Abstract
To examine the cerebellar contribution to human spatial navigation we used functional magnetic resonance imaging and virtual reality. Our findings show that the sensory-motor requirements of navigation induce activity in cerebellar lobules and cortical areas known to be involved in the motor loop and vestibular processing. By contrast, cognitive aspects of navigation mainly induce activity in a different cerebellar lobule (VIIA Crus I). Our results demonstrate a functional link between cerebellum and hippocampus in humans and identify specific functional circuits linking lobule VIIA Crus I of the cerebellum to medial parietal, medial prefrontal, and hippocampal cortices in nonmotor aspects of navigation. They further suggest that Crus I belongs to 2 nonmotor loops, involved in different strategies: place-based navigation is supported by coherent activity between left cerebellar lobule VIIA Crus I and medial parietal cortex along with right hippocampus activity, while sequence-based navigation is supported by coherent activity between right lobule VIIA Crus I, medial prefrontal cortex, and left hippocampus. These results highlight the prominent role of the human cerebellum in both motor and cognitive aspects of navigation, and specify the cortico-cerebellar circuits by which it acts depending on the requirements of the task.
Keywords: fMRI, functional connectivity, sequence learning, spatial memory, virtual reality
Introduction
The role of the cerebellum in motor control is well accepted (Manto et al. 2012) but how it contributes to cognitive functions has been and is still debated (see Timmann and Daum 2010; Buckner 2013; Koziol et al. 2013). Since the initial claim by Leiner et al. (1993), converging evidence from patients and brain imaging studies has revealed involvement of the cerebellum in various cognitive tasks in addition to motor ones (Stoodley et al. 2012). Further, anatomical and functional studies have identified connections between the cerebellum and nonmotor neocortical areas (Ramnani 2006; Strick et al. 2009; Buckner 2013). Navigation has proved an interesting paradigm to investigate multifaceted cerebellar functions as it involves both motor control and cognitive processes such as building mental representations of the external world (reviewed in Rondi-Reig and Burguiere 2005; Rochefort et al. 2013). The present study aims to highlight involvement of the cerebellum in different navigation strategies and to tease apart its contribution to the motor and cognitive aspects of navigation in humans.
Reports in patients with cerebellar pathologies have described dysfunctions of nonmotor processes, affecting a wide range of cognitive functions. These include altered social and emotional behavior and impaired mental performance (Schmahmann 1991; Fuentes and Bastian 2007; Molinari and Leggio 2007; Stoodley and Schmahmann 2009; Stoodley 2012). In addition, cerebellar activation has been observed in experiments with minimal motor demands including sensory tasks (Gao et al. 1996; Blakemore et al. 1999), linguistic tasks (Roskies et al. 2001; Noppeney and Price 2002; Xiang et al. 2003; Ravizza et al. 2006), executive function (Desmond et al. 1997; Desmond et al. 1998; Stoodley and Schmahmann 2009), working memory (Hayter et al. 2007), and spatial memory (Moffat et al. 2006; Stoodley et al. 2012; see also the review by Stoodley 2012).
The identification of multiple segregated neocortico-cerebellar circuits has further challenged the traditional view that the cerebellum is involved in motor control only (Ramnani 2006, 2012). While the motor loop has been described between the lobules V, VI, and VIII of the cerebellum and the primary motor cortex (Ramnani 2006, 2012; Krienen and Buckner 2009; Strick et al. 2009; O'Reilly et al. 2010; Prevosto et al. 2010) via the thalamus (Middleton and Strick 2001; Kelly and Strick 2003), viral tracing techniques in nonhuman primates and functional connectivity analyses in humans have also revealed 2 loops with nonmotor cortical areas, each involving lobule VIIA of the posterior cerebellum. The first one links lobule VIIA to the contralateral prefrontal cortex (Pfc) (Middleton and Strick 1997, 2001; Kelly and Strick 2003; Krienen and Buckner 2009; O'Reilly et al. 2010) and the second links it to the parietal cortex, mainly the contralateral inferior parietal lobule (Clower et al. 2001, 2005; O'Reilly et al. 2010; Prevosto et al. 2010).
Navigation tasks are particularly appropriate to explore both cognitive and motor roles of the cerebellum as they require encoding a representation of the environment and acquiring an adapted motor behavior towards a goal. In rodents, it has been shown that cerebellar dysfunctions yield impaired acquisition of navigation tasks (Petrosini et al. 1998; Rondi-Reig and Burguiere 2005), particularly highlighting the role of the cerebellum in procedural memory (Mandolesi et al. 2001; Torriero et al. 2007) and trajectory optimization (Rondi-Reig et al. 2002; Burguiere et al. 2005, 2010). Recently, cerebellar plasticity was also shown to be involved in shaping hippocampal representations of the environment through the processing of self-motion information (Rochefort et al. 2011). This result suggests that the cerebellum contributes to an anatomo-functional pathway through which the mental representation of space is built (reviewed in Rochefort et al. 2013).
Human navigation studies, combining virtual reality and functional neuroimaging, have reported activation in the cerebellum (e.g., Maguire et al. 1998), while focusing on hippocampal and cortical networks. Patient studies have also reported that cerebellar lesions lead to an alteration of visuospatial abilities with different characteristics depending on the side of the lesion (Malm et al. 1998; Molinari et al. 2004). When processing bidimensional complex figures mentally, patients with left sided lesions were able to process a limited number of items whereas patients with right cerebellar lesions were impaired in the correctness of their response (Molinari et al. 2004). Similarly, patients with lesions in the left hemisphere of the cerebellum showed deficits in cognitive operations in 3-dimensional (3D) space (Wallesch and Horn 1990). Recently, the medial left cerebellar lobule VII has been found to be activated during tasks including mental rotation (Stoodley et al. 2012).
Altogether those results suggest an involvement of the cerebellum in both motor and cognitive aspects of navigation, and suggest a functional interaction between the cerebellum and the hippocampus within spatial memory.
To investigate cerebellar involvement in motor and cognitive aspects of navigation, we recorded blood oxygen level-dependent (BOLD) activity in a virtual navigation task (the starmaze). This navigation task dissociates 2 cognitive navigation strategies: the place-based and the sequence-based strategies (Rondi-Reig et al. 2006; Arleo and Rondi-Reig 2007; Igloi et al. 2009). The place-based strategy, thought to rely on allocentric representations, was shown to depend on the right hippocampus (Igloi et al. 2010); the sequence-based strategy, thought to rely on sequential egocentric representations, appeared to depend on the left hippocampus (Igloi et al. 2010); (see also Ghaem et al. 1997; Mellet et al. 2000; Hartley et al. 2003; Iaria et al. 2003). Here, cerebellar activation was assessed as a function of cognitive strategy, taking into account movement-related confounds, and functional connectivity was analyzed to characterize possible interactions between cerebellum and hippocampus.
Materials and Methods
Participants
Nineteen male participants (aged 19–38, mean age 24.3) gave written consent and were paid for participating as approved by the local Research Ethics Committee (University College London, UK). All were right handed with normal or corrected to normal vision and reported to be in good health with no history of neurological disease. Two participants were excluded from scanning or further analysis due to failure to understand the task instructions.
Virtual Reality Design
We used a virtual reality starmaze designed with 3D StudioMax (Autodesk Fortune 1000, San Rafael, CA) and made interactive with Virtools (v3.5) (Dassault Systèmes, Suresnes, France). Every 200 ms, the position of the participant and his moving direction was registered in a Cartesian coordinate system. These records were analyzed to obtain the performed trajectory and the accuracy of the path at each trial.
The virtual reality maze comprised 5 central alleys forming a pentagon and 5 radiating alleys departing from the angles of the central pentagon (see Fig. 1A). Participants used a keypad to move their viewpoint forwards or backwards or to turn left or right; they could move around and perform rotations freely in all the alleys. Distant environmental cues surround the maze for orientation (see Igloi et al. 2010 for details).
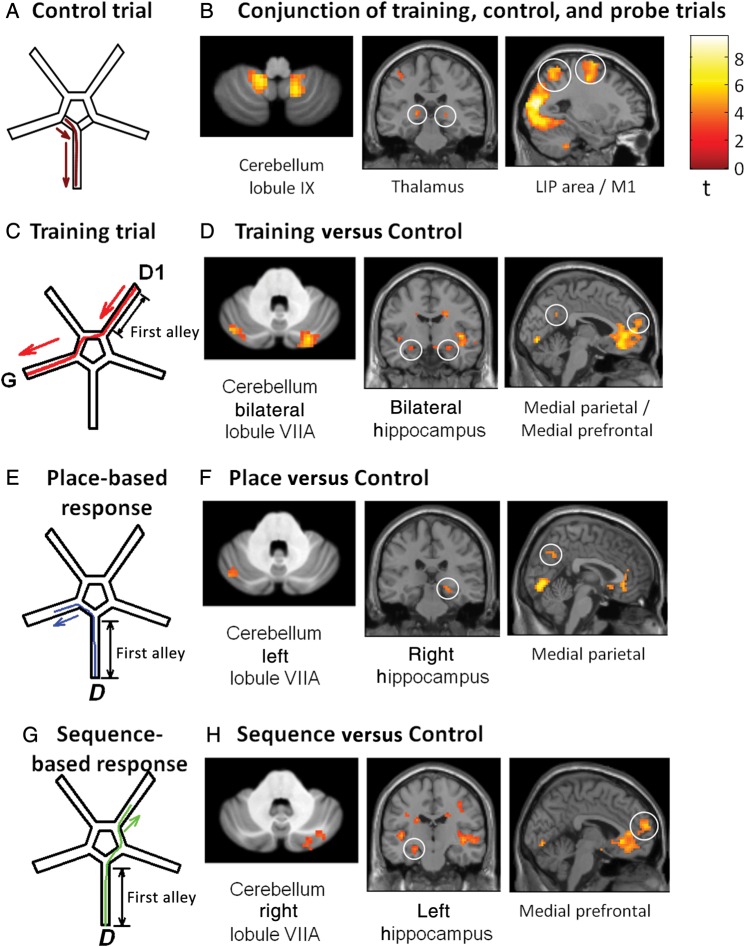
Figure 1.
(A) Subject's path in the control trials. (B) Results of the conjunction analysis including first alleys of successful training, control, and probe trials regardless of the strategy used, for the 17 participants. Left: bilateral activation of the posterior cerebellum lobule IX (peaks at MNI coordinates: −12, −58, −41 and 15, −57, −51). Center: bilateral activation of the thalamus in the white circles (peaks at: −18, −27, 3 and 21, −27, 0). Right: activation of the lateral intraparietal area (LIP) and the primary motor cortex in the white circles (peaks at: −21, −63, 60 and −24, −6, 63, respectively). (C) Training trials. D1: departure point for all training trials; G: goal; in red, the most direct path from the departure to the goal, which defines a successful training trial. (D) Activation of structures of interest for the successful training versus control trials at the group level (N = 17). Left: bilateral cerebellar lobule VIIA Crus I (peaks at MNI coordinates: 27, −81, −36 and −45, −72, −36). Center and right (adapted from Igloi et al. 2010): Bilateral hippocampus (peaks at: 30, −6, −15 and −21, −15, −15), along with medial prefrontal (−3, 42, 0) and medial parietal cortex (0, −54, 30). (E) Place-based path in a probe trial (in blue). D: departure point. (F) Group activation associated with place-based responses vs. control trials, in the 1st alley (N = 17). Left: left cerebellar lobule VIIA Crus I (−45 −72 −36). Center and Right (adapted from Igloi et al. 2010): Right hippocampus (24, −24, −9), along with medial parietal activation (3, −63, 36). (G) Sequence-based path in a probe trial (in green). D: departure point. (H) Group activation associated with sequence-based responses versus control trials, in the first alley (N = 17). Left: right Cerebellar lobule VIIA Crus I (36, −75, −39). Center and Right (adapted from Igloi et al. 2010): left hippocampus (−21, −15, −15) and medial prefrontal activation (−3, 60, 15).
Task Design
Participants were instructed to find a goal which would always be at the same place in the maze. The goal had no visible identifiers but, when reached, fireworks went off to indicate the successful end of the trial (feedback). Participants were told that the environment would not change during the experiment and that some trials would be terminated before feedback occurred. Before testing started, participants spent a few minutes moving freely in one alley of the environment to practice the motor aspects of the task.
The experiment was composed of 48 training trials, 16 probe trials and 16 control trials interleaved in pseudo-random order (see Supplementary Fig. 1 for trial order). For each trial, there was a time limit of 90 s. If participants did not reach the goal within 90 s the next trial was started. The only exception was the first training trial: if participants failed to reach the goal within 90 s, they were placed in the goal alley and were instructed to get to the goal by going straight ahead (as indicated by an arrow on the screen).
All training trials started from the same departure point (D1, Fig. 1C). Participants had to find the rewarded goal located in G (Fig. 1C). In training trials, successful navigation might be achieved using 2 different strategies: either by reproducing the sequence of body-turns (hereafter “sequence-based” strategy) or by memorizing the location of the goal relative to environmental cues (hereafter “place-based” strategy).
In probe trials, participants had to find the goal from 2 different departure points so that we could dissociate the use of either strategy according to the path chosen. From both probe departure points participants could perform place-based and sequence-based responses (see Igloi et al. 2010 for details). To avoid any deliberate strategy, participants were not informed of the existence of probe trials and these trials were not distinguished from the training ones in the time course of the experiment. In addition, the use of sequence-based or place-based strategy in a probe trial were both considered correct, so that a probe trial ended once the participant had navigated to an alley consistent with either strategy (Fig. 1E and G). However, to avoid (positive or negative) reinforcement of either strategy during probe trials and to avoid a subsequent bias towards one or the other strategy, probe trials always ended before participants would receive any feedback, in the middle of one of the final alleys. In order to get subjects used to prematurely ending trials, and not to alert them to the difference between probe and training trials, a proportion of the training trials (25%) and control trials (50%) also ended in the middle of the final alley.
Control trials consisted of a simple displacement task in a limited portion of the same maze, devoid of any landmark. Participants had to follow a straight alley and then perform one forced turn (to the right or to the left), the other alley being blocked by a wall (Fig. 1A). The unique turn prevents the subjects from encoding a sequence of body turns (which could be related to a sequence-based strategy) and the absence of landmarks avoids encoding environmental cues that could be related to a place-based strategy. To preserve the placement of the goal, the end alley of the control trials corresponded to a dead-end of the maze and the first alley corresponded to a central arm of the maze. In a featureless environment however, this is not distinguishable from the peripheral start alley used for other trials.
We focused our analyses mainly on activations before the first choice point of the trial, that is, in the first alley of the maze, so that activation patterns could be analyzed according to the strategy of the participant on that trial, as determined by their subsequent choices, but without any of the differences in behavior or stimuli which might result from making those choices (namely following different paths).
fMRI data Acquisition and Preprocessing
BOLD-sensitive T2*-weighted functional images were acquired on a 3T Siemens Allegra scanner using a gradient echo planar imaging (EPI) pulse sequence with the following parameters: time repetition = 3120 ms, time echo = 30 ms, flip angle = 90°, slice thickness = 2 mm, interslice gap = 1 mm, in-plane resolution = 3 × 3 mm, field of view = 192 mm2, 48 slices per volume covering the whole brain and cerebellum. The first 5 volumes were discarded to allow for T1 equilibration. The sequence was optimized to minimize signal dropouts in the medial temporal lobes (Weiskopf et al. 2006). Functional images were analyzed using SPM5 (www.fil.ion.ucl.ac.uk/spm/). This included standard preprocessing procedures: realignment, unwarping, slice timing to correct for differences in slice acquisition time, normalization (images were normalized to an EPI template specific to our sequence and scanner that was aligned to the MNI T1 template) and smoothing (with an isotropic 8-mm FWHM Gaussian kernel).
Cerebellum activations are displayed on the unbiased cerebellum atlas by Diedrichsen et al. (2006, 2009).
fMRI data Analysis
General Linear Model Analysis
fMRI time series were modeled using a general linear model which included separate regressors for the start alley, middle part, and final alley of every type of successful trial (i.e., training trials following the direct path shown in Fig. 1C, sequence-based responses for probe trials and place-based responses for probe trials). The training trials with indirect paths and probe trials for which no strategy could be identified (see supplementary materials of Igloi et al. 2010) were grouped into a separate regressor of the model. Two additional regressors modeled the first and second alleys of the control trials. This corresponds to eleven regressors of interest (i.e., 3 × 3 [training, sequence-based and place-based trials] × (start, middle, final alley) and 2 (control trials)). The model also included regressors based on estimates of head movement obtained from the realignment procedure. All regressors, except the movement parameters were convolved with the SPM hemodynamic response function. Data were high-pass filtered (cut-off period = 128 s).
At the single subject level, coefficients for each regressor were estimated by a least-mean-squares fit of the model to the time series. For each participant, we also contrasted the activation in the first alley of training and probe trials with the activation in the first alley of control trials. Three contrasts were thus performed: one for training trials, one for probe trials with sequence-based responses and one for probe trials with place-based responses. Further, we contrasted the activity of the probe trials' first alley between sequence-based and place-based responses.
The coefficients for each regressor were entered into a second-level conjunction analysis in order to assess the regions commonly involved in all types of trials. With such an analysis, we expect to highlight cortico-cerebellar networks related to sensory-motor requirements, independently of the various cognitive processes involved in the different experimental conditions. To facilitate their comparison with the activity related to the cognitive processes, we also report here the results of the second-level analysis performed on the 3 contrasts (training—control, place-based response—control, and sequence-based response—control, see Supplementary Table 1), although these were also detailed previously (Igloi et al. 2010). In the forebrain, only values surviving the uncorrected statistical threshold of P < 0.001 are reported (Table 1 and Supplementary Table 1). When focusing the analysis on the cerebellum, we also report regions surviving a more liberal threshold of P < 0.005 (Table 2).
Table 1.
Activation local maxima for the conjunction analysis (all P < 0.001)
| Forebrain area | Side | MNI coordinates |
Z-score | ||||
|---|---|---|---|---|---|---|---|
| Sup. Precentral s. (M1) | L | −24 | −3 | 72 | 5.23 | ||
| Sup. Precentral s. (FEF) | L | −30 | −12 | 48 | 4.97 | ||
| Sup. Precentral s. (FEF) | L | −21 | −3 | 48 | 4.92 | ||
| Sup. Precentral s. (FEF) | R | 30 | −3 | 51 | 5.12 | ||
| Sup. Precentral s. (M1) | R | 21 | 0 | 63 | 5.11 | ||
| Sup. Precentral s. | R | 15 | −12 | 69 | 4.29 | ||
| Sup. Precentral s. | L | −57 | 0 | 36 | 4.41 | ||
| Sup. Precentral s. | R | 48 | 3 | 30 | 3.41 | ||
| Lateral Intraparietal (LIP) | L | −21 | −63 | 60 | 6.94 | ||
| Lateral Intraparietal (LIP) | R | 30 | −75 | 33 | 6.53 | ||
| Intrapariatal Sulcus | L | −33 | −39 | 51 | 3.31 | ||
| Thalamus | L | −18 | −27 | 3 | 3.98 | ||
| Thalamus | R | 21 | −27 | 0 | 3.39 | ||
| Putamen | R | 21 | 9 | 12 | 3.71 | ||
| Cuneus | L | −30 | −81 | 21 | 7.43 | ||
| Cuneus | L | −27 | −87 | 15 | 7.04 | ||
| Cuneus | R | 30 | −87 | 12 | 6.40 | ||
| Lingual Gyrus | R | 33 | −78 | −9 | 6.47 | ||
| Cerebellum lobule | |||||||
| VI | R | 30 | −60 | −18 | 4.76 | ||
| VI | R | 27 | −66 | −18 | 4.62 | ||
| VIIIA → VIIIB | Vermis | 6 | −60 | −36 | 4.11 | ||
| VI | R | 12 | −72 | −18 | 4.07 | ||
| IX → VIIIB | Vermis | −3 | −60 | −39 | 4.06 | ||
| VIIIB → VIIB | Vermis | 0 | −72 | −27 | 3.73 | ||
| VIIIA | Vermis | 3 | −75 | −30 | 3.73 | ||
| VI → VI (Right) | Vermis, R | −6 | −75 | −18 | 3.42 | ||
| VIIIA | Vermis | 9 | −72 | −42 | 3.31 | ||
| IX → VIIIB | L | −12 | −48 | −51 | 6.03 | ||
| VIIIA → VIIIB | → | X | L | −30 | −42 | −45 | 4.73 |
| IX → VIIIB | R | 15 | −57 | −51 | 5.57 | ||
| X | R | 27 | −36 | −42 | 3.99 | ||
Note: L: Left; R: Right; V: Vermis; →: “activation extending to”; Sup: superior; s: sulcus; M1: primary motor area; FEF: frontal eye field.
Table 2.
Cerebellar activation for the contrasts: successful training trials versus control trials, place-based response versus control trials and sequence-based responses versus control trials and for the direct comparisons between place-based and sequence-based responses
| Side | MNI coordinates |
Z-score | |||
|---|---|---|---|---|---|
| Training versus control | |||||
| VIIA Crus I → VIIA Crus II | R | 27 | −81 | −36 | 4.25 |
| VIIA Crus I | L | −45 | −72 | −36 | 3.84 |
| VIIA Crus I | L | −33 | −81 | −33 | 2.9353 |
| I–IV | L | −9 | −42 | −15 | 2.6219 |
| Sequence-based versus control | |||||
| VIIA Crus I | R | 36 | −75 | −39 | 3.12 |
| VIIA Crus II | R | 27 | −78 | −39 | 3.047 |
| VIIA Crus II | R | 24 | −84 | −39 | 3.0298 |
| VIIA Crus I | R | 30 | −75 | −36 | 2.9704 |
| VIIA Crus I | R | 27 | −87 | −36 | 2.7704 |
| Place-based versus control | |||||
| VIIA Crus I | L | −45 | −72 | −36 | 3.28 |
| I–IV | L | −9 | −42 | −12 | 3.72 |
| I–IV | R | 9 | −33 | −12 | 3.4222 |
| VIIA Crus I | R | 27 | −81 | −33 | 2.7398 |
| Sequence-based versus place-based | |||||
| VIIa Crus I | R | 36 | −75 | −39 | 2.5841 |
| Place-based versus sequence-based | |||||
| VI | L | −36 | −42 | −33 | 4.4219 |
| VI → V → → X | L | −27 | −36 | −39 | 4.1354 |
| IX → X | R | 15 | −45 | −45 | 4.0973 |
| VI → VIIA Crus I | R | 12 | −78 | −21 | 3.555 |
| VI | L | −30 | −54 | −30 | 3.6505 |
| VI | L | −36 | −60 | −21 | 3.1794 |
| I–IV | 3 | −48 | 0 | 3.1505 | |
| VI | L | −15 | −66 | −21 | 3.1403 |
| VIIA Crus I → VI | R | 39 | −66 | −27 | 3.5479 |
| VI | R | 30 | −48 | −21 | 3.629 |
| VIIA Crus I | R | 36 | −72 | −27 | 3.3883 |
| VIIA Crus I | R | 39 | −75 | −24 | 3.2554 |
| VI | R | 30 | −63 | −30 | 3.4019 |
| VI | L | −6 | −78 | −21 | 2.7437 |
| VI | R | 33 | −60 | −27 | 3.0233 |
| VI | R | 36 | −54 | −24 | 2.7522 |
| IX | Vermis | −3 | −54 | −36 | 3.0283 |
| IX | −12 | −42 | −42 | 3.015 | |
| IX | L | −9 | −45 | −45 | 2.9809 |
| VI | L | −27 | −54 | −18 | 2.9401 |
| VI | R | 21 | −72 | −18 | 2.9566 |
| VIIA Crus II | R | 33 | −69 | −48 | 2.593 |
| VI | L | −30 | −54 | −30 | 3.6505 |
| VIIA Crus I | L | −45 | −72 | −27 | 2.7729 |
Note: This table reports all local maxima surviving an uncorrected threshold of P < 0.005 (in bold, P < 0.001).
Functional Connectivity Analysis on Regions of Interest (ROI) Delineated at the Individual Level
In a second step, we focused our analysis of the probe (place-based and sequence-based) trials on the activity of the bilateral cerebellum and hippocampus, as well as medial prefrontal and medial parietal cortices, since recent studies described anatomical connections between those latter structures and the cerebellum (Clower et al. 2001; Kelly and Strick 2003). With that aim, we defined spherical volumes of interest around a priori locations of these structures taken from the literature on navigation learning and mental representation of space, and checked that there was a group activation <10 mm around these coordinates in either contrast: place-based response—control, sequence-based response—control or training—control (see Supplementary Table 1). We then searched corresponding activation at the individual level: if a local maximum with uncorrected P < 0.05 was found <10 mm away from the group maximum, an individual ROI was defined as a 8-mm sphere around that maximum. Individual activation allowed us to define 6 ROIs, in the right and left hippocampus, right and left cerebellar lobule VIIA, the medial parietal cortex and the medial Pfc, in 13 subjects out of 17; the other 4 subjects for whom we could not find activation at the individual level for at least one region of interest were not included in the connectivity analysis. All coordinates are reported in MNI space.
We tested the functional connectivity between all regions of interest using a correlation matrix analysis. This analysis was performed on the deconvolved time series, extracted from the ROIs and restricted to the time periods when subjects were located in the first alley of the virtual environment, at the beginning of each trial. All these periods were concatenated to form a unique time series per region, subject and condition (place-based or sequence-based). For each subject and condition, correlations were computed between the time series of the 6 regions defined above. To make the correlations comparable between conditions, we computed them on time series of the same length. To avoid confounds due to different numbers of trials in the place-based and sequence-based conditions and variable durations spent in the first alley, correlations were computed on subsamples of data, the size of which was based on the shortest time series. Using bootstrap analysis, correlations between structures were computed using all possible subsamples of the shortest size in the initial time series. The correlation value was the average of all correlations obtained on subsamples.
Egocentric and allocentric scores correspond to a participant's spontaneous tendency to use the sequence-based and the place-based strategy respectively. Thus, the egocentric score corresponds to the number of sequence-based probe responses over the total number of probe tests. We computed the correlations between the values of the functional connectivity analysis for each participant and their egocentric and allocentric scores. The significant results (P < 0.05) are shown in Figure 2C and D.
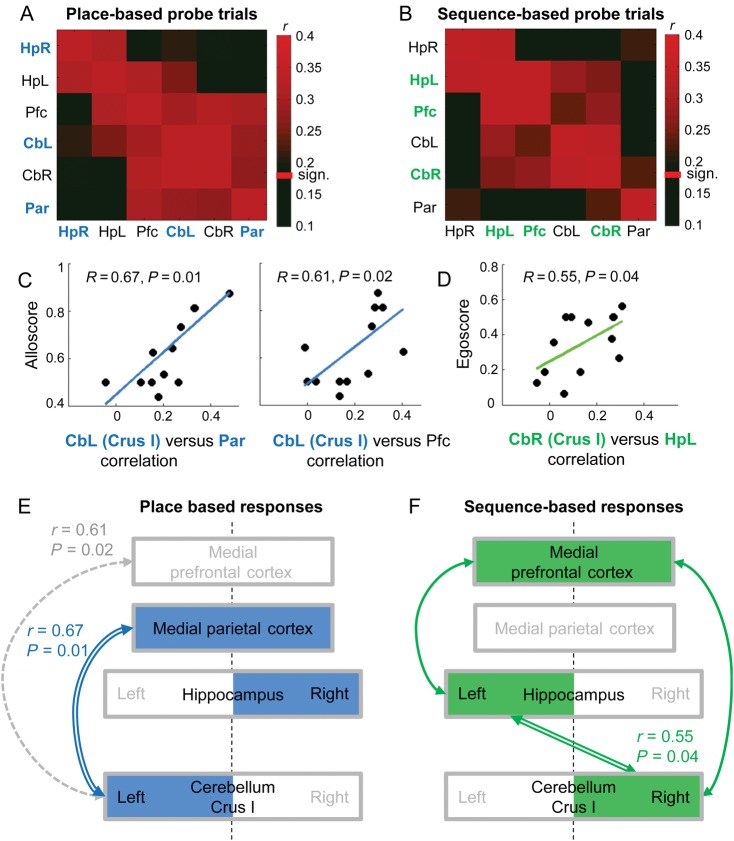
Figure 2.
(A and B) Correlation matrices between the structures of interest for place-based and sequence-based probe trials averaged over the 13 subjects included into the connectivity analysis. Correlation is significant above 0.18. The names of the structures found activated in the first alley of probe trials for place-based responses (when compared with control) are highlighted in bold and blue. Names of structures activated for sequence-based responses are highlighted in bold and green. (C) Positive correlation between the alloscore (i.e., tendency to use the place-based strategy) and the medial parietal-CbL (Crus I) correlation on all trials (N = 13) (D) Positive correlation between the egoscore (i.e., tendency to use the sequence-based strategy) and the left hippocampus-CbR (Crus I) correlation on all trials (N = 13).
Results
To minimize the impact of the different behaviors and stimulations which might occur along the different trials, all analyses were conducted in the first alley of the maze.
Conjunction and Contrast Analyses
The analysis in this section was performed on all 17 successful participants. We first focused on motor aspects of the tasks by determining the regions commonly activated during all types of trials (including training, control, and probe trials here) with a conjunction analysis (Friston et al. 1999). We found activation in the posterior cerebellum (cerebellar vermis VI, VIIIA, VIIIB; right lobules VI, IX, X; left lobules VIIIA, IX), primary motor cortex, lateral intraparietal area, and the thalamus (see Fig. 1B and Table 1).
We then contrasted training trials versus control trials to highlight activity related to cognitive processes. Unlike the conjunction analysis, this contrast showed increased activation in bilateral cerebellar lobules VIIA Crus I (Table 2), bilateral hippocampi and medial parietal and medial Pfc (Fig. 1D, Supplementary Table 2).
To further explore the specific areas involved in the place-based and sequence-based strategy, we split the analysis of probe trials according to the strategy. Contrasting probe trials in which place-based responses were made (so called place-based trials, see an example in Fig. 1E) versus control trials revealed activation in left cerebellar lobule VIIA Crus I (Table 2), right hippocampus, and bilateral medial parietal cortex (Fig. 1F and Supplementary Table 2). Contrasting probe trials in which a sequence-based response was made (then called sequence-based trials, Fig. 1G) versus control trials showed a complementary pattern with right cerebellar lobule VIIA Crus I (Table 2), left hippocampus, and left medial Pfc (Fig. 1H and Supplementary Table 2). Supplementary Figure 2 provides a plot of activity in all conditions for each region described above.
Directly contrasting sequence-based versus place-based trials revealed left-side lateralized activation of the parietal cortex (−51, −15, 24), left posterior insula (−51, −33, 6), medial Pfc (peak in the left hemisphere at −3, 60, 18 identical to the sequence-based vs. control maximum), and right lobule VIIA Crus I (subthreshold, see Table 2). Whereas the reverse contrast (place-based trials vs. sequence-based trials) showed an increased bilateral activation of the superior parieto-occipital sulcus (right: 18, −54, 15, left: −15, −57, 18), the right parahippocampus (24, −39, −6), posterior cerebellum (bilateral lobule VI, right lobule I–IV, IX, and VIIA and left VIIA subthreshold, see Table 2).
Functional Connectivity Between the Regions of Interest (ROI)
The analysis in this section was performed on the 13 subjects for whom 6 ROIs could be delineated at the individual level (4 subjects were excluded because no individual activation could be found in one of the ROIs at least, see Materials and Methods): left and right cerebellar lobules VIIA Crus I, left and right hippocampi, medial parietal, and medial prefrontal cortices. To investigate relationships between the time courses of activation in these regions depending on the navigation strategy, we performed a correlation analysis of the deconvolved time series corresponding to the first alley of each probe trial. Correlations were computed at the subject level and averaged across subjects to build connectivity matrices separately for place-based and sequence-based responses (see Fig. 2).
Place-based and sequence-based responses revealed different patterns of functional connectivity between those 6 structures, which do not merely correspond to the pattern of activation described previously. For both conditions, the hippocampus and the cerebellum showed strong correlations between left and right hemispheres (see HpR/HpL and CbL/CbR in Fig. 2A and B). We also observed consistently strong correlations between medial Pfc and left hippocampus (see Pfc/HpL in Fig. 2A,B). In place-based responses, the medial Pfc, although not activated, was strongly correlated with all other ROIs, except the right hippocampus (HpR). As for the regions activated in the place-based versus control contrast, only the left lobule VIIA Crus I and the medial parietal cortex were functionally connected (CbL/Par in Fig. 2A). In contrast, for sequence-based responses, the 3 key structures that were activated in the first alley when compared with control (i.e., right cerebellar lobule VIIA Crus I; left hippocampus and medial Pfc) all displayed significant correlated time-series (see CbR, HpL, and Pfc in Fig. 2B).
Beyond mere strategy-related activation and functional connectivity, we also assessed how these markers of activity correlated with behavior (alloscore and egoscore). Three correlations appeared significant (P < 0.05). Participants' spontaneous tendency to use a place-based strategy significantly correlated with the strength of the correlation between left lobule VIIA Crus I and the medial parietal cortex as well as with the strength of the correlation between left lobule VIIA Crus I and the medial Pfc (allocentric score, Fig. 2C). In contrast, a participant's tendency to use a sequence-based strategy correlated with the functional correlations between right lobule VIIA and left hippocampus (Fig. 2D).
Discussion
We investigated the implication of the cerebellum in 2 different navigation strategies, both dependent on the hippocampus. The left lobule VIIA Crus I, the right hippocampus and the medial parietal cortex are involved in place-based navigation, whereas the right lobule VIIA Crus I, the left hippocampus and the medial Pfc are implicated in sequential egocentric representation. In training trials, where both strategies are encoded in parallel (Igloi et al. 2009), both networks are simultaneously activated (bilateral lobules VIIA Crus I, bilateral hippocampi, medial prefrontal, and parietal cortices). These results support the idea that the left and right lobules VIIA Crus I of the cerebellum are part of 2 functional loops involved respectively in place-based and sequence-based navigation (Fig. 2E). This therefore suggests the existence of functional zones of the cerebellum involved in cognitive aspects of navigation.
Despite accumulating evidence supporting the idea that the cerebellum contributes to nonmotor function, it has been suggested that its implication in cognitive tasks such as navigation might be explained by task-related movements (Glickstein 2007), including eye movements (Timmann and Daum 2010). To assess whether the observed activation can be accounted for by task-related movements, we computed all results in the departure alley of the maze, where motor demands are identical across all trials (training, probe and control trial) and subjects. Thus, the contrasts between probe trials and control trials, used to highlight the regions supporting place- and sequence-based navigation, subtract out any common motor aspects.
This analysis also provides a way to explicitly look for movement-related activation common to all conditions, using a conjunction analysis. The identified activation of the cerebellar lobules VI, VIIIA,B, IX, X, the primary motor cortex and the lateral intraparietal, thalamus and visual areas is consistent with sensorimotor related network. Lobules VI and VIII form a motor loop with the primary motor and lateral intraparietal cortices (Prevosto et al. 2010; Ramnani 2012) via the thalamus (reviewed in Strick et al. 2009). Cerebellar lobules VI, VIIIA, and VIIIB have been previously found activated in relation to eyeblink (Timmann et al. 2003), eye movement and eye–hand coordination with visual feedback (Miall et al. 2001; Debaere et al. 2003), finger tapping, and somatosensorial aspect (VIIIB) (reviewed in Stoodley and Schmahmann 2009). Thus our activations in cerebellar lobules VI, VIIIA,B are consistent with the eye and finger movements performed by the subjects during the virtual navigation task and is also consistent with the activity observed in frontal and parietal eye fields (see Table 1; Lobel et al. 2001; Simon et al. 2002). Cerebellar lobules IX and X have been described as a target of visual (Glickstein et al. 1994) and vestibular projections (Shaikh et al. 2005). They have been recently implicated in optic flow processing in the absence of vestibular stimulation (Yakusheva et al. 2013). Their activation in our study is consistent with the dynamic visual stimulation induced by virtual navigation.
In contrast, none of the lobules VI, VIIIA,B, IX, X were significantly activated in the place- or sequence-based navigation compared with control trials. Equally, lobule VIIA Crus I, which was active in these conditions, has been associated with cognitive aspects of different tasks, such as executive function (Stoodley and Schmahmann 2009), working memory (N-back) (Stoodley et al. 2010), explicit learning of a sequence of finger movements (Lehéricy et al. 2005), or increases of performance accuracy during eye-hand synchrony (Miall et al. 2001). Directly contrasting the first alley of sequence versus place responses revealed a specific activation (subthreshold) of this lobule VII (Crus I). Contrasting the first alley of place versus sequence probe trials revealed additional activation of lobules VI, IX, and X suggesting a larger implication of the visual processing in the place-based strategy compared with the sequence-based strategy. This fits with our recent finding that place-based and sequence-based navigation are preferentially driven by landmark information and memory respectively (Cabral et al. 2014).
The structures activated in the first alley of probe trials for which subjects chose the place-based strategy (contrast place-based vs. control, see colored boxes in Fig. 2E) only partially tended to act in a coordinated fashion (see solid lines in Fig. 2E). Indeed, the time-course of activation in left lobule VIIA Crus I was significantly correlated with the time-course of activation in medial parietal cortex but not with that of the right hippocampus. This pattern of activation and correlations suggests that cerebellar lobule VIIA Crus I and medial parietal cortex perform related processing. Accordingly, the tendency of one subject to use the place-based strategy is correlated with the functional connectivity between cerebellum (Crus I) and the medial parietal cortex (precuneus). By contrast, the hippocampus may provide a complementary function in support of place-based responses. As reported previously (Igloi et al. 2010), the lateralized right activation of the hippocampus was observed whatever the side of the first turn, suggesting that this activation does not reflect the preparation of a left or of a right turn but rather the place-based representation (see also Spiers and Maguire 2006).
It has been suggested that the posterior parietal cortex supports transformation between egocentric representations viewed by the subject and allocentric representations stored in the hippocampal formation. Manipulation of spatial information (combination of sensory information and spatial memory) for the purposes of planning or navigation has been proposed to occur within the posterior parietal cortex, most likely within the precuneus (Byrne et al. 2007; Burgess 2008). Idiothetic information coming from the cerebellum (Rochefort et al. 2011) could then be processed by the parietal cortex; explaining the functional connection observed between the cerebellum (Crus I) and the medial parietal cortex. In addition, parietal cortex is a common target for projections from both hippocampal formation and cerebellum (Clower et al. 2001), which are coactivated in this condition. Although the medial Pfc is not significantly activated in the place-based strategy, we found that this structure was functionally connected to the left cerebellum (Crus I) and that the strength of this connection was a good marker of the participant's tendency to use the place-based strategy. This suggests that some regions might drive the behavior although they are not specifically more activated by the task.
In contrast, for sequence-based response, we found not only coactivation of right cerebellum VIIA Crus I, medial Pfc and left hippocampus but also correlated activity between those 3 structures. The functional connectivity observed between cerebellum VIIA Crus I and medial Pfc is in line with previously described anatomo-functional circuits (Kelly and Strick 2003; Krienen and Buckner 2009; O'Reilly et al. 2010; Buckner et al. 2011). Functional connectivity of cerebellum, prefrontal, and hippocampus is reminiscent of recent results on early motor sequence learning (Doyon et al. 2002; reviewed in Penhune and Steele 2012) and particularly when accurate spatio-temporal prediction of finger movements is involved (Onuki et al. 2015). Here we find this connectivity when the sequence is retrieved during the probe test. We additionally show that the connectivity between cerebellum (VIIA Crus I) and the left hippocampus is correlated with the tendency of the participant to use the sequence-based strategy. This suggests that, unlike classical motor sequence learning, this functional loop is involved in the use of a well-known spatio-temporal sequence.
In sum, we report functional coactivations between cerebellum and hippocampus in humans during spatial navigation, which suggest that the role of the cerebellum is not limited to sensori-motor processing. Rather, lobule VIIA combines with contralateral hippocampus and either the medial prefrontal or the medial parietal cortex to guide navigation using mnemonic representations of sequences or places.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Wayfinding grant of the EU, the Agence Nationale de la Recherche, the Fondation pour la Recherche Médicale (France) and the Medical Research Council and the Wellcome Trust (UK). C.F.D. is supported by the European Research Council (ERC-StG RECONTEXT 261177) and by The Netherlands Organisation for Scientific Research (NWO-Vidi 452-12-009). The group of L.R.R. is member of the Bio-Psy Labex and the ENP foundation.
Supplementary Material
Notes
We thank Joern Diedrichsen for useful discussions and the Wellcome Trust Centre for Neuroimaging at UCL for help and scanning facilities. Conflict of Interest: The authors declare no competing financial interests.
References
- Arleo A, Rondi-Reig L. 2007. Multimodal sensory integration and concurrent navigation strategies for spatial cognition in real and artificial organisms. J Integr Neurosci. 6(3):327–366. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. 1999. The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage. 10(4):448–459. [DOI] [PubMed] [Google Scholar]
- Buckner RL. 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 80(3):807–815. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. 2008. Spatial cognition and the brain. Ann N Y Acad Sci. 1124:77–97. [DOI] [PubMed] [Google Scholar]
- Burguiere E, Arleo A, Hojjati M, Elgersma Y, De Zeeuw CI, Berthoz A, Rondi-Reig L. 2005. Spatial navigation impairment in mice lacking cerebellar LTD: a motor adaptation deficit? Nat Neurosci. 8:1292–1294. [DOI] [PubMed] [Google Scholar]
- Burguiere E, Arabo A, Jarlier F, De Zeeuw CI, Rondi-Reig L. 2010. Role of the cerebellar cortex in conditioned goal-directed behavior. J Neurosci. 30:13265–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. 2007. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 114:340–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral HO, Vinck M, Fouquet C, Pennartz CMA, Rondi-Reig L, Battaglia FP. 2014. Oscillatory dynamics and place field maps reflect sequence and place memory processing in hippocampal ensembles under NMDA receptor control. Neuron. 81:402–415. [DOI] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. 2005. Basal ganglia and cerebellar inputs to “AIP”. Cereb Cortex. 15:913–920. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. 2001. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 21:6283–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. 2003. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 19(3):764–776. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Glover GH. 1998. Dissociation of frontal and cerebellar activity in a cognitive task: Evidence for a distinction between selection and search. Neuroimage. 7(4):368–376. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. 1997. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 17(24):9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. 2009. A probabilistic MR atlas of the human cerebellum. Neuroimage. 46:39–46. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Grafton S, Albert N, Hazeltine E, Ivry RB. 2006. Goal-selection and movement-related conflict during bimanual reaching movements. Cereb Cortex. 16:1729–1738. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. 2002. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA. 99:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. 1999. Multisubject fMRI studies and conjunction analyses. Neuroimage. 10:385–396. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. 2007. “Motor cognition”—what is it and is the cerebellum involved? Cerebellum. 6(3):232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong JH, Li JQ, Fox PT. 1996. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 272(5261):545–547. [DOI] [PubMed] [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M. 1997. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 8(3):739–744. [DOI] [PubMed] [Google Scholar]
- Glickstein M. 2007. What does the cerebellum really do? Curr Biol. 17(19):R824–R827. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Gerrits N, Kralj-Hans I, Mercier B, Stein J, Voogd J. 1994. Visual pontocerebellar projections in the macaque. J Comp Neurol. 349(1):51–72. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N. 2003. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 37(5):877–888. [DOI] [PubMed] [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N. 2007. Cerebellar contributions to working memory. Neuroimage. 36(3):943–954. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. 2003. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 23(13):5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. 2010. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci USA. 107:14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Zaoui M, Berthoz A, Rondi-Reig L. 2009. Sequential egocentric strategy is acquired as early as allocentric strategy: Parallel acquisition of these two navigation strategies. Hippocampus. 19:1199–1211. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. 2003. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, et al. 2013. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 13:151–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. 2009. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. 2005. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 102(35):12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. 1993. Cognitive and language functions of the human cerebellum. Trends Neurosci. 16(11):444–447. [DOI] [PubMed] [Google Scholar]
- Lobel E, Kahane P, Leonards U, Grosbras M, Lehericy S, Le Bihan D, Berthoz A. 2001. Localization of human frontal eye fields: anatomical and functional findings of functional magnetic resonance imaging and intracerebral electrical stimulation. J Neurosurg. 95(5):804–815. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. 1998. Knowing where and getting there: a human navigation network. Science. 280(5365):921–924. [DOI] [PubMed] [Google Scholar]
- Malm J, Kristensen B, Karlsson T, Carlberg B, Fagerlund M, Olsson T. 1998. Cognitive impairment in young adults with infratentorial infarcts. Neurology. 51(2):433–440. [DOI] [PubMed] [Google Scholar]
- Mandolesi L, Leggio MG, Graziano A, Neri P, Petrosini L. 2001. Cerebellar contribution to spatial event processing: involvement in procedural and working memory components. Eur J Neurosci. 14(12):2011–2022. [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-Garcia JM, da Guarda SN, Gerwig M, Habas C, Hagura N, Ivry RB, Marien P, et al. 2012. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 11(2):457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellet E, Briscogne S, Tzourio-Mazoyer N, Ghaem O, Petit L, Zago L, Etard O, Berthoz A, Mazoyer B, Denis M. 2000. Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. Neuroimage. 12(5):588–600. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H. 2001. The cerebellum coordinates eye and hand tracking movements. Nat Neurosci. 4(6):638–644. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. 1997. Cerebellar output channels. Int Rev Neurobiol. 41:4161–4182. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. 2001. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 21:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Elkins W, Resnick SM. 2006. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging. 27(7):965–972. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG. 2007. Cerebellar information processing and visuospatial functions. Cerebellum. 6(3):214–220. [DOI] [PubMed] [Google Scholar]
- Molinari M, Petrosini L, Misciagna S, Leggio MG. 2004. Visuospatial abilities in cerebellar disorders. J Neurol Neurosurg Psychiatry. 75(2):235–240. [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. 2002. A PET study of stimulus- and task-induced semantic processing. Neuroimage. 15(4):927–935. [DOI] [PubMed] [Google Scholar]
- Onuki Y, Van Someren EJ, De Zeeuw CI, Van der Werf YD. 2015. Hippocampal–cerebellar interaction during spatio-temporal prediction. Cereb Cortex. 25:313–321. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. 2010. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Steele CJ. 2012. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res. 226:579–591. [DOI] [PubMed] [Google Scholar]
- Petrosini L, Leggio MG, Molinari M. 1998. The cerebellum in the spatial problem solving: a co-star or a guest star? Prog Neurobiol. 56(2):191–210. [DOI] [PubMed] [Google Scholar]
- Prevosto V, Graf W, Ugolini G. 2010. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 20:214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. 2012. Frontal lobe and posterior parietal contributions to the cortico-cerebellar system. Cerebellum. 11:366–383. [DOI] [PubMed] [Google Scholar]
- Ramnani N. 2006. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 7:511–522. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. 2006. Cerebellar damage produces selective deficits in verbal working memory. Brain. 129:306–320. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L. 2011. Cerebellum shapes hippocampal spatial code. Science. 334:385–389. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Lefort JM, Rondi-Reig L. 2013. The cerebellum: a new key structure in the navigation system. Front Neural Circuits. 7:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L, Burguiere E. 2005. Is the cerebellum ready for navigation? Prog Brain Res. 148:201–212. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Le Marec N, Caston J, Mariani J. 2002. The role of climbing and parallel fibers inputs to cerebellar cortex in navigation. Behav Brain Res. 132(1):11–18. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. 2006. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci. 26:4071–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. 2001. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci. 13(6):829–843. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. 1991. An emerging concept—the cerebellar contribution to higher function. Arch Neurol-Chicago. 48(11):1178–1187. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. 2002. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 33(3):475–487. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. 2005. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 93(2):853–863. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. 2006. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 31(4):1826–1840. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. 2012. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 11(2):352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2009. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 44(2):489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. 2010. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 23:(1–2):65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. 2012. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage. 59(2):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. 2009. Cerebellum and nonmotor function. Annu Rev Neurosci. 32:413–434. [DOI] [PubMed] [Google Scholar]
- Timmann D, Daum I. 2010. How consistent are cognitive impairments in patients with cerebellar disorders? Behav Neurol. 23(1–2):81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Dimitrova A, Hein-Kropp C, Wilhelm H, Dorfler A. 2003. Cerebellar agenesis: clinical, neuropsychological and MR findings. Neurocase. 9(5):402–413. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Petrosini L, Caltagirone C. 2007. Cortical networks of procedural learning: evidence from cerebellar damage. Neuropsychologia. 45(6):1208–1214. [DOI] [PubMed] [Google Scholar]
- Wallesch CW, Horn A. 1990. Long-term effects of cerebellar pathology on cognitive functions. Brain Cogn. 14(1):19–25. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. 2006. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3T and 1.5T. NeuroImage. 33:493–504. [DOI] [PubMed] [Google Scholar]
- Xiang HD, Lin CY, Ma XH, Zhang ZQ, Bower JM, Weng XC, Gao JH. 2003. Involvement of the cerebellum in semantic discrimination: An fMRI study. Hum Brain Mapp. 18(3):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakusheva TA, Blazquez PM, Chen A, Angelaki DE. 2013. Spatiotemporal properties of optic flow and vestibular tuning in the cerebellar nodulus and uvula. J Neurosci. 33(38):15145–15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.