and P = 0.021, respectively) (Fig. 4A, left panel). After vaccination, we observed a significant rise in FcγRIIIa-V158-binding Abs to all three vaccine strains for both the HIV-negative and HIV-positive cohorts (P < 0.0001 for all). No significant difference in FcγRIIIa-V158-binding Abs between the HIV-negative and HIV-positive subjects was detected after vaccination, reflecting a larger increase in FcγRIIIa-V158-binding Abs in the HIV-positive group. Thus, these data suggest that the 2015 TIV induces Ab responses to vaccine-influenza antigens that are capable of engaging FcγRIIIa-V158.
FIG 4.
FcγRIIIa dimer-binding activity to vaccine and nonvaccine influenza virus strains. (A) FcγRIIIa-V158-binding Ab responses (left panel) and FcγRIIIa-F158-binding Ab responses (center panel) to the 2015 TIV vaccine-HAs [A/Switzerland/9715293/2013 (H3N2), A/California/7/2009 (H1N1), and B/Phuket/3073/2013] and FcγRIIIa-V158-binding Abs to three nonvaccine HAs (A/Perth/16/2009 (H3N2), A/New Caledonia/20/1999 (H1N1), and B/Florida/4/2006; right panel). Responses are with background subtracted (SIV gp120-coated wells) and normalized to 5 μg/ml of IVIG. Plasma was diluted 20 times before analysis (40 times before analysis with A/New Caledonia/20/1999). Baseline and postvaccine data were compared using Wilcoxon matched-pairs test. HIV-negative and HIV-positive groups were compared using the Mann-Whitney test. P values of <0.05 were considered significant. Lines and error bars indicate means and SEM, respectively. (B) Correlation of the HA-specific Abs binding to the high-affinity (V158) and low-affinity (F158) FcγRIIIa dimer, analyzed with nonparametric Spearman correlation. Data shown for all three vaccine HAs, i.e., A/South Australia/55/2014, A/California/7/2009, and B/Phuket/3073/2013. Results for all subjects (baseline and postvaccine) are shown in the graph. (C) Correlation between HI titers and FcγRIIIa-V158 dimer-binding Abs to the three vaccine antigens, A/Switzerland/9715293/2013 (A/South Australia/55/2014 used in HI assay), A/California/7/2009, and B/Phuket/3073/2013, analyzed with the nonparametric Spearman correlation. Results for all HIV-negative and HIV-positive subjects (baseline and postvaccine) are shown in the graph.
The use of recombinant dimeric FcγRs allows the interaction of different receptor forms to be studied. Indeed, more people are homozygous for the lower-affinity form (F158) of FcγRIIIa than for the higher-affinity form (V158) (9). We therefore measured the binding of HA-specific Abs to the FcγRIIIa-F158 dimer (Fig. 4A, center panel). Consistent with our FcγRIIIa-V158-binding data, we observed reduced FcγRIIIa-F158-binding Abs in HIV-positive subjects at baseline to A/Switzerland/9715293/2013 and B/Phuket/3073/2013 (P = 0.0017 and P = 0.044, respectively). However, no significant difference in FcγRIIIa-F158-binding Abs was detected after vaccination between the two groups. The 2015 TIV induced FcγRIIIa-F158-binding Abs in both groups and to all 3 vaccine antigens (P < 0.0001 for all, paired analyses pre- and postvaccination). We observed a strong positive correlation between induction of FcγRIIIa-V158-binding Abs and FcγRIIIa-F158-binding Abs to A/Switzerland/9715293/2013 (r = 0.90, P < 0.0001; Fig. 4B), A/California/7/2009 (r = 0.89, P < 0.0001; Fig. 4B), and B/Phuket/3073/2013 (r = 0.78, P < 0.0001; Fig. 4B).
The ability of non-NAbs with Fc-mediated functions to recognize HA of multiple strains is an important potential advantage of these Abs over strain-specific NAbs (26, 27). To evaluate the induction of cross-reactive FcγRIIIa-binding Abs by TIV, we tested the capacity of nonvaccine HA-specific Abs to bind to the FcγRIIIa-V158 dimer before and after vaccination (Fig. 4A, right panel). TIV vaccination significantly increased FcγRIIIa-V158-binding Abs to HAs from A/Perth/16/2009, an H3N2 subtype (HIV-negative and HIV positive, P < 0.0001), A/New Caledonia/20/1999, an H1N1 subtype (HIV positive, P = 0.0002), and B/Florida/4/2006 (HIV negative, P = 0.0005, and HIV positive, P < 0.0001). No significant change in FcγRIIIa-V158-binding Abs was seen in the HIV-negative group to HA from A/New Caledonia/20/1999. Thus, the 2015 TIV elicited broad serological responses with FcγRIIIa-binding activity, presumably reflecting the ability of FcγRIIIa-binding antibodies to bind to conserved regions of HA (26). We also observed a significant positive correlation between HI activity and FcγRIIIa-V158-binding activity for all three TIV vaccine strains (Fig. 4C), although the correlation was weakest for the novel H3N2 HA in the vaccine.
Induction of HA-specific NK cell line activating Abs by TIV.
We next evaluated if TIV induced Abs able to elicit degranulation (as measured by CD107a expression) of NK-92 cells, a human NK cell-like cell line transduced to express the high-affinity (V158) isoform of FcγRIIIa (Fig. 5A). We studied responses to HA from A/Switzerland/9715293/2013 (H3N2), since this antigen in the 2015 TIV was significantly different from that of H3N2 viruses in recent previous TIVs. A significant rise in A/Switzerland/9715293/2013-specific Abs that induced degranulation (CD107a expression) of NK-92 cells was observed after vaccination in both HIV-negative (P = 0.0033) and HIV-positive (P < 0.0001) subjects (Fig. 5B). Consistent with the FcγRIIIa dimer-binding assay, we observed lower A/Switzerland/9715293/2013-specific NK-92 cell-activating Abs at baseline in the HIV-positive group; however, this did not reach statistical significance (P = 0.11). However, a strong positive correlation was observed between NK-92 activation activity and FcγRIIIa-V158-binding activity (r = 0.78, P < 0.001; Fig. 5C).
A/Switzerland/9715293/2013 is a recent H3N2 antigenic variant. However, cross-reactive Abs able to mediate ADCC against immunologically novel influenza virus strains have been previously reported (26, 27). We therefore analyzed NK cell activation by serum Abs recognizing A/Switzerland/9715293/2013 in the presence or absence of baseline HI activity to this strain (Fig. 5D). Both prior to and after vaccination, we observed NK cell-activating Abs independently of baseline HI activity against A/South Australia/55/2014 [an A/Switzerland/9715293/2013 (H3N2)-like virus]. These results are consistent with previous work reflecting the broader recognition of HA antigens by functional non-NAbs compared to NAbs (12, 26, 28).
Induction of HA-specific antibody-dependent phagocytosis by TIV.
Fc-FcR interactions can mediate antiviral functions other than ADCC. ADP is mediated by FcγRIIa and may play a role in the prevention of viral infections such as HIV (29). To investigate if HIV infection alters the ADP Ab response to TIV vaccination, we assessed the ability of A/Switzerland/9715293/2013-specific Abs from HIV-negative and HIV-positive subjects to mediate phagocytosis of HA-coated beads in a THP-1 monocytic cell line that expresses FcγRIIa (Fig. 6A and B). We also assessed the FcγRIIa-H131-binding Ab response to A/Switzerland/9715293/2013 HA using a FcγRIIa-H131 dimer-binding assay (Fig. 6C). We detected significantly reduced ADP activity (percentage of THP-1 cells with internalized beads) in the HIV-positive group at baseline compared to the HIV-negative group (P = 0.0083; Fig. 6B). This was consistent with an observed reduction in baseline FcγRIIa-binding Abs in the HIV-positive group (P = 0.015; Fig. 6C). ADP activity increased in response to TIV in both cohorts; however, significantly lower ADP activity was observed in the HIV-positive group after vaccination than in the HIV-negative group (P = 0.0003; Fig. 6B). However, this was not reflected in the FcγRIIa-binding assay, in which no significant difference in FcγRIIa-H131-binding Abs between the HIV-negative and HIV-positive subjects was observed after vaccination (Fig. 6C). There was a significant correlation between the ADP-stimulating Abs in the functional assay and FcγRIIa-H131-binding Abs (r = 0.63, P < 0.001) to the novel HA from A/Switzerland/9715293/2013 (Fig. 6D). Furthermore, we observed that subjects with high serological HI activity generally had high ADP activity (Fig. 6E), although this observation failed to reach statistical significance.
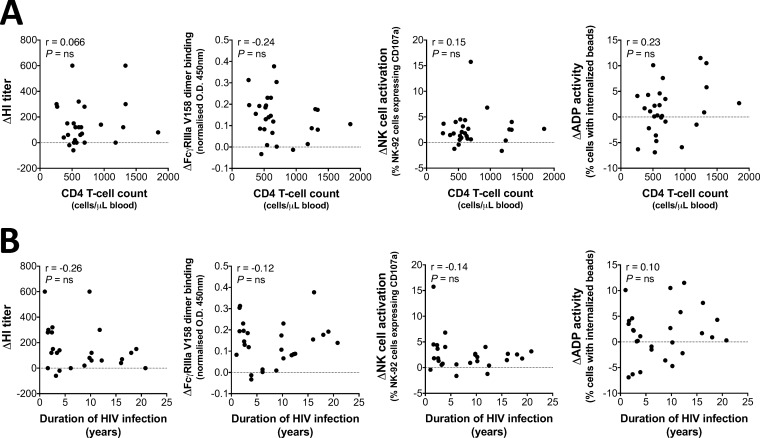
Correlations between CD4 T cell counts, duration of HIV infection, and Ab responses to TIV.
The characteristic depletion of CD4 T cells in untreated HIV infection has been associated with reduced Ab responses to TIV (18, 30). We therefore examined in HIV-positive subjects any relationship between baseline CD4 T cell counts (Fig. 7A) or the duration of HIV infection (Fig. 7B) and changes in HI activity, FcγRIIIa-V158-dimer binding, NK cell activation, and ADP activity to A/Switzerland/9715293/2013 following vaccination. No significant correlations were observed. These results likely reflect adequate treatment responsiveness to ART within our patient cohort, with generally high CD4 T cell counts observed in most individuals (mean, 729; range, 257 to 1,843).
FIG 7.
Correlations of CD4 T cell counts and HIV infection duration with functional Ab responses. (A) Correlation between CD4 T cell counts and the change in HI titer, FcγRIIIa-V158-dimer binding, NK cell activation, and ADP activity to A/Switzerland/9715293/2013 (postvaccine minus baseline response). (B) Correlation between the duration of HIV infection and the change in HI titer, FcγRIIIa-V158-dimer binding, NK cell activation, and ADP activity to A/Switzerland/9715293/2013 (postvaccine minus baseline response). Results for all 27 HIV-positive subjects are shown in the graphs. Analysis was by nonparametric Spearman's correlation.
DISCUSSION
NAbs clearly mediate protective immunity to influenza (31, 32); however, recent studies have suggested that Abs with Fc-mediated functions play an important additive role in protective immunity (11). We recruited subjects with and without underlying HIV infection to receive the 2015 TIV and studied functional Ab responses prior to and after vaccination. At baseline, HIV infection was associated with reduced Fc-mediated Ab functionality, despite similar serological HI activity to HIV-negative subjects. Although the reason for more-severe influenza infections in HIV-positive subjects is almost certainly multifactorial (20, 33–35), our results suggest that a reduction in Abs able to effectively interact with FcR may contribute to this increased risk. We detected NK cell-activating Abs to HA from the novel circulating influenza virus strain A/Switzerland/9715293/2013 in the majority (93%) of both HIV-negative and HIV-positive subjects in the absence of HI responses to this strain. This is consistent with previous studies showing that previous influenza exposures can elicit functional Abs that can cross-react with other influenza virus strains (26, 28). Indeed, highly cross-reactive, ADCC-mediating Abs are present in humans even to avian H5 and H7 in the absence of prior exposure (26, 28). Studies in both mice and macaques have shown a potential role for cross-reactive non-NAbs induced by prior seasonal influenza infections in the protection from pandemic influenza exposure (12, 36). These cross-reactive ADCC-mediating Abs can bind more conserved regions of the influenza HA, such as the stem (11, 26).
TIV is a partially effective strategy to prevent influenza, but its efficacy wanes in those in greatest need. Poor HI responses to TIV in HIV-positive subjects have been reported, but most studies analyzed cohorts prior to the widespread use of ART (18, 21).
In this study, we found that HI Abs to vaccine influenza virus strains increased equally after vaccination in the HIV-negative and HIV-positive cohorts. Furthermore, despite lower baseline levels of functional Abs in the HIV-positive group, both groups responded well to vaccination. Indeed, across several assays, the net increase in functional Ab responses to influenza increased to a greater extent in the HIV-positive group (albeit starting from a lower baseline level) than the HIV-negative group. These results are consistent with the use of ART, the generally high CD4 T cell count, and the undetectable HIV load in almost all subjects (Table 1). Indeed, the relative immunocompetence of our HIV-infected cohort precluded any significant correlations with responses to vaccination. Taken together, our results both support the use of ART in HIV-infected subjects to improve anti-influenza immunity and support the use of TIV in HIV-infected subjects in the current ART era since our cohort responded adequately to vaccination.
We acknowledge that the physiological relevance and protective role of ADCC and ADP Abs in anti-influenza immunity in humans require further study. Studies in mice and macaques suggest that ADCC Abs may assist in protection against or clearance of influenza virus infections (11, 12). Supporting a role for ADCC, Jegaskanda et al. found that the elderly had enhanced ADCC responses to the pandemic H1N1/09 virus strain prior to the epidemic. This presumably reflected distant prior infections with more closely related H1N1 strains and may contribute to reduced disease incidence within the elderly population during the 2009 pandemic (37). Ongoing studies such as the passive transfer of polyclonal immune serum in severe influenza infections (Clinical Trials registration no. NCT02287467) may help tease out a role for non-NAbs in control of human influenza infections.
We recognize that there are some limitations to our study. FcR-binding capacity and Ab-dependent NK cell activation are useful as surrogate markers of ADCC activity, but they do not directly quantitate Ab-mediated cell killing. We have, however, previously shown that Ab-mediated clearance of influenza virus-infected cells can be measured concurrently with NK cell activation (26). Further, we recently developed methods to study the Ab-mediated uptake of opsonized influenza virions, which in a separate study of influenza-specific immunity correlated well (r = 0.80, P = 0.0029) with the ADP assay employed here (43). Thus, we are confident that the assays we used have functional significance.
Our results suggest that HIV-infected subjects could derive significant benefit from influenza vaccination. However, the reported NK cell exhaustion and dysfunction in HIV-infected subjects (38–41), which only partially normalize after ART, may reduce the effectiveness of Abs with Fc-mediated functions. Further investigation into the FcγRIIIa surface expression on autologous NK cells and the TIV-induced autologous NK cell activation in HIV-infected subjects is warranted to help us better understand the effect of HIV infection on NK cell-mediated ADCC after influenza vaccination.
A number of further studies are suggested by this work. Although we observed increased anti-influenza immunity to nonvaccine strains after vaccination, further experiments with more-divergent influenza virus strains are required to assess the full breadth of TIV-induced functional non-NAb responses. The role of vaccination in boosting preexisting versus generating new non-NAb responses remains to be clarified. Technologies to define influenza-specific B cells and sequence their Ig genes allow a more detailed probe of preexisting versus new influenza-specific Ab responses and could be applied to non-NAb responses (42).
In conclusion, this study suggests that HIV-infected individuals have diminished functional Ab responses but that these can be restored with seasonal influenza vaccination. This highlights the importance of annual influenza vaccination within this at-risk group. Further investigation into the functional importance of Fc-mediated Ab immunity to influenza is warranted.
ACKNOWLEDGMENTS
We thank all healthy volunteers and clinical subjects who donated blood samples for this study. We thank Julie Silvers, Helen Kent, and the physicians at the Melbourne Sexual Health Centre for recruiting subjects and Thakshila Amarasena and Sheilajen Alcantara for assisting with blood sampling. We declare that we have no conflicts of interests.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Kunisaki KM, Janoff EN. 2009. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen C, Moyes J, Tempia S, Groom M, Walaza S, Pretorius M, Dawood H, Chhagan M, Haffejee S, Variava E, Kahn K, Tshangela A, von Gottberg A, Wolter N, Cohen AL, Kgokong B, Venter M, Madhi SA. 2013. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009-2011. Emerg Infect Dis 19:1766–1774. doi: 10.3201/eid1911.130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasker SA, Treanor JJ, Paxton WB, Wallace MR. 1999. Efficacy of influenza vaccination in HIV-infected persons. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 131:430–433. [DOI] [PubMed] [Google Scholar]
- 4.Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia-Sastre A, Moran TM, Palese P. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A 107:18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jegaskanda S, Reading PC, Kent SJ. 2014. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol 193:469–475. doi: 10.4049/jimmunol.1400432. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto G, Wright PF, Karzon DT. 1983. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis 148:785–794. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg SB, Six HR, Drake S, Couch RB. 1979. Cell cytotoxicity due to specific influenza antibody production in vitro after recent influenza antigen stimulation. Proc Natl Acad Sci U S A 76:4622–4626. doi: 10.1073/pnas.76.9.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol 166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. 1997. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest 100:1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ana-Sosa-Batiz F, Johnston AP, Liu H, Center RJ, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Kim JH, Michael NL, Kelleher AD, Stratov I, Kent SJ, Kramski M. 2014. HIV-specific antibody-dependent phagocytosis matures during HIV infection. Immunol Cell Biol 92:679–687. doi: 10.1038/icb.2014.42. [DOI] [PubMed] [Google Scholar]
- 11.DiLillo DJ, Tan GS, Palese P, Ravetch JV. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. 2013. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 87:5512–5522. doi: 10.1128/JVI.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jegaskanda S, Amarasena TH, Laurie KL, Tan HX, Butler J, Parsons MS, Alcantara S, Petravic J, Davenport MP, Hurt AC, Reading PC, Kent SJ. 2013. Standard trivalent influenza virus protein vaccination does not prime antibody-dependent cellular cytotoxicity in macaques. J Virol 87:13706–13718. doi: 10.1128/JVI.01666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moir S, Fauci AS. 2013. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev 254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
- 15.McVernon J, Laurie K, Nolan T, Owen R, Irving D, Capper H, Hyland C, Faddy H, Carolan L, Barr I, Kelso A. 2010. Seroprevalence of 2009 pandemic influenza A(H1N1) virus in Australian blood donors, October-December 2009. Euro Surveill 15(40):19678 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19678. [DOI] [PubMed] [Google Scholar]
- 16.Gong JH, Maki G, Klingemann HG. 1994. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8:652–658. [PubMed] [Google Scholar]
- 17.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. 2008. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 180:6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroon FP, van Dissel JT, de Jong JC, van Furth R. 1994. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 8:469–476. doi: 10.1097/00002030-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kelly D, Burt K, Missaghi B, Barrett L, Keynan Y, Fowke K, Grant M. 2012. Responses to pandemic ASO3-adjuvanted A/California/07/09 H1N1 influenza vaccine in human immunodeficiency virus-infected individuals. BMC Immunol 13:49. doi: 10.1186/1471-2172-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, Levandowski RA, Mican JM, Fauci AS. 2005. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis 191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 21.Chadwick EG, Chang G, Decker MD, Yogev R, Dimichele D, Edwards KM. 1994. Serologic response to standard inactivated influenza vaccine in human immunodeficiency virus-infected children. Pediatr Infect Dis J 13:206–211. doi: 10.1097/00006454-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Kohler I, Kouyos R, Bianchi M, Grube C, Wyrzucki A, Gunthard HF, Hangartner L. 2015. The impact of vaccination on the breadth and magnitude of the antibody response to influenza A viruses in HIV-infected individuals. AIDS 29:1803–1810. doi: 10.1097/QAD.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 23.Vigano A, Zuccotti GV, Pacei M, Erba P, Castelletti E, Giacomet V, Amendola A, Pariani E, Tanzi E, Clerici M. 2008. Humoral and cellular response to influenza vaccine in HIV-infected children with full viroimmunologic response to antiretroviral therapy. J Acquir Immune Defic Syndr 48:289–296. doi: 10.1097/QAI.0b013e3181632cda. [DOI] [PubMed] [Google Scholar]
- 24.Kroon FP, Rimmelzwaan GF, Roos MT, Osterhaus AD, Hamann D, Miedema F, van Dissel JT. 1998. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS 12:F217–F223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Agrati C, Gioia C, Castilletti C, Lapa D, Berno G, Puro V, Carletti F, Cimini E, Nisii C, Castellino F, Martini F, Capobianchi MR. 2012. Cellular and humoral immune responses to pandemic influenza vaccine in healthy and in highly active antiretroviral therapy-treated HIV patients. AIDS Res Hum Retroviruses 28:1606–1616. doi: 10.1089/aid.2011.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, Kent SJ. 2013. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 190:1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 27.Jegaskanda S, Vandenberg K, Laurie KL, Loh L, Kramski M, Winnall WR, Kedzierska K, Rockman S, Kent SJ. 2014. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis 210:1811–1822. doi: 10.1093/infdis/jiu334. [DOI] [PubMed] [Google Scholar]
- 28.Terajima M, Co MD, Cruz J, Ennis FA. 2015. High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 avian influenza A viruses in healthy US adults and older children. J Infect Dis 212:1052–1060. doi: 10.1093/infdis/jiv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med; 6(228):228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 30.Iorio AM, Francisci D, Camilloni B, Stagni G, De Martino M, Toneatto D, Bugarini R, Neri M, Podda A. 2003. Antibody responses and HIV-1 viral load in HIV-1-seropositive subjects immunised with either the MF59-adjuvanted influenza vaccine or a conventional non-adjuvanted subunit vaccine during highly active antiretroviral therapy. Vaccine 21:3629–3637. doi: 10.1016/S0264-410X(03)00408-0. [DOI] [PubMed] [Google Scholar]
- 31.Fox A, Mai le Q, Thanh le T, Wolbers M, Le Khanh Hang N, Thai PQ, Thi Thu Yen N, Minh Hoa le N, Bryant JE, Duong TN, Thoang DD, Barr IG, Wertheim H, Farrar J, Hien NT, Horby P. 2015. Hemagglutination inhibiting antibodies and protection against seasonal and pandemic influenza infection. J Infect 70:187–196. doi: 10.1016/j.jinf.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. 2010. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cagigi A, Nilsson A, Pensieroso S, Chiodi F. 2010. Dysfunctional B-cell responses during HIV-1 infection: implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect Dis 10:499–503. doi: 10.1016/S1473-3099(10)70117-1. [DOI] [PubMed] [Google Scholar]
- 34.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, Pahwa S. 2012. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood 120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez LA, Daniel A, Frank I, Tebas P, Boyer JD. 2014. Seroprotection of HIV-infected subjects after influenza A(H1N1) vaccination is directly associated with baseline frequency of naive T cells. J Infect Dis 210:646–650. doi: 10.1093/infdis/jiu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang Y, Banner D, Kelvin AA, Huang SS, Paige CJ, Corfe SA, Kane KP, Bleackley RC, Rowe T, Leon AJ, Kelvin DJ. 2012. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. J Virol 86:2229–2238. doi: 10.1128/JVI.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jegaskanda S, Laurie KL, Amarasena TH, Winnall WR, Kramski M, De Rose R, Barr IG, Brooks AG, Reading PC, Kent SJ. 2013. Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 208:1051–1061. doi: 10.1093/infdis/jit294. [DOI] [PubMed] [Google Scholar]
- 38.Parsons MS, Tang CC, Jegaskanda S, Center RJ, Brooks AG, Stratov I, Kent SJ. 2014. Anti-HIV antibody-dependent activation of NK cells impairs NKp46 expression. J Immunol 192:308–315. doi: 10.4049/jimmunol.1301247. [DOI] [PubMed] [Google Scholar]
- 39.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, Kramski M, Hearps AC, Cameron PU, Lewin SR, Crowe SM, Jaworowski A. 2012. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol 189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 40.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. 2003. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur J Immunol 33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 41.Leeansyah E, Zhou J, Paukovics G, Lewin SR, Crowe SM, Jaworowski A. 2010. Decreased NK Cell FcRgamma in HIV-1 infected individuals receiving combination antiretroviral therapy: a cross sectional study. PLoS One 5:e9643. doi: 10.1371/journal.pone.0009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheatley AK, Whittle JR, Lingwood D, Kanekiyo M, Yassine HM, Ma SS, Narpala SR, Prabhakaran MS, Matus-Nicodemos RA, Bailer RT, Nabel GJ, Graham BS, Ledgerwood JE, Koup RA, McDermott AB. 2015. H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J Immunol 195:602–610. doi: 10.4049/jimmunol.1402835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ana-Sosa-Batiz F, Vanderven H, Jegaskanda S, Johnston APR, Rockman S, Laurie K, Barr I, Reading P, Lichtfuss M, Kent SJ. Influenza-specific antibody-dependent phagocytosis. Plos One, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]