Abstract
Neurobiological research in genetically tractable organisms relies heavily on robust assays for behavioral phenotypes. The simple body plan of the nematode Caenorhabditis elegans makes it particularly amenable to the use of automated microscopy and image analysis to describe behavioral patterns quantitatively. This protocol first describes the preparation and use of media for growing and maintaining worms for tracking. The second part of the protocol describes how to prepare a single young adult worm for recording during video analysis. Although the protocol was developed for use in a single-worm tracker, it addresses factors important for the generation of reproducible, standardized images in all systems.
Materials
It is essential that you consult the appropriate Material Safety Data Sheets and your institution's Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPE: Please see the end of this article for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Nematode adult worms
Nematode growth medium (NGM) <R>
NGM, low-peptone
Follow the recipe for NGM with the following modifications: increase the agar to 20 g and decrease the peptone to 0.13 g
OP50 Escherichia coli
Equipment
Imaging setup, including stage with platform to support the sample
The hardware setup, including stage, camera, and light source, is shown in Figure 1.
Figure 1.
Hardware: (A) The stage, composed of x- and y-axis actuators and linear translation stages; (B) the camera; (C) the sample, a worm on an agar Petri dish; (D) a diffuser above a collimating Fresnel lens, to provide uniform illumination; (E) a high-intensity red light-emitting diode (LED) for illumination; (F) course and fine focusing knobs to focus the sample in view of the camera; (G) a rigid cage to center the illumination above the camera and eliminate vibrations caused by moving the stage.
Petri dishes, ≥5 cm
Worm tracking hardware and software package (e.g., Worm Tracker 2.0, available at http://www.mrc-lmb.cam.ac.uk/wormtracker/)
Method
Maintaining Strains
Prepare and pour fresh NGM into Petri dishes, and allow them to dry for 2 d with the lid on. (After drying, these NGM Petri dishes can be stored at 4°C for several days to retain their moisture; although, before use, they must be left out to reach room temperature.)
Seed each dish with three drops (~150 μL) of fresh OP50 bacteria. Leave them to dry, for 2 d, with the lid on.
Select six adult worms and transfer onto a dish.
Incubate the plate at 22°C.
Repeat Steps 1–4 every 3 d to maintain your strains.
Tracking
Prepare and pour fresh NGM into Petri dishes, and allow them to dry for 2 d with the lid on. (After drying, these NGM Petri dishes can be stored at 4°C for several days to retain their moisture; although, before use, they must be left out to reach room temperature.)
Seed each dish with three drops (~150 μL) of fresh OP50 bacteria. Leave them to dry, for 2 d, at room temperature with the lid on.
Approximately 16–18 h before tracking, select L4 worms from plates prepared in Steps 1–5 and transfer them to an NGM dish.
Store the plates at 22°C.
At 2 d before the experiment, prepare freshly poured low-peptone NGM dishes (these produce thin food lawns that produce less distortion in worm images). Leave them to dry, for 2 d, with the lid on.
At 30 min before each tracking experiment, seed each low-peptone dish with 20 μL of fresh OP50 bacteria. Leave them to dry with the lid on (30 min).
Select and transfer one worm to a plate (prepared in Step 11). Move the plate under the tracker. Wait 30 min for the worm to habituate, and record for 15 min at room temperature.
Record at least 20 worms (5 h of data). When performing multiple experiments on multiple trackers, they should be randomized in time and in choice of tracker. This helps avoid inconsistent spatial and temporal cues influencing behavior by driving their statistical effects into noise rather than amplifying them as signal. For similar reasons, it is best to space experiments out over several days using different experimenters.
Discussion
Background
Although most early work on C. elegans behavior was based on real-time observation, in recent years there has been increasing use of automated microscopy and video imaging analysis systems (known as “worm trackers”) for behavioral assays. These approaches offer a number of advantages over nonautomated methods. For example, many behavioral phentoypes have initially been described only in qualitative, imprecise terms (e.g., “weak kinker,” “loopy,” “coiler”). More quantitative assays lend precision to these descriptions and make it feasible to break behaviors down into components that can be related to particular neural functions. Another advantage is increased sensitivity, especially with regard to behaviors that occur over long timescales. There is also the potential for standardization between laboratories; if the same algorithms are used to score a behavior, data collected at different locations can in principle be compared as long as recording conditions are reproducibly controlled. Finally, an automated system (in particular a multiworm tracking microscope) offers the possibility of conducting high-throughput screens for mutants with complex behavioral phenotypes.
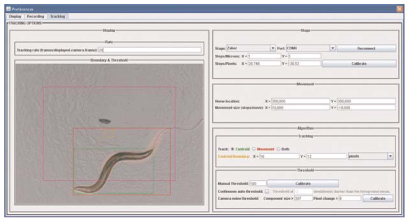
Worm trackers fall into two general types. Single-worm trackers follow individual animals at relatively high magnification, keeping them in the field of view by moving the camera or a motorized stage. Such systems have been used to study aspects of locomotion such as turning and speed (Pierce-Shimomura et al. 1999) as well as body posture and morphology (Karbowski et al. 2008). Multiworm trackers track multiple individuals in populations, by necessity at lower magnification, and usually focus on locomotion features. Generally speaking, single-worm trackers allow one to measure a greater number of parameters, whereas multiworm trackers offer much higher efficiency and throughput. Examples of single-worm trackers can be found in several published studies (e.g., Waggoner et al. 1998; Pierce-Shimomura et al. 1999; Hardaker et al. 2001; Feng et al. 2004; Geng et al. 2004; Cronin et al. 2005; Stephens et al. 2008). Another single-worm system, Worm Tracker 2.0, can be found on our website http://www.mrc-lmb.cam.ac.uk/wormtracker/. Imaging output from Worm Tracker 2.0.3.1 is shown in Figure 2.
Figure 2.
Single-worm tracking. This picture was taken from Worm Tracker 2.0.3.1. The largest connected component, the worm, is labeled in green. Motion is labeled in red. The tracker is attempting to keep the worm's centroid within the orange boundary and the worm's motion within the magenta boundary.
A protocol is also available for Illumination for Worm Tracking and Behavioral Imaging (Yemini et al. 2011a). A discussion of various methods for recording many worms simultaneously, including a protocol, can be found in Tracking Movement Behavior of Multiple Worms on Food (Yemini et al. 2011b). Other multiworm trackers have also been described (Ramot et al. 2008; Tsechpenakis et al. 2008).
Principles of Single-Worm Tracking
The benefit of single-worm over multiworm tracking is that it delivers videos with much higher spatial resolution—resolutions that enable neuronal tracking as well. Moreover, single-worm tracking opens up the door for stimulus targeting to specific locations of a worm on specific behavioral conditions. As such, image processing and computer vision to discover the worm must run as fast as possible. Among the simplest methods for worm localization, and the one seen most frequently in tracking software, is the following.
Incoming video is reduced to grayscale as worms present no additional information within color channels.
Video frames are dealt with individually and, using either a fixed or adaptive pixel value threshold, they are converted to a binary image of foreground and background pixels.
A connected-components algorithm (also known as particle or blob analysis) is run to label each group of connected foreground pixels with a separate numerical descriptor. The term “connected” usually refers to either four-connected pixels that touch only adjacently or eight-connected pixels that are permitted to touch diagonally as well.
Once the connected foreground pixels have been labeled, the “worm” is chosen to be either the largest group of connected pixels or a group that has an appropriate size and/or shape.
This “worm” is then tracked by moving the stage to keep it in view.
In general, refinements on the discovery algorithm described in point 3 include subtracting frames to determine motion and moving the stage to keep this motion in view. Motion tracking is necessary when illumination or other occlusions prevent accurate worm discovery using the more traditional approach of connected-components analysis. Additional refinements involve image processing to determine the worm’s contour, skeleton, or other descriptors that allow one to determine worm body part locations and/or behavior. These refinements are currently ad hoc as they can quickly approach the limit of what modern computers can perform in real time.
Recipe
Nematode Growth Medium (NGM)
| Reagent | Amount to add (for 1 L) | Final concentration |
|---|---|---|
| Agar | 17.0 g | 1.7% (w/v) |
| NaCl | 2.9 g | 50 mм |
| Peptone | 2.5 g | 0.25% (w/v) |
| CaCl2 (1 м) | 1 mL | 1 mм |
| Cholesterol (5 mg/mL) | 1 mL | 5 μg/mL |
| KH2PO4 (1 м) | 25 mL | 25 mм |
| MgSO4 (1 м) | 1 mL | 1 mм |
Mix the first three reagents and autoclave. After the mixture is cool, add the last four reagents.
Footnotes
Adapted from Imaging in Neuroscience (ed. Helmchen and Konnerth). CSHL Press, Cold Spring Harbor, NY, USA, 2011.
References
- Cronin CJ, Mendel JE, Mukhtar S, Kim YM, Stirbl RC, Bruck J, Sternberg PW. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet. 2005;6:5. doi: 10.1186/1471-2156-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Cronin CJ, Wittig JH, Jr, Sternberg PW, Schafer WR. An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics. 2004;5:115. doi: 10.1186/1471-2105-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W, Cosman P, Berry CC, Feng Z, Schafer WR. Automatic tracking, feature extraction and classification of C. elegans phenotypes. IEEE Trans Biomed Eng. 2004;51:1811–1820. doi: 10.1109/TBME.2004.831532. [DOI] [PubMed] [Google Scholar]
- Hardaker LA, Singer E, Kerr R, Zhou G, Schafer WR. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J Neurobiol. 2001;49:303–313. doi: 10.1002/neu.10014. [DOI] [PubMed] [Google Scholar]
- Karbowski J, Schindelman G, Cronin CJ, Seah A, Sternberg PW. Systems level circuit model of C. elegans undulatory locomotion: Mathematical modeling and molecular genetics. J Comput Neurosci. 2008;24:253–276. doi: 10.1007/s10827-007-0054-6. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D, Johnson BE, Berry TL, Jr, Carnell L, Goodman MB. The Parallel Worm Tracker: A platform for measuring average speed and drug-induced paralysis in nematodes. PLoS One. 2008;3:e2208. doi: 10.1371/journal.pone.0002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Johnson-Kerner B, Bialek W, Ryu WS. Dimensionality and dynamics in the behavior of C. elegans. PLoS Comput Biol. 2008;4:e1000028. doi: 10.1371/journal.pcbi.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsechpenakis G, Bianchi L, Metaxas D, Driscoll M. A novel computational approach for simultaneous tracking and feature extraction of C. elegans populations in fluid environments. IEEE Trans Biomed Eng. 2008;55:1539–1549. doi: 10.1109/TBME.2008.918582. [DOI] [PubMed] [Google Scholar]
- Waggoner LE, Zhou GT, Schafer RW, Schafer WR. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron. 1998;21:203–214. doi: 10.1016/s0896-6273(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Yemini E, Kerr RA, Schafer WR. Illumination for worm tracking and behavioral imaging. Cold Spring Harb Protoc. 2011a doi: 10.1101/pdb.prot067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemini E, Kerr RA, Schafer WR. Tracking movement behavior of multiple worms on food. Cold Spring Harb Protoc. 2011b doi: 10.1101/pdb.prot067025. [DOI] [PMC free article] [PubMed] [Google Scholar]