Abstract
In order to investigate the significance of antibiotics for the producing organism(s) in the natural habitat, we screened specimens of the polyporicolous fungus Hypocrea pulvinata growing on its natural hosts Piptoporus betulinus and Fomitopsis pinicola. Results showed that a particular group of nonribosomally biosynthesised antibiotic polypeptides, the peptaibiotics, which contain the nonproteinogenic marker amino acid α-aminoisobutyric acid (Aib), was produced in the natural habitat by the fungicolous producer and, consequently, released into the host. Using liquid chromatography coupled to electrospray high-resolution mass spectrometry we detected especially 19-, but also 11-, 18-, and 20-residue peptaibiotics in the five infected specimens analysed. Structures of peptaibiotics found were confirmed by analysing the peptaibiome of pure agar cultures obtained by single-ascospore isolation from the specimens. The 19-residue peptaibols were determined as deletion sequences of the trichosporins B lacking the Aib residue in position 6. Notably, 26 of the 28 peptaibiotics sequenced were novel; therefore the name ‘hypopulvins’ was introduced. Considering not only the ubiquity of both the two host species but also the highly specific association between H. pulvinata and P. betulinus/F. pinicola, and the abundance of this fungicolous species in north temperate regions of the world, a decisive role for the peptaibiotics detected in this study is predicted, which may act as mediators of the complex interactions between the basidiomycetous host and its fungicolous ascomycete ‘partner’. Structural analogies of the hypopulvins, particularly with other 18-, 19-, and 20-residue peptaibiotics, suggest that the hypopulvins are forming transmembrane ion channels and could thus support the hypothesis of a parasitic lifestyle of the fungicolous producer.
Keywords: Fungicolous fungi, HPLC/QTOF–ESI–HRMS, Hypocrea pulvinata, Metabolite profiling, Peptaibiotics, Peptaibols
Introduction
Natural products of fungal origin
As of 2010 approximately half a million natural products were known of which 60 000–80 000 are estimated to be of microbial origin. Approximately half of the latter display some kind of biological activity (Bérdy 2012). The fungal kingdom, which currently comprises more than 98 000 validly described species (Kirk et al. 2008) contributes some 30 000 natural products of which 15 000–16 000 are bioactive. Of these, around 11 250 originate from microscopic fungi (Bérdy 2012). Peptide antibiotics constitute a considerable part of those metabolites, including therapeutically important β-lactam antibiotics (penicillins, cephalosporins), which account for more than 65 % of the world antibiotics market. However, the significance of other, nonantibiotic peptide drugs of fungal origin is comparable – the immunosuppressant market is still dominated by the nonribosomal biosynthesised cyclosporine A (Elander 2003; Demain & Sanchez 2009).
Peptaibiotics – nonribosomally biosynthesised fungal peptide antibiotics containing α,α-dialkyl α-amino acids
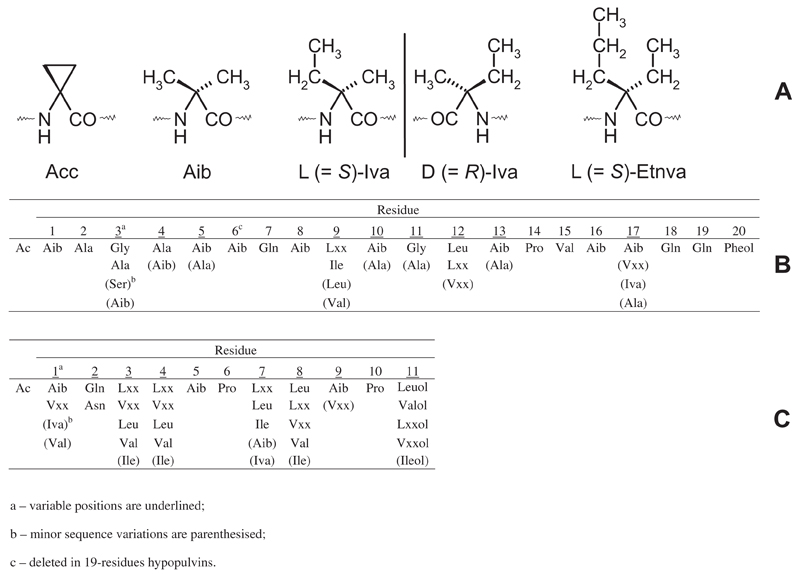
During the past two decades, a constantly growing group of peptide antibiotics, the peptaibiotics, have started to regain particular interest because of their unique bioactivities, resulting from their amphipathicity and helical conformations. Peptaibiotics are defined as nonribosomally biosynthesised, linear or cyclic polypeptide antibiotics of exclusively fungal origin, which (i) have a molecular weight between 500 and 2200 Da, thus containing 4–21 residues; (ii) show a high content of the marker α-aminoisobutyric acid (Aib) as well as other α,α-dialkylamino acids; (iii) are characterised by the presence of other nonproteinogenic amino acids and/or lipoamino acids; (iv) possess an acylated N-terminus, and (v) in the case of linear peptides, have a C-terminal residue that in large part consists of a free or acetylated amide-bonded β-amino alcohol. The C-terminus might also be an amine, amide, free amino acid, 2,5-diketopiperazine, or sugar alcohol. The majority of Aib-containing peptides carry a C-terminal residue representing a β-amino alcohol, and this subgroup is, therefore, referred to as peptaibols. As a result of the nonribosomal biosynthesis on large multifunctional peptide synthetases, highly complex mixtures of homologous and positionally isomeric peptides are produced. The latter phenomenon is commonly referred to as microheterogeneity, i.e., exchange of single or few amino acids or C-terminal amino alcohols, respectively (Degenkolb & Brückner 2008). Therefore, sophisticated, multidimensional analytical approaches such as HPLC coupled to tandem electrospray ionisation (ESI)-MSn or Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and chiral capillary gas-chromatography/selected ion monitoring electron impact MS became the methods of choice for structure elucidation of peptaibtiotics. To date, the presence of peptide-bound α,α-dialkyl α-amino acids, i.e., mostly Aib, but also d- and l-isovaline (Iva), l-α-ethylnorvaline (Etnva) as well as 1-aminocyclopropane-1-carboxylic acid (Acc) (for structures, see Fig 1), has been confirmed in acidic hydrolysates of more than 30 genera of fungi (Brückner et al. 2009). Among those the ubiquitous genus Trichoderma/Hypocrea, which currently contains approximately 200 validly described species (Jaklitsch 2009, 2011; Samuels & Ismaiel 2011; Jaklitsch & Voglmayr 2012; Jaklitsch et al. 2012; Samuels et al. 2012a, b) is generally regarded as the richest source of peptaibiotics (Degenkolb & Brückner 2008). Notably, d- and l-Iva may even occur in different positions of one and the same sequence, as was shown for clonostachin, neoefrapeptins, and integramides, for review see Degenkolb & Brückner (2008). Obviously these results contradict the still widespread belief that α,α-dialkyl α-amino acids do not or rarely occur in the biosphere and, if detected in the environment, are definitely of extraterrestrial origin. In fact fungal species, which contain peptaibiotic-producing strains, are ubiquitous and cosmopolitan, even occurring in marine, Arctic and Antarctic regions (Brückner et al. 2009). However, differentiation between biotically and abiotically synthesised Aib and homologues is possible by stable carbon and nitrogen isotopic composition (Elsila et al. 2011).
Fig 1. (A) Structures and configurations of α,α-dialkylamino acids found in peptaibiotics. (B) Building scheme of 20-residue SF1 peptaibiotics, produced by Hypocrea pulvinata. (C) General building scheme of 11-residue peptaibols found in SF4.
Bioactivities of peptaibiotics from Trichoderma/Hypocrea
In the past decade, many Trichoderma species, such as Trichoderma ovalisporum (Holmes et al. 2004), Trichoderma koningiopsis (Samuels et al. 2006a), and Trichoderma citrinoviride (Maddau et al. 2009) have gained increasing interest as potential biocontrol agents in plant protection. Further promising examples include Trichoderma paucisporum and Trichoderma theobromicola, displaying in vitro-activity against frosty pod rot of cocoa, Moniliophthora roreri (Samuels et al. 2006b), and Trichoderma martiale. In small-scale in situ field trials the latter proved highly effective against black pod rot of cocoa caused by Phytophthora palmivora (Hanada et al. 2009). The most successful story in cocoa biocontrol, however, is the recent approval of ‘Tricovab’ (Anonymous Novembro 2011/Fevereiro 2012), a formulation against Crinipellis (=Moniliophthora) perniciosa, the Witches’ broom pathogen, containing the hyperparasite Trichoderma stromaticum (Pomella et al. 2007). Although this species has been shown to produce five 18-residue peptaibols, trichostromaticins A-E (Degenkolb et al. 2006), the mechanism(s) contributing to its remarkable in vivo bioactivity have not been completely resolved yet. Peptaibiotics are prone to play a key role in the infection process of a host by a fungicolous species because of their unique ability of forming voltage-gated ion channels. This phenomenon is best described by the dipole flip-flop gating model (Menestrina et al. 1986). Their well-documented membrane activity, however, may also account for other striking bioactivities of peptaibiotics, such as neurolepsy (Berek et al. 2009), inhibition of amyloid β-peptide formation (Hosotani et al. 2007), inhibition of HIV-1 integrase (Singh et al. 2002), suppression of tumour cells, targeted Ca2+-mediated apoptosis and autophagy in human hepatocellular carcinoma cells (Shi et al. 2010), as well as induction of defence responses and systemic resistance in tobacco against tobacco mosaic virus (Luo et al. 2010), and programmed cell death in fungal plant pathogens (Shi et al. 2012).
In vivo-detection of peptaibiotics
Reports on the occurrence of peptaibiotics in the natural habitat of the producer(s) have sparsely been published so far. Most of almost 1000 individual sequences of peptaibiotics known to date have been sequenced in extracts of fungal cultures grown under artificial laboratory conditions. The first example of peptaibiotics obtained from natural specimens was the isolation of hypelcins A and B obtained from 2 kg of dried, crushed stromata of Hypocrea peltata (Fujita et al. 1984a; Matsuura et al. 1993, 1994). However, it appears that in recent years only a single contribution directly addressed the detection of peptaibols, viz. the 16-residue antiamoebins, under in vivo-conditions (Lehr et al. 2006) whereas other authors most probably have overlooked the presence of a fungicolous species in the examined material. Thus, the formation of peptaibiotics was accidentally ascribed to basidiomycetous hosts, which have rather been infested by an unrecognised fungicolous fungus, such as Sepedonium sp., for review see Degenkolb & Brückner (2008).
The present study is thus aimed at the question as to whether peptaibiotics formation under the conditions of the natural habitat of the producer(s) is either a rather infrequent or a more common phenomenon. If the latter hypothesis is true, peptaibiotics might, indeed, play a decisive role in the antibiotic-based colonisation and defence of the natural substrate, as was recently demonstrated for the antiamoebins from Stilbella fimetaria (Lehr et al. 2006). Given that this coprophilous species produces fungicidal amounts of peptaibols on herbivore dung, other highly specialised hypocrealean fungi occupying ecological niches must be considered as additional model systems for these investigations.
From an ecophysiological point of view, necrotrophic mycoparasites within the genus Trichoderma/Hypocrea are best suited for such study. This is due to the fact that a parallel formation and synergistic action of hydrolytic enzymes, above all as β-1,3-glucanase, and peptaibiotics have previously been attributed an important role in mycoparasitism between Trichoderma atroviride (originally misidentified as Trichoderma harzianum) and its fungal hosts such as Botrytis cinerea (Schirmböck et al. 1994; Lorito et al. 1996).
Choice of the model organism
Hypocrea pulvinata, the Ochre Cushion, a rather common fungus that occurs on decaying basidiomes of polypores in north temperate regions of the world (Jaklitsch 2011), was chosen as a first model organism. Notably, this polyporicolous species is assumed to display host specificity as it has only been unambiguously identified on the Birch Bracket Piptoporus betulinus and the Red-banded Bracket Fomitopsis pinicola so far. Records from other aphyllophoralean taxa such as Ganoderma sp. could not been confirmed in recent years; and the claimed association with Laetiporus sulphureus was recently shown to be based on misidentifications of the respective host (Jaklitsch 2011).
To prove our hypothesis of peptaibiotic production by members of the order Hypocreales under in vivo-conditions, basidiomes of P. betulinus and F. pinicola, infected with H. pulvinata, were collected from different locations. Cultures obtained from the respective teleomorphs were analysed using a peptaibiomics approach as described earlier (Krause et al. 2006; Degenkolb et al. 2012).
Materials and method
Chemicals
All solvents used, acetonitrile (MeCN), methanol (MeOH), CH2Cl2, and formic acid (FA), were of LC–MS grade from Sigma–Aldrich (Steinheim, Germany). Water was purified by a Merck-Millipore Milli-Q system (Schwalbach/Ts., Germany).
Isolation of pure agar cultures of anamorphs
Basidiomes of Piptoporus betulinus and Fomitopsis pinicola infected with Hypocrea pulvinata were collected from five different locations in Austria and Russia (I–V; Table 1). Pure agar cultures were obtained by single-ascospore isolations from the respective specimens as described by Jaklitsch (2009).
Table 1. Habitat and geographic origin of Hypocrea pulvinata isolates included in this study.
| Isolate | Host | Collecting information | Culture |
|---|---|---|---|
| I | Old basidiome of Piptoporus betulinus | Austria, Styria, Liezen, Untertal S Schladming, bog near Gasthof Tetter, 1020 m s.m., grid square 8648/1, 12 Jun. 2011, H. Voglmayr & Greilhuber | CBS 133228 |
| II | Basidiome of Piptoporus betulinus on Betula pendula | Austria, Lower Austria, Wien-Umgebung, Mauerbach, 31 Jul. 2011, W.M. Jaklitsch | CBS 133229 |
| III | Basidiome of Piptoporus betulinus on Betula pendula | Austria, Lower Austria, Wien-Umgebung, Mauerbach, 31 Jul. 2011, W.M. Jaklitsch | CBS 133230 |
| IV | Decaying basidiome of Piptoporus betulinus on Betula pendula | Russia, Republic of Buryatia, Bargusin district, Lake Baikal, ‘Holy Nose’ Peninsula; 53° 35'54.9〞 N, 108° 52'11.2〞 E; 24 Jul. 2011, A. Berg & H. Dörfelt | |
| V | Decaying basidiome of Fomitopsis pinicola on Pinus sibirica | Russia, Republic of Buryatia, Severo-Baykalsky district, Nizhneangarsk; 55° 46'39.4〞 N, 109° 35'49.9〞 E; 24 Jul. 2011, A. Berg & H. Dörfelt |
Extraction of specimens
Few milligram of teleomorph stromata were extracted with 40 ml of a mixture of CH2Cl2–MeOH (1:1v/v), centrifuged, the solvent was evaporated in vacuo (Rotavapor R-215, Büchi, Essen, Germany) or using a stream of nitrogen. For screening on the micrOTOF-Q II, the extracts were cleaned up over Sep-Pak Classic C18 cartridges (Waters, Eschborn, Germany) as described by Krause et al. (2006) whereas the crude extracts (redissolved in MeOH) were analysed directly using the more sensitive maXis QTOF. Hymenophores of apparently uninfected basidiomes of P. betulinus were investigated as a control.
Cultivation and extraction of pure cultures
Cultures of specimens I, II, and III were grown on potato dextrose agar (PDA) (Becton, Dickinson & Co., Heidelberg, Germany) at 23 °C for 6 d in Petri dishes of 9 cm diameter. These subcultures were used for inoculation of the main cultures on PDA. After 10 d of cultivation at 23 °C in the dark, ten fully-grown Petri dishes were extracted, the extracts combined, evaporated to dryness in vacuo, and the residues analysed as described below.
LC-MS analysis
Two QTOF systems, both from Bruker Daltonic (Bremen, Germany) controlled by HyStar v. 3.2 were used. Both instruments were equipped with an orthogonal ESI source, and coupled to a Dionex UltiMate 3000 UHPLC (Dionex, Idstein, Germany).
System 1: high-resolution micrOTOF-Q II mass spectrometer. For separation, an Acclaim 120 C8, 3 μm, 120 Å, 2.1 × 150 mm column (Dionex, Idstein, Germany) at a flow rate of 0.25 ml min−1, and a temperature of 35 °C was used. Eluent A consisted of H2O + 0.1 % FA, eluent B of 95 % MeCN + 0.1 % FA. Subsamples of 10 μl were injected. The column was held at 80 % A/20 % B for 5 min, then a gradient from 20 % B to 100 % over 55 min was applied. Thereafter, the column was held at 100 % B for 15 min, returned to the start conditions in 1 min, and finally equilibrated for 14 min.
Samples were screened for peptaibiotics in the positive ion mode using the following three-step routine procedure: A full scan was recorded from m/z 50 to 3000. This was followed by an in-source collision-induced dissociation (CID) scan from m/z 50 to 2000, recorded at energy of 150 eV. Finally, results of the in-source CID scan were verified by MS/MS experiments on selected precursor ions. For precursors <m/z 1000, a collision energy of 30 eV was applied, precursor ions in the m/z range from 1000 to 1500 were fragmented at a collision energy of 35 eV and precursor ions >m/z 1500 at a collision energy of 40 eV. The isolation width for MS/MS experiments was set to ±1 Da.
System 2: MaXis 3G QTOF mass spectrometer operated at a resolution of 40 000 full width at half maximum (FWHM). An Acquity UPLC® BEH300 C18, 1.7 μm, 2.1 × 150 mm column was used for separation, using H2O + 0.1 % FA (eluent A) and B of 100 % MeCN + 0.1 % FA. The flow rate was set to 0.3 ml min−1 and the temperature to 40 °C. The gradient started with 90 % A/10 % B at the time: 0 min and was changed to 50 % A/50 % B at time: 7 min, then to 30 % A/70 % B at time: 25 min, then raised to 100% B at time: 38 min and held at 100% B until time: 41 min before set to starting conditions from time 42 min to 46 min. Three microlitres were injected. MS were scanned in the m/z range 100–2000. Auto MS with precursor ion-dependent collision energy optimisation was used for fragmentation in the range of 10–65 eV.
Data interpretation was performed using the DataAnalysis v. 4.0 (Build 281) software (Bruker Daltonic, Bremen, Germany). Use of high-resolution ESI mass spectrometry allows the unequivocal sequencing of fragment ion series according to the Roepstorff/Fohlman/Biemann nomenclature. In cases where the isomeric amino acids (Leu/Ile and Val/Iva, respectively) or the corresponding amino alcohols (Leuol/Ileol) with the same elemental composition could not be distinguished, the abbreviations Lxx, Vxx, and Lxxol were used instead (Degenkolb et al. 2006, 2012; Krause et al. 2006).
Results
Screening of basidiomes infected by Hypocrea pulvinata
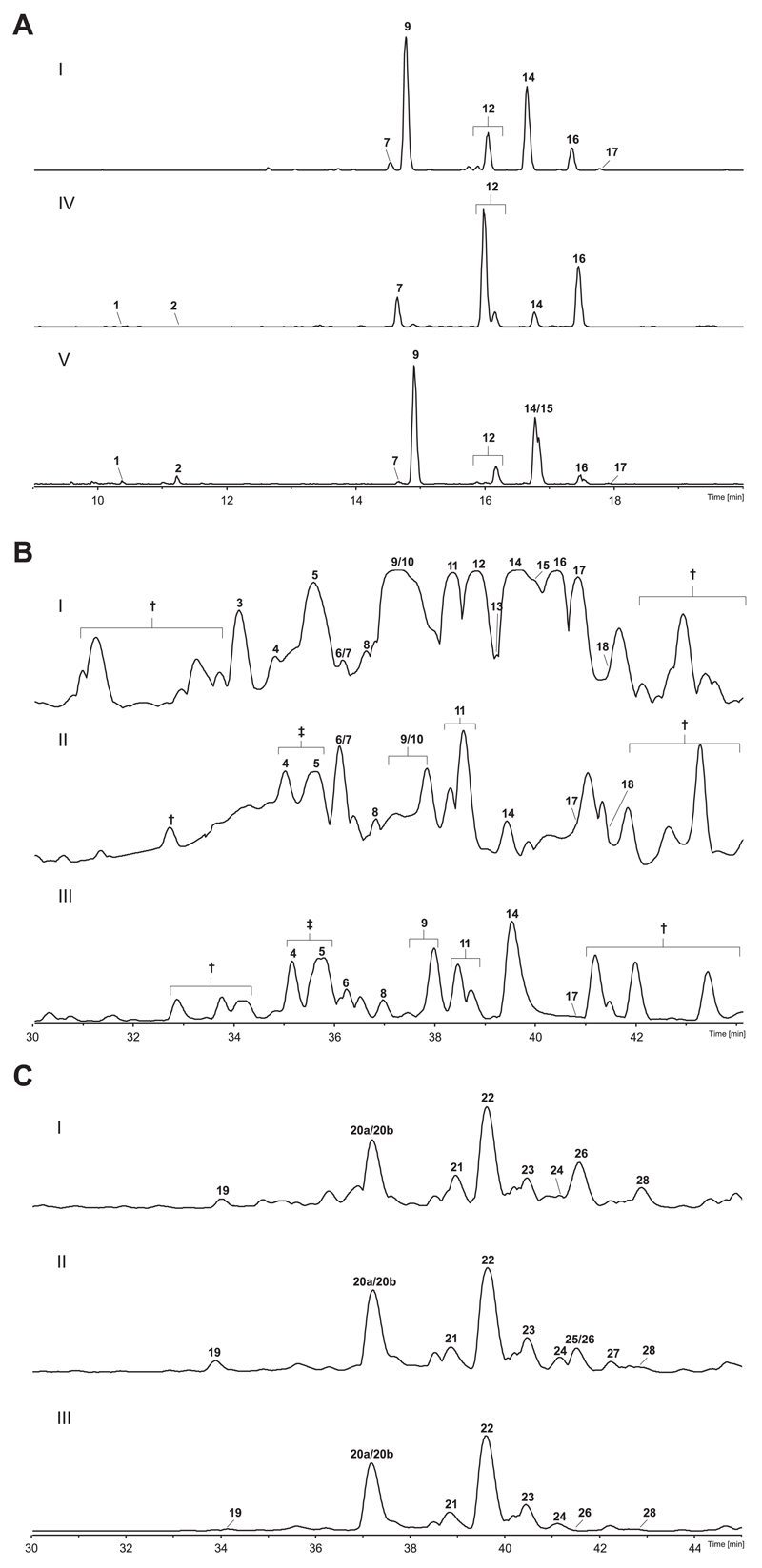
Biomass from the five infected samples investigated (Table 1) was shown to contain intense peaks of novel, especially 19-residue peptaibiotics, which we have named hypopulvins (HPVs). Analysis of two basidiomes from Piptoporus betulinus not infected by Hypocrea did not show any indications of peptaibiotics, thus verifying that they are produced by Hypocrea during infection of the basidiomes (data not shown). A comparison of base peak chromatograms (BPCs) is given in Fig 2A and B.
Fig 2. BPCs of (A) specimens I, IV, and V screened with the maxis TOF; (B) specimens I, II, and III screened with the micrOTOF-Q II; (C) plate cultures I, II, and III screened with the micrOTOF-Q II. † – no peptaibiotics; ‡ – coeluting peptaibiotics.
The peptaibiome of the teleomorph was dominated by 19-residue peptaibols (Tables 2a and 3a, Table S1a), as well as minor 18-residue deletion analogues (compounds 1 and 2), only detected in isolates IV and V.
Table 2a. Sequences of 18- and 19-residue peptaibiotics detected in specimens IeV of Hypocrea pulvinata.
| No. | tR [min] | Residue |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | – | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |||
| 1a | 10.5 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | – |
| 2a | 11.2 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | – |
| 3 | 34.1–34.2 | Ac | Aib | Ala | Ser | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 4 | 34.7–34.9 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Ala | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 5 | 35.6–35.9 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Vxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 6 | 36.2 | Ac | Aib | Ala | Ala | Ser | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 7 | 36.3 | Ac | Aib | Ala | Ala | Ala | Ala | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 8 | 36.7–36.9 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Ala | Gly | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Pheol |
| 9 | 37.2–37.4 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 10 | 37.3–37.5 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Lxxol |
| 11 | 38.3–38.4 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Pheol |
| 12 | 38.7–38.9 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 13 | 39.2 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 14 | 39.5–39.7 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 15 | 39.9 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Lxxol |
| 16 | 40.4–40.5 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| 17 | 40.8–41.1 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Pheol |
| 18 | 41.3–41.6 | Ac | Aib | Ala | Ala | Ala | Vxx | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| No. | Compound identical or positionally isomeric with | References | ||||||||||||||||||||
| 1a | new (deletion sequence of 9) | |||||||||||||||||||||
| 2a | new (deletion sequence of 14, 15, and 16) | |||||||||||||||||||||
| 3 | new (trichosporin B-Ia-[Aib]6) | Iida et al. 1990 | ||||||||||||||||||||
| 4 | new (trichosporin B-IIIa-[Aib]6, [Aib]9 → [Ala]9) | Iida et al. 1990 | ||||||||||||||||||||
| 5 | new (C-terminal pentadecapeptide resembles polysporin A with [Vxx]8 → [Lxx]8, N-terminal: trichosporin B-pentapeptide) | New et al. 1996 | ||||||||||||||||||||
| 6 | new (C-terminal pentadecapeptide resembles trichosporins B-Ia and B-IIIa with [Gly]10 → [Ala]10) | Iida et al. 1990 | ||||||||||||||||||||
| 7 | new (trichosporin B-IIIc-[Aib]6) | Iida et al. 1990 | ||||||||||||||||||||
| 8 | new (C-terminal pentadecapeptide resembles polysporin D with [Aib]9 → [Ala]9, N-terminal: trichosporin B-pentapeptide) | New et al. 1996 | ||||||||||||||||||||
| 9 | new (trichosporin B-IIIa-[Aib]6) | Iida et al. 1990 | ||||||||||||||||||||
| 10 | new (isomer of 9: [Pheol]19 → [Lxxol]19; C-terminal octapeptide found in some of the hypelcins A and stilbolflavins B) | Matsuura et al. 1993; Jaworski & Brückner 2001 | ||||||||||||||||||||
| 11 | new (trichosporin B-IVb-[Aib]6) | Iida et al. 1990 | ||||||||||||||||||||
| 12 | new (cf. 9; trichosporin B-IIIa-[Aib]6) | Iida et al. 1990 | ||||||||||||||||||||
| 13 | new (N-terminal hexadecapeptide: cf. trichosporins B IIIa/IVb; decapeptide [Aib]9-[Gln]18: cf. tricholongin B I) | Iida et al. 1990; Rebuffat et al. 1991 | ||||||||||||||||||||
| 14 | new (trichosporin B-IIIa-[Aib]6, [Gly]10 → [Ala]10) | Iida et al. 1990 | ||||||||||||||||||||
| 15 | new (cf. 14: [Pheol]19 → [Lxxol]19; cf. 10: [Gly]10 → [Ala]10; trichosporin B-IIIa-[Aib]6, [Gly]10 → [Ala]10, [Pheol]19 → [Lxxol]19) | Iida et al. 1990 | ||||||||||||||||||||
| 16 | new (positional isomer of 14) | Iida et al. 1990 | ||||||||||||||||||||
| 17 | new (positional isomer of 18) | |||||||||||||||||||||
| 18 | new (cf. 14: [Aib]5 → [Vxx]5) | |||||||||||||||||||||
Detected in specimens IV and V, only.
Variable residues are underlined. Minor sequence variants are underlined in the individual sequences.
Table 3a. Comparison of the peptaibiotic pattern of specimens I–V.
| Compound |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 2a | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| tR [min] | 10.5 | 11.2 | 34.1–34.2 | 34.7–34.9 | 35.6–35.9 | 36.2 | 36.3 | 36.7–36.9 | 37.2–37.4 | |
| [M + Na]+ | n.d. | n.d. | 1904.0666 | 1874.0586 | 1874.0624 | 1918.0835 | 1874.0788 | 1888.0757 | 1888.0711 | |
| [M + H]+ | 1732.9987 | 1747.0138 | 1882.0893 | 1852.0790 | 1852.0776 | 1896.0999 | 1852.0743 | 1866.0911 | 1866.1009 | |
| I | n.d. | n.d. | + | + | + | + | + | + | + | |
| II | n.d. | n.d. | n.d. | (+) | (+) | + | (+) | + | + | |
| III | n.d. | n.d. | n.d. | n.d. | n.d. | (+) | n.d. | + | + | |
| IVb | (+) | (+) | (+) | (+) | + | (+) | + | n.d. | + | |
| Vb | + | + | (+) | (+) | + | (+) | + | n.d. | + | |
| Compound | ||||||||||
| 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| tR [min] | 37.3–37.5 | 38.3–38.4 | 38.7–38.9 | 39.2 | 39.5–39.7 | 39.9 | 40.4–40.5 | 40.8–41.1 | 41.3–41.6 | |
| [M + Na]+ | 1854.0799 | 1902.0918 | 1888.0783 | 1918.1024 | 1902.0898 | 1868.0893 | 1902.0974 | 1916.1049 | 1916.1051 | |
| [M + H]+ | 1832.1076 | 1880.1072 | 1866.0997 | 1896.1048 | 1880.1187 | 1846.1231 | 1880.1177 | 1894.1248 | 1894.1233 | |
| I | + | + | + | + | (+) | (+) | + | + | + | |
| II | + | + | + | n.d. | n.d. | (+) | + | + | (+) | |
| III | n.d. | + | + | n.d. | n.d. | n.d. | + | (+) | n.d. | |
| IVb | n.d. | + | + | n.d. | + | + | + | (+) | (+) | |
| Vb | + | + | + | + | + | + | + | (+) | n.d. | |
n.d.: not detected.
+: positive.
(+): weakly positive.
Detected by DTU maXis gradient, only.
Interpolated from DTU maXis gradient using higher injection volume.
All compounds display the typical characters of the peptaibol subfamily 1 (SF1: Table 4, Fig 1) the largest one of the nine subfamilies, as introduced by Chugh & Wallace (2001). Accordingly, in HPV’s, one Gln residue is found in position 6, and another two towards the C-terminus, in position 17 and 18. A highly conserved Pro residue is located in position 13 of the peptide chain. Many sequences have a Gly or Ala residue in position 10. Most of the HPV’s terminate in phenylalaninol (Pheol), only two in Lxxol. At least four, at most seven, residues are occupied by an Aib residue. Qualitative differences between the teleomorphs observed with the maXis and the micrOTOF-Q II might be explained by the different screening dates, considering that the peptaibiome of a living specimen is subjected to dynamic changes.
Table 4. Structural variation of 19-residue peptaibiotics produced by the five H. pulvinata isolates investigated.
| Residue |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| Ac | Aib | Ala | Ala | Ala | Aib | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol |
| (Ser)b | (Ser) | (Ala) | (Vxx) | (Ala) | Ala | (Vxx) | (Lxx) | (Vxx) | (Lxxol) | ||||||||||
| (Vxx) | (Ser) | ||||||||||||||||||
Variable positions are underlined.
Minor sequence variations are parenthesised.
Screening of Hypocrea pulvinata plate cultures
Pure agar cultures were only obtained from single ascospores of specimens I, II, and III (Table 1), whereas specimens IV and V did not contain viable ascospores anymore. However, BPCs of the three plate cultures screened are highly similar (Fig 2C). Comparing the peptaibiotic pattern of specimens vs. plate culture (Tables 2b and 3b, Table S1b), 19-residue peptaibols were also found predominantly in the cultures although their microheterogeneity was less pronounced:
Table 2b. Sequences of 11-, 19-, and 20-residue peptaibiotics detected in plate cultures of Hypocrea pulvinata I–III.
| No. | tR [min] | Residue |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | – | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | ||||||
| 19 | 34.0–34.2 | Ac | Aib | Ala | Ala | Ser | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol | |||
| 20a | 37.0–37.2 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Vxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol | |||
| 20b | 37.0–37.3 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Gly | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol | |||
| 21 | 38.9–39.0 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol | |||
| 22 | 39.6–39.7 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol | |||
| 23 | 40.2–40.5 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Aib | Gln | Gln | Pheol | |||
| 24 | 41.0–41.1 | Ac | Aib | Ala | Ala | Ala | Aib | – | Gln | Aib | Lxx | Aib | Ala | Lxx | Aib | Pro | Lxx | Aib | Aib | Gln | Gln | Pheol | |||
| Residue |
|||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||||||
| 25 | 41.3–41.6 | Ac | Aib | Ala | Aib | Ala | Aib | Aib | Gln | Aib | Vxx | Aib | Gly | Lxx | Aib | Pro | Lxx | Aib | Aib | Gln | Gln | Pheol | |||
| 26 | 41.5 | Ac | Aib | Gln | Lxx | Lxx | Aib | Pro | Lxx | Lxx | Aib | Pro | Lxxol | ||||||||||||
| 27 | 42.3–42.5 | Ac | Aib | Ala | Aib | Ala | Aib | Aib | Gln | Aib | Vxx | Aib | Gly | Lxx | Aib | Pro | Lxx | Aib | Aib | Glu | Gln | Pheol | |||
| 28 | 42.9 | Ac | Vxx | Gln | Lxx | Lxx | Aib | Pro | Lxx | Lxx | Aib | Pro | Lxxol | ||||||||||||
| No. | Compound identical or positionally isomeric with | References | |||||||||||||||||||||||
| 19 | new (cf. 6: [Ala]10 → [Gly]10) | ||||||||||||||||||||||||
| 20a | new (trichosporin B-IIId -[Aib]6) | Iida et al. 1990 | |||||||||||||||||||||||
| 20b | new (cf. 9) | ||||||||||||||||||||||||
| 21 | new (cf. 13) | ||||||||||||||||||||||||
| 22 | new (cf. 14) | ||||||||||||||||||||||||
| 23 | new (cf. 14) | ||||||||||||||||||||||||
| 24 | new (cf. 22, [Vxx]14→ [Lxx]14) | ||||||||||||||||||||||||
| 25 | new (trichosporin B-IIId: [Vxx]15 → [Lxx]15) | Iida et al. 1990 | |||||||||||||||||||||||
| 26 | cf. trichorovins XIII, XIV; hypomurocins A-V, A-Va; | Iida et al. 1995; Wada et al. 1995; Becker et al. 1997 | |||||||||||||||||||||||
| trichorozin IV; trichobrachins III: I, J; trichobrachins III: 16a, 17, 18; | Brückner et al. 1993; Krause et al. 2007 | ||||||||||||||||||||||||
| trichobrachins C-1, C-2; Tv29-11-V b | Ruiz et al. 2007; Mukherjee et al. 2011 | ||||||||||||||||||||||||
| 27 | new | ||||||||||||||||||||||||
| 28 | cf. Trichofumin B, Tv29-11-VI, hypojecorin A-22 and A-23 | Berg et al. 2003; Mukherjee et al. 2011 | |||||||||||||||||||||||
| Degenkolb et al. 2012 | |||||||||||||||||||||||||
Variable residues are underlined in the table header. Minor sequence variants are underlined in the individual sequences.
Table 3b. Comparison of the peptaibiotic pattern of plate cultures I–III.
| Compound |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 20a | 20b | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | |
| tR [min] | 34.0–34.2 | 37.0–37.2 | 37.0–37.3 | 38.9–39.0 | 39.6–39.7 | 40.2–40.5 | 41.0–41.1 | 41.3–41.6 | 41.5 | 42.3–42.5 | 42.9 |
| [M + Na]+ | 1904.0757 | n.d. | 1888.0737 | n.d. | 1902.0868 | 1902.0864 | 1916.1030 | 1987.1402 | 1189.7938 | 1988.1451 | 1203.8111 |
| [M + H]+ | 1882.0812 | 1852.0721 | 1866.0909 | 1896.0874 | 1880.1063 | 1880.1065 | 1894.1222 | 1965.1576 | 1211.7767 | 1966.1576 | 1225.7929 |
| I | + | (+) | + | (+) | + | + | + | n.d. | + | n.d. | + |
| II | + | (+) | + | (+) | + | + | + | + | + | + | + |
| III | + | (+) | + | (+) | + | + | + | n.d. | (+) | n.d. | (+) |
The pattern of 19-residue peptaibols was less diverse, and amino acid exchanges were only found in positions 4, 8, 10, and 14 (Table 2b).
In contrast to what has been observed for the specimens, two new 20-residue minor compounds, (25) and (27), representing the building scheme of trichosporins B (Iida et al. 1990, 1993), were detected. Another two minor compounds, 11-residue peptaibols (26) and (28), display the structural characteristics of the growing subfamily 4 (SF4: (Chugh & Wallace 2001; Degenkolb et al. 2012)).
Considerable microheterogeneity, including positional isomerism, of long-chain peptaibiotics was observed in both stromata and cultures: Sequences (4), (5), (7), and (20a) represent positional isomers of m/z 1852.0721 [M + H]+, whereas sequences (8), (9), (12), and (20b) display m/z 1866.0909 ([M + H]+). Five sequences (11), (14), (16), (22), and (23) represent positional isomers of m/z 1880.1064 ([M + H]+); whereas another three (17), (18), and (24) are of m/z 1894.1222 ([M + H]+). Further, Ser-containing positional isomers of m/z 1882.085 [M + H]+ are represented by (3) and (19), whereas sequences (6), (13), and (21) display m/z 1896.0874 [M + H]+.
Discussion
General remarks
The most notable result of this investigation is the unequivocal confirmation of peptaibiotic biosynthesis in the natural habitat of a fungicolous fungus, indicating that these are required for infection. We here present the first example of in vivo-production of peptaibiotics by a fungicolous fungus growing on either of its natural hosts. Considering (i) the ubiquity of both the two host species (ii) the highly specific association between Hypocrea pulvinata and Piptoporus betulinus/Fomitopsis pinicola, and (iii) the abundance of this fungicolous species in north temperate regions of the world including Europe, North America, Japan, and Russia (Jaklitsch 2011), it seems reasonable to postulate a decisive role for the peptaibiotics detected in this study:
They are hypothesised to be one of the mediators of the complex interactions between the basidiomycetous host and its fungicolous ascomycete ‘partner’ (see below).
Twenty-four of the 26 new HPVs produced by H. pulvinata are 19-residue peptaibols, basically representing deletion sequences (Δ Aib6) of the 20-residue trichosporins B (Iida et al. 1990, 1993) and polysporins A–D (New et al. 1996), both isolated from strains of Trichoderma polysporum. This deletion, however, is predicted not to negatively influence the bioactivity of these long-chain peptaibols as all important structural features, which comply with the requirements for the formation of transmembrane ion channels in artificial lipid bilayer membranes, are still present. Generally, 18-, 19-, and 20-residue peptaibiotics display a higher membrane pore formation activity by several orders of magnitude in comparison to smaller peptaibols consisting of less than 17 residues (Grigoriev et al. 2003).
Importance of different structurally conserved amino acid residues for the bioactivity of peptaibiotics – general remarks
Aib and Iva residues strongly promote the formation of helical structures (α- or 310-helices, and even mixed forms) (Toniolo & Benedetti 1991), which is due to the steric constraints imposed by the geminal alkyl groups of the Cα-atom (Chugh & Wallace 2001).
The significance of glutamine residues
Gln residues in position 6 or 7 were postulated to play a key role for ion channel stabilisation (Fox & Richards 1982), as they are located in the pore lumen, lining it together with Gln17–18 and Gly10 and Pro13 carbonyls of the adjoining transmembrane helices (Duclohier 2004). Molle et al. (1996) demonstrated that replacement of Gln7 by Ala7 resulted in complete loss of channel-forming activity, which supports this hypothesis. This phenomenon was explained by the inability of this nonpolar residue to form side-chain H-bonds. Interhelix H-bonds are thought to play a decisive role in the stabilisation of channel-forming helix bundles. This hypothesis was corroborated by the replacement of Gln7 by Asn7, restoring nearly initial functional properties. In contrast, replacement of Gln7 by Ser7 resulted in a significantly reduced voltage dependence and shorter channel lifetime. This was explained by the formation of much weaker H-bonds by the –CH2OH group of Ser7 (Molle et al. 1996).
The decisive role of proline residues
Prolines in position 13 or 14 of the peptide chain create bends of the helical structure, thus affecting the (i) probability of ion channel formation, (ii) lifetime of the channel, and (iii) occurrence of multilevel conductance (Nagaoka et al. 1996).
For the 20-residue alamethicins (ALM) F30, proline in position 14 which corresponds to Pro13 of the 19-residue HPV’s was demonstrated to display optimal channel activity whereas synthetic analogues bearing Pro in positions 11, 12, 13, 15, 16, and 17 formed considerably less stable ion channels (Kaduk et al. 1997). A proline residue in position 14 of ALM F30 is essential for (i) haemolysis of human erythrocytes, (ii) stimulation of catecholamine secretion from bovine adrenal chromaffin cells, and (iii) induction of metabolic activity in bovine aortic endothelial cells (Dathe et al. 1998). Surprisingly, it was reported that Gln7, Gly11, and Pro14 are not essential for channel formation but substitution of any of these residues reduced (i) the number of conductance levels and (ii) significantly decreased their lifetimes (Kaduk et al. 1998).
Role of the C-terminal substituents
Aromatic residues such as the C-terminal Pheol, which are located in the membrane interface between the hydrophilic lipid head groups and the hydrophobic fatty acid chains, have been postulated to stabilise the polypeptide transmembrane segments (Wallace 2000).
The two compounds (10) and (15) carrying a C-terminal Lxxol residue resemble some of the structural properties of two other 20-residue peptaibol mixtures – hypelcins A from stromata of Hypocrea peltata (Fujita et al. 1984a; Matsuura et al. 1993) and stilboflavins B from Stilbella flavipes CBS 146.81 (Jaworski & Brückner 2001). Hypelcins A were shown to (i) act as uncouplers of oxidative phosphorylation in rat liver mitochondria (Takaishi et al. 1980), (ii) exhibit antibacterial and antifungal activity, including growth inhibition of the shiitake mushroom Lentinus edodes, (iii) exert contractile action on guinea pig ileum (Fujita et al. 1984b), and (iv) induce ion channels in planar bilayer lipid membranes. Remarkably, the substitution of Pheol for Leuol and Ileol, respectively, resulted in prolonged open channel lifetime (Koide et al. 1997). Stilboflavins were reported to exhibit weak antibiotic activity against grampositive bacteria and cause haemolysis of sheep erythrocytes (Jaworski & Brückner 2001).
The two 18-residue sequences, compounds (1) and (2), exhibit a deletion of the C-terminal amino alcohol residue. Compared to the amount of the major 19-residue peptaibols, their possible contribution to the postulated bioactivity of the mixture of HPVs is regarded negligible. Despite this, truncated sequences of SF1 peptaibols have been reported before:
19-residue peptaibiotics, trichobrachins I (TB I) from Trichoderma ghanense CBS 936.69 (syn. Trichoderma parceramosum) lacking the C-terminal Pheol residue, were shown to originate from 20-residue trichobrachins II (TB II) by enzymatic degradation (Krause et al. 2007). Two minor desPheol-compounds F30 representing 1.3 % of the ALM mixture from Trichoderma arundinaceum CBS 123793 (formerly known as Trichoderma ‘viride’ NRRL 3199) have been detected by nonaqueous capillary electrophoresis (NACE) coupled to electrospray mass spectrometry (Psurek et al. 2006). Two further 17-residue minor compounds lacking the Gln residue in position 18 are predicted to be present; however, the intensity of their diagnostic CID-fragment ions was too low for sequencing.
Multiple bioactivities of the structurally related trichosporins B
The structurally similar trichosporins B (Fig 1, Tables 2a and b) were isolated from the culture filtrate of Trichoderma polysporum TMI 60146, a destructive mycoparasite of Lentinus edodes (Fujita et al. 1988; Iida et al. 1993). To date, a multitude of biological activities has been reported for trichosporins B, including uncoupling of the respiratory activity of rat liver mitochondria (Fujita et al. 1988; Okuda et al. 1994), Ca2+-dependent catecholamine secretion from bovine adrenal medullary chromaffin cells (Tachikawa et al. 1991, 1995, 1996), formation of voltage-gated ion channels (Nagaoka et al. 1995), and antitrypanosomal activity (Iwatsuki et al. 2010).
Minor, 11-residue peptaibols coproduced by Hypocrea pulvinata
The two 11-residue compounds (26) and (28) are typical representatives of the growing SF4 (Chugh & Wallace 2001; Degenkolb et al. 2012). Apparently, a complex mixture of homologues and positional isomers was detected in the plate culture of H. pulvinata I, whereas only trace amounts were found in isolates II and III. However, 11-residue peptaibols were absent in the teleomorphs. Typical signals, indicative of 11-residue peptaibols, viz. pseudomolecular ions m/z 1147.7/1169.7, 1161.7/1183.7, and 1175.8/1197.8 (all [M + H]+/[M + Na]+) were found. Due to the rather low intensity of these diagnostic ions, which is partly caused by their coelution with major 19-residue peptaibols, only two major peptaibols (26) and (28) could be sequenced (Table 2b, Table S1b). The huge number of positional isomeric SF4 11-residue sequences described to date (Brückner et al. 1993; Krause et al. 2007; Mukherjee et al. 2011; Degenkolb et al. 2012) would require sophisticated methods for chiral sequence analysis (Becker et al. 1997; Jaworski & Brückner 2001) in order to evaluate their novelty or recurrence, respectively. Basically, SF4 11-residue sequences may contribute to the bioactivity of H. pulvinata. The extent of this contribution cannot be evaluated; however, it is regarded as minor because of the comparatively low abundance of these 11-residue peptaibols. Biological activities reported for homologous and/or positional isomeric SF4 11-residue peptaibols comprise induction of voltage-gated ion channels in lipid bilayers (Iida et al. 1995; Wada et al. 1995, 1996), haemolysis of rat erythrocytes (Becker et al. 1997), cytotoxicity towards KB cancer cells (Ruiz et al. 2007), and inhibition of grampositive bacteria (Becker et al. 1997; Berg et al. 2003; Krause et al. 2007).
Thoughts about nonribosomal biosynthesis and module skipping
Only the single anamorphic isolate II has been shown to produce the 20-residue peptaibols (25) and (27), indicating the presence of a respective 20-module nonribosomal peptide synthetase (NRPS). It is thus surprising to detect both 19- and 20-residue peptaibols in this isolate, indicating that a 20-module NRPS may produce 19-residue peptaibols as major products. The exclusive 19-residue content of the other isolates could be interpreted with the deletion of module 6 to a 19-module multienzyme, or by module skipping during peptide elongation. Module skipping could be related to functional properties of this module with respect to binding or processing of Aib intermediates. Likewise Hypocrea pulvinata might harbour 19-module synthetases, while isolate II has a gain of function insertion mutagenesis to exclusively arrive at 20 modules. The 19-module NRPS detected by genomic sequencing in Hypocrea atroviridis had been associated with 19- and 20-residue atroviridins from Trichoderma atroviride (Komon-Zelakowska et al. 2007), but the strain used in that study has later been shown to produce only the 19-residue trichorzianins (Stoppacher et al. 2007), and this NRPS thus has been identified as trichorzianine synthetase. As in 19-residue HPVs, the first Gln of trichorzianins is found in position 6, clearly resulting from a deletion of the 6th module found in a 20-module NRPS synthetase (as paracelsin synthetase, HvD, unpubl. data). We cannot decide on this without knowledge of the respective gene structures of the anamorphs.
Conclusion
Taken these findings together, we dare to predict a mycoparasitic lifestyle of the host-specific polyporicolous2 Hypocrea pulvinata:
It has been demonstrated by in vitro-studies, that chitinases and β-1,3-glucanases act synergistically with peptaibiotics in inhibiting spore germination and hyphal elongation of Botrytis cinerea. In the strain ATCC 36042 (=CBS 391.92), which was originally identified as Trichoderma harzianum (el Hajji et al. 1987) but later shown to belong to Trichoderma atroviride (Kuhls et al. 1996), parallel formation of hydrolytic enzymes and 19-residue antifungal trichorzianins A and B is triggered in the presence of cell walls of plant-pathogenic fungi (Schirmböck et al. 1994). Trichorzianins have previously been shown to form voltage-gated ion channels in planar lipid bilayers (Molle et al. 1987), modify the membrane permeability of liposomes, and they are active against Rhizoctonia solani and Phythophthora cactorum. Based on these results, a model of how peptaibiotics such as trichorzianins and hydrolases interact synergistically was proposed: First, the host cell wall is digested enzymatically; thereafter peptaibiotics will penetrate the cell membrane in order to form ion channels. Cell leakage reduces the ability of the host to effectively repair its cell wall. Eventually, inhibition of chitin and β-glucan synthesis further amplifies the destructive effect of chitinases and β-1,3-glucanases (Lorito et al. 1996). These mechanisms, however, may also account for the recently published induction of programmed cell death in plant fungal pathogens (Shi et al. 2012) caused by the 20-residue peptaibol trichokonin VI (=gliodeliquescin A: (Brückner & Przybylski 1984)), from Trichoderma koningii, Trichoderma pseudokoningii, and Trichoderma (syn. Gliocladium) deliquescens,3 the anamorph of Hypocrea lutea (Jaklitsch 2011). The presence of peptaibiotics was also shown to play a role in the induction of plant defence responses (Viterbo et al. 2007).
The data presented here (i) substantiate the hypothesis of a mycoparasitic lifestyle for H. pulvinata, (ii) show the potential for a possible application as a biological control agent, and (iii) indicate the presence of obviously different HPV synthetases (NRPSs) in isolates I, II, and III.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.funbio.2012.10.003.
Acknowledgements
This study was supported by the Hessian Ministry for Science and Art by a grant from the LOEWE-Schwerpunkt Programme ‘Insect Biotechnology’ to Andreas Vilcinskas. DTU acknowledges the grant from the Danish Research Council (FI 2136-08-0023) for the maXis QTOF system, and MYCORED (EC KBBE-2007-222690-2) for supporting Anita Iversen. Walter M. Jaklitsch is grateful for support by the Austrian Science Fund (project P22081-B17).
We are indebted to Kim N. Kirchhoff (Department of Animal Ecology and Systematics, Systematics and Biodiversity Group, Justus-Liebig University Gießen) for her assistance in translation of a reference in Portuguese.
Abbreviations
- QTOF-MS
quadrupole time-of-flight mass spectrometry
Footnotes
Aib has also been detected in an oatmeal agar plate culture of the polyporicolous Cosmospora berkeleyana CBS 234.70 (syn. Acremonium berkleyanum, syn. A. butyri) isolated from the Tinder Polypore Fomes fomentarius (Brückner et al. 2009).
Gliodeliquescin A was isolated from Gliocladium deliquescens NRRL 1086 (Brückner et al. 1989) and not from NRRL 3091 (Brückner & Przybylski 1984). According to phylogenetic data (18S rRNA, ITS 1 and 2), G. deliquescens NRRL 1086 (=CBS 228.48 = ATCC 10097) has been reidentified as G. viride (www.straininfo.net/strains/260309).
References
- Anonymous, Novembro 2011/Fevereiro. Ministério da agricultura, pecuária e abastecimento (Mapa)/comissão executiva do plano da lavoura cacaueira (Ceplac). Ministério da agricultura aprovou registro do tricovab para combate à vassoura-de-bruxa. Jornal de Cacau. 2012;6:5. [Google Scholar]
- Becker D, Kiess M, Brückner H. Structures of peptaibol antibiotics hypomurocin A and B from the ascomycetous fungus Hypocrea muroiana Hino et Katsumoto. Liebigs Annalen Recueil. 1997:767–772. [Google Scholar]
- Bérdy J. Thoughts and facts about antibiotics: where we are now and where we are heading. Journal of Antibiotics 65: 385–395 Corrigendum. Journal of Antibiotics. 2012;65:441. doi: 10.1038/ja.2012.54. [DOI] [PubMed] [Google Scholar]
- Berek I, Becker A, Schröder H, Härtl A, Höllt V, Grecksch G. Ampullosporin A, a peptaibol from Sepedonium ampullosporum HKI-0053 with neuroleptic-like activity. Behavioural Brain Research. 2009;203:232–239. doi: 10.1016/j.bbr.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Berg A, Grigoriev PA, Degenkolb T, Neuhof T, Härtl A, Schlegel B, Gräfe U. Isolation, structure elucidation and biological activities of trichofumins A, B, C and D, new 11 and 13mer peptaibols from Trichoderma sp. HKI 0276. Journal of Peptide Science. 2003;9:810–816. doi: 10.1002/psc.498. [DOI] [PubMed] [Google Scholar]
- Brückner H, Przybylski M. Methods for the rapid detection, isolation and sequence determination of “peptaibols” and other Aib-containing peptides of fungal origin. I. Gliodeliquescin A from Gliocladium deliquescens. Chromatographia. 1984;19:188–199. [Google Scholar]
- Brückner H, Wunsch P, Kussin C. Production of polypeptide antibiotics by molds of the genus Gliocladium. In: Aubry A, Marraud M, Vitoux B, editors. Second Forum on Peptides. Vol. 174. Colloque INSERM/John Libbey Eurotext Ltd; London: 1989. pp. 103–106. [Google Scholar]
- Brückner H, Kripp T, Kieß M. Polypeptide antibiotics trichovirin and trichobrachin: sequence determination and total synthesis. In: Brandenburg D, Ivanov V, Voelter W, editors. Chemistry of Peptides and Proteins. Proceedings of the 7th USSR–FRG Symposium on Chemistry of Peptides and Proteins, Dilizhan, USSR, September 23–30, 1989, and of the 8th FRG–USSR Symposium on Chemistry of Peptides and Proteins, Aachen, FRG; September 29–October, 3, 1991; Aachen: Mainz Verlag; 1993. pp. 357–373. [Google Scholar]
- Brückner H, Becker D, Gams W, Degenkolb T. Aib and Iva in the biosphere: neither rare nor necessarily extraterrestrial. Chemistry and Biodiversity. 2009;6:38–56. doi: 10.1002/cbdv.200800331. [DOI] [PubMed] [Google Scholar]
- Chugh JK, Wallace BA. Peptaibols: models for ion channels. Biochemical Society Transactions. 2001;29:565–570. doi: 10.1042/bst0290565. [DOI] [PubMed] [Google Scholar]
- Dathe M, Kaduk C, Tachikawa E, Melzig MF, Wenschuh H, Bienert M. Proline at position 14 of alamethicin is essential for hemolytic activity, catecholamine secretion from chromaffin cells and enhanced metabolic activity in endothelial cells. Biochimica et Biophysica Acta. 1998;1370:175–183. doi: 10.1016/s0005-2736(97)00260-5. [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Brückner H. Peptaibiomics: towards a myriad of bioactive peptide containing Cα-dialkylamino acids? Chemistry and Biodiversity. 2008;5:1817–1843. doi: 10.1002/cbdv.200890171. [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Gräfenhan T, Berg A, Nirenberg HI, Gams W, Brückner H. Peptaibiomics: screening for polypeptide antibiotics (peptaibiotics) from plant-protective Trichoderma species. Chemistry and Biodiversity. 2006;3:593–610. doi: 10.1002/cbdv.200690063. [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Karimi Aghcheh R, Dieckmann R, Neuhof T, Baker SE, Druzhinina IS, Kubicek CP, Brückner H, von Döhren H. The production of multiple small peptaibol families by single 14-module peptide synthetases in Trichoderma/Hypocrea. Chemistry and Biodiversity. 2012;9:499–535. doi: 10.1002/cbdv.201100212. [DOI] [PubMed] [Google Scholar]
- Demain AL, Sanchez S. Microbial drug discovery: 80 years of progress. Journal of Antibiotics. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclohier H. Helical kink and channel behaviour: a comparative study with the peptaibols alamethicin, trichotoxin and antiamoebin. European Biophysics Journal. 2004;33:169–174. doi: 10.1007/s00249-003-0383-y. [DOI] [PubMed] [Google Scholar]
- Elander RP. Industrial production of β-lactam antibiotics. Applied Microbiology and Biotechnology. 2003;61:385–392. doi: 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- Elsila JE, Callahan MP, Glavin DP, Dworkin JP, Brückner H. Distribution and stable isotopic composition of amino acids from fungal peptaibiotics: assessing the potential for meteoritic contamination. Astrobiology. 2011;11:123–133. doi: 10.1089/ast.2010.0505. [DOI] [PubMed] [Google Scholar]
- Fox RO, Jr, Richards FM. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-Å resolution. Nature. 1982;300:325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Fujita T, Takaishi Y, Ogawa T, Tokimoto K. Fungal metabolites. 1. Isolation and biological activities of hypelcins A and B (growth inhibitors against Lentinus edodes) from Hypocrea peltata. Chemical and Pharmaceutical Bulletin. 1984a;32:1822–1828. [Google Scholar]
- Fujita T, Takaishi Y, Matsuura K, Takeda Y, Yoshioka Y, Brückner H. Further investigation of peptide antibiotic hypelcin A: isolation and structures of hypelcins A-I, A-II, A-III, and A-IV. Chemical and Pharmaceutical Bulletin. 1984b;32:2870–2873. doi: 10.1248/cpb.32.2870. [DOI] [PubMed] [Google Scholar]
- Fujita T, Iida A, Uesato S, Takaishi Y, Shingu T, Saito M, Morita M. Structural elucidation of trichosporin-B-Ia, IIIa, IIId, and V from Trichoderma polysporum. Journal of Antibiotics. 1988;41:814–818. doi: 10.7164/antibiotics.41.814. [DOI] [PubMed] [Google Scholar]
- Grigoriev PA, Schlegel B, Kronen M, Berg A, Härtl A, Gräfe U. Differences in membrane pore formation by peptaibols. Journal of Peptide Science. 2003;9:763–768. doi: 10.1002/psc.502. [DOI] [PubMed] [Google Scholar]
- el Hajji M, Rebuffat S, Lecommandeur D, Bodo B. Isolation and sequence determination of trichorzianines A, antifungal peptides from Trichoderma harzianum. International Journal of Peptide and Protein Research. 1987;29:207–215. doi: 10.1111/j.1399-3011.1987.tb02247.x. [DOI] [PubMed] [Google Scholar]
- Hanada RE, Pomella AWV, Soberanis W, Loguercio LL, Pereira JO. Biocontrol potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora) of cacao. Biological Control. 2009;50:143–149. [Google Scholar]
- Holmes KA, Schroers H-J, Thomas SE, Evans HC, Samuels GJ. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycological Progress. 2004;3:199–210. [Google Scholar]
- Hosotani N, Kumagai K, Honda S, Ito A, Shimatani T, Saji I. SPF-5506-A4, a new peptaibol inhibitor of amyloid β-peptide formation produced by Trichoderma sp. Journal of Antibiotics. 2007;60:184–190. doi: 10.1038/ja.2007.20. [DOI] [PubMed] [Google Scholar]
- Iida A, Okuda M, Uesato S, Takaishi Y, Shingu T, Morita M, Fujita T. Fungal metabolites. Part 3. Structural elucidation of antibiotic peptides, trichosporin-B-lllb, -lllc, -IVb, -IVc, -IVd, -Vla and -Vlb from Trichoderma polysporum. Application of fast-atom bombardment mass spectrometry/mass spectrometry to peptides containing a unique Aib-Pro peptide bond. Journal of the Chemical Society, Perkin Transactions. 1990;1:3249–3255. [Google Scholar]
- Iida J, Iida A, Takahashi Y, Takaishi Y, Nagaoka Y, Fujita T. Fungal metabolites. Part 5. Rapid structure elucidation of antibiotic peptides, minor components of trichosporin Bs from Trichoderma polysporum. Application of linked-scan and continuous-flow fast-atom bombardment mass spectrometry. Journal of the Chemical Society, Perkin Transactions. 1993;1:357–365. [Google Scholar]
- Iida A, Sanekata M, Wada S-I, Fujita T, Tanaka H, Enoki A, Fuse G, Kanai M, Asami K. Fungal metabolites. XVIII. New membrane-modifying peptides, trichorozins I–IV, from the fungus Trichoderma harzianum. Chemical and Pharmaceutical Bulletin. 1995;43:392–397. doi: 10.1248/cpb.43.392. [DOI] [PubMed] [Google Scholar]
- Iwatsuki M, Kinoshita Y, Niitsuma M, Hashida J, Mori M, Ishiyama A, Namatame M, Nishihara-Tsukashima A, Nonaka K, Masuma R, Otoguro K, et al. Antitrypanosomal peptaibiotics, trichosporins B-VIIa and B-VIIb, produced by Trichoderma polysporum FKI-4452. Journal of Antibiotics. 2010;63:331–333. doi: 10.1038/ja.2010.41. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. European species of Hypocrea part I. The green-spores species. Studies in Mycology. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM. European species of Hypocrea part II: species with hyaline ascospores. Fungal Diversity. 2011;48:1–250. doi: 10.1007/s13225-011-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. Hypocrea britdaniae and H. foliicola: two remarkable new European species. Mycologia. 2012;104:925–941. doi: 10.3852/11-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:1213–1221. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A, Brückner H. Sequences of polypeptide antibiotics stilboflavins, natural peptaibols libraries of the mold Stilbella flavipes. Journal of Peptide Science. 2001;7:433–447. doi: 10.1002/psc.335. [DOI] [PubMed] [Google Scholar]
- Kaduk C, Duclohier H, Dathe M, Wenschuh H, Beyermann M, Molle G, Bienert M. Influence of proline position upon the channel activity of alamethicin. Biophysical Journal. 1997;72:2151–2159. doi: 10.1016/S0006-3495(97)78858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaduk C, Dathe M, Bienert M. Functional modifications of alamethicin ion channels by substitution of glutamine 7, glycine 11 and proline 14. Biochimica et Biophysica Acta. 1998;1373:137–146. doi: 10.1016/s0005-2736(98)00100-x. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. Dictionary of the Fungi. 10th edn. CABI International; Wallingford: 2008. [Google Scholar]
- Koide N, Asami K, Fujita T. Ion-channels formed by hypelcins, antibiotic peptides, in planar bilayer lipid membranes. Biochimica et Biophysica Acta. 1997;1326:47–53. doi: 10.1016/s0005-2736(97)00005-9. [DOI] [PubMed] [Google Scholar]
- Komon-Zelakowska M, Neuhof T, Dieckmann R, von Döhren H, Herrera-Estrella A, Kubicek CP, Druzhinina IS. Formation of atroviridin by Hypocrea atroviridis is conidiation associated and positively regulated by blue light and the G-protein GNA3. Eukaryotic Cell. 2007;6:2332–2342. doi: 10.1128/EC.00143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, Kirschbaum J, Brückner H. Peptaibiomics: an advanced, rapid and selective analysis of peptaibiotics/peptaibols by SPE/LC-ES-MS. Amino Acids. 2006;30:435–443. doi: 10.1007/s00726-005-0275-9. [DOI] [PubMed] [Google Scholar]
- Krause C, Kirschbaum J, Brückner H. Peptaibiomics: microheterogeneity, dynamics, and sequences of trichobrachins, peptaibiotics from Trichoderma parceramosum BISSETT (T. longibrachiatum RIFAI) Chemistry and Biodiversity. 2007;4:1083–1102. doi: 10.1002/cbdv.200790098. [DOI] [PubMed] [Google Scholar]
- Kuhls K, Lieckfeldt E, Samuels GJ, Kovacs W, Meyer W, Petrini O, Gams W, Börner T, Kubicek CP. Molecular evidence that the asexual industrial fungus Trichoderma reesei is a clonal derivative of the ascomycete Hypocrea jecorina. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7755–7760. doi: 10.1073/pnas.93.15.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr N-A, Meffert A, Antelo L, Sterner O, Anke H, Weber RWS. Antiamoebins, myrocin B and the basis of antifungal antibiosis in the coprophilus fungus Stilbella erythrocephala (syn. S. fimetaria) FEMS Microbial Ecology. 2006;55:106–112. doi: 10.1111/j.1574-6941.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- Lorito M, Farkas V, Rebuffat S, Bodo B, Kubicek CP. Cell wall synthesis is a major target of mycoparasitic antagonism by Trichoderma harzianum. Journal of Bacteriology. 1996;178:6382–6385. doi: 10.1128/jb.178.21.6382-6385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhang D-D, Dong X-W, Zhao P-B, Chen L-L, Song X-Y, Wang X-J, Chen X-L, Shi M, Zhang Y-Z. Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against tobacco mosaic virus. FEMS Microbiology Letters. 2010;313:120–126. doi: 10.1111/j.1574-6968.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- Maddau L, Cabras A, Franceschini A, Linaldeddu BT, Crobu S, Roggio T, Pagnozzi D. Occurrence and characterization of peptaibols from Trichoderma citrinoviride, an endophytic fungus of cork oak, using electrospray ionization quadrupole time-of-flight mass spectrometry. Microbiology. 2009;155:3371–3381. doi: 10.1099/mic.0.030916-0. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Yesilada A, Iida A, Takaishi Y, Kanai M, Fujita T. Fungal metabolites. Part 8. Primary structures of antibiotic peptides, hypelcin A-I, A-Il, A-III, A-IV, A-V, A-VI, A-VII, A-VIII and A-IX from Hypocrea peltata. Journal of the Chemical Society, Perkin Transactions. 1993;1:381–387. [Google Scholar]
- Matsuura K, Shima O, Takeda Y, Takaishi Y, Nagaoka Y, Fujita T. Fungal metabolites. XV. Primary structures of antibiotic peptides, hypelcins B-I, B-II, B-III, B-IV and B-V, from Hypocrea peltata. Application of electrospray mass spectrometry and electrospray mass spectrometry/mass spectrometry. Chemical and Pharmaceutical Bulletin. 1994;42:1063–1069. doi: 10.1248/cpb.42.1063. [DOI] [PubMed] [Google Scholar]
- Menestrina G, Voges K-P, Jung G, Boheim G. Voltage-dependent channel formation by rods of helical polypeptides. Journal of Membrane Biology. 1986;93:111–132. doi: 10.1007/BF01870804. [DOI] [PubMed] [Google Scholar]
- Molle G, Duclohier H, Spach G. Voltage-dependent and multi-state ionic channels induced by trichorzianines, antifungal peptides related to alamethicin. FEBS Letters. 1987;224:208–212. doi: 10.1016/0014-5793(87)80449-0. [DOI] [PubMed] [Google Scholar]
- Molle G, Dugast J-Y, Spach G, Duclohier H. Ion channel stabilization of synthetic alamethicin analogs by rings of inter-helix H-bonds. Biophysical Journal. 1996;70:1669–1675. doi: 10.1016/S0006-3495(96)79729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Wiest A, Ruiz N, Keightley A, Moran-Diez ME, McCluskey K, Pouchus YF, Kenerley CM. Two classes of new peptaibols are synthesized by a single non-ribosomal peptide synthetase of Trichoderma virens. Journal of Biological Chemistry. 2011;286:4544–4554. doi: 10.1074/jbc.M110.159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka Y, Iida A, Kambara T, Tachikawa E, Asami K, Fujita T. Effect of lipophilicity of trichosporin-Bs on ion-channel formation and catecholamine-releasing activity. Biological and Pharmaceutical Bulletin. 1995;18:640–642. doi: 10.1248/bpb.18.640. [DOI] [PubMed] [Google Scholar]
- Nagaoka Y, Iida A, Kambara T, Asami K, Tachikawa E, Fujita T. Role of proline-residue in the channel-forming and catecholamine-releasing activities of the peptaibol, trichosporin-B-VIa. Biochimica et Biophysica Acta. 1996;1283:31–36. doi: 10.1016/0005-2736(96)00070-3. [DOI] [PubMed] [Google Scholar]
- New AP, Eckers C, Haskins NJ, Neville WA, Elson S, Hueso-Rodríguez JA, Rivera-Sagredo A. Structures of polysporins A–D, four new peptaibols isolated from Trichoderma polysporum. Tetrahedron Letters. 1996;37:3039–3042. [Google Scholar]
- Okuda M, Iida A, Uesato S, Nagaoka Y, Fujita T, Takaishi Y, Terada H. Fungal metabolites. X. The effect of peptide antibiotics, trichosporin-Bs, on the respiratory activity of mitochondria. Biological and Pharmaceutical Bulletin. 1994;17:482–485. doi: 10.1248/bpb.17.482. [DOI] [PubMed] [Google Scholar]
- Pomella AWV, Jorge de Souza T, Niella GR, Bateman RP, Hebbar PK, Loguercio LL, Lumsden DR. Trichoderma stromaticum for management of witches’ broom in Brazil. In: Vincent C, Goettel MS, Lazarovits G, editors. Biological Control: a global perspective. CABI International; Wallingford/AAFC: 2007. pp. 210–217. [Google Scholar]
- Psurek A, Neusüß C, Degenkolb T, Brückner H, Balaguer E, Imhof D, Scriba GKE. Detection of new amino acid sequences of alamethicins F30 by nonaqueous capillary electrophoresis-mass spectrometry. Journal of Peptide Science. 2006;12:279–290. doi: 10.1002/psc.720. [DOI] [PubMed] [Google Scholar]
- Rebuffat S, Prigent Y, Auvin-Guette C, Bodo B. Tricholongins BI and BII, 19-residue peptaibols from Trichoderma longibrachiatum. Solution structure from two-dimensional NMR spectroscopy. European Journal of Biochemistry. 1991;201:661–674. doi: 10.1111/j.1432-1033.1991.tb16327.x. [DOI] [PubMed] [Google Scholar]
- Ruiz N, Wielgosz-Colin G, Poirier L, Grovel O, Petit KE, Mohamed-Benkada M, Robiou du Pont T, Bissett J, Vérité P, Barnathan G, Pouchus YF. New trichobrachins, 11-residue peptaibols from a marine strain of Trichoderma longibrachiatum. Peptides. 2007;28:1351–1358. doi: 10.1016/j.peptides.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Ismaiel A. Hypocrea peltata: a mycological Dr Jekyll and Mr Hyde? Mycologia. 2011;103:616–630. doi: 10.3852/10-227. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Dodd S, Lu B-S, Petrini O, Schroers H-J, Druzhinina IS. The Trichoderma koningii aggregate species. Studies in Mycology. 2006a;56:67–133. doi: 10.3114/sim.2006.56.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ, Suarez C, Solis K, Holmes KA, Thomas SE, Ismaiel A, Evans HC. Trichoderma theobromicola and T. paucisporum: two new species isolated from cacao in South America. Mycological Research. 2006b;110:381–392. doi: 10.1016/j.mycres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Ismaiel A, de Souza J, Chaverri P. Trichoderma stromaticum and its overseas relatives. Mycological Progress. 2012a;11:215–254. [Google Scholar]
- Samuels GJ, Ismaiel A, Mulaw TB, Szakacs G, Druzhinina IS, Kubicek CP, Jaklitsch WM. The Longibrachiatum clade of Trichoderma: a revision with new species. Fungal Diversity. 2012b;55:77–108. doi: 10.1007/s13225-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmböck M, Lorito M, Wang Y-L, Hayes CK, Arisan-Atac I, Scala F, Harman GE, Kubicek CP. Parallel formation and synergism of hydrolytic enzymes and peptaibols antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Applied and Environmental Microbiology. 1994;60:4364–4370. doi: 10.1128/aem.60.12.4364-4370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Wang H-N, Xie S-T, Luo Y, Sun C-Y, Chen X-L, Zhang Y-Z. Antimicrobial peptaibols, novel suppressors of tumor cells, targeted calcium-mediated apoptosis and autophagy in human hepatocellular carcinoma cells. Molecular Cancer. 2010;9:26. doi: 10.1186/1476-4598-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Chen L, Wang X-W, Zhang T, Zhao P-B, Song X-Y, Sun C-Y, Chen X-L, Zhou B-C, Zhang Y-Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology. 2012;158:166–175. doi: 10.1099/mic.0.052670-0. [DOI] [PubMed] [Google Scholar]
- Singh BS, Herath K, Guan Z, Zink DL, Dombrowski AW, Polishook JD, Silverman KC, Lingham RB, Felock PJ, Hazuda DJ. Integramides A and B, two novel non-ribosomal linear peptides containing nine Cα-methyl amino acids produced by fungal fermentations that are inhibitors of HIV-1 integrase. Organic Letters. 2002;4:1431–1434. doi: 10.1021/ol025540a. [DOI] [PubMed] [Google Scholar]
- Stoppacher R, Reithner B, Omann M, Zeilinger S, Krska R, Schuhmacher R. Profiling of trichorzianins in culture samples of Trichoderma atroviride by liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21:3963–3970. doi: 10.1002/rcm.3301. [DOI] [PubMed] [Google Scholar]
- Tachikawa E, Nogimori K, Takahashi S, Mizuma K, Itoh K, Kashimoto T, Nagaoka Y, Iida A, Fujita T. Pathway for Ca2+ influx into cells by trichosporin-B-VIa, an α-aminoisobutyric acid-containing peptide, from the fungus Trichoderma polysporum. Biochimica et Biophysica Acta. 1996;1282:140–148. doi: 10.1016/0005-2736(96)00052-1. [DOI] [PubMed] [Google Scholar]
- Tachikawa E, Takahashi S, Mizuma K, Kadhimoto T, Nagaoka Y, Iida A, Fujita T. Properties of trichosporon-B-VIa-induced catecholamine secretion from bovine adrenal chromaffin cells. Biological and Pharmaceutical Bulletin. 1995;18:1165–1167. doi: 10.1248/bpb.18.1165. [DOI] [PubMed] [Google Scholar]
- Tachikawa E, Takahashi S, Furumachi K, Kashimoto T, Iida A, Nagaoka Y, Fujita T, Takaishi Y. Trichosporin-B-III, an α-aminoisobutyric acid-containing peptide, causes Ca2+-dependent catecholamine secretion from adrenal medullary chromaffin cells. Molecular Pharmacology. 1991;40:790–797. [PubMed] [Google Scholar]
- Takaishi T, Terada H, Fujita T. The effect of two new peptide antibiotics, the hypelcins, on mitochondrial function. Experientia. 1980;36:550–552. doi: 10.1007/BF01965794. [DOI] [PubMed] [Google Scholar]
- Toniolo C, Benedetti E. The polypeptide 310-helix. Trends in Biochemical Sciences. 1991;16:350–353. doi: 10.1016/0968-0004(91)90142-i. [DOI] [PubMed] [Google Scholar]
- Viterbo A, Wiest A, Brotman Y, Chet I, Kenerley C. The 18mer peptaibols from Trichoderma virens elicit plant defence responses. Molecular Plant Pathology. 2007;8:737–746. doi: 10.1111/j.1364-3703.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Wada S-I, Iida A, Akimoto N, Kanai M, Toyama N, Fujita T. Fungal metabolites. XIX. Structural elucidation of channel-forming peptides, trichorovins I–XIV, from the fungus Trichoderma viride. Chemical and Pharmaceutical Bulletin. 1995;43:910–915. doi: 10.1248/cpb.43.910. [DOI] [PubMed] [Google Scholar]
- Wada S-I, Iida A, Asami K, Fujita T. Ion-channel forming property of trichorovin-XII, an 11-residue peptaibols from the fungus Trichoderma viride, in planar lipid bilayer membranes. Bioorganic and Medical Chemistry Letters. 1996;6:2275–2278. [Google Scholar]
- Wallace BA. Common structural features in gramicidin and other ion channels. BioEssays. 2000;22:227–234. doi: 10.1002/(SICI)1521-1878(200003)22:3<227::AID-BIES4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.